Abstract
Mammals that hibernate experience extreme metabolic states and body temperatures as they transition between euthermia, a state resembling typical warm blooded mammals, and prolonged torpor, a state of suspended animation where the brain receives as low as 10% of normal cerebral blood flow. Transitions into and out of torpor are more physiologically challenging than the extreme metabolic suppression and cold body temperatures of torpor per se. Mammals that hibernate show unprecedented capacities to tolerate cerebral ischemia, a decrease in blood flow to the brain caused by stroke, cardiac arrest or brain trauma. While cerebral ischemia often leads to death or disability in humans and most other mammals, hibernating mammals suffer no ill effects when blood flow to the brain is dramatically decreased during torpor or experimentally induced during euthermia. These animals, as adults, also display rapid and pronounced synaptic flexibility where synapses retract during torpor and rapidly re-emerge upon arousal. A variety of coordinated adaptations contribute to tolerance of cerebral ischemia in these animals. In this review we discuss adaptations in heterothermic mammals that may suggest novel therapeutic targets and strategies to protect the human brain against cerebral ischemic damage and neurodegenerative disease.
Keywords: Cerebral ischemia, Hibernation, Neurodegeneration, Stroke, Torpor
1. Introduction
Hibernation is identified by a prolonged state of energy conservation, termed torpor, that allows heterothermic mammals to tolerate the limited resource availability encountered in extreme environments (Drew et al., 2007). During the hibernation season, hibernating animals experience multiple bouts of torpor that are interrupted by brief periods of euthermia. Torpor can be divided into three phases, onset, maintenance and arousal, which are followed by a period of interbout euthermia. During onset and maintenance of torpor, animals exhibit overall metabolic suppression, decreased body temperature, reduction in heart rate, reduction in cerebral blood flow, decreased oxygen consumption, lowered respiratory rates and suppressed immune responses (Barger et al., 2003; Barnes, 1989; Buck and Barnes, 2000a; Drew et al., 2009; Drew et al., 2004; Drew et al., 2002; Toien et al., 2001). During arousal from torpor, rapid blood reperfusion is accompanied by enormous oxygen consumption and elevation in body temperature. Inflammatory and immune responses resume, and the capacity to scavenge free radicals is increased (Barnes, 1989; Bouma et al., 2011; Prendergast et al., 2002; Toien et al., 2001). Interbout euthermia is characterized by metabolism, blood flow and body temperature typical of a homeothermic mammal of similar size (Drew et al., 2004).
In the hibernating phase, an animal’s oxygen demand is decreased to as low as 2% of euthermic oxygen demand and cerebral blood flow is decreased to as low as 10% of the euthermic phase (Drew et al., 2009; Drew et al., 2002). Even when not hibernating, arctic ground squirrels (AGS) (Urocitellus parryii) can tolerate at least 10 min of global ischemia without detectable neuronal injury (Figures 1 and 2) (Dave et al., 2009; Dave et al., 2006). Understanding the mechanism by which hibernators tolerate such a drastic reduction in cerebral blood flow and achieve this profound metabolic suppression has the potential to facilitate the design of novel therapies for diseases or conditions where interruptions in blood flow to the brain are inherent. In this review, we discuss the major mechanisms by which cerebral ischemic injury may be avoided during hibernation. We also present the current understanding of how the brain of hibernating species are protected from cerebral ischemia when euthermic.
Figure 1. Schematic diagram depicting the adaptations used to minimize the cognitive and energetic costs of arousal.
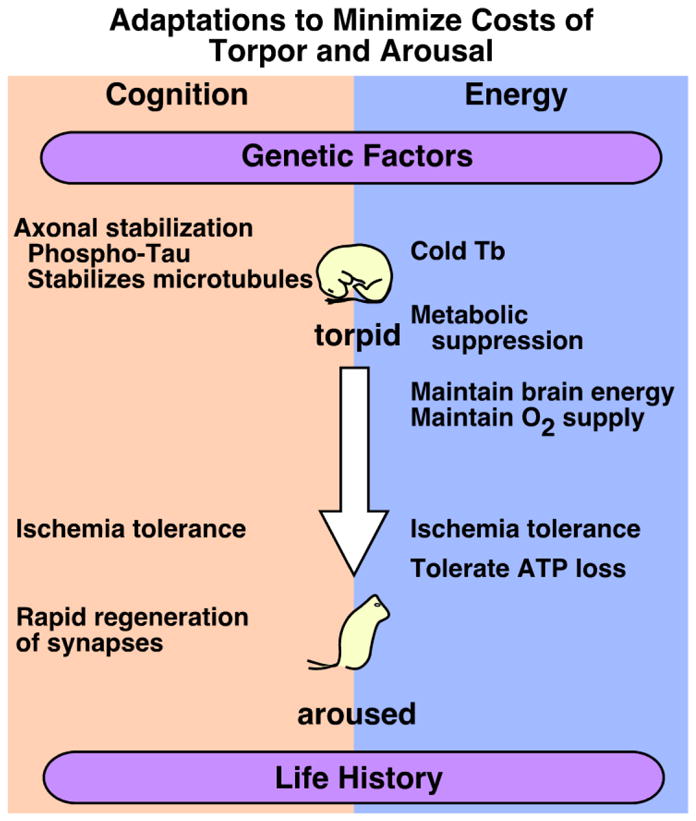
Genetic factors and potentially life history contribute to the ability to tolerate multiple arousals from the torpid state without cognitive deficits or cellular pathology. Rapid regeneration of synaptic profiles during arousal prevents cognitive deficits and likely contributes to improved mnemonic function 24 h after arousal. Cold body temperature (Tb) combined with metabolic suppression during torpor conserves energy. Overall maintenance of cellular homeostasis during arousal promotes neuronal cell survival and contributes to ischemia tolerance in hibernating species.
We also present data that support our hypothesis (Figure 1) that hibernators have evolved physiological adaptations as a result of selective pressures associated with transitions into and out of torpor. These adaptations may have materialized as tolerance to cerebral ischemia during euthermia and an increased capacity for adult synaptic plasticity.
2. Torpor
Decreased body temperature and metabolic suppression are the two main characteristics of torpor (Drew et al., 2007; Drew et al., 2009). In very small hibernators, metabolic suppression may be regulated by temperature-dependent passive processes. In contrast, in relatively large hibernators metabolic suppression clearly precedes the temperature drop, as detected by greatly diminished oxygen consumption, suggesting a large contribution of temperature-independent processes (Drew et al., 2007; Drew et al., 2009; Geiser, 2004; Toien et al., 2011). Understanding the mechanism of metabolic suppression during torpor is important because combining features of metabolic suppression with therapeutic hypothermia may provide better protection against cellular damage following cerebral ischemia and brain trauma in humans.
The physiological mechanisms that initiate torpor in hibernating mammals are poorly understood. These mechanisms are believed to involve interactions between neural systems regulating endogenous timing, metabolism, and homeostasis. In a recent study it was observed that activation of A1AR within the CNS is necessary and sufficient to induce torpor in arctic ground squirrels in a seasonally dependent manner (Jinka et al., 2011). Delivery of the adenosine A1 receptor (A1AR) antagonist cyclopentyltheophylline into the lateral ventricle of AGS reversed spontaneous entrance into torpor (Jinka et al., 2011). Moreover, delivery of the A1AR agonist N(6)-cyclohexyladenosine (CHA) induced torpor in all AGS tested during the mid-hibernation season but did not induce torpor in any of the AGSs tested during the summer “off-season”. Torpor within the hibernation season was specific to A1AR activation as the A3AR agonist failed to induce torpor while the A2AAR antagonist failed to reverse the spontaneous onset of torpor. Still missing, however, is an understanding of how the brain coordinates a decrease in whole animal metabolic rate. We hypothesize that central A1AR activation suppresses thermogenesis and that the gain in this pathway is increased during the hibernation season to lower the hypothalamic temperature that will stimulate thermogenesis (i.e., decreases the lower critical temperature). It then follows that torpid metabolic rate represents the basal metabolic rate in the torpid state.
The rate of cooling during onset of torpor is slower than during drug-induced hypothermia due to postural adjustments, decreases in respiratory frequency and profound vasoconstriction in the lower extremities (Drew et al., 2012; Milsom et al., 2001). This reduced cooling rate is consistent with decreased thermal conductance, which is reported to be lower for torpid animals than for euthermic animals (Jinka et al., 2011; Snyder and Nestler, 1990). During torpor, when body temperature can fall to as low as 0 °C without stimulating thermogenesis, a new equilibrium between heat loss and production may define the minimal torpid metabolic rate within the thermoneutral zone of a hibernating animal (Buck and Barnes, 2000b). How thermal conductance is regulated or when it is decreased is unclear. Gain in the neural pathways that coordinate heat retention may increase seasonally similar to the gain in the pathways that suppress thermogenesis.
2.1 Hypothermia
Hypothermia is one of the main features of torpor. During torpor the extent of the drop in body temperature varies from species to species. In some extreme hibernators, such as AGS, core body temperature reaches 0 °C in equilibrium with the ambient temperature. Hypothermia is shown to protect brain against ischemic injury in preclinical and clinical studies (Bernard et al., 2002; Hypothermia after Cardiac Arrest Study, 2002; Krieger and Yenari, 2004; Yenari and Hemmen, 2010). Thus, the profound hypothermia observed during torpor likely provides some degree of neuroprotection in hibernating animals. Several mechanisms are involved in hypothermia-induced neuroprotection. Cerebral blood flow is linearly decreased when core temperature decreases from 37 °C to 18 °C. Mild hypothermia prevents cerebral ischemia induced early postreperfusion hyperemia and late postreperfusion hypoperfusion, both of which are believed to contribute to cerebral ischemic damage (Yenari et al., 2008). Hypothermia prevents cerebral ischemia-induced excitotoxicity, a major mechanism of neuronal death following cerebral ischemia. Baker et al. demonstrated that intraischemic hypothermia blunts the extracellular release of glutamate in the core region following permanent focal cerebral ischemia, suggesting the role of hypothermia in reducing exitotoxicity (Baker et al., 1995). Another study observed lower glutamate and dopamine release in striatum following endothelin-1-induced focal cerebral ischemia in hypothermia treated group (Van Hemelrijck et al., 2003). The fact that the effects of hypothermia and N-methyl-D-aspartate (NMDA) antagonist MK-801 do not show additive protective effects against focal cerebral ischemia when used in combination supports the hypothesis that both of them prevent cerebral ischemic damage by reducing excitotoxicity (Frazzini et al., 1994).
