SUMMARY
Although improving glucose metabolism by inhibition of pyruvate dehydrogenase kinase 4 (PDK4) might prove beneficial in the treatment of type 2 diabetes or diet-induced obesity, it might induce detrimental effects by inhibiting fatty acid oxidation. PPARα agonists are often used to treat dyslipidemia in patients, especially in type 2 diabetes. Combinational treatment with a PDK4 inhibitor and PPARα agonists may prove beneficial. However, PPARα agonists may be less effective in the presence of a PDK4 inhibitor because PPARα agonists induce PDK4 expression. In the present study, the effects of clofibric acid, a PPARα agonist, on blood and liver lipids were determined in wild type and PDK4 knockout mice fed a high fat diet. As expected, treatment of wild type mice with clofibric acid resulted in less body weight gain, smaller epididymal fat pads, greater insulin sensitivity, and lower levels of serum and liver triacylglycerol. Surprisingly, rather than decreasing the effectiveness of clofibric acid, PDK4 deficiency enhanced the beneficial effects of clofibric acid on hepatic steatosis, lowered blood glucose levels, and did not prevent the positive effects of clofibric acid on serum triacylglycerols and free fatty acids. The metabolic effects of clofibric acid are therefore independent of the induction of PDK4 expression. The additive beneficial effects on hepatic steatosis may be due to induction of increased capacity for fatty acid oxidation and partial uncoupling of oxidative phosphorylation by clofibric acid and a reduction in the capacity for fatty acid synthesis by PDK4 deficiency.
Keywords: Diet-induced obesity, Peroxisome proliferator-activated receptor α, Pyruvate dehydrogenase complex, Pyruvate dehydrogenase kinase, Clofibric acid
INTRODUCTION
Fibrates are clinically used to decrease plasma triacylglycerol and increase the level of high-density lipoprotein (HDL)-cholesterol [1]. These actions of fibrates, believed to be due to altered expression of apolipoproteins [2, 3] and peroxisomal and mitochondrial fatty acid oxidation enzymes [4], reduce the risk of cardiovascular disease, improve insulin sensitivity, and reduce adiposity [5]. Most of the effects of fibrates are mediated by activation of the nuclear receptor peroxisome proliferator-activated receptor α (PPARα), a transcription factor responsible for regulation of genes involved in lipid metabolism. Activation of PPARα by fibrates also ameliorates hepatic steatosis in rodents [6, 7].
Regulation of the activity of the pyruvate dehydrogenase complex (PDC) is important for glucose homeostasis and the control of fuel selection by tissues [8]. When blood glucose levels are elevated by carbohydrate intake, PDC is relatively dephosphorylated and active to promote glucose disposal and the synthesis and storage of fatty acids as triacylglycerols. When glucose levels are low because of fasting, PDC is highly phosphorylated and inactive to inhibit oxidation of gluconeogenic substrates (pyruvate, lactate, and alanine) and promote glucose synthesis and fatty acid oxidation. The phosphorylation state and therefore the activity of PDC [9] are set by the relative activities of the four pyruvate dehydrogenase kinases (PDKs) and two pyruvate dehydrogenase phosphatases (PDPs). Of the enzymes that modulate PDC activity, PDK4 is of special interest because its expression is markedly increased by fasting [10] and studies with PDK4 knockout mice [11] have shown PDK4 is important for maintaining fasting blood glucose levels.
Fibrates are effective for the treatment of dyslipidemia, one of the major problems of diabetes. The possibility that PDK4 inhibitors may also prove useful in the treatment of type 2 diabetes obesity was suggested by our previous studies [12, 13]. Since PPARα agonists increase PDK4 expression in muscle, heart, kidney, and liver [14, 15] which may mediate some of the effects of fibrates, there is concern that fibrates may be less effective in the presence of a PDK4 inhibitor. This study was designed to test whether the lipid lowering effects of clofibric acid, a PPARα agonist, are affected by PDK4 deficiency. Surprisingly, PDK4 deficiency enhanced the beneficial effects of clofibric acid on hepatic steatosis and did not prevent its hypolipidemic effects. These findings suggest the metabolic effects of clofibric acid are independent of the induction of PDK4 expression. Therefore, a PDK4 inhibitor and a fibrate could potentially be used in combination to lower blood glucose and ameliorate hepatic steatosis.
RESULTS
Effects of clofibric acid and PDK4 deficiency on body weight, tissue weights, and food consumption of mice fed a high saturated fat (HSF) diet
As we found in a previous study [13], PDK4 knockout mice gained less weight and had smaller livers than wild type mice fed the HSF diet (Table 1). Clofibric acid also reduced body weight and epididymal fat pad weight but, as expected, increased liver weight in both wild type and PDK4 knockout mice (Table 1). Body weight gains were almost completely prevented by clofibric acid in both wild type and PDK4 knockout mice (Table 1). Epididymal fat pad weights were likewise reduced to a similar extent in both groups. The reduction in liver size caused by PDK4 deficiency was completely overcome by clofibric acid (Table 1). Food consumption was not affected by clofibric acid in either the wild type (1.8 ± 0.1 vs. 2.1 ± 0.2 g per day for non-treated and clofibric acid-treated mice, respectively) or the PDK4 knockout mice (1.8 ± 0.1 vs. 2.0 ± 0.1 g per day for non-treated and clofibric acid-treated mice, respectively). In spite of this, the development of obesity was largely prevented by the addition of clofibric acid to the diet of both the wild type and the PDK4 knockout mice (Figure 1). Food efficiency in terms of weight gain per amount of food consumed was therefore markedly attenuated by clofibric acid in both the wild type and the PDK4 knockout mice.
Table 1.
Body and tissue weights of fasted wild type (WT) and PDK4 knockout (PDK4 KO) mice fed a HSF diet with and without clofibric acid
| Genotype | Clofibric acid |
Body weight gain | Final body weight |
Epididymal fat pads |
Liver | |
|---|---|---|---|---|---|---|
|
g/first 14 weeks |
g/second 14 weeks |
g | % of body weight | |||
| WT | − | 15.0 ± 0.8 | 13.1 ± 1.3 | 48.3 ± 1.8 | 7.5 ± 0.3 | 3.8 ± 0.3 |
| WT | + | 4.8 ± 1.7*† | 36.3 ± 2.6* | 4.8 ± 0.8* | 5.2 ± 0.1*† | |
| PDK4 KO | − | 12.8 ± 0.9 * | 9.8 ± 0.8* | 41.1 ± 1.5* | 6.5 ± 0.2 | 3.0 ± 0.1 * |
| PDK4 KO | + | 2.6 ± 0.3*† | 32.5 ± 1.3*† | 3.8 ± 0.6*† | 5.2 ± 0.2*† | |
Data are presented as mean ± S.E., n = 12 for wild type and PDK4 knockout mice fed on a HSF diet. Diet of half of the wild type mice and half of the PDK4 knockout mice was supplemented with clofibric acid after the first 14 weeks of feeding.
