Abstract
Pneumococcal and Salmonella typhi infections are two major diseases for children in developing countries. For typhoid fever, licensed Vi polysaccharide vaccines are ineffective in children <2 year-old. While investigational Vi conjugate vaccines have been shown effective in clinical trials, they are currently only available to restricted areas. Pneumococcal capsular polysaccharide conjugate vaccines are highly effective in children, but suffer from some limitations including cost and limited serotype coverage. We have previously shown that a fusion conjugate vaccine, consisting of pneumococcal fusion protein PsaA and pneumolysoid (PdT) conjugated to a polysaccharide, results in enhanced antibody and CD4+ Th17 cell responses as well as protection against pneumococcal colonization and disease in mice. Here we applied this approach to develop a bivalent vaccine against pneumococcus and S. typhi. Two species-conserved pneumococcal antigens (SP1572 or SP2070) were fused to the nonhemolytic pneumolysoid PdT. SP1572-PdT was then conjugated to Vi polysaccharide and SP2070-PdT was conjugated to the pneumococcal cell wall polysaccharide (CWPS; also conserved). Mice immunized with this bivalent conjugate were protected against pneumococcal colonization and sepsis challenges, and made anti-Vi antibody concentrations higher by 40 fold compared to mice that received equimolar mixtures of the antigens. An enhanced killing of Vi-bearing Salmonellae in vitro was demonstrated from plasma of mice that received the fusion conjugate but not the mixture of antigens. Our results support further evaluation of this bivalent immunogen for the prevention of pneumococcal colonization and disease, and of typhoid fever.
Keywords: Streptococcus pneumoniae, Salmonella typhi, vaccine, Vi polysaccharide
INTRODUCTION
Infections by Salmonella enterica serovar typhi (S. typhi) and Streptococcus pneumoniae (pneumococcus) are major causes of morbidity and mortality in childhood, especially in developing countries. Typhoid fever [caused by S. typhi] is a major threat to children, particularly in southeast and central Asia, Latin America and Africa. More than 20 million cases are estimated each year, accounting for over 200,000 deaths, primarily in children under 5 years age [1–3]. With respect to pneumococcus, estimates suggest 14.5 million cases of invasive disease occur worldwide in children under 5 years of age and over 820,000 deaths [4]. Both of these pathogens afflict children in the same epidemiologic context and there remains an unmet need to develop an affordable and effective vaccine for use in developing countries. A bivalent vaccine that targets both pathogens could represent an economical solution to this problem.
The capsular polysaccharide Vi of S. typhi is an important virulence factor and also a protective antigen [5–7]. Both pure polysaccharide and conjugated Vi had been tested in clinical trials and shown to be protective against typhoid fever [8, 9]. Vi is a linear polymer composed of (α1-4)-2-deoxy-2-N-acetyl galacturonic acid moieties and is a thymus-independent antigen. Children under 2 years of age do not respond to immunization with Vi. In contrast, immunization with Vi conjugated to the nontoxic recombinant exotoxin A of Pseudomonas aeruginosa (Vi-rEPA) produces protective levels of serum anti-Vi IgG in infants and young children [9, 10]. Vi-protein conjugates using other carrier proteins such as CRM197, tetanus toxoid, diphtheria toxoid, cholera toxins, the B subunit of the heat labile toxin of Escherichia coli, recombinant outer membrane protein of Klebsiella pneumoniae and ion-regulated outer-membrane proteins of S. typhi have been evaluated in preclinical studies, and some have been tested in humans [11–18]. A conjugate typhoid vaccine is licensed but only available in restricted areas and not widely used[19]. Another vaccine approach is oral immunization with the attenuated Ty21a strain, which provides comparable protection to the Vi polysaccharide vaccine [20, 21] but likewise is not approved for young children.