In addition, several studies demonstrated that hypothermia suppresses cerebral ischemia-induced oxidative stress in different models of cerebral ischemia (Karibe et al., 1994; Katz et al., 2004). Studies have also demonstrated that hypothermia prevents oxidative stress induced cellular damage such as DNA fragmentation following cerebral ischemia (Ji et al., 2007; Nakamura et al., 1999; Phanithi et al., 2000). Phanithi et al. observed that hypothermia treatment decreases post-ischemic expression of Fas (a type-II transmembrane protein of the tumor necrosis factor family) and caspase-3 (Phanithi et al., 2000). Hypothermia also decreases the release of mitochondrial cytochrome-c following focal cerebral ischemia (Yenari et al., 2002). Mild hypothermia (33° C) is shown to increase expression of anti-apoptotic protein Bcl-2 following global cerebral ischemia (Zhang et al., 2001). In complementary fashion, hypothermia appears to activate cell survival pathways (Yenari et al., 2008).
Hypothermia also reduces post-ischemic inflammation. Maier et al demonstrated that hypothermia-treated animals exhibit significantly less activity of the inflammatory marker myeloperoxidase in the peri-core region following focal cerebral ischemia when measured at 24 h post-ischemia (Maier et al., 1998). Post-stroke activation of transcription factor Nuclear factor-kappa B (NF-κB) leads to the expression of many inflammatory genes involved in stroke damage (Yenari and Han, 2006). Mild hypothermia treatment is able to suppress post-ischemic activation of NF-κB (Han et al., 2003; Webster et al., 2009). Post-ischemic hypothermia is able to lower interleukin-18 mRNA and protein level as well as decrease microglia activation in the developing brain (Fukui et al., 2006). This accumulation of evidence suggests that hypothermia may play a role in neuroprotection during torpor.
2.2 Excitotoxicity
The brain consumes an excessive amount of oxygen for its mass because large amounts of oxygen are required to maintain ionic gradients across neuronal plasma membranes (Boveris and Chance, 1973). Ischemia-induced depletion of cellular ATP prevents neurons from maintaining membrane potential, resulting in neuronal depolarization. The loss of neuronal membrane potential results in increased intracellular calcium concentrations, leading to activation of calcium-dependent processes as well as to the massive release of neurotransmitters, including the excitatory neurotransmitter glutamate (Doyle et al., 2008; Lipton, 1999b). Increased glutamate concentration in synaptic clefts activates NMDA and α-amino-3-hydroxy-5 methyl-4-isoxazolepropionic acid (AMPA) receptors, causing excitotoxic calcium influx (Lipton, 1999b). Pharmacological inhibition of NMDA receptors during early reperfusion following cerebral ischemia is shown to reduce ischemic damage in preclinical studies (Lipton, 1999b).
Channel arrest, a term coined to describe overall down-regulation of ion channels, enhances resistance to modeled ischemia in brain tissue from torpid ground squirrels (Doll et al., 1991). Hippocampal slices harvested from torpid ground squirrels or ground squirrels during interbout euthermia are demonstrated to resist injury during modeled ischemia (Ross et al., 2006). Resistance to injury due to oxygen and nutrient deprivation, however, persists for a longer period of time in culture in slices harvested from torpid animals (Ross et al., 2006). Furthermore, the Na+/K+ ATPase inhibitor ouabain induced significantly higher cell death in chronic hippocampal slices from interbout euthermic AGS (ibeAGS) compared to hibernating AGS (hAGS). The resistance to ouabain-induced toxicity suggests that channel arrest is more pronounced, and the requirement for ATP to maintain ionic gradients is significantly diminished during torpor. However, some degree of ion channel arrest persists during interbout euthermia since hippocampal slices from both hAGS and ibeAGS resist the excitotoxic effects of 500 μM NMDA and 20 mM KCl (NMDA/KCl) better than do ischemia-sensitive rats. Again, resistance to this excitotoxic challenge persisted longer in hippocampal slices obtained from hAGS than ibeAGS, supporting the observation that channel arrest is more pronounced and persistent during torpor. The phenomenon of channel arrest is also observed during euthermia in arctic ground squirrels (see section 3: Euthermic phase for details). It should be noted that more direct electrophysiological studies are needed for in depth characterization of the phenomenon of channel arrest in heterothermic animals during torpor and euthermia.
Downregulation of NMDA receptors (NMDAR) in the torpid state contributes to channel arrest in hAGS. NMDAR play an important role in excitotoxicity. NMDAR are composed of NMDAR1 (NR1), a variety of NMDAR2 (NR2) subunits (NR2A-D), and NMDAR3 (NR3). NMDAR activity is regulated by either phosphorylation of NR1 and NR2 subunits or by internalization of NR1 (Forrest et al., 1994; Liu and Zhang, 2000; Nakanishi, 1992; Sakimura et al., 1995; Zhao et al., 2006b). Glutamate-induced Ca2+ influx is suppressed in hippocampal slices from hAGS and ibeAGS compared to rat; however, the NMDAR-mediated contribution of glutamate-induced Ca2+ influx is suppressed more in hippocampal slices from hAGS than in ibeAGS (Zhao et al., 2006c). Down-regulation of NMDAR in hAGS may be due, in part, to decreased phosphorylation of NR1 in hAGS compared to ibeAGS and rats. To rule out the possibility of an epiphenomenon, more direct mechanistic studies using molecular biology tools are needed. Similar observations of channel arrest are made in other ischemia / anoxia-tolerant species such as turtles (Chrysemys picta). Anoxia-tolerant turtles exhibit silencing / down- regulation of NMDAR activity by its dephosphorylation (Bickler et al., 2000). A previous study demonstrated that ischemia tolerance can be induced in mice by knocking out the NR2A subunit (Morikawa et al., 1998). The phenomenon of excitotoxicity during hibernation and arousal is not well understood and warrants further study. Overall, the literature suggests that hibernating animals preserve neuronal ion homeostasis and decrease excitotoxicity by decreasing NMDAR activity during hibernation.
2.3 Synaptogenesis
Another interesting feature of hibernation is maintenance and regeneration of synapses. Spines and synaptic structures retract from disuse and cold temperatures (Fu et al., 2007; Kirov et al., 2004; Roelandse and Matus, 2004); however, hibernating species may limit temperature dependent loss of synaptic profiles. During torpor, dendritic branching and synaptic profiles (e.g., spine densities) decrease in complexity, size and numbers, but rapidly re-emerge upon arousal (Magarinos et al., 2006; Popov and Bocharova, 1992; Popov et al., 1992; von der Ohe et al., 2006). Synaptogenesis may contribute to cognitive enhancement observed 24h after arousal from torpor in AGS (Weltzin et al., 2006). A linear relationship exists between body temperature and loss of dendritic branches and synaptic profiles during torpor; however, retraction is slower than in nonhibernating species such as mice (von der Ohe et al., 2006). Hippocampal slices from mice show rapid loss of actin-based motility followed by loss of the entire dendritic spine structure at later times (Kirov et al., 2004; Roelandse and Matus, 2004). By contrast, in the hibernating Golden-mantled ground squirrel (Spermophilus lateralis), decreasing temperature from 17°C to 7°C does not produce further decreases in hippocampal CA3 spine density or other related parameters (von der Ohe et al., 2006). The limited temperature dependence of synaptic restructuring in these CA3 apical dendrites as well as incomplete loss of synaptic profiles at 7°C in other brain regions suggests that hibernators may limit temperature dependent loss of synaptic profiles (von der Ohe et al., 2006).
Paired helical filament-like hyperphosphorylation of the microtubule-associated protein tau may be one mechanism to stabilize the cytoskeleton and synaptic structure during torpor (Arendt et al., 2003). This interpretation supports the view that while tau hyperphosphorylation is a prelude to neurofibrillary tangles a histopathological hallmark of Alzheimers disease (AD) polymerization of hyperphosphorylated tau has a positive effect on microtubule assembly and is not likely the cause of cognitive decline (Alonso et al., 2001; Alonso Adel et al., 2006). Hyperphosphorylation of tau in hibernating European ground squirrels (Spermophilus citellus) reverses rapidly upon arousal from torpor (Arendt et al., 2003). Suppression of cerebral metabolism leads to regional cooling in brains of patients with AD and cold-induced inhibition of serine-threonine protein phosphatase 2A may allow hyperphosphorylated tau to accumulate in AD (Planel et al., 2004). Hibernating animals are temperature compensated in a way that avoids cold-induced inhibition of the phosphatases necessary for reversal of tau phosphorylation (Su et al., 2008). In this way, these animals may be able to reap benefits from tau phosphorylation such as stabilization of the cytoskeleton, but avoid pathological events caused by failed phosphatase activity.
Stabilization of the cytoskeleton during torpor may contribute to rapid regeneration of synapses during arousal. The rate of dendrite extension is rapid during arousal as compared to the rate in developing primate embryo or adult rat exposed to 4 months of environmental enrichment (Duffy and Rakic, 1983; Greenough et al., 1986; Magarinos et al., 2006; Markham and Greenough, 2004; von der Ohe et al., 2006). Mechanisms to minimize synaptic regression during torpor and to rapidly regenerate synapses upon arousal may contribute to successful entrance into and exit from hibernation without neuronal death.
2.4 Oxidative stress
A cerebral ischemia-induced limitation of substrates and increase in intracellular ions such as calcium leads to increased free radical release from mitochondria (Doyle et al., 2008; Lipton, 1999a; Niizuma et al., 2010). Ischemia also increases nitric oxide production by activating nitric oxide synthase (Doyle et al., 2008; Love, 1999; Samdani et al., 1997). Supra-physiological (i.e. pathological) levels of free radicals can damage proteins, lipids and nucleic acids leading to impaired cellular functions. Oxidative stress can deplete cellular NAD+ by activating the DNA repair enzyme poly (ADP-ribose) polymerase-1 (PARP-1) (Strosznajder et al., 2010). Depletion of NAD+ leads to impaired NAD+-dependent processes such as glycolysis, the tricarboxylic acid cycle and mitochondrial respiration leading to suppressed ATP production and ultimately cellular energy failure and cell death (Doyle et al., 2008; Skaper, 2003). Oxidative stress can also activate matrix metalloproteases, damaging the vascular wall and resulting in increased blood-brain barrier permeability (Crack and Taylor, 2005; Doyle et al., 2008; Romanic et al., 1998). In brief, ischemia-induced oxidative stress plays a crucial role in contributing to cell death.