P < 0.05 relative to non-treated wild type mice.
P < 0.05 relative to non-treated PDK4 knockout mice.
Figure 1. Effects of clofibric acid and PDK4 deficiency on body weights of mice fed HSF diet.
Body weights of wild type mice (squares) and PDK4 knockout mice (triangle) fed the HSF diet with (closed symbols) and without (open symbols) clofibric acid. Clofibric acid was added to the diets of half of the wild type mice and half of PDK4 knockout mice at the 14th week of the feeding period. Data are presented as means ± S.E. with n = 6 mice per group at the start and 14th week of the experiment; n = 6 mice per group at the end of the experiment. *P < 0.05 relative to wild type mice fed the HSF diet with clofibric acid and PDK4 knockout mice fed the HSF diet with or without clofibric acid.
Since PPARα activation induces expression of uncoupling proteins (UCP) in several tissues [16–20], dissipation of energy by heat as a consequence of uncoupling of oxidative phosphorylation may be responsible for reduced food efficiency and less weight gain in fibrate-treated rats [21, 22]. That clofibric acid induces the expression of UCP3 in the liver of wild type mice fed the HSF diet in fed state was confirmed by quantitative real-time PCR (Table 2). PDK4 deficiency alone was without effect but supplementation of the diet of PDK4 knockout mice with clofibric acid produced an even greater relative amount of UCP3 (Table 2). Therefore, uncoupling and the resulting reduced food efficiency may contribute to the additive effects produced by clofibric acid treatment and PDK4 deficiency.
Table 2.
UCP3 mRNA expression in livers of wild type (WT) and PDK4 knockout (PDK4 KO) mice fed a HSF diet with and without clofibric acid
| Genotype | Clofibric acid |
Liver |
|---|---|---|
| fold change | ||
| WT | − | 1.0 ± 0.1 |
| WT | + | 59.0 ± 4.8*† |
| PDK4 KO | − | 0.5 ± 0.2 |
| PDK4 KO | + | 82.2 ± 5.2*†# |
Data are presented as mean ± S.E. relative to nontreated wild type mice. n = 4 for wild type and PDK4 knockout mice fed on a HSF diet with and without clofibric acid. UCP3 mRNA was measured by quantitative real-time PCR as described in the Materials and Methods section.
P < 0.05 relative to non-treated wild type mice.
P < 0.05 relative to non-treated PDK4 knockout mice.
P < 0.05 relative to clofibric acid-treated wild type mice.
Effects of clofibric acid and PDK4 deficiency on blood glucose levels and ketone bodies in mice fed the HSF diet
As expected [13], wild type mice fed the high saturated fat diet exhibited fasting hyperglycemia (Table 3) whereas PDK4 knockout mice remained euglycemic. Supplementing the diet with clofibric acid had no effect on blood glucose in the wild type mice and the PDK4 knockout mice (Table 3). As also expected [12], serum ketone bodies were significantly greater in PDK4 knockout mice than in wild type mice (Table 3) and clofibric acid increased the level further in both groups. Thus, PDK4 deficiency and clofibric acid produce additive effects on serum ketone bodies.
Table 3.
Blood glucose, triacylglycerol, free fatty acid, and ketone body levels of fasted wild type (WT) and PDK4 knockout (PDK4 KO) mice fed a HSF diet with and without clofibric acid
| Genotype | Clofibric acid | Blood glucose |
Triacylglycerol | Free fatty acid |
Ketone bodies |
|---|---|---|---|---|---|
| mg/dl | mmole/l | ||||
| WT | − | 143 ± 5 | 48 ± 5 | 0.48 ± 0.10 | 0.20 ± 0.02 |
| WT | + | 134 ± 7 | 29 ± 1*† | 0.37 ± 0.07 | 0.58 ± 0.10* |
| PDK4 KO | − | 90 ± 9*# | 71 ± 9* | 0.67 ± 0.09 | 0.46 ± 0.05* |
| PDK4 KO | + | 105 ± 7 *# | 29 ± 1*† | 0.29 ± 0.05 † | 1.09 ± 0.14*†# |
Data are presented as mean ± S.E., n = 5 or 6 for wild type and PDK4 knockout mice fed on a HSF diet with and without clofibric acid.
P < 0.05 relative to non-treated wild type mice.
P < 0.05 relative to non-treated PDK4 knockout mice.
P < 0.05 relative to clofibric acid-treated wild type mice.
Effects of clofibric acid and PDK4 deficiency on serum lipids and hepatic steatosis in mice fed the HSF diet
Supplementing the diet with clofibric acid lowers serum triacylglycerol (Table 3). In contrast, PDK4 deficiency caused a modest increase in serum triacylglycerol and a trend toward higher free fatty acid levels (Table 3). In spite of this, the combination of clofibric acid and PDK4 deficiency significantly reduced both serum triacylglycerol and free fatty acids (Table 3). The beneficial effects of clofibric acid on serum lipids were therefore greater in PDK4 knockout mice than wild type mice fed the HSF diet.
Hepatic steatosis induced by the HSF diet (Figure 2A) was attenuated by PDK4 deficiency (Figure 2B), as reported previously [13]. Supplementing the diet of wild type mice with clofibric acid also reduced the liver fat content (Figure 2C). The combination of PDK4 deficiency and treatment with clofibric acid resulted in the lowest amount of liver fat (Figure 2D). These findings were confirmed by quantitative analysis of the amounts of liver triacylglycerol (Table 4). Supplementing the diet with clofibric acid and PDK4 deficiency reduced liver triacylglycerol. Consistent with the histological analysis, the lowest amount of triacylglycerol was found in the liver of clofibric acid-treated PDK4 knockout mice.
