Abstract
BACKGROUND
Recent studies on GTPases have suggested that reduced Duo and Cdc42 transcript expression is involved in dendritic spine loss in schizophrenia. In murine models, Duo and Cdc42 phosphorylate PAK1, which modifies the activity of regulatory myosin light chain (MLC) and cofilin by altering their phosphorylation. Therefore, we hypothesized that in schizophrenia abnormal Duo and Cdc42 expression result in changes in MLC and/or cofilin phosphorylation, which may alter actin cytoskeleton dynamics underlying dendritic spine maintenance.
METHODS
We performed Western blot protein expression analysis in postmortem brains from patients diagnosed with schizophrenia and a comparison group. We focused our studies in the anterior cingulate cortex (ACC) (n=33 comparison group; n=36 schizophrenia) and dorsolateral prefrontal cortex (DLPFC) (n=29 comparison group; n=35 schizophrenia).
RESULTS
In both ACC and DLPFC, we found a reduction of Duo expression and PAK1 phosphorylation in schizophrenia. Cdc42 protein expression was decreased in ACC, but not in DLPFC. In ACC, we observed decreased PAK1 phosphorylation and increased MLC (pMLC) phosphorylation, while in DLPFC pMLC remained unchanged.
DISCUSSION
These data suggest a novel mechanism that may underlie dendritic spine loss in schizophrenia. The increase in pMLC seen in ACC may be associated with dendritic spine shrinkage. The lack of an effect on pMLC in DLPFC suggests that in schizophrenia PAK1 downstream pathways are differentially affected in these cortical areas.
Keywords: kalirin, GTPases, cofilin, LIMK, cytoskeleton, dendritic spines
Introduction
Schizophrenia is a chronic psychiatric illness that affects approximately 1% of the world’s population. A consistent finding in postmortem brain in patients with schizophrenia is a reduction in dendritic spine density (1–4), although the cause of dendritic spine loss remains unknown. One hypothesis proposes abnormalities of glutamatergic pathway connectivity in schizophrenia. Decreased presynaptic glutamatergic input may contribute to NMDA receptor hypofunction and cytoskeletal reorganization, which could in turn result in reduced number of dendritic spines. Supporting this model, several proteins that stabilize the actin cytoskeleton, including Reelin, Fragile X mental retardation protein (FMRP) and disrupted in schizophrenia-1 (DISC-1) have been shown to be abnormally expressed in the brain in schizophrenia (5–7). Because actin filament remodeling is critical for dendritic spine formation, maintenance and plasticity (8), understanding the molecular pathways that can regulate actin cytoskeleton dynamics may permit the elucidation of the mechanism of dendritic spine loss in schizophrenia.
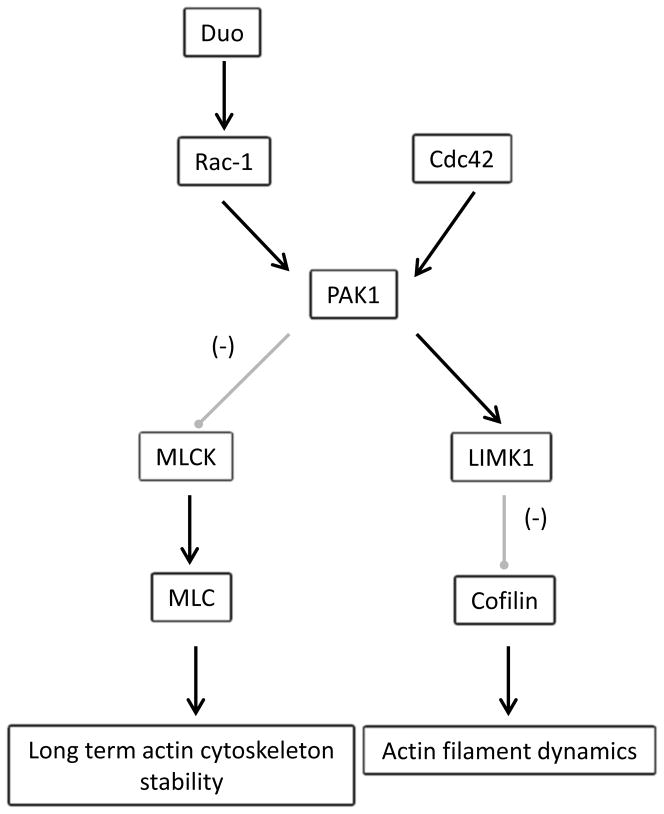
Small GTP binding proteins of the Rho family, such as Rac-1 and Cdc42, are known to regulate actin dynamics and newly formed actin filament stability (9). Recently, it has been reported that calcium influx through the NMDA receptor activates Duo (murine Kalirin-7), a Rho guanine nucleotide exchange factor (GEF) that directly regulates Rac-1 activity, acting as a linking factor between synaptic proteins and the cytoskeleton (10). A pathway downstream of Duo involves activation of PAK1, a regulator of actin cytoskeleton dynamics, and provides a possible mechanistic link to synaptic activity and circuit plasticity, both of which are abnormal in schizophrenia (7, 9, 11). Supporting the suggestion that Duo may underlie some abnormalities seen in schizophrenia, Kalirin-7 (Kal-7) knockout mice have dendritic spine loss and behavioral abnormalities felt to be similar to those seen in this illness (12, 13), and postmortem brain studies have found decreased mRNA expression of Duo and Cdc42 in DLPFC in schizophrenia (14). Interestingly, both Duo and Cdc42 activate PAK1 (15), leading to cytoskeletal rearrangement through two downstream pathways. PAK1 stabilizes actin filament dynamics through its interaction with LIMK1 and downstream inhibition of cofilin, an actin depolymerization agent (9). PAK1 also inhibits myosin light chain kinase (MLCK), an enzyme that specifically phosphorylates MLC (16). MLC phosphorylation has been shown to be critical for long term structural actin cytoskeleton stability, which underlies molecular processes leading to long term memory consolidation (17) (Fig. 1).
Figure 1.
Duo/Cdc42 pathways. NMDA receptor activity drives calcium influx into the dendritic spine, which in turn activates CAMKII which can phosphorylate Duo. Activated Duo drives Rac-1 mediated phosphorylation of PAK1. Cdc42 activity also results in PAK1 phosphorylation in a Rac-1 independent pathway. Activated PAK1 has opposite effects on different downstream pathways: it inhibits MLCK in turn decreasing phosphorylation of MLC, but also activates LIMK which inhibits cofilin, an actin depolymerizing agent. CAMKII: Calcium/calmodulin dependent kinase II, Rac-1: Ras-related C3 botulinum toxin substrate 1, Cdc42: cell division cycle 42. PAK1: p-21 activated kinase 1, MLCK: myosin light chain kinase, MLC: regulatory myosin light chain. LIMK1: LIM domain kinase.