In AGS, oxidative stress is absent during torpor due to suppressed oxidative metabolism (Orr et al., 2009). To determine the antioxidant status of hibernating animals during hibernation and during arousal, the dynamics of the antioxidants ascorbate and glutathione in two species of hibernating ground squirrels (AGS and 13-lined ground squirrels: Spermophilus tridecemlineatus) were determined (Drew et al., 1999). During hibernation, plasma ascorbate levels were drastically increased (3 – 4 fold) in both hibernating species studied. Ascorbate levels were returned to pre-hibernating / euthermic levels during arousal when reactive oxygen species generation was expected to peak during maximum oxygen consumption. A similar trend was observed in ascorbate levels in AGS cerebrospinal fluid (CSF). In contrast, brain ascorbate levels were lowered by 10 – 15% in both species during hibernation. Similar high plasma ascorbate levels were reported in hibernating woodchuck (Marmota imonax) (Bito and Roberts, 1974). High levels of ascorbate in plasma during hibernation suggest that ascorbate is poised to redistribute to protect tissues at the time of arousal when free radical generation is expected to peak.
In addition to small molecule antioxidants, attempts have been made to determine antioxidant enzyme levels during hibernation. In another species of hibernating ground squirrel, Citellus citellus, the levels of superoxide dismutase (SOD) were similar in a variety of tissues in the hibernating and active states, except the levels were higher in spleen during hibernation (Petrovic et al., 1983). Similar results were also observed in the 13-lined ground squirrel. The activities and levels of the two intracellular isoforms of SOD (CuZnSOD and MnSOD) were not different between active and hibernating squirrels (Page et al., 2009). However, SOD activity was significantly higher immediately after arousal in all tissues studied except brown adipose tissue. In a follow up study, the same group reported that CuZnSOD activity in brains of hibernating ground squirrel was higher compared with active animals (Petrovic et al., 1986). The activities of antioxidant defense system enzymes SOD, catalase, glutathione peroxidase and glutathione reductase are increased in the brains of Citellus citellus when animals are exposed to cold temperatures (Buzadzic et al., 1997). In hibernating Syrian hamsters (Mesocricetus auratus), an extracellular protein of 260-kDa exhibiting SOD-like activity was suggested to play a role in tolerance from oxidative stress during arousal from torpor (Okamoto et al., 2006).
Overall, the literature suggests that the levels of antioxidant defense molecules are increased in hibernating animals either during hibernation or at the time of arousal. It is plausible that increased antioxidant capacity during hibernation and at the time of arousal may enhance tissue protection in hibernating animals.
2.5 Inflammatory responses
Cellular as well as cytokine-mediated inflammatory responses play an important role in ischemia-induced brain damage. Cellular inflammatory responses following cerebral ischemia include activation of microglia and astrocytes and infiltration of blood cells, primarily neutrophils, into the brain (del Zoppo, 2010; Doyle et al., 2008; Taoufik and Probert, 2008). Depletion of neutrophils and suppression of leukocyte adhesion lowers ischemic damage in animal models of cerebral ischemia (Bowes et al., 1993; Dawson et al., 1996; Drew et al., 2001; Zhang et al., 1994). Tumor necrosis factor (TNF), interleukin 1 (IL-1), and interleukin 6 (IL-6) are three major cytokines that participate in ischemic damage (del Zoppo, 2010; Doyle et al., 2008). The role of TNF in exacerbating ischemic injury is controversial. Exogenous delivery of TNF into ischemic tissue is shown to aggravate injury (Barone et al., 1997; Taoufik and Probert, 2008). Antibodies to TNF and TNF binding proteins afford protection against ischemic damage, while TNF knock-out mice show reduced ischemic damage (Martin-Villalba et al., 2001; Taoufik and Probert, 2008). In contrast, mice deficient in two TNF receptors exhibited increased ischemic damage (Bruce et al., 1996; Taoufik and Probert, 2008). In general, excessive inflammatory response against ischemic tissue is responsible for increased damage.
During hibernation, the concentration of leukocytes in the circulation drops drastically. In AGS, the leukocyte count drops by more than 90% (Drew et al., 2001; Toien et al., 2001), while during arousal, the leukocyte count is restored rapidly to normal levels (Drew et al., 2001; Toien et al., 2001). Moreover, leukopoietic activity in bone marrow is drastically reduced during hibernation (Drew et al., 2001; Szilagyi and Senturia, 1972). Furthermore, there are large numbers of mature leukocytes stored in the bone marrow during hibernation (Drew et al., 2001; Szilagyi and Senturia, 1972). These data suggest that stored leukocytes rapidly enter the circulation restoring their levels upon arousal. Expression of intercellular adhesion molecule-1 (ICAM-1) on rat microvascular endothelial cells is increased when exposed to plasma from hibernating 13-lined ground squirrels (Drew et al., 2001; Yasuma et al., 1997), suggesting that increased ICAM-1 expression may be responsible for margination or extravasation of leukocytes from the circulation (Yasuma et al., 1997). In addition, antibody production from leukocytes is drastically reduced during hibernation (Drew et al., 2001; McKenna and Musacchia, 1968; Sidky and Auerbach, 1968). In hibernating hamsters and ground squirrels, fat-soluble factors released from brown adipose tissue can act as immunosuppressants during hibernation (Atanassov et al., 1995; Drew et al., 2001; Sidky et al., 1969). Low body temperature promotes storage of lymphocytes in secondary lymphoid organs, a response that depends on a temperature-dependent decline in sphingosine-1-phosphate expression (Bouma et al., 2011). Overall, these data suggest that suppressed inflammatory pathways during hibernation may be, in part, responsible for absence of brain damage in aroused hibernating animals. In particular, cerebral leukocyte invasion is a significant contributor to deleterious neuroinflammation after stroke (Liesz et al., 2011). Further work is necessary to understand the mechanisms that cause hibernation associated lymphopenia which may have direct implications for reducing neuronal damage in stroke.
2.6 Cell death pathways
Cell death following ischemic insult occurs by necrosis, apoptosis and autophagy (Doyle et al., 2008; Taoufik and Probert, 2008). It appears that all of these processes work in parallel, because cells have been observed to switch from one type of death to another. Ionic imbalance, excitotoxicity, increased oxidative stress, DNA damage, protease activation, mitochondrial dysfunction, and the release of pro-apoptotic molecules from mitochondria are major triggers for cell death pathways (Doyle et al., 2008; Taoufik and Probert, 2008).
At ambient temperatures below about 30 °C torpor is interrupted by brief periods of euthermia (Dausmann et al., 2004). What induces these arousals is unknown although they represent significant energy demand. For torpid hibernators housed near their thermoneutral zone, i.e., a range of ambient termperature where torpid metabolic rate is minimal, about 70% of energy reserves required for the entire hibernation season are consumed during arousal and subsequent episodes of euthermia [reviewed in (Heldmaier et al., 2004)]. During this period of high metabolic demand, hemoglobin-O2 saturation (or sO2) falls from a mean of 86% to a minimum of 57% without producing neuronal histopathology or oxidative stress in AGS (Ma et al., 2005). Humans and other homeothermic mammals require sO2 of greater than 95% to maintain healthy levels of tissue oxygenation. The partial pressure of arterial oxygen (Pao2) also falls in AGS from about 60 mmHg to as low as 7 mmHg. The affinity of hemoglobin for oxygen differs among species (Maginniss and Milsom, 1994) and in golden-mantled ground squirrels a higher affinity for oxygen, especially during torpor, means animals maintain high sO2 at lower Pao2. However, this is not the case for AGS where a low sO2 indicates that tissue oxygenation does not keep pace with demand during arousal.
Hypoxia inducible factor 1 α (HIF1α) is a transcription activator that is stabilized under hypoxic conditions. HIF1α activates target genes involved in ischemic preconditioning and hypoxia / ischemia adaptation (Ratan et al., 2004). Owing to robust oxygen demand during arousal, animals experience severe depletion in arterial O2 tension (Pao2) and sO2 as discussed above (Ma et al., 2005). Lower PaO2 and sO2 during arousal stabilizes HIF1α (Ma et al., 2005). Activation of extracellular signal-regulated protein kinase (ERK) and c-Jun N-terminal kinase / stress-activated protein kinase (JNK) is observed during arousal of AGS, and the levels of activated ERK and JNK are correlated with levels of HIF1α during arousal (Zhu et al., 2005). Moreover, inhibition of JNK reduced baseline survival of AGS hippocampal neurons in acute hippocampal slices, demonstrating that JNK promotes neuronal survival in this species (Christian et al., 2008). An earlier study found decreased phosphorylation of Akt, a pro-survival protein, in the brain and muscle of hibernating 13-lined ground squirrels (Cai et al., 2004). Fleck and Carey observed that expression of antiapoptotic proteins is increased during hibernation in enterocytes of 13-lined ground squirrels (Fleck and Carey, 2005). Overall, these studies demonstrate that during arousal, stabilized HIF1α and other mechanisms activate cell survival pathways. Chen and colleagues demonstrated that, in the greater horseshoe bat (Rhinolophus ferrumequinum), a majority of the 17 genes that are overexpressed during hibernation are responsible for regulation of apoptosis, neuronal growth, signal transduction, and neuroprotection (Chen et al., 2008). More work is required to investigate isozyme specific effects of MAPK signaling and to explore downstream consequences of chronically high expression of HIF 1α.
These studies also demonstrate that biochemically, the brains of hibernators resemble a preconditioned-like state that protects the brain from ischemia / reperfusion injury. Unlike preconditioning, AGS retain ischemia tolerance and do not require a sublethal stimulus to induce tolerance. Ischemic preconditioning was found to induce changes in gene expression consistent with suppression of metabolic pathways and immune responses and reduction of ion channel activity (Stenzel-Poore et al., 2003), all of which are characteristic of hibernation and thought to contribute to neuroprotection in the hibernating state (Drew et al., 2001). Stenzel-Poore et al. (2007) suggest that preconditioning is associated with reprogramming of the cytotoxic cellular response. This reprogrammed response resembles the naïve response in hibernating species. Overall, activation of cell survival pathways during hibernation and /or arousal may protect brains of hibernators from ischemia / reperfusion injury.