Current pneumococcal vaccines target the polysaccharide capsule by the inclusion of individual polysaccharide-protein conjugates for some of the more common capsular types associated with invasive disease. To date, there have been three licensed conjugate vaccines, comprising valencies of 7, 10 or 13 [22–24]. While these vaccines are highly effective against strains bearing the included capsular serotypes, their high cost and complexity of manufacture represent an important hurdle for widespread use. Furthermore, particularly in developing countries, the prevailing serotypes are not always well covered by the existing vaccines [25]. Finally, the rapid emergence of serotypes not included in the vaccine has been observed in several countries, including the US and in Europe [26], potentially threatening the long-term efficacy of this approach.
Thus, alternative vaccine strategies are being sought. It has long been recognized that antibodies to noncapsular antigens conserved widely within the S. pneumoniae species could protect mice against pneumococcal invasive disease [27, 28]. Some of these vaccine candidates have progressed to clinical trials [29, 30]. More recently, work in mice has revealed the existence of an antibody-independent, CD4+ Th17-mediated mechanism of protection against pneumococcal colonization [31–33]. It has been argued that a protein-based vaccine that could confer antibody-mediated immunity to invasive disease and Th17-mediated protection against nasopharyngeal colonization, may represent an attractive alternative to pneumococcal conjugate vaccines, by providing a two-pronged mechanism of protection [34, 35].
We have described a fusion conjugate construct, consisting of a conserved pneumococcal cell wall polysaccharide (CWPS) conjugated to the fused pneumococcal surface adhesin A (PsaA) and the non-hemolytic pneumolysin mutant PdT (W433F, D385N, and C428G). This construct elicited both antibody and Th17 cell responses to proteins and conferred protection against both invasive disease and colonization [36]. Here we applied this approach to two conserved protective pneumococcal proteins and S. typhi Vi polysaccharide for the development of a vaccine candidate targeting both pneumococcus and S. typhi.
RESULTS
Selection of the pneumococcal protein components
Based on the observation that a killed S. pneumoniae whole cell preparation administered intranasally (SPWCV) protects mice against colonization in a CD4+ T cell dependent manner [32, 37, 38], we fractionated SPWCV to identify protein antigens that elicit the highest IL-17A responses from splenocytes of immunized animals (data not shown). Two antigens proved highly potent at eliciting IL-17A responses from SPWCV-immunized animals and are known to be conserved: SP1572 (pneumococcal protective protein A), a non-heme iron-containing ferritin previously evaluated in mouse models of colonization and disease [39, 40] and SP2070, a surface-exposed glucose-6-phosphate isomerase against which age-dependent increases in antibodies have been demonstrated in humans [41]. These two proteins were then tested in an intranasal immunization model using cholera toxin as an adjuvant, and they conferred protection against nasopharyngeal pneumococcal carriage (data not shown).
Fusion conjugates consisting of Vi polysaccharide conjugated to the fusions of the two pneumococcal proteins to PdT confer protection against pneumococcal colonization but not against sepsis challenge
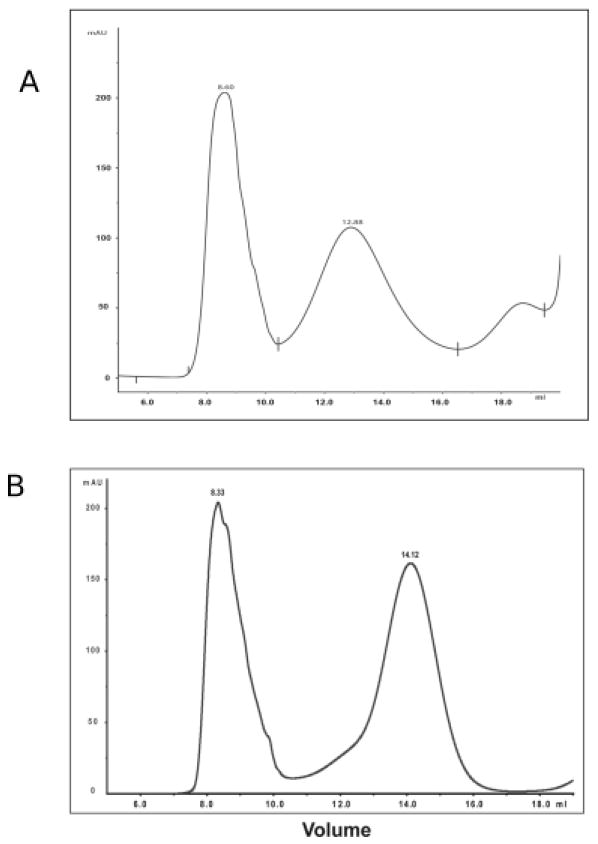
DNA fragments encoding SP1572 or SP2070 fused to PdT with a polylinker GSGGGGS were generated through PCR and cloned into pQE30 vector as described in the Materials and Methods section. After transformation, His-tagged proteins were purified from E. coli using a Ni-NTA column (Qiagen), and peak fractions were combined and run through a Sepharose S200 gel filtration column for further purification. Proteins were then conjugated to Vi using a modification of the method described in [15]. The Vi-conjugates were separated from free protein and Vi by passage through a Superose 6 column (Fig 1A and 1B). The Vi-conjugates SP1572-PdT-Vi and SP2070-PdT-Vi had protein:Vi ratios of 1:1.4 and 1:1.7 mg/mg , respectively, determined by the BCA assay of protein and concentration of O-acetyl of Vi [42].