In this study, given previous findings of dendritic spine abnormalities in schizophrenia, we sought to explore molecular changes that might underlie this observation. Accordingly, we focused on potential disruption of the PAK1 pathway in schizophrenia. We assayed by Western blot analysis proteins associated with this pathway in the DLPFC and ACC from subjects with schizophrenia and a comparison group. We found that the Duo/PAK1 pathway is disrupted in the ACC and DLPFC in schizophrenia, suggesting that abnormal regulation of this pathway may have a direct effect on cytoskeletal proteins which could underlie dendritic spine loss in this illness.
Methods and materials
Subjects, tissue acquisition and preparation
Samples from the ACC and DLPFC were obtained from the Mount Sinai Medical Center brain collection, as previously described (18) (Table 1). The same subjects were used in both DLPFC and ACC studies, except 5 additional subjects were available for study in the ACC experiments.
Table 1.
Subjects.
| DLPFC | ACC | |||
|---|---|---|---|---|
| Comparison | Schizophrenia | Comparison | Schizophrenia | |
| n | 29 | 35 | 33 | 36 |
| F/M | 17/12 | 11/24 | 19/14 | 11/25 |
| Age | 78.1 ± 2.7 | 74.4 ± 2.0 | 77.8 ± 2.4 | 74.2 ± 1.9 |
| pH | 6.43 ± 0.05 | 6.37 ± 0.05 | 6.43 ± 0.04 | 6.38 ± 0.05 |
| PMI (h) | 8.2 ± 1.3 | 12.6 ± 1.1 | 8.3 ± 1.2 | 13.4 ± 1.3 |
| On/Off Rx | 0/29 | 24/11 | 0/33 | 25/11 |
ACC: Anterior cingulate cortex, DLPFC: Dorsolateral prefrontal cortex. F: female, M: male, PMI (h): Postmortem interval in hours. Off medication indicates patients that had not received antipsychotic medications for 6 weeks or more at the time of death.
Briefly, patients diagnosed with schizophrenia using DSM-III-R criteria (19) were recruited prospectively. Each patient had a documented history of psychotic symptoms before the age of 40, and at least 10 years of hospitalization with a diagnosis of schizophrenia made by two clinicians. The subjects were evaluated using multiple instruments and clinical assessments including NINCDS-AIREN criteria for vascular dementia; NINCDS, DSMIV and CERAD for dementia; consensus criteria for a clinical diagnosis of probable or possible diffuse Lewy body disease; UPDRS for Parkinson’s disease; clinical assessment for frontotemporal dementia; medical history for psychiatric illnesses; history of drug or alcohol abuse; and tests of cognition including the MMSE and CDR. In addition, each brain was examined neuropathologically by systematized macro- and microscopic evaluation using CERAD guidelines. The subjects studied did not show sufficient neuropathological evidence to meet criteria for neurodegenerative disorders including Alzheimer’s disease (20). Comparison subjects were also evaluated for and free from psychiatric illnesses, history of substance abuse and neurodegenerative disorders. Exclusion criteria included substance abuse, suicide, or coma for more than six hours before death. Next of kin consent to perform an autopsy on the body and brain for diagnostics and research purposes was obtained for each subject.
At time of autopsy, grey matter from DLPFC (area 9) and ACC (area 32) of the left hemisphere was dissected. The tissue was pulverized using small amounts of liquid nitrogen and stored at −80°C. Samples were then reconstituted and homogenized in 5mM Tris-HCl, pH 7.4, 0.32M sucrose and a protease inhibitor tablet (Complete Mini, Roche Diagnostics, Manheim Germany) using a Power Gen 125 homogenizer (Thermo Fisher Scientific, Rockford, Illinois, USA). The homogenate was assayed for protein concentration using a BCA protein assay kit (Thermo Fisher Scientific, Rockford, Illinois, USA) and stored at −80°C until use. In some experiments, not every subject was available for study.
Rodent antipsychotic drug treatment
Male Sprague-Dawley rats (250g) were treated with haloperidol decanoate for 9 months. Rats were housed in pairs and injected intramuscularly every three weeks, for a total of 12 injections, with vehicle (sesame oil) or 28.5mg/kg of haloperidol decanoate in sesame oil. This dose was chosen based on previous reports in the literature (21–24). Rats were sacrificed by decapitation and the brains were immediately removed, dissected on wet ice, and the left anterior cortex was collected and stored at −80°C until it was prepared for Western blot analysis as described above. Ten haloperidol treated and ten control rats were used for each experiment. These experiments were carried out according to UAB guidelines and all procedures complied with IACUC regulations.
Western blot analysis
Western blot analyses were performed as previously described (18, 25). Briefly, samples were diluted in ultrapure water and a reducing buffer to a concentration of 20μg of protein per 10 μl and denatured at 70°C for 10 minutes. For each subject, 20μg total protein per lane was loaded in duplicate into a 4–12% gradient polyacrylamide bis-tris gel (Invitrogen, Carlsbad, California). Subjects were randomly distributed among blots. The antibody concentration and the amount of protein loaded were optimized for each protein to ensure that detection was within the linear range of the assay and that the primary antibody was present in excess. The coefficient of variation for each assay ranged from 6.5 to 26% (Table S1 in the Supplement).
After electrophoresis, samples were transferred to PVDF membranes using Bio-Rad semi-dry transblotters (Hercules, California). Membranes were blocked with Li-Cor blocking buffer (Lincoln, Nebraska) for total proteins or 5% bovine serum albumin (BSA) in phosphate buffer solution (PBS) for phospho-proteins for one hour at room temperature. Blots were then probed with primary antisera diluted in Li-Cor or 1% BSA buffer (Table S1 in the Supplement) overnight at 4°C, except for loading controls that were incubated for one hour at room temperature (26). Membranes were washed twice for fifteen minutes in PBS and probed for one hour at room temperature with goat anti-mouse, goat anti-rabbit or rabbit anti-goat IR-Dye 670 or 800cw labeled secondary antibody diluted in Li-Cor or 1% BSA buffer. After two 15minute washes in PBS, membranes were imaged using a Li-Cor Odyssey scanner. Loading control proteins (VCP, 110kDa and β-tubulin, 55kDa) were chosen for each assay based on the molecular weight of the target protein to avoid potential interference based on similar sizes. No significant differences in either VCP or β-tubulin expression between diagnosis groups were found in DLPFC or ACC (Figure S1 in the Supplement). Full blot images for all proteins studied are shown in Figure S2 in the Supplement.
Data analysis
Boxes were manually placed around each band of interest to obtain integrated intensity values using Odyssey 3.0 analytical software (Li-Cor, Lincoln, Nebraska). Intra-lane background was subtracted. For each band of interest, the value was normalized to the in-lane value of β-tubulin or VCP. For each subject, duplicate normalized data were averaged and the resulting values were used for statistical analysis.