2.7 Adenosine A1 receptors
A1AR activation induces ischemia tolerance in homeothermic species through activation of phospholipase C, production of diacylglycerol and subsequent activation of protein kinase C, epsilon (PKCε) (Di-Capua et al., 2003; Lange-Asschenfeldt et al., 2004; Raval et al., 2003). The role of A1AR activation was also demonstrated in an in vitro model of anoxic preconditioning (Perez-Pinzon et al., 1996). In other models of ischemic preconditioning A1AR activation was shown to confer neuroprotection via opening of ATP-sensitive potassium channels (Heurteaux et al., 1995; Reshef et al., 2000). Anoxia tolerance in certain species of turtle also requires adenosine binding to AlAR (Perez-Pinzon et al., 1993).
Although seasonal sensitization of A1AR signaling is clearly poised to contribute to ischemia tolerance, a role for adenosine in ischemia tolerance in AGS has not been observed. Resistance to ischemic injury in vivo or in vitro is independent of the hibernation season (Christian et al., 2008; Dave et al., 2006, 2009). Moreover, the delay in anoxic depolarization is not reversed by an A1AR antagonist (Dave et al., unpublished), nor is ischemia tolerance attenuated by an A1AR antagonist despite an OGD-induced increase in adenosine release in brain slices harvested from AGS during the hibernation season (Drew et al., unpublished). Overall, these studies suggest that activation of A1AR is not necessary for the robust ischemia tolerance in hibernators.
2.8 Other neuroprotective mechanisms
Cerebral blood flow falls to ischemic-like levels during torpor without apparent neuronal histopathology or neurological deficits (Frerichs and Hallenbeck, 1998a). Unlike ischemia, however, torpid hibernators do not experience deficits in brain energy or O2 supply because the decreased blood flow meets the needs of decreased metabolic demand. This suggests that hibernators are not in an ischemic state once they have entered torpor (Henry et al., 2007; Lust et al., 1989). This balance between supply and demand may wane, however, during the metabolically demanding process of arousal (Ma et al., 2005). Although energy charge is not affected by arousal in hamsters (Lust et al., 1989) low sO2 in AGS suggests that this species experiences deficient O2 delivery during arousal. Nonetheless, “reperfusion” during arousal from torpor is not injurious (Ma et al., 2005). In contrast, reperfusion of ischemic tissues in nonhibernating mammals can induce vasospasm, with subsequent disruption in blood flow as well as inflammatory and pro-oxidative and pro-nitrosative processes that contribute to tissue damage and poor recovery following stroke and cardiac arrest (Hossmann, 1997; Palomares and Cipolla, 2011).
Consistent with previous studies (Krilowicz, 1985), torpid AGS have higher levels of blood ketones than 24 h-aroused AGS (Christian et al., 2008). Rapid processing of ketone bodies during the first 24 h of arousal indicates that ketones are an important source of energy during arousal and may play a neuroprotective role (Andrews, 2007; Maalouf et al., 2007). Further work is needed to understand the relationship between ketone catabolism and neuroprotection in hibernation.
3. Euthermic phase
In vitro studies demonstrate resistance to modeled ischemia in hippocampus from both hAGS and ibeAGS compared to the more ischemia-sensitive rat (Ross et al., 2006). Brains of ibeAGS retain a significant capacity to tolerate energy deprivation even when not in a torpid state. Tolerance in ibeAGS appears to involve channel arrest, but of a more limited scope than the channel arrest that occurs during torpor in hAGS. Relative to rat, glutamate-induced Ca2+ influx is suppressed in both hAGS and ibeAGS and membrane expression of the essential NR1 subunit for NMDAR is lower in hAGS and ibeAGS than in rat (Zhao et al., 2006a).
These in vitro observations of tolerance were extended to model ischemia into the in vivo condition by using a clinically relevant model of cardiac arrest. Using this model, it was demonstrated that summer euthermic AGS resist injury from an ischemic insult even when the body temperature is maintained at 37 °C (Dave et al., 2009; Dave et al., 2006). Based on hippocampal slices harvested from hibernating and euthermic 13-lined ground squirrels, Frerichs and Hallenbeck observed that at 36°C the duration of ischemia tolerated by slices prepared from active ground squirrels did not differ from slices prepared from rats, and slices from both rats and active ground squirrels were more vulnerable to modeled I/R than slices prepared from torpid ground squirrels (Frerichs and Hallenbeck, 1998b). Later it was demonstrated that slices from euthermic AGS tolerate oxygen / glucose deprivation better than do rats, even at 37°C (Ross et al., 2006). These studies thus suggest that ischemia tolerance exists in AGS not only during torpor / hibernation but also during euthermia and that the degree of tolerance in AGS may not be matched by all hibernating species. Surprisingly, an earlier study demonstrated that euthermic AGS maintained lower PaO2 and elevated PaCO2 compared to rat (Ma et al., 2005). It is possible that owing to chronic mild degrees of anoxia and hypercapnia, ischemia-tolerant pathways are active in euthermic AGS. In support of this speculation, euthermic AGS brains displayed HIF1α protein levels that were as high as levels observed after arousal and more than double the levels observed in tissue from hibernating AGS (Ma et al., 2005). Upregulation of HIF1α and HIF1α-regulated gene expression plays an important role in hypoxia-induced ischemic tolerance (Bergeron et al., 2000; Bernaudin et al., 2002; Bruer et al., 1997; Gidday et al., 1994; Jones and Bergeron, 2001; Miller et al., 2001; Prass et al., 2003; Ruscher et al., 2002).
In addition, we have observed that in vivo, and in hippocampal slices, ischemic depolarization is delayed in euthermic AGS when compared to rats, supporting the idea that channel arrest is present to some extent even in euthermic AGS (Dave et al., 2009). It should be noted that we had not observed any spontaneous torpor in these summer active animals. Neuronal depolarization during ischemia represents a collapse of ion homeostasis ultimately leading to neuronal death (Kaminogo et al., 1998). Blocking or delaying ischemic depolarization can significantly improve ischemia recovery (Anderson et al., 2005; Takeda et al., 2003). Also, inhibition of PKCε during in vitro ischemia reversed the delay in ischemic depolarization in slices from euthermic AGS but had no effect on the timing of depolarization in slices from rats (Dave et al., 2009). PKCε activation inhibits Na+/K+-ATPase and voltage-gated sodium channel (VGSC) (Chen et al., 2005; Nowak et al., 2004), both key players in ion homeostasis and its collapse during ischemia. This observation indicates that PKCε activation is required for the robust ion homeostasis in euthermic AGS brain tissue during experimental ischemia. It is possible that in AGS neurons, PKCε is poised to regulate this balance by decreasing VGSC and Na+/K+-ATPase function. Activation of PKCε plays an essential role in induction of ischemia tolerance in many organs including brain and heart (Perez-Pinzon, 2007; Savitz and Fisher, 2007). In summary, we and others have demonstrated that, ischemia tolerance pathways are chronically active in euthermic hibernators.
4. Summary
Mammals capable of hibernation represent a robust example of tolerance to cerebral ischemia that is unmatched by any other model of ischemia tolerance. Hibernation is characterized by profound decreases in metabolic demand and body temperature as well as decreased blood flow does not produce an ischemic state. Brief, interbout arousals occur frequently throughout the hibernation season and appear to be the most physiologically challenging aspect of heterothermy where blood flow may not meet cellular demands. Moreover, synapses lost during prolonged torpor rapidly return during interbout arousal. Thus, tolerance to rapid changes in blood flow and metabolic demand as well as for the capacity to stabilize and regenerate synapses likely evolved as means for successful hibernation (Figure 1). Humans, in contrast, suffer significant consequences in response to limited blood flow where they experience significant injury in response to ischemia and reperfusion, in addition to having limited capacity for synapse regeneration. There is much to be learned about cellular, molecular and systems-wide mechanisms that protect heterothermic mammals from ischemia and promote synaptic regeneration upon arousal. Currently, little is known about these mechanisms; however, clear appreciation of the degree of ischemia tolerance these animals display opens the door for further in depth mechanistic studies. Better understanding of regulatory mechanisms will guide discovery of therapeutics designed to mimic natural means of ischemia tolerance and adult synaptic plasticity.