Figure 1.
Preparation of SP1572-PdT-Vi (A) and SP2070-PdT-Vi (B) conjugates. SP1572-PdT and SP2070-PdT were purified from E. coli and then conjugated to Vi as described in Materials and Methods. Products were run through a Superose 6 column and fractions from void volume (which contain conjugated material) were collected to separate conjugate from free proteins.
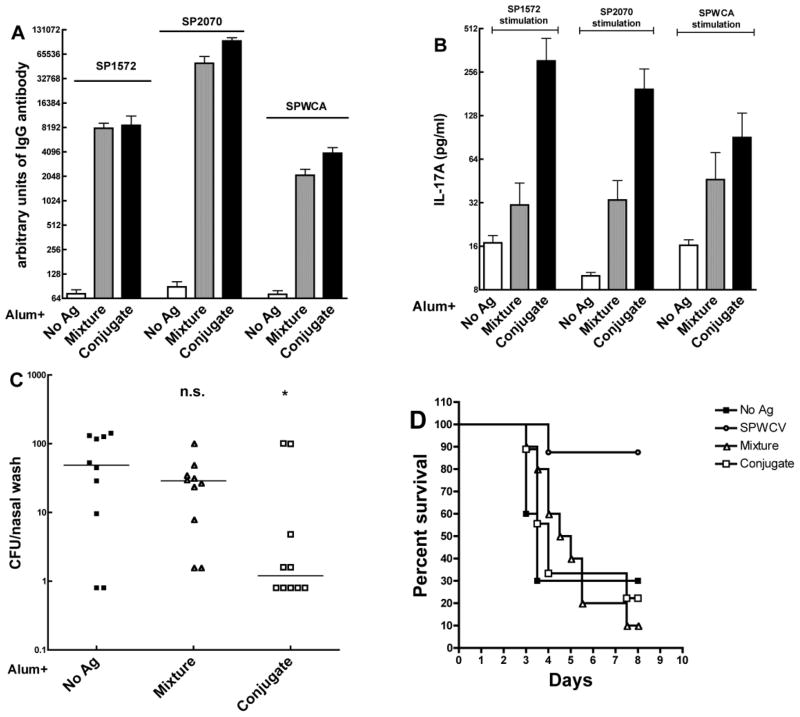
For immunization, the two Vi-conjugates were mixed in a 1:1 ratio (based on protein content. Mice were immunized subcutaneously three times at two-week intervals with either alum alone, the combination of both Vi fusion conjugates adsorbed onto alum, or the equimolar mixture of all the uncoupled components of the fusion conjugates (referred to hereafter as mixture). Mice in the Vi-conjugate group had similar levels of anti-SP1572, SP2070 or SPWCA IgG antibodies in the plasma (Figure 2A). Whole blood from mice immunized with the combination of Vi-conjugates and stimulated with either individual protein or with SPWCA made significantly higher IL-17A than the whole blood from mice in the alum or mixture groups (Figure 2B). The concentration of Vi antibody was over 40 times higher in mice that received the combination of fusion conjugates than in mice that received the mixture of antigens with alum (data not shown).