Figure 2. Effect of clofibric acid and PDK4 deficiency on hepatic steatosis in mice fed the HSF diet.
Microscopic photographs demonstrating the histological appearance of the livers of wild type (A) and PDK4 knockout (B) mice fed the HSF diet or wild type (C) and PDK4 knockout (D) mice fed the HSF diet with clofibric acid. Tissue sections were prepared from the livers of the overnight-fasted mice and stained with Oil red O. The material stained red corresponds to fat droplets. Original magnification was ×200. Similar results were obtained with liver samples from four different mice of each group.
Table 4.
Concentration of triacylglycerol in livers and gastrocnemius muscles of overnight-fasted wild type (WT) and PDK4 knockout (PDK4 KO) mice fed a HSF diet with and without clofibric acid
| Genotype | Clofibric acid |
Liver | Gastrocnemius |
|---|---|---|---|
| mg/g wet weight | |||
| WT | − | 235 ± 18 | 90 ± 8 |
| WT | + | 114 ± 24*† | 49 ± 8* |
| PDK4 KO | − | 185 ± 9* | 64 ± 7* |
| PDK4 KO | + | 72 ± 11 *† | 33 ± 5*† |
Data are presented as mean ± S.E., n = 6 for wild type and PDK4 knockout mice fed on a HSF diet with and without clofibric acid. Concentration of triacylglycerol was measured as described in the Materials and Methods section.
P < 0.05 relative to non-treated wild type mice.
P < 0.05 relative to non-treated PDK4 knockout mice.
Similar to the findings with liver, dietary clofibric acid and PDK4 deficiency decreased the amount of triacylglycerol in gastrocnemius muscles (Table 4). As with the liver, these effects were additive, the lowest amount of triacylglycerol was induced in the gastrocnemius muscle by the combination of clofibric acid and PDK4 deficiency. These findings show that this desirable effect of clofibric acid on hepatic steatosis is not dependent on up regulation of PDK4.
Effects of clofibric acid and PDK4 deficiency on insulin sensitivity in mice fed the HSF diet
Wild type mice fed the high saturated fat diet for 20 weeks were insulin resistant as measured by insulin levels (not shown) and the insulin tolerance test (Figure 3). As reported previously [13], PDK4 knockout mice fed the high saturated fat diet were significantly more insulin sensitive than wild type mice. Supplementing the diet with clofibric acid also improved insulin sensitivity in the wild type mice but the effect was not additive with the increase in insulin sensitivity caused by PDK4 deficiency (Figure 3).
Figure 3. Effect of clofibric acid and PDK4 deficiency on insulin sensitivity in mice fed the HSF diet.
Insulin tolerance test (ITT) of wild type (squares) and PDK4 knockout (triangles) mice fed the HSF diet with (closed symbols) and without (open symbols) clofibric acid. At the 20th week of feeding, insulin (1U/kg of body weight) was administrated to 5 h-fasted mice by intraperitoneal injection. Blood glucose levels were measured at the indicated time points. Data are presented as mean ± S.E. with n = 4 mice per group. *P < 0.05 relative to wild type mice fed the HSF diet.
Effects of clofibric acid and PDK4 deficiency on the PDC activity in livers and gastrocnemius muscles of mice fed the HSF diet
Since clofibric acid was expected to induce PDK4 in the liver, we anticipated a lower PDC activity state (% of the complex in the active, dephosphorylated state) in the liver of clofibric acid-treated wild type mice compared to non-treated mice. Surprisingly, clofibric acid significantly increased the PDC activity state (Table 5). Contrary to our previous study which indicated no effect of PDK4 deficiency on the PDC activity in livers of mice fed a different high fat diet [12], PDK4 deficiency resulted in a significantly higher PDC activity state in the livers of the mice fed the HSF used in this study (Table 5). The highest PDC activity state occurred in PDK4 knockout mice treated with clofibric acid (Table 5). These findings beg the question of whether expression or activities of the pyruvate dehydrogenase phosphatases may be affected by clofibric acid, but this was not investigated in this study. In contrast to liver, neither PDK4 deficiency nor clofibric acid treatment increased the PDC activity state in the gastrocnemius muscles of wild type and PDK4 knockout mice (Table 5).
Table 5.
PDC activity in livers and gastrocnemius muscles of fasted wild type (WT) and PDK4 knockout (PDK4 KO) mice fed a HSF diet with and without clofibric acid
| Genotype | Clofibric acid |
Liver | Gastrocnemius |
|---|---|---|---|
| % Active | |||
| WT | − | 0.6 ± 0.2 | 1.6 ± 0.2 |
| WT | + | 7.6 ± 0.5*† | 1.5 ± 0.6 |
| PDK4 KO | − | 3.1 ± 0.4* | 2.3 ± 0.3 |
| PDK4 KO | + | 9.0 ± 1.5*† | 1.9 ± 0.4 |
Data are presented as mean ± S.E., n = 5 or 6 for wild type and PDK4 knockout mice fed on a HSF diet with and without clofibric acid. % Active PDC was determined as described in the Materials and Methods section.
P < 0.03 relative to non-treated wild type mice.
P < 0.01 relative to non-treated PDK4 knockout mice.
Effects of clofibric acid on mitochondrial enzymes in livers of mice fed the HSF diet
In an effort to understand the basis for the increase in PDC activity state in the liver of clofibric acid treated mice, the protein amounts of the four PDKs were measured by Western blot analysis. No effect of clofibric acid or PDK4 deficiency was observed on the amounts of PDK1, PDK2, or PDK3 protein (data not shown). As expected, PDK4 protein was totally absent from the livers of PDK4 knockout mice and was induced to high amounts by clofibric acid in the liver of wild type mice in fed state (Figure 4). However, the treatment of clofibric acid did not increase the amount of PDK4 protein in the liver of wild type mice fed the HSF diet in fasting state (Figure 4). The same was found for PDK4 expression in gastrocnemius muscles (data not shown). These findings may explain at least in part why the PDC activity was not suppressed in livers of clofibric acid-treated wild type mice in the fasting state. The mechanism by which the treatment of clofibric acid increases the PDC activity remains to be elucidated.