Acknowledgments
This work was supported by the US Army Research Office W911NF-05-1-0280, The US Army Medical Research and Materiel Command 05178001, the National Institute of Neurological Disorders and Stroke NS041069-06, R15NS070779, NS45676-01, NS054147-01, and NS34773. We thank Dr. Brant D. Watson for critical reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alonso A, Zaidi T, Novak M, Grundke-Iqbal I, Iqbal K. Hyperphosphorylation induces self-assembly of tau into tangles of paired helical filaments/straight filaments. Proc Natl Acad Sci USA. 2001;98:6923–6928. doi: 10.1073/pnas.121119298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso Adel C, Li B, Grundke-Iqbal I, Iqbal K. Polymerization of hyperphosphorylated tau into filaments eliminates its inhibitory activity. Proc Natl Acad Sci USA. 2006;103:8864–8869. doi: 10.1073/pnas.0603214103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson TR, Jarvis CR, Biedermann AJ, Molnar C, Andrew RD. Blocking the anoxic depolarization protects without functional compromise following simulated stroke in cortical brain slices. J Neurophysiol. 2005;93:963–979. doi: 10.1152/jn.00654.2004. [DOI] [PubMed] [Google Scholar]
- Andrews MT. Advances in molecular biology of hibernation in mammals. Bioessays. 2007;29:431–440. doi: 10.1002/bies.20560. [DOI] [PubMed] [Google Scholar]
- Arendt T, Stieler J, Strijkstra AM, Hut RA, Rudiger J, Van der Zee EA, Harkany T, Holzer M, Hartig W. Reversible paired helical filament-like phosphorylation of tau is an adaptive process associated with neuronal plasticity in hibernating animals. J Neurosci. 2003;23:6972–6981. doi: 10.1523/JNEUROSCI.23-18-06972.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atanassov CL, Naegeli HU, Zenke G, Schneider C, Kramarova LI, Bronnikov GE, Van Regenmortel MH. Anti-lymphoproliferative activity of brown adipose tissue of hibernating ground squirrels is mainly caused by AMP. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1995;112:93–100. doi: 10.1016/0742-8413(95)00000-3. [DOI] [PubMed] [Google Scholar]
- Baker CJ, Fiore AJ, Frazzini VI, Choudhri TF, Zubay GP, Solomon RA. Intraischemic hypothermia decreases the release of glutamate in the cores of permanent focal cerebral infarcts. Neurosurgery. 1995;36:994–1001. doi: 10.1227/00006123-199505000-00016. discussion 1001–1002. [DOI] [PubMed] [Google Scholar]
- Barger JL, Brand MD, Barnes BM, Boyer BB. Tissue-specific depression of mitochondrial proton leak and substrate oxidation in hibernating arctic ground squirrels. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1306–1313. doi: 10.1152/ajpregu.00579.2002. [DOI] [PubMed] [Google Scholar]
- Barnes BM. Freeze avoidance in a mammal: body temperatures below 0 degree C in an Arctic hibernator. Science. 1989;244:1593–1595. doi: 10.1126/science.2740905. [DOI] [PubMed] [Google Scholar]
- Barone FC, Arvin B, White RF, Miller A, Webb CL, Willette RN, Lysko PG, Feuerstein GZ. Tumor necrosis factor-alpha. A mediator of focal ischemic brain injury. Stroke. 1997;28:1233–1244. doi: 10.1161/01.str.28.6.1233. [DOI] [PubMed] [Google Scholar]
- Bergeron M, Gidday JM, Yu AY, Semenza GL, Ferriero DM, Sharp FR. Role of hypoxia-inducible factor-1 in hypoxia-induced ischemic tolerance in neonatal rat brain. Ann Neurol. 2000;48:285–296. [PubMed] [Google Scholar]
- Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- Bernaudin M, Nedelec AS, Divoux D, MacKenzie ET, Petit E, Schumann-Bard P. Normobaric hypoxia induces tolerance to focal permanent cerebral ischemia in association with an increased expression of hypoxia-inducible factor-1 and its target genes, erythropoietin and VEGF, in the adult mouse brain. J Cereb Blood Flow Metab. 2002;22:393–403. doi: 10.1097/00004647-200204000-00003. [DOI] [PubMed] [Google Scholar]
- Bickler PE, Donohoe PH, Buck LT. Hypoxia-induced silencing of NMDA receptors in turtle neurons. J Neurosci. 2000;20:3522–3528. doi: 10.1523/JNEUROSCI.20-10-03522.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bito LZ, Roberts JC. The effects of hibernation on the chemical composition of cerebrospinal and intraocular fluids, blood plasma and brain tissue of the woodchuck (Marmota monax) Comp Biochem Physiol A Comp Physiol. 1974;47:183–193. doi: 10.1016/0300-9629(74)90063-2. [DOI] [PubMed] [Google Scholar]
- Bouma HR, Kroese FG, Kok JW, Talaei F, Boerema AS, Herwig A, Draghiciu O, van Buiten A, Epema AH, van Dam A, Strijkstra AM, Henning RH. Low body temperature governs the decline of circulating lymphocytes during hibernation through sphingosine-1-phosphate. Proc Natl Acad Sci USA. 2011;108:2052–2057. doi: 10.1073/pnas.1008823108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973;134:707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowes MP, Zivin JA, Rothlein R. Monoclonal antibody to the ICAM-1 adhesion site reduces neurological damage in a rabbit cerebral embolism stroke model. Exp Neurol. 1993;119:215–219. doi: 10.1006/exnr.1993.1023. [DOI] [PubMed] [Google Scholar]
- Bruce AJ, Boling W, Kindy MS, Peschon J, Kraemer PJ, Carpenter MK, Holtsberg FW, Mattson MP. Altered neuronal and microglial responses to excitotoxic and ischemic brain injury in mice lacking TNF receptors. Nat Med. 1996;2:788–794. doi: 10.1038/nm0796-788. [DOI] [PubMed] [Google Scholar]
- Bruer U, Weih MK, Isaev NK, Meisel A, Ruscher K, Bergk A, Trendelenburg G, Wiegand F, Victorov IV, Dirnagl U. Induction of tolerance in rat cortical neurons: hypoxic preconditioning. FEBS Lett. 1997;414:117–121. doi: 10.1016/s0014-5793(97)00954-x. [DOI] [PubMed] [Google Scholar]
- Buck CL, Barnes BM. Effects of ambient temperature on metabolic rate, respiratory quotient, and torpor in an arctic hibernator. Am J Physiol Regul Integr Comp Physiol. 2000a;279:R255–262. doi: 10.1152/ajpregu.2000.279.1.R255. [DOI] [PubMed] [Google Scholar]
- Buck CL, Barnes BM. Effects of ambient temperature on metabolic rate, respiratory quotient, and torpor in an arctic hibernator. Am J Physiol Regul Integr Comp Physiol. 2000b;279:R255–262. doi: 10.1152/ajpregu.2000.279.1.R255. [DOI] [PubMed] [Google Scholar]
- Buzadzic B, Blagojevic D, Korac B, Saicic ZS, Spasic MB, Petrovic VM. Seasonal variation in the antioxidant defense system of the brain of the ground squirrel (Citellus citellus) and response to low temperature compared with rat. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1997;117:141–149. doi: 10.1016/s0742-8413(97)00061-3. [DOI] [PubMed] [Google Scholar]
- Cai D, McCarron RM, Yu EZ, Li Y, Hallenbeck J. Akt phosphorylation and kinase activity are down-regulated during hibernation in the 13-lined ground squirrel. Brain Res. 2004;1014:14–21. doi: 10.1016/j.brainres.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Chen J, Yuan L, Sun M, Zhang L, Zhang S. Screening of hibernation-related genes in the brain of Rhinolophus ferrumequinum during hibernation. Comp Biochem Physiol B Biochem Mol Biol. 2008;149:388–393. doi: 10.1016/j.cbpb.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Chen Y, Cantrell AR, Messing RO, Scheuer T, Catterall WA. Specific modulation of Na+ channels in hippocampal neurons by protein kinase C epsilon. J Neurosci. 2005;25:507–513. doi: 10.1523/JNEUROSCI.4089-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian SL, Ross AP, Zhao HW, Kristenson HJ, Zhan X, Rasley BT, Bickler PE, Drew KL. Arctic ground squirrel (Spermophilus parryii) hippocampal neurons tolerate prolonged oxygen-glucose deprivation and maintain baseline ERK1/2 and JNK activation despite drastic ATP loss. J Cereb Blood Flow Metab. 2008;28:1307–1319. doi: 10.1038/jcbfm.2008.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crack PJ, Taylor JM. Reactive oxygen species and the modulation of stroke. Free Radic Biol Med. 2005;38:1433–1444. doi: 10.1016/j.freeradbiomed.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Dausmann KH, Glos J, Ganzhorn JU, Heldmaier G. Physiology: hibernation in a tropical primate. Nature. 2004;429:825–826. doi: 10.1038/429825a. [DOI] [PubMed] [Google Scholar]
- Dave KR, Anthony Defazio R, Raval AP, Dashkin O, Saul I, Iceman KE, Perez-Pinzon MA, Drew KL. Protein kinase C epsilon activation delays neuronal depolarization during cardiac arrest in the euthermic arctic ground squirrel. J Neurochem. 2009;110:1170–1179. doi: 10.1111/j.1471-4159.2009.06196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave KR, Prado R, Raval AP, Drew KL, Perez-Pinzon MA. The arctic ground squirrel brain is resistant to injury from cardiac arrest during euthermia. Stroke. 2006;37:1261–1265. doi: 10.1161/01.STR.0000217409.60731.38. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Ruetzler CA, Carlos TM, Kochanek PM, Hallenbeck JM. Polymorphonuclear leukocytes and microcirculatory perfusion in acute stroke in the SHR. Keio J Med. 1996;45:248–252. doi: 10.2302/kjm.45.248. [DOI] [PubMed] [Google Scholar]
- del Zoppo GJ. Acute anti-inflammatory approaches to ischemic stroke. Ann N Y Acad Sci. 2010;1207:143–148. doi: 10.1111/j.1749-6632.2010.05761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di-Capua N, Sperling O, Zoref-Shani E. Protein kinase C-epsilon is involved in the adenosine-activated signal transduction pathway conferring protection against ischemia-reperfusion injury in primary rat neuronal cultures. J Neurochem. 2003;84:409–412. doi: 10.1046/j.1471-4159.2003.01563.x. [DOI] [PubMed] [Google Scholar]