Figure 2.
Immunogenicity and evaluation of protection against pneumococcal colonization and aspiration/sepsis by SP1572-PdT-Vi and SP2070-PdT-Vi fusion conjugate. Mice were immunized subcutaneously three times two-weeks apart. Mice were bled two weeks after last immunization to analyze antibody levels in plasma and IL-17A responses in whole blood. (A). Antibody responses to SP1572, SP2070 and SPWCA in adjuvant alone (white bar), mixture (equimolar concentration, gray bar) or conjugate (combination of SP1572-PdT-Vi and SP2070-PdT-Vi, black bar) immunized mice. The antibody titers in mixture and conjugate groups were similar. (B). IL-17A responses following ex vivo stimulation with SP1572, SP2070 or SPWCA. Mice in the conjugate group had significantly higher IL-17A responses to all stimuli compared to mice in the mixture group. Bars represent mean value with SEM. (C). Protection against pneumococcal colonization challenge. Mice were challenged intranasally with the pneumococcal serotype 6B 0603 strain and the colonization density was determined 10 days later. Mice that received the combination of fusion conjugates had significantly lower densities of colonization than mice that received alum alone (P=0.043 by Mann-Whitney U analysis) whereas mice that received the mixture of all antigens were not significantly protected (P=0.28). Each symbol represents the density of colonization of an individual mouse and the horizontal line represents the median density of colonization. (D). Mice were challenged with 106 cfu of WU2 strain by inducing aspiration under light anesthesia and onset of illness/death monitored for 8 days. No significant protection by immunization with the Vi-conjugate or the mixture was observed, whereas mice that received the whole cell vaccine were significantly protected against illness/death compared to mice that received alum alone (P=0.01 by Kaplan-Meier analysis).
Two different pneumococcal challenge models were used to evaluate the protective capacity of these vaccines: a) a colonization model using a serotype 6B clinical isolate strain 603 which causes asymptomatic carriage and does not cause bacteremic disease in mice [43] and b) a lung inhalation model in which lightly anesthetized mice are given a nasal inoculum containing a serotype 3 strain which causes lung infection, bacteremia and sepsis [36]. As shown in Figure 2C, when these mice were intranasally challenged with serotype 6B strain 0603 in the colonization model, the pneumococcal colony-forming units (cfu) recovered in the nasal wash from mice of the Vi-conjugate group were significantly lower than from mice of the mixture group or those that received alum alone. However, the combination of two Vi fusion conjugates did not offer protection against death compared to either alum or the mixture (Figure 2D) in the lung inhalation model.
Immunization with a combination of Vi- and CWPS-fusion conjugates confers protection against both nasopharyngeal colonization and aspiration pneumonia/ sepsis
We have demonstrated previously that immunization with a conjugate of the cell wall polysaccharide (CWPS) of S. pneumoniae to a fusion protein of PsaA and PdT confers protection against colonization and sepsis, in a Th17- and antibody-dependent fashion, respectively [36, 44], We therefore reasoned that the combination of both approaches may be necessary to develop a vaccine candidate that elicits protection against both colonization and invasive disease. We hypothesized that a combination of a Vi and CWPS conjugates using the protein antigens SP1572 and SP2070 would be protective against pneumococcal colonization and sepsis, while also eliciting antibody responses to the Vi component. To test this hypothesis, SP1572-PdT was conjugated to Vi as described above and SP2070-PdT was conjugated to CWPS as described before [36], using 1-cyano-4-dimethylaminopyridinium tetrafluoroborate (CDAP); both conjugates were separated on a Superose 6 column. SP1572-PdT-Vi had a protein:Vi ratio of 1:1.6 mg/mg and SP2070-PdT-CWPS had a ratio of 1:0.65 mg/mg.