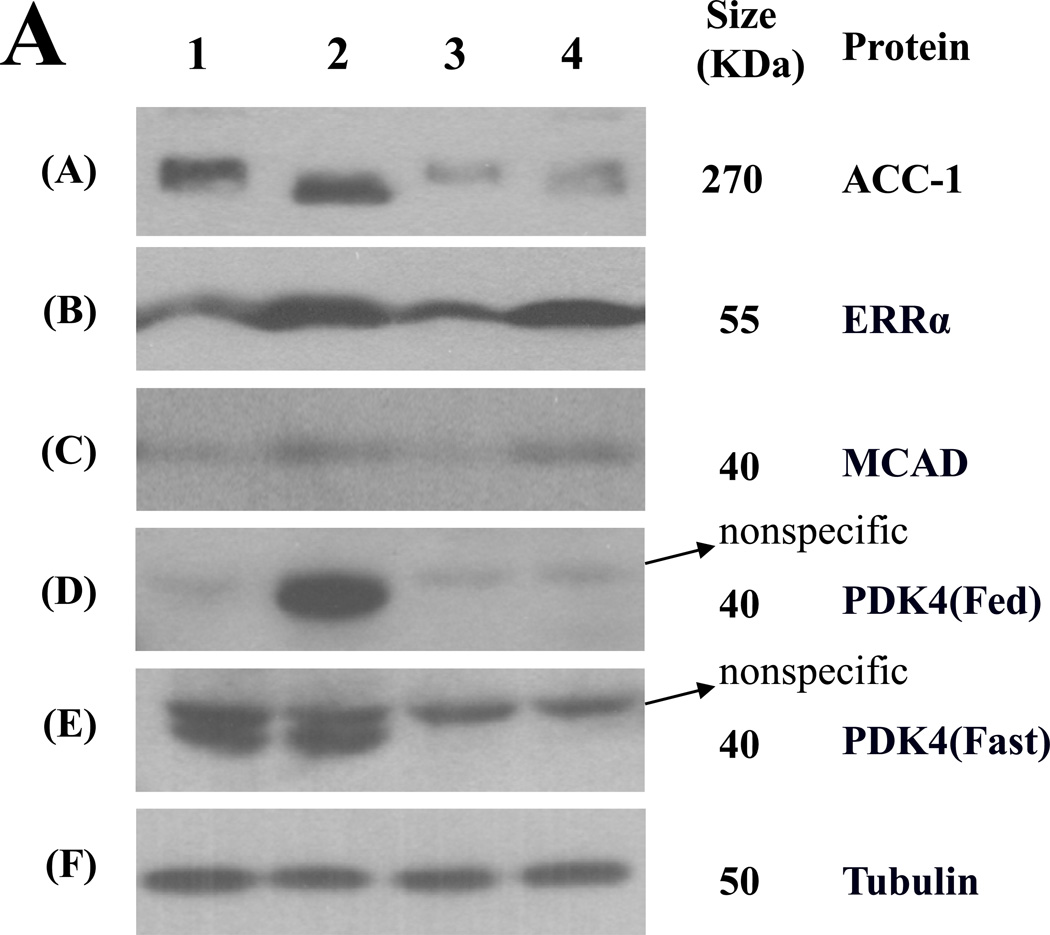
Figure 4. Effect of clofibric acid and PDK4 deficiency on expression of liver enzymes in mice fed the HSF diet.
(A) Representative Western blots are shown for the amounts of acetyl-CoA carboxylase 1 (ACC-1), estrogen-related receptor α (ERRα), medium chain acyl-CoA dehydrogenase (MCAD), and PDK4 in the livers of wild type or PDK4 knockout mice fed the HSF diet with and without clofibric acid. Tubulin served as a loading control. Amount of all enzymes was measured in the samples prepared from the livers of fasted mice except PDK4 whose amount was measured in the samples prepared from the livers of both fed and fasted mice. The Western blot analysis was performed as described in the Materials and Methods section. 1, wild type mice fed the HSF diet; 2, wild type mice fed the HSF diet with clofibric acid; 3, PDK4 knockout mice fed the HSF diet; 4, PDK4 knockout mice fed the HSF diet with clofibric acid. (B) Histograms constructed from data obtained by the Western blot analysis. Data are presented as mean ± S.E. with n = 4 mice per group. *P < 0.05 relative to wild type mice fed the HSF diet. **P < 0.05 relative to PDK4 knockout mice fed the HSF diet.
Effects of clofibric acid and PDK4 deficiency on the liver capacity for fatty acid oxidation and synthesis in mice fed the HSF diet
To determine the mechanism by which clofibric acid reduces the hepatic steatosis induced by the HSF diet feeding, the protein amounts of selected enzymes involved in lipid metabolism were measured (Figure 4). Clofibric acid increased the amounts of medium chain acyl-CoA dehydrogenase (MCAD) in the livers of both wild type and PDK4 knockout mice, consistent with the known stimulation of fatty acid oxidation by PPARα agonists [23]. Consistent with increases in fatty acid oxidation enzymes, the protein amounts of estrogen-related receptor α (ERRα), a positive transcriptional factor for MCAD expression [24] and PDK4 expression [25], were significantly increased by clofibric acid in the livers of both genotypes (Figure 4). As expected from our previous study [13], the amount of acetyl-CoA carboxylase 1 (ACC1), the rate-limiting enzyme of fatty acid synthesis was decreased in the livers of PDK4 knockout mice without and with supplementation of the diet with clofibric acid (Figure 4).
Effects of clofibric acid and PDK4 deficiency on acetyl-CoA and coenzyme A levels in livers of mice fed the HSF diet
Expansion of the coenzyme A pool size is believed to be part of the mechanism by which clofibric acid promotes fatty acid oxidation in the liver [26, 27]. Supplementing the HSF diet with clofibric acid had this effect in both wild type and PDK4 knockout mice fed the HSF diet (Table 6). Although not statistically significant, a trend towards higher acetyl-CoA levels in livers of clofibric acid-treated mice of both genotype groups was found (Table 6). It is also notable that PDK4 deficiency did not change the levels of coenzyme A and acetyl-CoA or the ratio of acetyl-CoA to coenzyme A with or without treatment with clofibric acid. These findings suggest that flux through PDC is probably not differentially regulated by the acetyl-CoA to CoA ratio in the different treatments used in this study and that the expansion of the coenzyme A pool in response to clofibric acid is not linked to up regulation of PDK4 expression.
Table 6.