- Doll CJ, Hochachka PW, Reiner PB. Channel arrest: implications from membrane resistance in turtle neurons. Am J Physiol Regul Integr Comp Physiol. 1991;261:R1321–1324. doi: 10.1152/ajpregu.1991.261.5.R1321. [DOI] [PubMed] [Google Scholar]
- Doyle KP, Simon RP, Stenzel-Poore MP. Mechanisms of ischemic brain damage. Neuropharmacology. 2008;55:310–318. doi: 10.1016/j.neuropharm.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew K, Zuckerman J, Shenk P, Bogren L, Jinka T, Moore J. Hibernation: a natural model of tolerance to cerebral ischemia/reperfusion. In: Gidday J, Perez-Pinzon M, Zhang J, editors. Innate Neuroprotection for Stroke. Springer; New York, NY: 2012. In Press. [Google Scholar]
- Drew KL, Buck CL, Barnes BM, Christian SL, Rasley BT, Harris MB. Central nervous system regulation of mammalian hibernation: implications for metabolic suppression and ischemia tolerance. J Neurochem. 2007;102:1713–1726. doi: 10.1111/j.1471-4159.2007.04675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew KL, Christian SL, Jinka TR, Hollen L, Dehn J. “Natural” tolerance in hibernators: Can we learn from physiological and preconditioning against ischemic or hypoxic brain injury? Research Signpost; Trivandrum, Kerala, India: 2009. pp. 1–44. [Google Scholar]
- Drew KL, Harris MB, LaManna JC, Smith MA, Zhu XW, Ma YL. Hypoxia tolerance in mammalian heterotherms. J Exp Biol. 2004;207:3155–3162. doi: 10.1242/jeb.01114. [DOI] [PubMed] [Google Scholar]
- Drew KL, Osborne PG, Frerichs KU, Hu Y, Koren RE, Hallenbeck JM, Rice ME. Ascorbate and glutathione regulation in hibernating ground squirrels. Brain Res. 1999;851:1–8. doi: 10.1016/s0006-8993(99)01969-1. [DOI] [PubMed] [Google Scholar]
- Drew KL, Rice ME, Kuhn TB, Smith MA. Neuroprotective adaptations in hibernation: therapeutic implications for ischemia-reperfusion, traumatic brain injury and neurodegenerative diseases. Free Radic Biol Med. 2001;31:563–573. doi: 10.1016/s0891-5849(01)00628-1. [DOI] [PubMed] [Google Scholar]
- Drew KL, Toien O, Rivera PM, Smith MA, Perry G, Rice ME. Role of the antioxidant ascorbate in hibernation and warming from hibernation. Comp Biochem Physiol C Toxicol Pharmacol. 2002;133:483–492. doi: 10.1016/s1532-0456(02)00118-7. [DOI] [PubMed] [Google Scholar]
- Duffy CJ, Rakic P. Differentiation of granule cell dendrites in the dentate gyrus of the rhesus monkey: a quantitative Golgi study. J Comp Neurol. 1983;214:224–237. doi: 10.1002/cne.902140210. [DOI] [PubMed] [Google Scholar]
- Fleck CC, Carey HV. Modulation of apoptotic pathways in intestinal mucosa during hibernation. Am J Physiol Regul Integr Comp Physiol. 2005;289:R586–R595. doi: 10.1152/ajpregu.00100.2005. [DOI] [PubMed] [Google Scholar]
- Forrest D, Yuzaki M, Soares HD, Ng L, Luk DC, Sheng M, Stewart CL, Morgan JI, Connor JA, Curran T. Targeted disruption of NMDA receptor 1 gene abolishes NMDA response and results in neonatal death. Neuron. 1994;13:325–338. doi: 10.1016/0896-6273(94)90350-6. [DOI] [PubMed] [Google Scholar]
- Frazzini VI, Winfree CJ, Choudhri HF, Prestigiacomo CJ, Solomon RA. Mild hypothermia and MK-801 have similar but not additive degrees of cerebroprotection in the rat permanent focal ischemia model. Neurosurgery. 1994;34:1040–1045. doi: 10.1227/00006123-199406000-00013. discussion 1045–1046. [DOI] [PubMed] [Google Scholar]
- Frerichs KU, Hallenbeck JM. Hibernation in ground squirrels induces state and species-specific tolerance to hypoxia and aglycemia: an in vitro study in hippocampal slices. J Cereb Blood Flow Metab. 1998a;18:168–175. doi: 10.1097/00004647-199802000-00007. [DOI] [PubMed] [Google Scholar]
- Frerichs KU, Hallenbeck JM. Hibernation in ground squirrels induces state and species-specific tolerance to hypoxia and aglycemia: an in vitro study in hippocampal slices. J Cereb Blood Flow Metab. 1998b;18:168–175. doi: 10.1097/00004647-199802000-00007. [DOI] [PubMed] [Google Scholar]
- Fu Z, Lee SH, Simonetta A, Hansen J, Sheng M, Pak DT. Differential roles of Rap1 and Rap2 small GTPases in neurite retraction and synapse elimination in hippocampal spiny neurons. J Neurochem. 2007;100:118–131. doi: 10.1111/j.1471-4159.2006.04195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui O, Kinugasa Y, Fukuda A, Fukuda H, Tskitishvili E, Hayashi S, Song M, Kanagawa T, Hosono T, Shimoya K, Murata Y. Post-ischemic hypothermia reduced IL-18 expression and suppressed microglial activation in the immature brain. Brain Res. 2006;1121:35–45. doi: 10.1016/j.brainres.2006.08.121. [DOI] [PubMed] [Google Scholar]
- Geiser F. Metabolic rate and body temperature reduction during hibernation and daily torpor. Annu Rev Physiol. 2004;66:239–274. doi: 10.1146/annurev.physiol.66.032102.115105. [DOI] [PubMed] [Google Scholar]
- Gidday JM, Fitzgibbons JC, Shah AR, Park TS. Neuroprotection from ischemic brain injury by hypoxic preconditioning in the neonatal rat. Neurosci Lett. 1994;168:221–224. doi: 10.1016/0304-3940(94)90455-3. [DOI] [PubMed] [Google Scholar]
- Greenough WT, McDonald JW, Parnisari RM, Camel JE. Environmental conditions modulate degeneration and new dendrite growth in cerebellum of senescent rats. Brain Res. 1986;380:136–143. doi: 10.1016/0006-8993(86)91437-x. [DOI] [PubMed] [Google Scholar]
- Han HS, Karabiyikoglu M, Kelly S, Sobel RA, Yenari MA. Mild hypothermia inhibits nuclear factor-kappaB translocation in experimental stroke. J Cereb Blood Flow Metab. 2003;23:589–598. doi: 10.1097/01.WCB.0000059566.39780.8D. [DOI] [PubMed] [Google Scholar]
- Heldmaier G, Ortmann S, Elvert R. Natural hypometabolism during hibernation and daily torpor in mammals. Respir Physiol Neurobiol. 2004;141:317–329. doi: 10.1016/j.resp.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Henry PG, Russeth KP, Tkac I, Drewes LR, Andrews MT, Gruetter R. Brain energy metabolism and neurotransmission at near-freezing temperatures: in vivo (1)H MRS study of a hibernating mammal. J Neurochem. 2007;101:1505–1515. doi: 10.1111/j.1471-4159.2007.04514.x. [DOI] [PubMed] [Google Scholar]
- Heurteaux C, Lauritzen I, Widmann C, Lazdunski M. Essential role of adenosine, adenosine A1 receptors, and ATP-sensitive K+ channels in cerebral ischemic preconditioning. Proc Natl Acad Sci USA. 1995;92:4666–4670. doi: 10.1073/pnas.92.10.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossmann KA. Reperfusion of the brain after global ischemia: hemodynamic disturbances. Shock. 1997;8:95–101. doi: 10.1097/00024382-199708000-00004. discussion 102–103. [DOI] [PubMed] [Google Scholar]
- Hypothermia after Cardiac Arrest Study G. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- Ji X, Luo Y, Ling F, Stetler RA, Lan J, Cao G, Chen J. Mild hypothermia diminishes oxidative DNA damage and pro-death signaling events after cerebral ischemia: a mechanism for neuroprotection. Front Biosci. 2007;12:1737–1747. doi: 10.2741/2185. [DOI] [PubMed] [Google Scholar]
- Jinka TR, Toien O, Drew KL. Season primes the brain in an arctic hibernator to facilitate entrance into torpor mediated by adenosine A1 receptors. J Neurosci. 2011;31:10752–10758. doi: 10.1523/JNEUROSCI.1240-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones NM, Bergeron M. Hypoxic preconditioning induces changes in HIF-1 target genes in neonatal rat brain. J Cereb Blood Flow Metab. 2001;21:1105–1114. doi: 10.1097/00004647-200109000-00008. [DOI] [PubMed] [Google Scholar]
- Kaminogo M, Suyama K, Ichikura A, Onizuka M, Shibata S. Anoxic depolarization determines ischemic brain injury. Neurol Res. 1998;20:343–348. doi: 10.1080/01616412.1998.11740529. [DOI] [PubMed] [Google Scholar]
- Karibe H, Chen SF, Zarow GJ, Gafni J, Graham SH, Chan PH, Weinstein PR. Mild intraischemic hypothermia suppresses consumption of endogenous antioxidants after temporary focal ischemia in rats. Brain Res. 1994;649:12–18. doi: 10.1016/0006-8993(94)91043-x. [DOI] [PubMed] [Google Scholar]
- Katz LM, Young AS, Frank JE, Wang Y, Park K. Regulated hypothermia reduces brain oxidative stress after hypoxic-ischemia. Brain Res. 2004;1017:85–91. doi: 10.1016/j.brainres.2004.05.020. [DOI] [PubMed] [Google Scholar]
- Kirov SA, Petrak LJ, Fiala JC, Harris KM. Dendritic spines disappear with chilling but proliferate excessively upon rewarming of mature hippocampus. Neuroscience. 2004;127:69–80. doi: 10.1016/j.neuroscience.2004.04.053. [DOI] [PubMed] [Google Scholar]
- Krieger DW, Yenari MA. Therapeutic hypothermia for acute ischemic stroke: what do laboratory studies teach us? Stroke. 2004;35:1482–1489. doi: 10.1161/01.STR.0000126118.44249.5c. [DOI] [PubMed] [Google Scholar]
- Krilowicz BL. Ketone body metabolism in a ground squirrel during hibernation and fasting. Am J Physiol Regul Integr Comp Physiol. 1985;249:R462–470. doi: 10.1152/ajpregu.1985.249.4.R462. [DOI] [PubMed] [Google Scholar]
- Lange-Asschenfeldt C, Raval AP, Dave KR, Mochly-Rosen D, Sick TJ, Perez-Pinzon MA. Epsilon protein kinase C mediated ischemic tolerance requires activation of the extracellular regulated kinase pathway in the organotypic hippocampal slice. J Cereb Blood Flow Metab. 2004;24:636–645. doi: 10.1097/01.WCB.0000121235.42748.BF. [DOI] [PubMed] [Google Scholar]