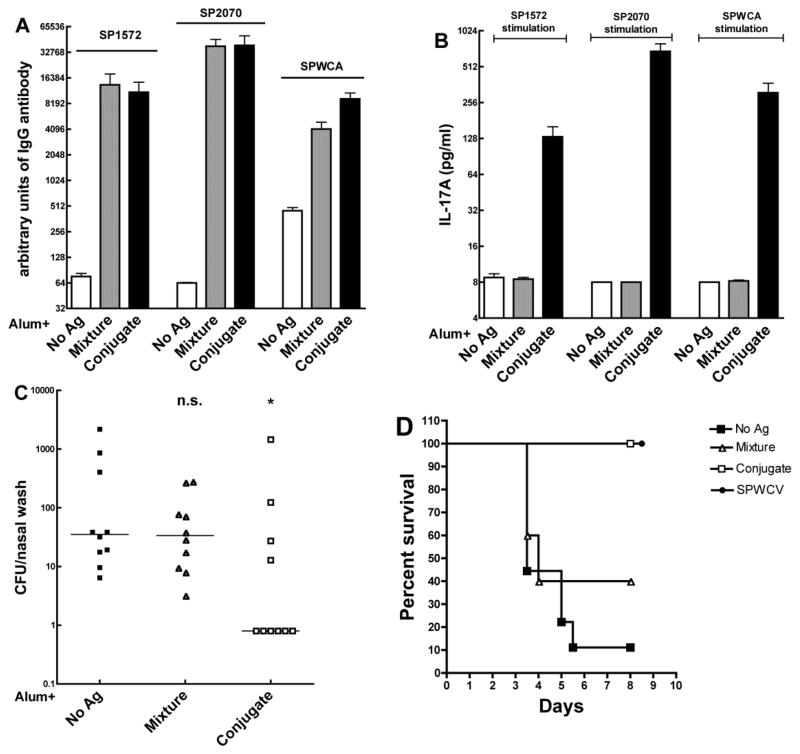
Mice were subcutaneously immunized with the combination of the Vi conjugate SP1572-PdT-Vi and the CWPS-conjugate SP2070-PdT-CWPS with alum, the mixture of all components with alum or alum alone. As shown in Figure 3A, mice in the combined CWPS- and Vi-conjugate group had similar antibody level responses to SP1572 and SP2070 but significantly higher antibody response to SPWCA. Stimulation of whole blood from the CWPS- and Vi- conjugates group also resulted in significantly greater IL-17A responses to either of the protein antigens (SP1572 or SP2070) and to SPWCA than mice in the alum or the mixture groups, whose responses to these stimuli were barely detectable (Figure 3B).
Figure 3.
Immunogenicity and evaluation of protection against pneumococcal colonization and aspiration/sepsis by the combination of SP1572-PdT-Vi and SP2070-PdT-CWPS fusion conjugates. (A). Antibody responses to SP1572, SP2070 and SPWCA in adjuvant alone (white bar), mixture (equimolar concentration, gray bar) or conjugate (combination of SP1572-PdT-Vi and SP2070-PdT-CWPS, black bar) immunized mice. The antibody responses to the proteins or SPWCA from mice in the mixture and conjugate group were similar. (B). IL-17A responses to SP1572, SP2070 or SPWCA. Mice in the conjugate group made significantly higher IL-17A in response to all stimuli than mice in the mixture group or mice immunized with alum alone. Bars represent mean value with SEM. (C). Protection against pneumococcal colonization. The same group of mice was challenged with strain 0603 intranasally and nasal washes collected 10 days later. Mice immunized with the combination of CWPS- and Vi-conjugates were significantly protected against colonization when compared to mice that received alum alone (P=0.036 by Mann-Whitney U) whereas mice that were immunized with the mixture of all components were not protected (P=0.63). (D). Mice immunized with either the SPWCV or the combination of CWPS and Vi fusion conjugates were significantly protected against aspiration pneumonia/sepsis due to strain WU2 (P=0.002 by Kaplan Meier respectively vs. mice immunized with alum alone) whereas mice that received the mixture alone were not protected (P=0.29).