Free CoA and acetyl-CoA level in the livers of fasted wild type (WT) and PDK4 knockout (PDK4 KO) mice fed a HSF diet with and without clofibric acid
| Genotype | Clofibric acid |
Free CoA | Acetyl-CoA | Acetyl-CoA/CoA |
|---|---|---|---|---|
| nmole / g of liver wet weight | ||||
| WT | − | 158 ± 6 | 134 ± 14 | 0.8 ± 0.1 |
| WT | + | 235 ± 7*† | 179 ± 16 | 0.8 ± 0.1 |
| PDK4 KO | − | 169 ± 9 | 130 ± 19 | 0.8 ± 0.1 |
| PDK4 KO | + | 225 ± 10*† | 177 ± 24 | 0.8 ± 0.1 |
Data are presented as mean ± S.E., n = 5 or 6 for wild type and PDK4 knockout mice fed on a HSF diet with and without clofibric acid.
P < 0.01 relative to non-treated wild type mice.
P < 0.01 relative to non-treated PDK4 knockout mice.
DISCUSSION
We originally thought that up regulation of PDK4 by clofibric acid might be responsible for the beneficial effects of clofibric acid on serum and hepatic lipid levels. This notion was based on previous findings that: (a) clofibric acid and other PPARα ligands, e.g. Wy-14,643, increase PDK4 expression in major tissues of the body [14, 15] and (b) PDK4 deficiency increases PDC activity which increases pyruvate oxidation at the expense of fatty acid oxidation [11]. However, the data presented here with PDK4 knockout mice show that induction of PDK4 expression is not necessary for the beneficial effects of clofibric acid on lipid levels. Indeed, clofibric acid is more effective in PDK4 knockout mice, suggesting that the induction of PDK4 that normally occurs in response to clofibric acid may oppose the beneficial effects of this compound on lipid levels.
As expected, wild type mice fed the HSF diet accumulated large amounts of fat and became obese. Clofibric acid prevented the development of obesity by the mice. As reported previously [13], PDK4 deficiency also attenuated the weight gain induced by the HSF diet. Although not statistically significant, a trend toward further attenuation in weight gain was found in clofibric acid-treated PDK4 knockout mice compared to clofibric acid-treated wild type mice. In agreement with these effects on body weight, clofibric acid, PDK4 deficiency, and combination of both factors reduced epididymal fat pad weight. Since the mice of the four groups consumed the same amounts of food, less weight gain in clofibric acid-treated wild type and PDK4 knockout mice was not due to less calorie intake. Indeed, food efficiency determined by weight gain relative to food intake was markedly reduced in mice fed the diet supplemented with clofibric acid. Since uncoupling proteins (UCP) are induced by PPARα activation [16–21] and clofibric acid induced UCP3 remarkably in wild type mice and even more remarkably in PDK4 knockout mice, dissipation of energy by heat as a consequence of uncoupling of oxidative phosphorylation may be responsible for controlling weight gain in the clofibric acid-treated mice.
Activation of PPARα by PPARα agonists increases the enzymatic capacity for fatty acid oxidation [28]. Established mechanisms include induction of mitochondrial and peroxisomal fatty acid oxidation enzymes [29], induction of malonyl-CoA decarboxylase which relieves inhibition of CPT-1 by malonyl-CoA [30], and expansion of the size of coenzyme A pool [31]. Evidence that clofibric acid acts at least in part via these mechanisms was found in this study. However, there is also evidence that PPARα agonists can increase the capacity for de novo lipogenesis [32]. Therefore, clofibric acid may also dissipate energy via a futile cycle created by de novo fatty acid synthesis followed by fatty acid oxidation. However, loss of energy via this futile cycle should be reduced in clofibric acid-treated PDK4 knockout mice because fatty acid synthesis enzymes are reduced in PDK4 knockout mice [13].
Both clofibric acid and PDK4 deficiency reduced liver fat accumulation and the combination further reduced the amount, suggesting the effects of PDK4 deficiency and clofibric acid on hepatic steatosis are additive with independent mechanisms. As expected, clofibric acid increased the amounts of MCAD, a key mitochondrial fatty acid oxidation enzyme. In agreement with the histological observation, PDK4 deficiency did not reduce the increased amounts of MCAD in the livers of clofibric acid-treated mice. Moreover, ERRα which is known to increase MCAD expression [24] was also increased in the livers of clofibric acid-treated wild type and PDK4 knockout mice. Since PDK4 deficiency reduces fat accumulation in the liver at least in part by decreasing the amounts of enzymes involved in fatty acid synthesis [13], the amount of ACC1 was measured. Clofibric acid did not alter the amount of ACC1, but PDK4 deficiency caused a significant reduction. ACC1 was also reduced in clofibric acid-treated PDK4 knockout mice. Both clofibric acid treatment and PDK4 deficiency increased serum ketone bodies. In the case of clofibric acid, this likely reflects the increase in enzymatic capacity for fatty acid oxidation. In PDK4 deficiency, the mechanism is less certain but may be due to stimulation of ketone body formation from fatty acids as a consequence of inhibition of the citric acid cycle. Previous studies have shown that PDK4 deficiency limits the synthesis of oxaloacetate in the liver [11]. PDK4 deficiency causes greater PDC activity which results in greater oxidation of pyruvate by peripheral tissues, thereby reducing the release of three carbon compounds into the blood and therefore reducing the amount of pyruvate available for the synthesis of oxaloacetate in the liver. Limiting citric acid cycle by the availability of oxaloacetate directs acetyl-CoA produced by fatty acid oxidation into ketone body formation. Since oxidation of fatty acids via the citric acid produces more ATP than oxidation of fatty acids to ketone bodies, larger amounts of fatty acids have to be oxidized to meet the energy needs of the liver in PDK4 knockout out animals.
In summary, the findings reported here suggest that clofibric acid ameliorates body weight gain and hepatic steatosis by partially uncoupling oxidative phosphorylation and increasing the capacity for fatty acid oxidation. Up regulation of PDK4 is not required for this effect. Indeed, PDK4 deficiency complements the action of clofibrate by decreasing the capacity for fatty acid synthesis and perhaps also by promoting the oxidation of fatty acids to ketone bodies. These findings suggest that a PDK4 inhibitor and a fibrate could potentially be used in combination to reduce body weight and prevent hepatic steatosis in patients with type 2 diabetes or diet-induced obesity.