- Liesz A, Zhou W, Mracsko E, Karcher S, Bauer H, Schwarting S, Sun L, Bruder D, Stegemann S, Cerwenka A, Sommer C, Dalpke AH, Veltkamp R. Inhibition of lymphocyte trafficking shields the brain against deleterious neuroinflammation after stroke. Brain. 2011;134:704–720. doi: 10.1093/brain/awr008. [DOI] [PubMed] [Google Scholar]
- Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999a;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999b;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhang J. Recent development in NMDA receptors. Chinese medical journal. 2000;113:948–956. [PubMed] [Google Scholar]
- Love S. Oxidative stress in brain ischemia. Brain Pathol. 1999;9:119–131. doi: 10.1111/j.1750-3639.1999.tb00214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lust WD, Wheaton AB, Feussner G, Passonneau J. Metabolism in the hamster brain during hibernation and arousal. Brain Res. 1989;489:12–20. doi: 10.1016/0006-8993(89)90003-6. [DOI] [PubMed] [Google Scholar]
- Ma YL, Zhu X, Rivera PM, Toien O, Barnes BM, LaManna JC, Smith MA, Drew KL. Absence of cellular stress in brain after hypoxia induced by arousal from hibernation in Arctic ground squirrels. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1297–1306. doi: 10.1152/ajpregu.00260.2005. [DOI] [PubMed] [Google Scholar]
- Maalouf M, Sullivan PG, Davis L, Kim DY, Rho JM. Ketones inhibit mitochondrial production of reactive oxygen species production following glutamate excitotoxicity by increasing NADH oxidation. Neuroscience. 2007;145:256–264. doi: 10.1016/j.neuroscience.2006.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS, Saboureau M, Pevet P. Rapid and reversible changes in intrahippocampal connectivity during the course of hibernation in European hamsters. Proc Natl Acad Sci USA. 2006;103:18775–18780. doi: 10.1073/pnas.0608785103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maginniss LA, Milsom WK. Effects of hibernation on blood oxygen transport in the golden-mantled ground squirrel. Respir Physiol. 1994;95:195–208. doi: 10.1016/0034-5687(94)90116-3. [DOI] [PubMed] [Google Scholar]
- Maier CM, Ahern K, Cheng ML, Lee JE, Yenari MA, Steinberg GK. Optimal depth and duration of mild hypothermia in a focal model of transient cerebral ischemia: effects on neurologic outcome, infarct size, apoptosis, and inflammation. Stroke. 1998;29:2171–2180. doi: 10.1161/01.str.29.10.2171. [DOI] [PubMed] [Google Scholar]
- Markham JA, Greenough WT. Experience-driven brain plasticity: beyond the synapse. Neuron Glia Biol. 2004;1:351–363. doi: 10.1017/s1740925x05000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Villalba A, Hahne M, Kleber S, Vogel J, Falk W, Schenkel J, Krammer PH. Therapeutic neutralization of CD95-ligand and TNF attenuates brain damage in stroke. Cell Death Differ. 2001;8:679–686. doi: 10.1038/sj.cdd.4400882. [DOI] [PubMed] [Google Scholar]
- McKenna JM, Musacchia XJ. Antibody formation in hibernating ground squirrels (Citrellus tridecemlineatus) Proc Soc Exp Biol Med. 1968;129:720–724. doi: 10.3181/00379727-129-33408. [DOI] [PubMed] [Google Scholar]
- Miller BA, Perez RS, Shah AR, Gonzales ER, Park TS, Gidday JM. Cerebral protection by hypoxic preconditioning in a murine model of focal ischemia-reperfusion. Neuroreport. 2001;12:1663–1669. doi: 10.1097/00001756-200106130-00030. [DOI] [PubMed] [Google Scholar]
- Milsom WK, Zimmer MB, Harris MB. Vagal control of cardiorespiratory function in hibernation. Exp Physiol. 2001;86:791–796. doi: 10.1111/j.1469-445x.2001.tb00046.x. [DOI] [PubMed] [Google Scholar]
- Morikawa E, Mori H, Kiyama Y, Mishina M, Asano T, Kirino T. Attenuation of focal ischemic brain injury in mice deficient in the epsilon1 (NR2A) subunit of NMDA receptor. J Neurosci. 1998;18:9727–9732. doi: 10.1523/JNEUROSCI.18-23-09727.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Miyamoto O, Yamagami S, Toyoshima T, Negi T, Itano T, Nagao S. The chronic cell death with DNA fragmentation after post-ischaemic hypothermia in the gerbil hippocampus. Acta Neurochir (Wien) 1999;141:407–412. doi: 10.1007/s007010050317. discussion 412–403. [DOI] [PubMed] [Google Scholar]
- Nakanishi S. Molecular diversity of glutamate receptors and implications for brain function. Science. 1992;258:597–603. doi: 10.1126/science.1329206. [DOI] [PubMed] [Google Scholar]
- Niizuma K, Yoshioka H, Chen H, Kim GS, Jung JE, Katsu M, Okami N, Chan PH. Mitochondrial and apoptotic neuronal death signaling pathways in cerebral ischemia. Biochim Biophys Acta. 2010;1802:92–99. doi: 10.1016/j.bbadis.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak G, Bakajsova D, Clifton GL. Protein kinase C-epsilon modulates mitochondrial function and active Na+ transport after oxidant injury in renal cells. Am J Physiol Renal Physiol. 2004;286:F307–316. doi: 10.1152/ajprenal.00275.2003. [DOI] [PubMed] [Google Scholar]
- Okamoto I, Kayano T, Hanaya T, Arai S, Ikeda M, Kurimoto M. Up-regulation of an extracellular superoxide dismutase-like activity in hibernating hamsters subjected to oxidative stress in mid- to late arousal from torpor. Comp Biochem Physiol C Toxicol Pharmacol. 2006;144:47–56. doi: 10.1016/j.cbpc.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Orr AL, Lohse LA, Drew KL, Hermes-Lima M. Physiological oxidative stress after arousal from hibernation in Arctic ground squirrel. Comp Biochem Physiol A Mol Integr Physiol. 2009;153:213–221. doi: 10.1016/j.cbpa.2009.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page MM, Peters CW, Staples JF, Stuart JA. Intracellular antioxidant enzymes are not globally upregulated during hibernation in the major oxidative tissues of the 13-lined ground squirrel Spermophilus tridecemlineatus. Comp Biochem Physiol A Mol Integr Physiol. 2009;152:115–122. doi: 10.1016/j.cbpa.2008.09.032. [DOI] [PubMed] [Google Scholar]
- Palomares SM, Cipolla MJ. Vascular Protection Following Cerebral Ischemia and Reperfusion. Journal of neurology & neurophysiology. 2011;2011 doi: 10.4172/2155-9562.s1-004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Pinzon MA. Mechanisms of neuroprotection during ischemic preconditioning: lessons from anoxic tolerance. Comp Biochem Physiol A Mol Integr Physiol. 2007;147:291–299. doi: 10.1016/j.cbpa.2006.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Pinzon MA, Lutz PL, Sick TJ, Rosenthal M. Adenosine, a “retaliatory” metabolite, promotes anoxia tolerance in turtle brain. J Cereb Blood Flow Metab. 1993;13:728–732. doi: 10.1038/jcbfm.1993.93. [DOI] [PubMed] [Google Scholar]
- Perez-Pinzon MA, Mumford PL, Rosenthal M, Sick TJ. Anoxic preconditioning in hippocampal slices: role of adenosine. Neuroscience. 1996;75:687–694. doi: 10.1016/0306-4522(96)00311-9. [DOI] [PubMed] [Google Scholar]
- Petrovic VM, Milic B, Spasic M, Saicic Z. Copper-zinc containing and manganese containing superoxide dismutase in the ground squirrel/Citellus citellus/--the effect of hibernation. Free Radic Res Commun. 1986;1:339–346. doi: 10.3109/10715768609080973. [DOI] [PubMed] [Google Scholar]
- Petrovic VM, Saicic Z, Milic B, Spasic M, Radojicic R. Distribution of superoxide dismutase in the ground squirrel (Citellus citellus): effect of the hibernation and arousal. Comp Biochem Physiol B. 1983;75:699–700. doi: 10.1016/0305-0491(83)90119-0. [DOI] [PubMed] [Google Scholar]
- Phanithi PB, Yoshida Y, Santana A, Su M, Kawamura S, Yasui N. Mild hypothermia mitigates post-ischemic neuronal death following focal cerebral ischemia in rat brain: immunohistochemical study of Fas, caspase-3 and TUNEL. Neuropathology. 2000;20:273–282. doi: 10.1046/j.1440-1789.2000.00346.x. [DOI] [PubMed] [Google Scholar]
- Planel E, Miyasaka T, Launey T, Chui DH, Tanemura K, Sato S, Murayama O, Ishiguro K, Tatebayashi Y, Takashima A. Alterations in glucose metabolism induce hypothermia leading to tau hyperphosphorylation through differential inhibition of kinase and phosphatase activities: implications for Alzheimer’s disease. J Neurosci. 2004;24:2401–2411. doi: 10.1523/JNEUROSCI.5561-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov VI, Bocharova LS. Hibernation-induced structural changes in synaptic contacts between mossy fibres and hippocampal pyramidal neurons. Neuroscience. 1992;48:53–62. doi: 10.1016/0306-4522(92)90337-2. [DOI] [PubMed] [Google Scholar]
- Popov VI, Bocharova LS, Bragin AG. Repeated changes of dendritic morphology in the hippocampus of ground squirrels in the course of hibernation. Neuroscience. 1992;48:45–51. doi: 10.1016/0306-4522(92)90336-z. [DOI] [PubMed] [Google Scholar]
- Prass K, Scharff A, Ruscher K, Lowl D, Muselmann C, Victorov I, Kapinya K, Dirnagl U, Meisel A. Hypoxia-induced stroke tolerance in the mouse is mediated by erythropoietin. Stroke. 2003;34:1981–1986. doi: 10.1161/01.STR.0000080381.76409.B2. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Freeman DA, Zucker I, Nelson RJ. Periodic arousal from hibernation is necessary for initiation of immune responses in ground squirrels. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1054–1062. doi: 10.1152/ajpregu.00562.2001. [DOI] [PubMed] [Google Scholar]
- Ratan RR, Siddiq A, Aminova L, Lange PS, Langley B, Ayoub I, Gensert J, Chavez J. Translation of ischemic preconditioning to the patient: prolyl hydroxylase inhibition and hypoxia inducible factor-1 as novel targets for stroke therapy. Stroke. 2004;35:2687–2689. doi: 10.1161/01.STR.0000143216.85349.9e. [DOI] [PubMed] [Google Scholar]