As predicted by the IL-17A assay, mice in the combined conjugate group were significantly protected against colonization compared with mice in the alum group (p=0.036, Fig 3C), whereas mice in the mixture group were not significantly protected. Similarly, the mice in the combined conjugate group were fully protected against type 3 pneumococcal aspiration/sepsis (p=0.002) whereas immunization with the mixture conferred no survival advantage when compared to immunization with alum alone (Fig 3D).
Immunization with Vi and CWPS conjugates induces functional anti-Vi antibodies
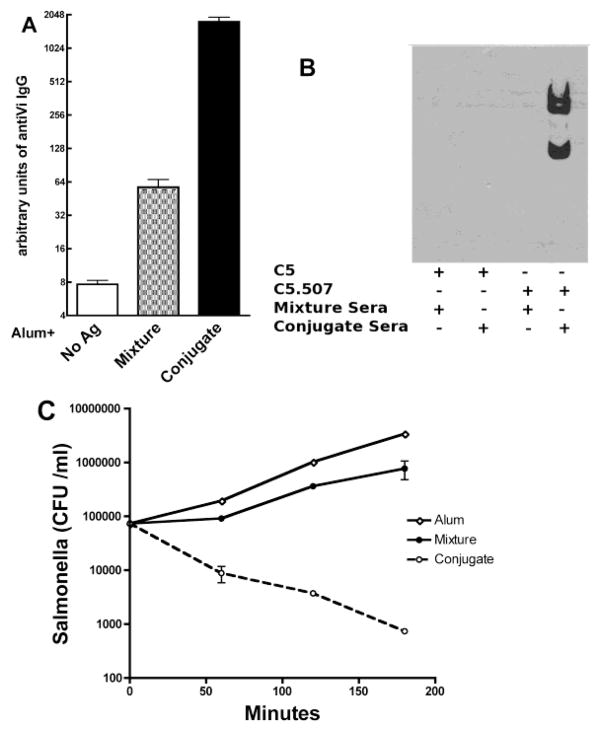
Because S. typhi does not infect mice, immunogenicity and in vitro killing assays were used to determine the ability of the fusion conjugates to elicit functional antibodies against this organism. As shown in Figure 4A, and as expected for an uncoupled polysaccharide, immunization with the mixture of all antigens increased the anti-Vi antibody concentration only slightly (4-fold) compared with immunization with alum alone; in contrast, mice in the combined CWPS- and VI-conjugate group demonstrated over 40-fold increases in anti-Vi IgG plasma concentration than mice that received the mixture of antigens, a response that is similar to that induced by immunization with the two Vi conjugates (data not shown). This result suggests that substitution of the 2070-PdT-CWPS conjugate for the 2070-PdT-Vi conjugate did not significantly reduce or interfere with Vi antibody production.
Figure 4.
Plasma samples from mice immunized with the combination of Vi and CWPS fusion conjugates bind to Vi and promote antibody-mediated killing of Vi-expressing Salmonella strain. (A). ELISA measured the plasma concentrations of anti-Vi IgG. Mice immunized with the combination of the two fusion conjugates had significantly higher plasma concentrations of anti-Vi IgG than mice immunized with the mixture of all antigens (P<0.0001 by Mann-Whitney U). (B) Western blotting analysis revealed that plasma from conjugate-immunized mice binds to Vi-bearing S. typhimurium (C5.507 strain) whereas no binding could be observed with plasma from mixture-immunized mice; no binding could be observed to the non Vi-bearing Salmonella typhimurium strain C5, confirming specificity of the immune response. (C) Sera from mice immunized with the CWPS and Vi fusion conjugates enhanced killing of Vi-expressing Salmonella. Plasma from mice immunized with the two fusion conjugates or with the mixture of all components were used in an killing assay using differentiated human HL-60 cells and strain C5.507. Bacterial growth was observed over a 3-hour period. C5.507 strain incubated with plasma from conjugate-immunized mice demonstrated a 2-log reduction in bacterial CFU, whereas no killing could be observed when plasma from mixture-immunized mice was used. The figure shows one representative result from 3 independent experiments.