Data were analyzed using Statistica software (Statsoft, Tulsa, Oklahoma). Correlation analyses were performed to determine associations between the dependent variables and tissue pH, age and postmortem interval (PMI). One-way analysis of covariance (ANCOVA) was used to analyze the data when significant correlations with potential covariates were found, otherwise one-way analysis of variance (ANOVA) was used. For the rat experiment, one-way ANOVA was used. For all tests α=0.05.
Results
Proteins of the Duo/Rac-1/PAK-1 pathway are abnormally expressed in frontal cortex in schizophrenia
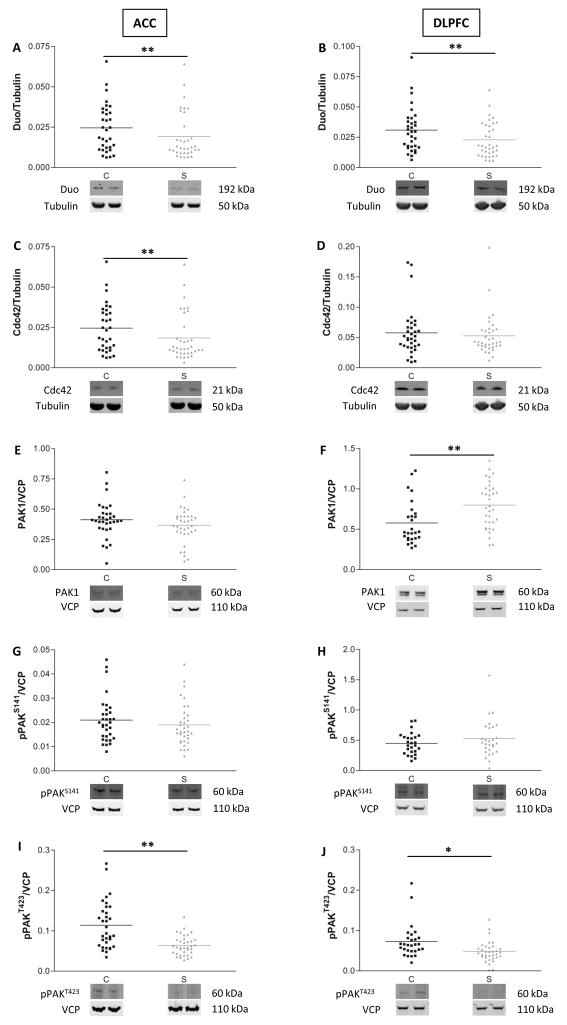
An earlier study reported decreased transcript levels of Duo and Cdc42 in DLPFC in schizophrenia (14). Because this report relied on transcript expression, we first determined if the protein expression levels of Duo and Cdc42 are also abnormal in schizophrenia. Western blot analysis of Duo and Cdc42 expression revealed decreased Duo in both ACC and DLPFC in schizophrenia (Fig. 2A, B. ACC: F(1,47)=10.9, p=0.002; DLPFC: F(1,45)= 10, p=0.003) while Cdc42 expression was decreased only in ACC in these subjects (Fig. 2C, D. ACC: F(1,45)=8.2, p=0.006; DLPFC: F(1,41)=2.6, p=NS).
Figure 2.
Decreased Duo expression is associated with decreased phosphorylation of PAK1 in frontal cortex in schizophrenia. Scatter plots of protein expression levels in ACC and DLPFC of comparison subjects (C) and patients with schizophrenia (S). Duo (A, B) expression is decreased in both areas in schizophrenia, while Cdc42 (C, D) expression is reduced in ACC. PAK1 is elevated in DLPFC but not in ACC (E, F). PAK1 phosphorylated on serine 141 (pPAKS141) was not changed (G, H), but in both ACC and DLPFC decreased expression of PAK1 phosphorylated at threonine 423 (pPAKT423) was seen (I, J). *=p<0.05, **=p<0.01, one-way ANOVA. Data are expressed as a ratio of the optical density value for the protein of interest to the optical density of the β-tubulin or VCP band from the same subject.
Because Cdc42 and Duo activate PAK1 directly and indirectly, respectively, we next sought to determine if decreased expression of these proteins is in turn associated with diminished activation of PAK1. Activation of PAK1 is a two-step process including autophosphorylation of a regulatory domain at serine 144, followed by phosphorylation of the catalytic domain at threonine 423 (27). Western blot analysis showed that total PAK1 expression was unchanged in ACC but was increased in DLPFC in schizophrenia (Fig. 2E, F. ACC: F(1,66)=1.8, p=NS; DLPFC: F(1,59)=9.6, p=0.003), while autophosphorylation of PAK at serine 141 was unchanged in both areas (pPAKS141, Fig. 2G, H). On the other hand, autophosphorylation at the catalytic site threonine 423 was significantly reduced in both areas in schizophrenia (pPAKT423, Fig. 2I, J. ACC: F(1,66)=17.4, p=0.0001; DLPFC: F(1,53)=14.1, p=0.0004), consistent with decreased PAK1 activity in these brain regions in this illness.
Myosin light chain phosphorylation is increased in ACC but not DLPFC in schizophrenia
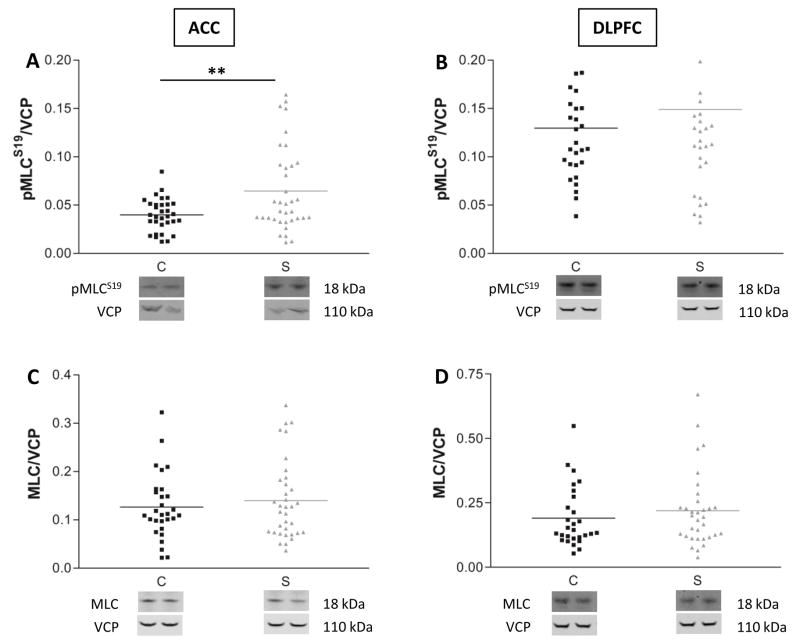
In rodents, PAK phosphorylation leads to inhibition of MLCK, an enzyme known to phosphorylate MLC at serine 19 (pMLCS19) (28, 29). Therefore, we hypothesized that the decrease in PAK1 activity seen in schizophrenia would result in increased MLCK activity with a concomitant increase in MLC phosphorylation. Our expression analyses showed that MLC phosphorylation was increased in ACC in schizophrenia (Fig. 3A F(1,67)=10, p=0.002), although there was no change in MLC phosphorylation in DLPFC (Fig. 3B F(1,61)=1.07, p=NS). Total MLC expression was unchanged in both areas (Fig. 3C, D). To see if pMLCS19 levels directly correlated with pPAKT423 expression, we evaluated within-subject correlations of these measures (Figure S3 in the Supplement). We did not find a linear correlation between pMLCS19 and pPAKT423 expression levels in either diagnostic group.