- Raval AP, Dave KR, Mochly-Rosen D, Sick TJ, Perez-Pinzon MA. Epsilon PKC is required for the induction of tolerance by ischemic and NMDA-mediated preconditioning in the organotypic hippocampal slice. J Neurosci. 2003;23:384–391. doi: 10.1523/JNEUROSCI.23-02-00384.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reshef A, Sperling O, Zoref-Shani E. The adenosine-induced mechanism for the acquisition of ischemic tolerance in primary rat neuronal cultures. Pharmacol Ther. 2000;87:151–159. doi: 10.1016/s0163-7258(00)00045-0. [DOI] [PubMed] [Google Scholar]
- Roelandse M, Matus A. Hypothermia-associated loss of dendritic spines. J Neurosci. 2004;24:7843–7847. doi: 10.1523/JNEUROSCI.2872-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanic AM, White RF, Arleth AJ, Ohlstein EH, Barone FC. Matrix metalloproteinase expression increases after cerebral focal ischemia in rats: inhibition of matrix metalloproteinase-9 reduces infarct size. Stroke. 1998;29:1020–1030. doi: 10.1161/01.str.29.5.1020. [DOI] [PubMed] [Google Scholar]
- Ross AP, Christian SL, Zhao HW, Drew KL. Persistent tolerance to oxygen and nutrient deprivation and N-methyl-D-aspartate in cultured hippocampal slices from hibernating Arctic ground squirrel. J Cereb Blood Flow Metab. 2006;26:1148–1156. doi: 10.1038/sj.jcbfm.9600271. [DOI] [PubMed] [Google Scholar]
- Ruscher K, Freyer D, Karsch M, Isaev N, Megow D, Sawitzki B, Priller J, Dirnagl U, Meisel A. Erythropoietin is a paracrine mediator of ischemic tolerance in the brain: evidence from an in vitro model. J Neurosci. 2002;22:10291–10301. doi: 10.1523/JNEUROSCI.22-23-10291.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakimura K, Kutsuwada T, Ito I, Manabe T, Takayama C, Kushiya E, Yagi T, Aizawa S, Inoue Y, Sugiyama H, et al. Reduced hippocampal LTP and spatial learning in mice lacking NMDA receptor epsilon 1 subunit. Nature. 1995;373:151–155. doi: 10.1038/373151a0. [DOI] [PubMed] [Google Scholar]
- Samdani AF, Dawson TM, Dawson VL. Nitric oxide synthase in models of focal ischemia. Stroke. 1997;28:1283–1288. doi: 10.1161/01.str.28.6.1283. [DOI] [PubMed] [Google Scholar]
- Savitz SI, Fisher M. Prophylactic neuroprotection. Curr Drug Targets. 2007;8:846–849. doi: 10.2174/138945007781077382. [DOI] [PubMed] [Google Scholar]
- Sidky YA, Auerbach R. Effect of hibernation on the hamster spleen immune reaction in vitro. Proc Soc Exp Biol Med. 1968;129:122–127. doi: 10.3181/00379727-129-33265. [DOI] [PubMed] [Google Scholar]
- Sidky YA, Daggett LR, Auerbach R. Brown fat: its possible role in immunosuppression during hibernation. Proc Soc Exp Biol Med. 1969;132:760–763. doi: 10.3181/00379727-132-34305. [DOI] [PubMed] [Google Scholar]
- Skaper SD. Poly(ADP-Ribose) polymerase-1 in acute neuronal death and inflammation: a strategy for neuroprotection. Ann N Y Acad Sci. 2003;993:217–228. doi: 10.1111/j.1749-6632.2003.tb07532.x. [DOI] [PubMed] [Google Scholar]
- Snyder GK, Nestler JR. Relationships between body temperature, thermal conductance, Q10 and energy metabolism during daily torpor and hibernation in rodents. J Comp Physiol B. 1990;159:667–675. doi: 10.1007/BF00691712. [DOI] [PubMed] [Google Scholar]
- Stenzel-Poore MP, Stevens SL, Xiong Z, Lessov NS, Harrington CA, Mori M, Meller R, Rosenzweig HL, Tobar E, Shaw TE, Chu X, Simon RP. Effect of ischaemic preconditioning on genomic response to cerebral ischaemia: similarity to neuroprotective strategies in hibernation and hypoxia-tolerant states. Lancet. 2003;362:1028–1037. doi: 10.1016/S0140-6736(03)14412-1. [DOI] [PubMed] [Google Scholar]
- Strosznajder RP, Czubowicz K, Jesko H, Strosznajder JB. Poly(ADP-ribose) metabolism in brain and its role in ischemia pathology. Mol Neurobiol. 2010;41:187–196. doi: 10.1007/s12035-010-8124-6. [DOI] [PubMed] [Google Scholar]
- Su B, Wang X, Drew KL, Perry G, Smith MA, Zhu X. Physiological regulation of tau phosphorylation during hibernation. J Neurochem. 2008;105:2098–2108. doi: 10.1111/j.1471-4159.2008.05294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szilagyi JE, Senturia JB. A comparison of bone marrow leukocytes in hibernating and nonhibernating woodchucks and ground squirrels. Cryobiology. 1972;9:257–261. doi: 10.1016/0011-2240(72)90044-2. [DOI] [PubMed] [Google Scholar]
- Takeda Y, Namba K, Higuchi T, Hagioka S, Takata K, Hirakawa M, Morita K. Quantitative evaluation of the neuroprotective effects of hypothermia ranging from 34 degrees C to 31 degrees C on brain ischemia in gerbils and determination of the mechanism of neuroprotection. Crit Care Med. 2003;31:255–260. doi: 10.1097/00003246-200301000-00040. [DOI] [PubMed] [Google Scholar]
- Taoufik E, Probert L. Ischemic neuronal damage. Curr Pharm Des. 2008;14:3565–3573. doi: 10.2174/138161208786848748. [DOI] [PubMed] [Google Scholar]
- Toien O, Blake J, Edgar DM, Grahn DA, Heller HC, Barnes BM. Hibernation in black bears: independence of metabolic suppression from body temperature. Science. 2011;331:906–909. doi: 10.1126/science.1199435. [DOI] [PubMed] [Google Scholar]
- Toien O, Drew KL, Chao ML, Rice ME. Ascorbate dynamics and oxygen consumption during arousal from hibernation in Arctic ground squirrels. Am J Physiol Regul Integr Comp Physiol. 2001;281:R572–583. doi: 10.1152/ajpregu.2001.281.2.R572. [DOI] [PubMed] [Google Scholar]
- Van Hemelrijck A, Vermijlen D, Hachimi-Idrissi S, Sarre S, Ebinger G, Michotte Y. Effect of resuscitative mild hypothermia on glutamate and dopamine release, apoptosis and ischaemic brain damage in the endothelin-1 rat model for focal cerebral ischaemia. J Neurochem. 2003;87:66–75. doi: 10.1046/j.1471-4159.2003.01977.x. [DOI] [PubMed] [Google Scholar]
- von der Ohe CG, Darian-Smith C, Garner CC, Heller HC. Ubiquitous and temperature-dependent neural plasticity in hibernators. J Neurosci. 2006;26:10590–10598. doi: 10.1523/JNEUROSCI.2874-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster CM, Kelly S, Koike MA, Chock VY, Giffard RG, Yenari MA. Inflammation and NFkappaB activation is decreased by hypothermia following global cerebral ischemia. Neurobiol Dis. 2009;33:301–312. doi: 10.1016/j.nbd.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weltzin MM, Zhao HW, Drew KL, Bucci DJ. Arousal from hibernation alters contextual learning and memory. Behav Brain Res. 2006;167:128–133. doi: 10.1016/j.bbr.2005.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuma Y, McCarron RM, Spatz M, Hallenbeck JM. Effects of plasma from hibernating ground squirrels on monocyte-endothelial cell adhesive interactions. Am J Physiol. 1997;273:R1861–1869. doi: 10.1152/ajpregu.1997.273.6.R1861. [DOI] [PubMed] [Google Scholar]
- Yenari M, Kitagawa K, Lyden P, Perez-Pinzon M. Metabolic downregulation: a key to successful neuroprotection? Stroke. 2008;39:2910–2917. doi: 10.1161/STROKEAHA.108.514471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yenari MA, Han HS. Influence of hypothermia on post-ischemic inflammation: role of nuclear factor kappa B (NFkappaB) Neurochem Int. 2006;49:164–169. doi: 10.1016/j.neuint.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Yenari MA, Hemmen TM. Therapeutic hypothermia for brain ischemia: where have we come and where do we go? Stroke. 2010;41:S72–74. doi: 10.1161/STROKEAHA.110.595371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yenari MA, Iwayama S, Cheng D, Sun GH, Fujimura M, Morita-Fujimura Y, Chan PH, Steinberg GK. Mild hypothermia attenuates cytochrome c release but does not alter Bcl-2 expression or caspase activation after experimental stroke. J Cereb Blood Flow Metab. 2002;22:29–38. doi: 10.1097/00004647-200201000-00004. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Chopp M, Li Y, Zaloga C, Jiang N, Jones ML, Miyasaka M, Ward PA. Anti-ICAM-1 antibody reduces ischemic cell damage after transient middle cerebral artery occlusion in the rat. Neurology. 1994;44:1747–1751. doi: 10.1212/wnl.44.9.1747. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Sobel RA, Cheng D, Steinberg GK, Yenari MA. Mild hypothermia increases Bcl-2 protein expression following global cerebral ischemia. Brain Res Mol Brain Res. 2001;95:75–85. doi: 10.1016/s0169-328x(01)00247-9. [DOI] [PubMed] [Google Scholar]
- Zhao HW, Christian SL, Castillo MR, Bult-Ito A, Drew KL. Distribution of NMDA receptor subunit NR1 in arctic ground squirrel central nervous system. J Chem Neuroanat. 2006a;32:196–207. doi: 10.1016/j.jchemneu.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao HW, Ross AP, Christian SL, Buchholz JN, Drew KL. Decreased NR1 phosphorylation and decreased NMDAR function in hibernating Arctic ground squirrels. J Neurosci Res. 2006b;84:291–298. doi: 10.1002/jnr.20893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao HW, Ross AP, Christian SL, Buchholz JN, Drew KL. Decreased NR1 phosphorylation and decreased NMDAR function in hibernating Arctic ground squirrels. J Neurosci Res. 2006c;84:291–298. doi: 10.1002/jnr.20893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Smith MA, Perry G, Wang Y, Ross AP, Zhao HW, Lamanna JC, Drew KL. MAPKs are differentially modulated in arctic ground squirrels during hibernation. J Neurosci Res. 2005;80:862–868. doi: 10.1002/jnr.20526. [DOI] [PubMed] [Google Scholar]