MATERIALS AND METHODS
Animals
Experimental protocols were approved by the Animal Care and Use Committee of the Indiana University School of Medicine. PDK4 knockout mice were generated as previously described [11]. At 5 weeks of age, groups of 12 male wild type and 12 male PDK4 knockout mice were housed with two mice per cage under controlled temperature (23 ± 2°C) and a 12-h light/dark cycle (lights on at 7 a.m. and off at 7 p.m.). The mice were fed a high saturated fat (HSF) diet (catalog no. D12330; Research Diets, New Brunswick, NJ) that was low in carbohydrate and high in saturated fat (58 % by calories). According to the supplier, the fatty acids present in the HSF diet were 93.3 % saturated, 2.4 % monounsaturated, and 4.3 % polyunsaturated. After 14 weeks on the HSF diet, 0.5 % clofibric acid (197777, Sigma-Aldrich, St. Louis, MO) was added to the diet of half of the mice of each group. Based on their measured food consumption, the mice consumed about 10 mg of clofibric acid per day. Body weights of the mice were determined weekly. Food consumption was monitored during the 16th week of the feeding period. The experiment was terminated after 28 weeks of feeding. After overnight fasting (from 3 p.m. to 8 a.m.), blood was taken from the tail for the measurement of glucose and the mice were anesthetized with pentobarbital (60 mg/kg of body weight). Blood was drawn from the inferior vena cava to measure serum metabolites. Gastrocnemius muscle, liver, heart, and epididymal fat pad were harvested, as rapidly as possible in the order given, immediately freeze-clamped with Wollenberger tongs at the temperature of liquid nitrogen, powdered under liquid nitrogen with a mortar and pestle, and stored at −85°C for analysis. Small pieces of the liver were also collected and snap-frozen in liquid nitrogen for histological analysis.
Measurement of metabolite concentrations
Triacylglycerols in sera or tissues were determined with the L-type TG H assay kit from Wako Chemical (Richmond, VA); free fatty acids with the Half Micro Assay kit from Roach Diagnostics (Indianapolis, IN). Tissue levels of CoA and acetyl-CoA were determined as described by Michal and Bergmeyer [33].
Measurement of the PDC activity
Pulverized tissues were homogenized in an extraction buffer [34] containing 30 mM HEPES-KOH, pH 7.5, 0.5 mM thiamine pyrophosphate, 3 % (v/v) Triton X-100, 5 mM EDTA, 2 % (v/v) bovine serum, 5 mM dithiothreitol, 10 µM tosyl phenylalanyl chloromethyl ketone, 10 µg/ml trypsin inhibitor, 1 µM of leupeptin, 2 mM deoxycholic acid, and 50 mM potassium fluoride. The supernatants were obtained by centrifugation at 10,000 × g for 10 min at 4°C. Total and actual PDC activities were measured as previously described [11]. PDC activity is expressed as the percentage of actual activity relative to total activity.
Insulin tolerance test (ITT)
At the 20th week of feeding, ITT were performed after food had been withheld from the mice for 5 h (9 a.m. to 2 p.m.). Insulin (1 U/kg of body weight, Humulin R; Eli Lilly, Indianapolis, IN) was given by intraperitoneal injection. Tail blood glucose levels were measured at 0, 15, 30, and 60 min.
Histochemistry of the livers
Histological examination of the liver was performed by the Immunohistochemistry Laboratory of Indiana University School of Medicine. The liver sections were stained with Oil Red O.
Western blot analysis
Tissue powder prepared under liquid nitrogen was homogenized with radio-immunoprecipitation assay (RIPA) buffer containing 50 mM Tris/HCl (pH 7.4), 150 mM NaCl, 0.25 % (v/v) deoxycholic acid, 1 % (v/v) Nonidet P-40, 1 mM EDTA, 10 µM tosyl phenylalanyl chloromethyl ketone, 10 µg/ml trypsin inhibitor, 1 mM phenylmethylsulfonyl fluoride, 2 µg/ ml aprotinin, 3.5 mM bis-benzamidine, 50 mM potassium fluoride, and 0.4 mM sodium orthovanadate. Tissue extracts were obtained by centrifugation at 13400 × g for 10 min at 4° C. Protein concentration was determined by the Bio-Rad assay. Equal amounts of protein were separated on SDS-polyacrylamide gels, transferred to a nitrocellulose membrane by the wet blotting method, and probed with antibodies directed against estrogen-related receptor α (ERRα; 07-662, Millipore, Billerica, MA), medium chain acyl-CoA dehydrogenase (MCAD; sc-50587, Santa Cruz Biotechnology, Santa Cruz, CA), acetyl-CoA carboxylase 1 (ACC-1; 07-439, Upstate, Lake Placid, NY), tubulin (ab6046, Abcam, Cambridge, MA), and PDK1 (KAP16 PK112,Stressgen, Plymouth Meeting, PA), PDK2 (sc-100534, Santa Cruz Biotechnology, Santa Cruz, CA), PDK3 (H00005165-M01, Abnova, Walnut, CA), and PDK4 [11]. The amounts of bound antibodies were assessed by the peroxidase activity of horseradish peroxidase-conjugated secondary antibody as detected by chemiluminescence with Lumi-light western blotting substrate (Roche Diagnostics, Indianapolis, IN).
Quantitative real-time PCR analysis
Total RNA was isolated from frozen liver samples with an RNAqueous®-4PCR Kit (Applied Biosystems/Ambion, Austin, TX, USA). RNA integrity was checked by agarose gel electrophoresis. Validated primer sets for DNA amplification were obtained from SABiosciences (Frederick, MD, USA): uncoupling protein 3 (GenBank ID: NM_009464), and Tubulin β1 (GenBank ID: NM_001080971).
With Brilliant® II SYBR® Green QRT-PCR, AffinityScript™ Mix, 2-Step reagent (Stratagene, La Jolla, CA, USA), 3 µg of RNA from each sample were reverse transcribed to cDNA, and then a proper amount of the cDNA (decided by standard curve generated for each gene analyzed) was used as template for qPCR with the Mx3005P Quantitative PCR System (Stratagene, La Jolla, CA, USA). A threshold cycle (Ct value) was obtained for each amplification curve. After normalized with the Ct value obtained for Tubulin β1 in the same samples, the resulting ΔCt values were used to obtain relative fold change in expression of the indicated gene in each treated group relative to that in the control group.