Figure 3.
Decreased PAK1 phosphorylation is associated with increased MLC phosphorylation. Protein expression levels of the active phosphorylated form of MLC (pMLCS19) (A, B) and total MLC (C, D) were quantified in ACC and DLPFC. Data are expressed as a ratio of the optical density value for the protein of interest to the optical density of VCP from the same patient. **=p<0.01, one-way ANOVA. C=comparison subjects, S=schizophrenia subjects.
LIMK1/cofilin pathway is not abnormal in schizophrenia
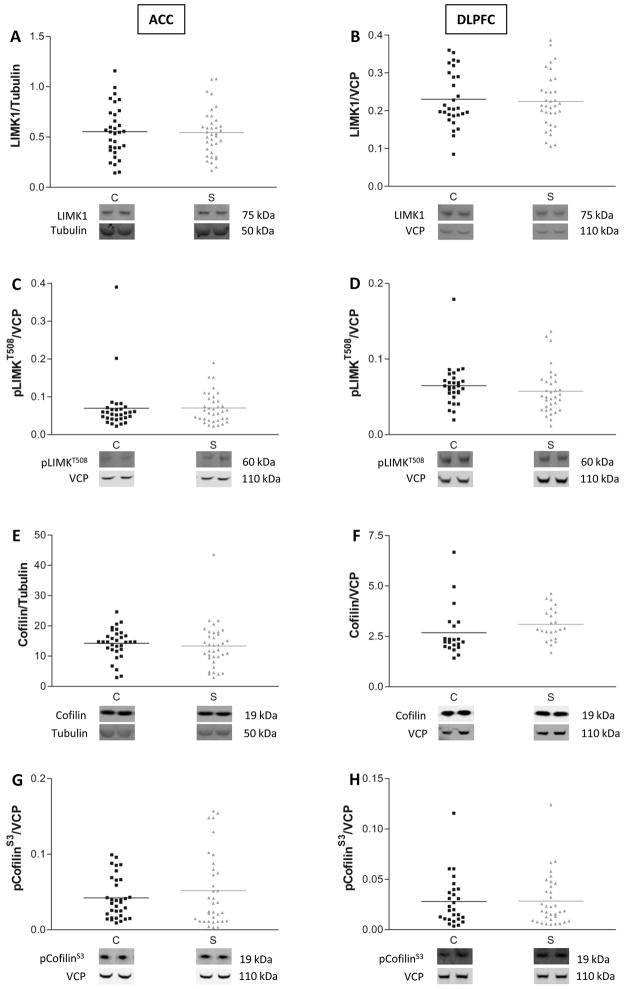
Another downstream target of PAK1 is LIMK1. In contrast to its action on MLCK, PAK1 phosphorylation of LIMK1 at threonine 508 (pLIMK1T508) results in its activation (30). Active LIMK1 then phosphorylates and inactivates cofilin, a protein that stabilizes actin filaments and regulates dendritic spine development (31). Given that PAK1 activity is decreased in schizophrenia, we tested the hypothesis that LIMK1/cofilin downstream pathway would be affected as well. We found no change in expression of total (Fig. 4A, B) or phosphorylated LIMK1 (Fig. 4C, D) in schizophrenia. Expression of total cofilin (Fig. 4E, F) and its phosphorylated form (Fig. 4G, H) were also unchanged in both cortical areas, suggesting that in these brain areas, abnormal expression of PAK1 is not associated with alterations of the LIMK1/cofilin pathway.
Figure 4.
LIMK1/cofilin pathway is unaffected in schizophrenia. Total (A, B) and phosphorylated (pLIMK1T508) LIMK1 protein levels in ACC and DLPFC of patients diagnosed with schizophrenia are unchanged relative to comparison subjects (C, D). Similarly, no differences are found in expression of the downstream effector cofilin (E, F) and its phosphorylated form (pCofilinS3) (G, H). C=comparison subjects, S=schizophrenia. Data are expressed as the ratio of the optical density value for the protein of interest to the optical density for β-tubulin or VCP from the same subject.
Although postmortem interval (PMI) varied among subjects, we did not find an association between PMI and the expression of any of the phospho-proteins we assayed (Figure S4 in the Supplement). Additionally, no correlations were found between age at time of death (AOD) and the expression levels of any of the proteins studied (Figures S5 and S6 in the Supplement).
Protein expression in brain tissue of rats treated with antipsychotic medication
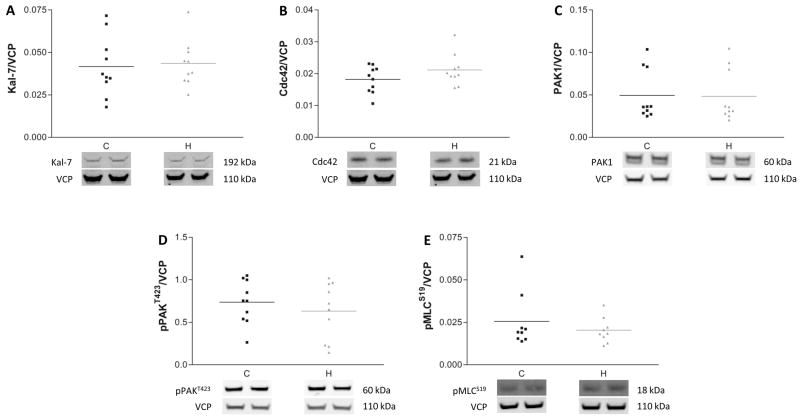
Because most of the schizophrenia subjects used in this study were receiving antipsychotic treatment at the time of death, we tested the possibility that chronic treatment with antipsychotic medications may explain the differences in protein expression we observed in schizophrenia. We measured protein expression in frontal cortex from rats chronically treated with haloperidol compared to a vehicle treated control group. Consistent with previous studies on mRNA expression in brains of monkeys treated with antipsychotics (14), we found no differences in protein expression of any of the proteins we studied in the human experiments (Fig. 5). In addition, we compared protein expression levels in schizophrenia patients on or off antipsychotic medications for more than 6 weeks at the time of death. These post-hoc analyses showed no significant changes associated with medication status for the proteins studied in either cortical area (Figures S7 and S8 in the Supplement). Taken together, these data suggest that chronic antipsychotic drug treatment likely does not account for the protein expression changes we found in schizophrenia.
Figure 5.
Chronic treatment of rats with haloperidol does not affect protein expression in frontal cortex. Western blot analysis of Kalirin-7 (Kal-7, A), Cdc42 (B), PAK1 (C), pPAKT423 (D) and pMLCS19 (E) revealed no differences in the protein expression levels between vehicle (C) and haloperidol (H) treated rats. Data are expressed as a ratio of the optical density value for the protein of interest to the optical density of the band for VCP from the same animal.
Discussion
Dendritic spine loss has been reported in schizophrenia (2–4, 32, 33) yet the underlying mechanism of this observation has not been determined. Murine studies have demonstrated that a loss of afferent input can result in reduced number of dendritic spines (34–37). In addition, reduced excitatory presynaptic input (1, 4, 38, 39) and diminished neuronal soma volume (1, 40–42) have been reported in schizophrenia. On the other hand, developmental findings have suggested that decreased axonal input is a consequence of dendritic spine loss (43), which in schizophrenia may be caused by synaptic overpruning, a hypothesis that has been previously proposed (44–47). Our studies support a hypothesis that intrinsic molecular pathways critical for dendritic spine structural and molecular maintenance are abnormal in schizophrenia.
The actin cytoskeleton and its regulating proteins are essential for synapse formation, maturation, stability and plasticity (9, 11, 17, 48–52). Previous findings have suggested cytoskeletal dysfunction underlying dendritic spine loss (7, 14, 53, 54), supported by previous proteomic studies (55), we suggest a downstream molecular pathways that might lead to actin rearrangement in schizophrenia. Here, we report abnormalities in a GTPase downstream pathway resulting in increased MLC phosphorylation, demonstrating modifications in proteins that directly interact with the actin cytoskeleton in schizophrenia.
Selective disruption of myosin II in the hippocampus has been reported to cause deficient long term memory consolidation (17) and dendritic spine abnormalities (48, 51), both of which have been noted in schizophrenia (56). MLC phosphorylation is critical for myosin II activation, a key step for synaptic transmission and maintenance of synaptic structure (48, 51, 57). Our findings suggest a mechanism where decreased phosphorylation of PAK1 increases MLC phosphorylation, which in turn may increase the rate of actin depolymerization, and lead to dendritic spine collapse in schizophrenia. In support of this model, studies in rodents have found diminished dendritic spine dynamics and density as a result of decreased Rac-1 activity (58, 59), and increased neurite retraction after activating MLC either by decreasing its dephosphorylation or by overexpressing a dominant active form (60). Therefore, abnormalities in signaling leading to MLC phosphorylation may account for some of the synaptic changes seen in this illness. Although our results suggest a possible mechanism underlying synaptic dysfunction in schizophrenia, it is important to note that these changes were found in total cortical homogenates, and not at the cellular level. Further studies are necessary to define mechanisms at the cellular level.
The LIMK1/cofilin pathway is also downstream of PAK, and is involved in actin filament stability. Disruption of LIMK1/cofilin causes abnormal dendritic spine morphology and decreased basal neurotransmitter release (61) but is not critical for long term potentiation, memory formation, retrieval or consolidation (9, 11). Given that we found abnormal PAK phosphorylation in both ACC and DLPFC in schizophrenia, we investigated the possibility of disruption of this pathway as well. The lack of abnormality of LIMK1/cofilin we found in schizophrenia is also found in Fragile X syndrome, suggesting that these illnesses may be associated with disruption of long term actin cytoskeleton stability as opposed to immediate actin filament regulation (11).
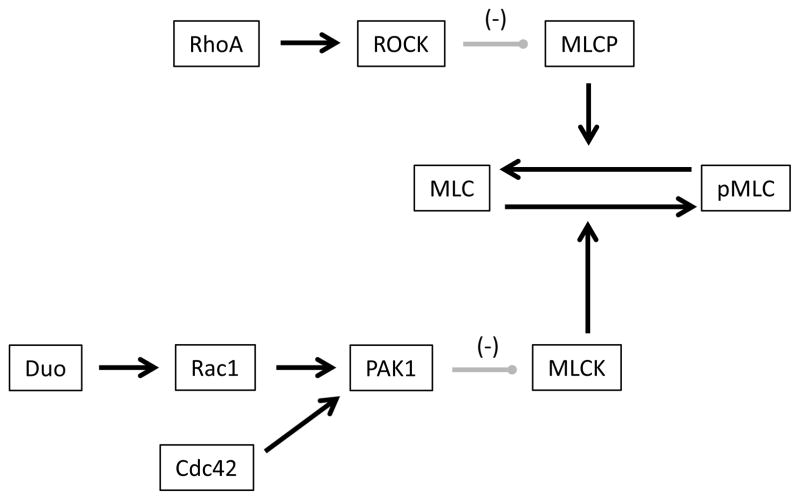
Interestingly, there is a lack of MLC phosphorylation changes in DLPFC despite Duo/PAK1 pathway abnormalities. A possible explanation of this discrepancy is suggested by the increase in total PAK1 that we found only in DLPFC. Similar data have been reported in Fragile X syndrome, where it was proposed that since PAK follows third order kinetics, the increase in substrate (PAK1) and decrease in drivers (Duo) would result in decreased phosphorylation at the catalytic site of PAK (11). This model, however, does not explain the increase in PAK1 we found in DLPFC in schizophrenia, which may be due to abnormal trafficking on microtubule associated proteins (62) or myosin motors. Alternatively, the lack of increased MLC phosphorylation might be the result of a compensatory increase in myosin light chain phosphatase (MLCP) activity. MLCP inactivates MLC by driving its dephosphorylation (63). Activation of MLCP is inhibited by the RhoA/ROCK pathway (64, 65). Increased MLCK activity may drive a compensatory downregulation of the RhoA/ROCK pathway in the DLPFC to increase MLCP activity and maintain MLC phosphorylation (Fig. 6). In support of this, previous studies in the prefrontal cortex in schizophrenia have found decreased expression of RhoA mRNA (14), which could lead to upregulation of MLCP function. Alternatively, loss of dendritic spines in DLPFC in schizophrenia may be a result of differential changes in PAK1 downstream pathways, such as actin branching mechanisms involving the Arp2/3 complex (66), or actin polymerization regulators such as cortactin (67).
Figure 6.
Schematic representation of MLC phosphorylation regulation by PAK1 and the Rho/ROCK pathways. Duo/Rac-1/PAK1 and Cdc42/PAK1 pathways both lead to MLCK inhibition and a decrease in MLC phosphorylation. RhoA/ROCK pathway activation results in MLCP inhibition, thus increasing MLC phosphorylation. RhoA: Ras homolog A, ROCK: Rho-associated protein kinase 1, MLCP: myosin light chain phosphatase.