Statistical analysis
Values are presented as mean ± S.E. with the indicated number of independent samples. The statistical significance of differences between groups was determined with one-way ANOVA corrected by the Tukey-Kramer method or the Student’s t-test. P values less than 0.05 were considered to be statistically significant.
ACKNOWLEDGMENTS
We thank Dr. N.H. Jeoung at Catholic University of Daegu (South Korea) for helpful discussion. This work was supported by grants to R.A. Harris from the National Institutes of Health (DK47844) and a VA Merit Review Grant.
Current address of B. Hwang is Wisconsin Institutes for Medical Research, room 6168, 1111 Highland Avenue, Madison, WI 53705, U.S.A.
Abbreviations
- PDC
pyruvate dehydrogenase complex
- PDK4
pyruvate dehydrogenase kinase isoenzyme 4
- CoA
coenzyme A
- PPARα
peroxisome proliferator-activated receptor α
- MCAD
medium chain acyl-CoA dehydrogenase.
Footnotes
Disclosures: No conflicts of interest are declared by the authors.
REFERENCES
- 1.Filippatos T, Milionis HJ. Treatment of hyperlipidaemia with fenofibrate and related fibrates. Expert Opin Investig Drugs. 2008;17:1599–1614. doi: 10.1517/13543784.17.10.1599. [DOI] [PubMed] [Google Scholar]
- 2.Hertz R, Bishara-Shieban J, Bar-Tana J. Mode of action of peroxisome proliferators as hypolipidemic drugs. Suppression of apolipoprotein C-III. J Biol Chem. 1995;270:13470–13475. doi: 10.1074/jbc.270.22.13470. [DOI] [PubMed] [Google Scholar]
- 3.Vu-Dac N, Schoonjans K, Kosykh V, Dallongeville J, Fruchart JC, Staels B, Auwerx J. Fibrates increase human apolipoprotein A-II expression through activation of the peroxisome proliferator-activated receptor. J Clin Invest. 1995;96:741–750. doi: 10.1172/JCI118118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mannaerts GP, Debeer LJ, Thomas J, De Schepper PJ. Mitochondrial and peroxisomal fatty acid oxidation in liver homogenates and isolated hepatocytes from control and clofibrate-treated rats. J Biol Chem. 1979;254:4585–4595. [PubMed] [Google Scholar]
- 5.Guerre-Millo M, Gervois P, Raspe E, Madsen L, Poulain P, Derudas B, Herbert JM, Winegar DA, Willson TM, Fruchart JC, et al. Peroxisome proliferator-activated receptor alpha activators improve insulin sensitivity and reduce adiposity. J Biol Chem. 2000;275:16638–16642. doi: 10.1074/jbc.275.22.16638. [DOI] [PubMed] [Google Scholar]
- 6.Seo YS, Kim JH, Jo NY, Choi KM, Baik SH, Park JJ, Kim JS, Byun KS, Bak YT, Lee CH, et al. PPAR agonists treatment is effective in a nonalcoholic fatty liver disease animal model by modulating fatty-acid metabolic enzymes. J Gastroenterol Hepatol. 2008;23:102–109. doi: 10.1111/j.1440-1746.2006.04819.x. [DOI] [PubMed] [Google Scholar]
- 7.Harano Y, Yasui K, Toyama T, Nakajima T, Mitsuyoshi H, Mimani M, Hirasawa T, Itoh Y, Okanoue T. Fenofibrate, a peroxisome proliferator-activated receptor alpha agonist, reduces hepatic steatosis and lipid peroxidation in fatty liver Shionogi mice with hereditary fatty liver. Liver Int. 2006;26:613–620. doi: 10.1111/j.1478-3231.2006.01265.x. [DOI] [PubMed] [Google Scholar]
- 8.Randle PJ. Metabolic fuel selection: general integration at the whole-body level. Proc Nutr Soc. 1995;54:317–327. doi: 10.1079/pns19950057. [DOI] [PubMed] [Google Scholar]
- 9.Harris RA, Bowker-Kinley MM, Huang B, Wu P. Regulation of the activity of the pyruvate dehydrogenase complex. Adv Enzyme Regul. 2002;42:249–259. doi: 10.1016/s0065-2571(01)00061-9. [DOI] [PubMed] [Google Scholar]
- 10.Wu P, Blair PV, Sato J, Jaskiewicz J, Popov KM, Harris RA. Starvation increases the amount of pyruvate dehydrogenase kinase in several mammalian tissues. Arch Biochem Biophys. 2000;381:1–7. doi: 10.1006/abbi.2000.1946. [DOI] [PubMed] [Google Scholar]
- 11.Jeoung NH, Wu P, Joshi MA, Jaskiewicz J, Bock CB, Depaoli-Roach AA, Harris RA. Role of pyruvate dehydrogenase kinase isoenzyme 4 (PDHK4) in glucose homoeostasis during starvation. Biochem J. 2006;397:417–425. doi: 10.1042/BJ20060125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeoung NH, Harris RA. Pyruvate dehydrogenase kinase-4 deficiency lowers blood glucose and improves glucose tolerance in diet-induced obese mice. Am J Physiol Endocrinol Metab. 2008;295:E46–E54. doi: 10.1152/ajpendo.00536.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hwang B, Jeoung NH, Harris RA. Pyruvate dehydrogenase kinase isoenzyme 4 (PDHK4) deficiency attenuates the long-term negative effects of a high-saturated fat diet. Biochem J. 2009;423:243–252. doi: 10.1042/BJ20090390. [DOI] [PubMed] [Google Scholar]
- 14.Motojima K, Seto K. Fibrates and statins rapidly and synergistically induce pyruvate dehydrogenase kinase 4 mRNA in the liver and muscles of mice. Biol Pharm Bull. 2003;26:954–958. doi: 10.1248/bpb.26.954. [DOI] [PubMed] [Google Scholar]
- 15.Wu P, Peters JM, Harris RA. Adaptive increase in pyruvate dehydrogenase kinase 4 during starvation is mediated by peroxisome proliferator-activated receptor alpha. Biochem Biophys Res Commun. 2001;287:391–396. doi: 10.1006/bbrc.2001.5608. [DOI] [PubMed] [Google Scholar]