To address the possibility that chronic antipsychotic treatment that the subjects with schizophrenia had received could alter the expression or phosphorylation of the proteins we studied, we tested the effect of long term administration of haloperidol in rats, and found that none of the proteins that we studied were changed by this treatment. These data, together with a lack of difference in protein expression levels in schizophrenia patients on or off antipsychoatic medication for more than 6 weeks at the time of death, suggest that treatment with antipsychotics is not likely to account for the differences we found in this study.
In summary, we found reduced levels of expression of key components of a cytoskeletal regulation pathway in frontal cortical areas in schizophrenia. Disruption of the Duo/Rac-1/PAK1 and Cdc42/PAK1 pathways in ACC, presumably leading to an increase in MLC phosphorylation, suggests a mechanism to explain cytoskeletal dysfunction in schizophrenia, and may reflect an underlying mechanism to explain dendritic spine loss in this illness.
Supplementary Material
Acknowledgments
The authors thank Drs. Lulu Y. Chen, Christopher S. Rex and Robert McCullumsmith for their helpful scientific discussions, Dr. Adam Funk for his technical assistance and the Alabama Brain Collection. This work was supported by National Institutes of Health (NIH) Grant MH53327 (JHMW), MH064673 (VH) and MH066392 (VH).
Footnotes
Financial Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lewis DA, Glantz LA, Pierri JN, Sweet RA. Altered cortical glutamate neurotransmission in schizophrenia: evidence from morphological studies of pyramidal neurons. Ann N Y Acad Sci. 2003 Nov;1003:102–112. doi: 10.1196/annals.1300.007. [DOI] [PubMed] [Google Scholar]
- 2.Glantz LA, Lewis DA. Dendritic spine density in schizophrenia and depression. Arch Gen Psychiatry. 2001 Feb;58(2):203. doi: 10.1001/archpsyc.58.2.203. [DOI] [PubMed] [Google Scholar]
- 3.Garey LJ, Ong WY, Patel TS, Kanani M, Davis A, Mortimer AM, et al. Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. J Neurol Neurosurg Psychiatry. 1998 Oct;65(4):446–453. doi: 10.1136/jnnp.65.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000 Jan;57(1):65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- 5.Fatemi SH, Stary JM, Earle JA, Araghi-Niknam M, Eagan E. GABAergic dysfunction in schizophrenia and mood disorders as reflected by decreased levels of glutamic acid decarboxylase 65 and 67 kDa and Reelin proteins in cerebellum. Schizophr Res. 2005 Jan 1;72(2–3):109–122. doi: 10.1016/j.schres.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 6.Chai X, Forster E, Zhao S, Bock HH, Frotscher M. Reelin stabilizes the actin cytoskeleton of neuronal processes by inducing n-cofilin phosphorylation at serine3. J Neurosci. 2009 Jan 7;29(1):288–299. doi: 10.1523/JNEUROSCI.2934-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayashi-Takagi A, Takaki M, Graziane N, Seshadri S, Murdoch H, Dunlop AJ, et al. Disrupted-in-Schizophrenia 1 (DISC1) regulates spines of the glutamate synapse via Rac1. Nat Neurosci. 2010 Mar;13(3):327–332. doi: 10.1038/nn.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hotulainen P, Hoogenraad CC. Actin in dendritic spines: connecting dynamics to function. J Cell Biol. 2010 May 17;189(4):619–629. doi: 10.1083/jcb.201003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rex CS, Chen LY, Sharma A, Liu J, Babayan AH, Gall CM, et al. Different Rho GTPase-dependent signaling pathways initiate sequential steps in the consolidation of long-term potentiation. J Cell Biol. 2009 Jul 13;186(1):85–97. doi: 10.1083/jcb.200901084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Penzes P, Johnson RC, Alam MR, Kambampati V, Mains RE, Eipper BA. An isoform of kalirin, a brain-specific GDP/GTP exchange factor, is enriched in the postsynaptic density fraction. J Biol Chem. 2000 Mar 3;275(9):6395–6403. doi: 10.1074/jbc.275.9.6395. [DOI] [PubMed] [Google Scholar]
- 11.Chen LY, Rex CS, Babayan AH, Kramar EA, Lynch G, Gall CM, et al. Physiological activation of synaptic Rac>PAK (p-21 activated kinase) signaling is defective in a mouse model of fragile X syndrome. J Neurosci. 2010 Aug 18;30(33):10977–10984. doi: 10.1523/JNEUROSCI.1077-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma XM, Kiraly DD, Gaier ED, Wang Y, Kim EJ, Levine ES, et al. Kalirin-7 is required for synaptic structure and function. J Neurosci. 2008 Nov 19;28(47):12368–12382. doi: 10.1523/JNEUROSCI.4269-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie Z, Cahill ME, Radulovic J, Wang J, Campbell SL, Miller CA, et al. Hippocampal phenotypes in kalirin-deficient mice. Mol Cell Neurosci. 2011 Jan;46(1):45–54. doi: 10.1016/j.mcn.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hill JJ, Hashimoto T, Lewis DA. Molecular mechanisms contributing to dendritic spine alterations in the prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2006 Jun;11(6):557–566. doi: 10.1038/sj.mp.4001792. [DOI] [PubMed] [Google Scholar]
- 15.Manser E, Leung T, Salihuddin H, Zhao ZS, Lim L. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature. 1994 Jan 6;367(6458):40–46. doi: 10.1038/367040a0. [DOI] [PubMed] [Google Scholar]
- 16.Kamm KE, Stull JT. Dedicated myosin light chain kinases with diverse cellular functions. J Biol Chem. 2001 Feb 16;276(7):4527–4530. doi: 10.1074/jbc.R000028200. [DOI] [PubMed] [Google Scholar]
- 17.Rex CS, Gavin CF, Rubio MD, Kramar EA, Chen LY, Jia Y, et al. Myosin IIb regulates actin dynamics during synaptic plasticity and memory formation. Neuron. 2010 Aug 26;67( 4):603–617. doi: 10.1016/j.neuron.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Funk AJ, Rumbaugh G, Harotunian V, McCullumsmith RE, Meador-Woodruff JH. Decreased expression of NMDA receptor-associated proteins in frontal cortex of elderly patients with schizophrenia. Neuroreport. 2009 Jul 15;20(11):1019–1022. doi: 10.1097/WNR.0b013e32832d30d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bauer D, Gupta D, Harotunian V, Meador-Woodruff JH, McCullumsmith RE. Abnormal expression of glutamate transporter and transporter interacting molecules in prefrontal cortex in elderly patients with schizophrenia. Schizophr Res. 2008 Sep;104(1–3):108–120. doi: 10.1016/j.schres.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Purohit DP, Davidson M, Perl DP, Powchik P, Haroutunian VH, Bierer LM, et al. Severe cognitive impairment in elderly schizophrenic patients: a clinicopathological study. Biol Psychiatry. 1993 Feb 15;33(4):255–260. doi: 10.1016/0006-3223(93)90291-k. [DOI] [PubMed] [Google Scholar]
- 21.Kashihara K, Sato M, Fujiwara Y, Harada T, Ogawa T, Otsuki S. Effects of intermittent and continuous haloperidol administration on the dopaminergic system in the rat brain. Biol Psychiatry. 1986 Jun;21(7):650–656. doi: 10.1016/0006-3223(86)90126-5. [DOI] [PubMed] [Google Scholar]
- 22.Harte MK, Bachus SB, Reynolds GP. Increased N-acetylaspartate in rat striatum following long-term administration of haloperidol. Schizophr Res. 2005 Jun 15;75(2–3):303–308. doi: 10.1016/j.schres.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Mithani S, Atmadja S, Baimbridge KG, Fibiger HC. Neuroleptic-induced oral dyskinesias: effects of progabide and lack of correlation with regional changes in glutamic acid decarboxylase and choline acetyltransferase activities. Psychopharmacology (Berl) 1987;93(1):94–100. doi: 10.1007/BF02439593. [DOI] [PubMed] [Google Scholar]
- 24.Gunne LM, Haggstrom JE. Reduction of nigral glutamic acid decarboxylase in rats with neuroleptic-induced oral dyskinesia. Psychopharmacology (Berl) 1983;81(3):191–194. doi: 10.1007/BF00427260. [DOI] [PubMed] [Google Scholar]
- 25.Kristiansen LV, Patel SA, Haroutunian V, Meador-Woodruff JH. Expression of the NR2B-NMDA receptor subunit and its Tbr-1/CINAP regulatory proteins in postmortem brain suggest altered receptor processing in schizophrenia. Synapse. 2010 Jul;64(7):495–502. doi: 10.1002/syn.20754. [DOI] [PubMed] [Google Scholar]
- 26.Bauer DE, Haroutunian V, McCullumsmith RE, Meador-Woodruff JH. Expression of four housekeeping proteins in elderly patients with schizophrenia. J Neural Transm. 2009 Apr;116(4):487–491. doi: 10.1007/s00702-008-0143-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chong C, Tan L, Lim L, Manser E. The mechanism of PAK activation. Autophosphorylation events in both regulatory and kinase domains control activity. J Biol Chem. 2001 May 18;276(20):17347–17353. doi: 10.1074/jbc.M009316200. [DOI] [PubMed] [Google Scholar]
- 28.Sanders LC, Matsumura F, Bokoch GM, de Lanerolle P. Inhibition of myosin light chain kinase by p21-activated kinase. Science. 1999 Mar 26;283(5410):2083–2085. doi: 10.1126/science.283.5410.2083. [DOI] [PubMed] [Google Scholar]
- 29.Zhang H, Webb DJ, Asmussen H, Niu S, Horwitz AF. A GIT1/PIX/Rac/PAK signaling module regulates spine morphogenesis and synapse formation through MLC. J Neurosci. 2005 Mar 30;25(13):3379–3388. doi: 10.1523/JNEUROSCI.3553-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edwards DC, Sanders LC, Bokoch GM, Gill GN. Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nat Cell Biol. 1999 Sep;1(5):253–259. doi: 10.1038/12963. [DOI] [PubMed] [Google Scholar]
- 31.Bernstein BW, Bamburg JR. ADF/cofilin: a functional node in cell biology. Trends Cell Biol. 2010 Apr;20(4):187–195. doi: 10.1016/j.tcb.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sweet RA, Henteleff RA, Zhang W, Sampson AR, Lewis DA. Reduced dendritic spine density in auditory cortex of subjects with schizophrenia. Neuropsychopharmacology. 2009 Jan;34(2):374–389. doi: 10.1038/npp.2008.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Law AJ, Weickert CS, Hyde TM, Kleinman JE, Harrison PJ. Reduced spinophilin but not microtubule-associated protein 2 expression in the hippocampal formation in schizophrenia and mood disorders: molecular evidence for a pathology of dendritic spines. Am J Psychiatry. 2004 Oct;161(10):1848–1855. doi: 10.1176/ajp.161.10.1848. [DOI] [PubMed] [Google Scholar]
- 34.McKinney RA, Capogna M, Durr R, Gahwiler BH, Thompson SM. Miniature synaptic events maintain dendritic spines via AMPA receptor activation. Nat Neurosci. 1999 Jan;2(1):44–49. doi: 10.1038/4548. [DOI] [PubMed] [Google Scholar]
- 35.McNeill TH, Brown SA, Hogg E, Cheng HW, Meshul CK. Synapse replacement in the striatum of the adult rat following unilateral cortex ablation. J Comp Neurol. 2003 Dec 1;467(1):32–43. doi: 10.1002/cne.10907. [DOI] [PubMed] [Google Scholar]
- 36.Cheng HW, Rafols JA, Goshgarian HG, Anavi Y, Tong J, McNeill TH. Differential spine loss and regrowth of striatal neurons following multiple forms of deafferentation: a Golgi study. Exp Neurol. 1997 Oct;147(2):287–298. doi: 10.1006/exnr.1997.6618. [DOI] [PubMed] [Google Scholar]
- 37.Ingham CA, Hood SH, van Maldegem B, Weenink A, Arbuthnott GW. Morphological changes in the rat neostriatum after unilateral 6-hydroxydopamine injections into the nigrostriatal pathway. Exp Brain Res. 1993;93(1):17–27. doi: 10.1007/BF00227776. [DOI] [PubMed] [Google Scholar]
- 38.Garey LJ, Von Bussmann KA, Hirsch SR. Decreased numerical density of kainate receptor-positive neurons in the orbitofrontal cortex of chronic schizophrenics. Exp Brain Res. 2006 Aug;173(2):234–242. doi: 10.1007/s00221-006-0396-8. [DOI] [PubMed] [Google Scholar]
- 39.Garey L. When cortical development goes wrong: schizophrenia as a neurodevelopmental disease of microcircuits. J Anat. 2010 Oct;217(4):324–333. doi: 10.1111/j.1469-7580.2010.01231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sweet RA, Bergen SE, Sun Z, Sampson AR, Pierri JN, Lewis DA. Pyramidal cell size reduction in schizophrenia: evidence for involvement of auditory feedforward circuits. Biol Psychiatry. 2004 Jun 15;55(12):1128–1137. doi: 10.1016/j.biopsych.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 41.Sweet RA, Pierri JN, Auh S, Sampson AR, Lewis DA. Reduced pyramidal cell somal volume in auditory association cortex of subjects with schizophrenia. Neuropsychopharmacology. 2003 Mar;28(3):599–609. doi: 10.1038/sj.npp.1300120. [DOI] [PubMed] [Google Scholar]