- 16.Tsuboyama-Kasaoka N, Takahashi M, Kim H, Ezaki O. Up-regulation of liver uncoupling protein-2 mRNA by either fish oil feeding or fibrate administration in mice. Biochem Biophys Res Commun. 1999;257:879–885. doi: 10.1006/bbrc.1999.0555. [DOI] [PubMed] [Google Scholar]
- 17.Barbera MJ, Schluter A, Pedraza N, Iglesias R, Villarroya F, Giralt M. Peroxisome proliferator-activated receptor alpha activates transcription of the brown fat uncoupling protein-1 gene. A link between regulation of the thermogenic and lipid oxidation pathways in the brown fat cell. J Biol Chem. 2001;276:1486–1493. doi: 10.1074/jbc.M006246200. [DOI] [PubMed] [Google Scholar]
- 18.Young ME, Patil S, Ying J, Depre C, Ahuja HS, Shipley GL, Stepkowski SM, Davies PJ, Taegtmeyer H. Uncoupling protein 3 transcription is regulated by peroxisome proliferator-activated receptor (alpha) in the adult rodent heart. FASEB J. 2001;15:833–845. doi: 10.1096/fj.00-0351com. [DOI] [PubMed] [Google Scholar]
- 19.Wensaas AJ, Rustan AC, Rokling-Andersen MH, Caesar R, Jensen J, Kaalhus O, Graff BA, Gudbrandsen OA, Berge RK, Drevon CA. Dietary supplementation of tetradecylthioacetic acid increases feed intake but reduces body weight gain and adipose depot sizes in rats fed on high-fat diets. Diabetes Obes Metab. 2009;11:1034–1049. doi: 10.1111/j.1463-1326.2009.01092.x. [DOI] [PubMed] [Google Scholar]
- 20.Lanni A, Mancini FP, Sabatino L, Silvestri E, Franco R, De Rosa G, Goglia F, Colantuoni V. De novo expression of uncoupling protein 3 is associated to enhanced mitochondrial thioesterase-1 expression and fatty acid metabolism in liver of fenofibrate-treated rats. FEBS Lett. 2002;525:7–12. doi: 10.1016/s0014-5793(02)02828-4. [DOI] [PubMed] [Google Scholar]
- 21.Park MK, Lee HJ, Hong SH, Choi SS, Yoo YH, Lee KI, Kim DK. The increase in hepatic uncoupling by fenofibrate contributes to a decrease in adipose tissue in obese rats. J Korean Med Sci. 2007;22:235–241. doi: 10.3346/jkms.2007.22.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gustafson LA, Kuipers F, Wiegman C, Sauerwein HP, Romijn JA, Meijer AJ. Clofibrate improves glucose tolerance in fat-fed rats but decreases hepatic glucose consumption capacity. J Hepatol. 2002;37:425–431. doi: 10.1016/s0168-8278(02)00212-x. [DOI] [PubMed] [Google Scholar]
- 23.Ouali F, Djouadi F, Bastin J. Effects of fatty acids on mitochondrial beta-oxidation enzyme gene expression in renal cell lines. Am J Physiol Renal Physiol. 2002;283:F328–f334. doi: 10.1152/ajprenal.00324.2001. [DOI] [PubMed] [Google Scholar]
- 24.Vega RB, Kelly DP. A role for estrogen-related receptor alpha in the control of mitochondrial fatty acid beta-oxidation during brown adipocyte differentiation. J Biol Chem. 1997;272:31693–31699. doi: 10.1074/jbc.272.50.31693. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Ma K, Sadana P, Chowdhury F, Gaillard S, Wang F, McDonnell DP, Unterman TG, Elam MB, Park EA. Estrogen-related receptors stimulate pyruvate dehydrogenase kinase isoform 4 gene expression. J Biol Chem. 2006;281:39897–39906. doi: 10.1074/jbc.M608657200. [DOI] [PubMed] [Google Scholar]
- 26.Voltti H, Savolainen MJ, Jauhonen VP, Hassinen IE. Clofibrate-induced increase in coenzyme A concentration in rat tissues. Biochem J. 1979;182:95–102. doi: 10.1042/bj1820095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skrede S, Halvorsen O. Increased biosynthesis of CoA in the liver of rats treated with clofibrate. Eur J Biochem. 1979;98:223–229. doi: 10.1111/j.1432-1033.1979.tb13180.x. [DOI] [PubMed] [Google Scholar]
- 28.Gulick T, Cresci S, Caira T, Moore DD, Kelly DP. The peroxisome proliferator-activated receptor regulates mitochondrial fatty acid oxidative enzyme gene expression. Proc Natl Acad Sci U S A. 1994;91:11012–11016. doi: 10.1073/pnas.91.23.11012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Green S, Wahli W. Peroxisome proliferator-activated receptors: finding the orphan a home. Mol Cell Endocrinol. 1994;100:149–153. doi: 10.1016/0303-7207(94)90294-1. [DOI] [PubMed] [Google Scholar]
- 30.Lee GY, Kim NH, Zhao ZS, Cha BS, Kim YS. Peroxisomal-proliferator-activated receptor alpha activates transcription of the rat hepatic malonyl-CoA decarboxylase gene: a key regulation of malonyl-CoA level. Biochem J. 2004;378:983–990. doi: 10.1042/BJ20031565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brass EP. Interaction of carnitine and propionate with pyruvate oxidation by hepatocytes from clofibrate-treated rats: importance of coenzyme A availability. J Nutr. 1992;122:234–240. doi: 10.1093/jn/122.2.234. [DOI] [PubMed] [Google Scholar]
- 32.Knight BL, Hebbachi A, Hauton D, Brown AM, Wiggins D, Patel DD, Gibbons GF. A role for PPARalpha in the control of SREBP activity and lipid synthesis in the liver. Biochem J. 2005;389:413–421. doi: 10.1042/BJ20041896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Michal G, Bergmeyer HU. [The enzymatic analysis of coenzyme A.] Biochim Biophys Acta. 1963;67:599–616. doi: 10.1016/0006-3002(63)91870-5. [DOI] [PubMed] [Google Scholar]
- 34.Nakai N, Sato Y, Oshida Y, Fujitsuka N, Yoshimura A, Shimomura Y. Insulin activation of pyruvate dehydrogenase complex is enhanced by exercise training. Metabolism. 1999;48:865–869. doi: 10.1016/s0026-0495(99)90220-2. [DOI] [PubMed] [Google Scholar]