- 42.Sweet RA, Bergen SE, Sun Z, Marcsisin MJ, Sampson AR, Lewis DA. Anatomical evidence of impaired feedforward auditory processing in schizophrenia. Biol Psychiatry. 2007 Apr 1;61(7):854–864. doi: 10.1016/j.biopsych.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 43.Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005 Nov;6(11):877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- 44.Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res. 1982;17(4):319–334. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 45.Keshavan MS, Anderson S, Pettegrew JW. Is schizophrenia due to excessive synaptic pruning in the prefrontal cortex? The Feinberg hypothesis revisited. J Psychiatr Res. 1994 May-Jun;28(3):239–265. doi: 10.1016/0022-3956(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 46.Pettegrew JW, Keshavan MS, Minshew NJ. 31P nuclear magnetic resonance spectroscopy: neurodevelopment and schizophrenia. Schizophr Bull. 1993;19(1):35–53. doi: 10.1093/schbul/19.1.35. [DOI] [PubMed] [Google Scholar]
- 47.Pettegrew JW, Minshew NJ. Molecular insights into schizophrenia. J Neural Transm Suppl. 1992;36:23–40. doi: 10.1007/978-3-7091-9211-5_3. [DOI] [PubMed] [Google Scholar]
- 48.Rubio MD, Johnson R, Miller CA, Huganir RL, Rumbaugh G. Regulation of synapse structure and function by distinct myosin II motors. J Neurosci. 2011 Jan 26;31(4):1448–1460. doi: 10.1523/JNEUROSCI.3294-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fischer M, Kaech S, Knutti D, Matus A. Rapid actin-based plasticity in dendritic spines. Neuron. 1998 May;20(5):847–854. doi: 10.1016/s0896-6273(00)80467-5. [DOI] [PubMed] [Google Scholar]
- 50.Fischer M, Kaech S, Wagner U, Brinkhaus H, Matus A. Glutamate receptors regulate actin-based plasticity in dendritic spines. Nat Neurosci. 2000 Sep;3(9):887–894. doi: 10.1038/78791. [DOI] [PubMed] [Google Scholar]
- 51.Ryu J, Liu L, Wong TP, Wu DC, Burette A, Weinberg R, et al. A critical role for myosin IIb in dendritic spine morphology and synaptic function. Neuron. 2006 Jan 19;49(2):175–182. doi: 10.1016/j.neuron.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 52.Chen LY, Rex CS, Casale MS, Gall CM, Lynch G. Changes in synaptic morphology accompany actin signaling during LTP. J Neurosci. 2007 May 16;27(20):5363–5372. doi: 10.1523/JNEUROSCI.0164-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cahill ME, Xie Z, Day M, Photowala H, Barbolina MV, Miller CA, et al. Kalirin regulates cortical spine morphogenesis and disease-related behavioral phenotypes. Proc Natl Acad Sci U S A. 2009 Aug 4;106(31):13058–13063. doi: 10.1073/pnas.0904636106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xie Z, Photowala H, Cahill ME, Srivastava DP, Woolfrey KM, Shum CY, et al. Coordination of synaptic adhesion with dendritic spine remodeling by AF-6 and kalirin-7. J Neurosci. 2008 Jun 11;28(24):6079–6091. doi: 10.1523/JNEUROSCI.1170-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.English JA, Dicker P, Focking M, Dunn MJ, Cotter DR. 2-D DIGE analysis implicates cytoskeletal abnormalities in psychiatric disease. Proteomics. 2009 Jun;9(12):3368–3382. doi: 10.1002/pmic.200900015. [DOI] [PubMed] [Google Scholar]
- 56.Van Snellenberg JX. Working memory and long-term memory deficits in schizophrenia: is there a common substrate? Psychiatry Res. 2009 Nov 30;174(2):89–96. doi: 10.1016/j.pscychresns.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 57.Korobova F, Svitkina T. Molecular architecture of synaptic actin cytoskeleton in hippocampal neurons reveals a mechanism of dendritic spine morphogenesis. Mol Biol Cell. 2010 Jan 1;21(1):165–176. doi: 10.1091/mbc.E09-07-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nakayama AY, Harms MB, Luo L. Small GTPases Rac and Rho in the maintenance of dendritic spines and branches in hippocampal pyramidal neurons. J Neurosci. 2000 Jul 15;20( 14):5329–5338. doi: 10.1523/JNEUROSCI.20-14-05329.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tashiro A, Yuste R. Regulation of dendritic spine motility and stability by Rac1 and Rho kinase: evidence for two forms of spine motility. Mol Cell Neurosci. 2004 Jul;26(3):429–440. doi: 10.1016/j.mcn.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 60.Amano M, Chihara K, Nakamura N, Fukata Y, Yano T, Shibata M, et al. Myosin II activation promotes neurite retraction during the action of Rho and Rho-kinase. Genes to Cells. 1998 Mar;3(3):177–188. doi: 10.1046/j.1365-2443.1998.00181.x. [DOI] [PubMed] [Google Scholar]
- 61.Meng Y, Zhang Y, Tregoubov V, Janus C, Cruz L, Jackson M, et al. Abnormal spine morphology and enhanced LTP in LIMK-1 knockout mice. Neuron. 2002 Jul 3;35(1):121–133. doi: 10.1016/s0896-6273(02)00758-4. [DOI] [PubMed] [Google Scholar]
- 62.Li C, Zheng Y, Qin W, Tao R, Pan Y, Xu Y, et al. A family-based association study of kinesin heavy chain member 2 gene (KIF2) and schizophrenia. Neurosci Lett. 2006 Oct 23;407(2):151–155. doi: 10.1016/j.neulet.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 63.Hartshorne DJ. Myosin phosphatase: subunits and interactions. Acta Physiol Scand. 1998 Dec;164(4):483–493. doi: 10.1046/j.1365-201X.1998.00447.x. [DOI] [PubMed] [Google Scholar]
- 64.Zhao ZS, Manser E. PAK and other Rho-associated kinases--effectors with surprisingly diverse mechanisms of regulation. Biochem J. 2005 Mar 1;386(Pt 2):201–214. doi: 10.1042/BJ20041638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sandquist JC, Swenson KI, Demali KA, Burridge K, Means AR. Rho kinase differentially regulates phosphorylation of nonmuscle myosin II isoforms A and B during cell rounding and migration. J Biol Chem. 2006 Nov 24;281(47):35873–35883. doi: 10.1074/jbc.M605343200. [DOI] [PubMed] [Google Scholar]
- 66.Vadlamudi RK, Li F, Barnes CJ, Bagheri-Yarmand R, Kumar R. p41-Arc subunit of human Arp2/3 complex is a p21-activated kinase-1-interacting substrate. EMBO Rep. 2004 Feb;5(2):154–160. doi: 10.1038/sj.embor.7400079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grassart A, Meas-Yedid V, Dufour A, Olivo-Marin JC, Dautry-Varsat A, Sauvonnet N. Pak1 phosphorylation enhances cortactin-N-WASP interaction in clathrin-caveolin-independent endocytosis. Traffic. 2010 Aug;11(8):1079–1091. doi: 10.1111/j.1600-0854.2010.01075.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.