Cell-binding and in vitro killing assays were then performed. S. typhimurium strains C5 and C5.507 (the C5 strain engineered to express the Vi polysaccharide, both kind gifts of Drs. Dougan and Hale, The Wellcome Sanger Trust Institute, Cambridge UK) [13] were incubated with a 1:50 dilution of plasma from mice immunized with the mixture or CWPS- and Vi-conjugate. As shown in the Western blot of Figure 4B, binding of mouse antibody could only be observed when the C5.507 strain was incubated with plasma originating from mice in the combined conjugate group. This suggests that antibody elicited by the combined conjugate vaccine binds to the Vi-polysaccharide present on a whole organism and that the response is specific for this polysaccharide.
A killing assay using differentiated HL-60 cells was then performed to test the neutralizing capacity of these antibodies. While there was no appreciable killing when the Vi-negative C5 strain was incubated with plasma from mice immunized with either the mixture or the combined conjugate vaccine (data not shown), rapid and effective killing (almost 2-log reduction) could be observed when the Vi-positive strain C5.507 was pre-incubated with plasma from combined conjugate-immunized mice (Figure 4C). Similar results were obtained when human blood neutrophils purified from healthy volunteers were used in place of the HL-60 cells (data not shown). Thus, these results demonstrate that immunization with the combination of CWPS and Vi fusion conjugates elicits functionally active antibodies directed against Vi.
DISCUSSION
The data support the development of a parenteral bivalent vaccine against pneumococcus and S. typhi, with aluminum hydroxide as adjuvant. The ability of Vi conjugates to elicit functional antibodies against the Vi capsular polysaccharide has been established in both animal models and clinical studies, and the results presented here are consistent with these findings. Addition of the CWPS conjugate resulted in an effective immunogen against pneumococcal pneumonia/sepsis by an encapsulated strain of serotype 3. Additionally, by making use of the fusion conjugate approach, we were also able to elicit Th17 responses to two pneumococcal proteins that are associated with protection against nasopharyngeal colonization, as demonstrated here.
The efficacy of pneumococcal capsular polysaccharide conjugate vaccines in the prevention of invasive disease has been well established [45]. More recently, concern has been raised regarding the phenomenon of serotype replacement, whereby strains carrying capsular polysaccharides not included in the seven-valent conjugate vaccine increased in frequency following the introduction of the conjugate vaccine [26]. To a large extent, the emergence of these non-vaccine type strains is the direct consequence of the success of the vaccines in reducing nasopharyngeal colonization by vaccine-type strains. This impact on colonization also underlies the overall success of the conjugate vaccines in the U.S., where 2/3 of all prevented invasive disease cases are the consequence of an indirect effect, or herd protection [45].
Based on these data, we adopted the premise that for a noncapsular pneumococcal vaccine candidate to be viable, it should provide protection against disease but also against colonization [34]. Building on recent studies by our group and others [31, 34, 46] that have implicated a critical role of Th17 cells in the control of pneumococcal colonization, we identified pneumococcal protein antigens that confer protection against carriage by this mechanism. The two proteins we identified, SP1572 and SP2070, are conserved (over 90% amino acid sequence identity) in all the presently sequenced pneumococcal strains (n=42) and thus may be expected to provide protection against a wide range of pneumococci. Unlike capsular PS, CWPS of pneumococcus is highly conserved across all serotypes, with either one or two phosphorylcholine moieties per repeat unit[47]. Antibodies directed against components of CWPS have been shown to be protective in some animal models [48–50] and intranasal vaccination with CWPS is protective in murine colonization and sepsis models [44]. Here we showed that a bivalent vaccine consisting of a combination of two fusion conjugates of SP1572-PdT-Vi and SP2070-PdT-CWPS elicits Th17 responses and provides protection against pneumococcal nasopharyngeal colonization whereas the uncoupled components adsorbed onto alum neither induced this response nor conferred protection.
This vaccine also could be expected to confer protection against typhoid fever in young children. Currently licensed typhoid vaccines include the live Ty21a strain given orally and the purified Vi polysaccharide administered parenterally. The oral vaccine is not approved for young children and the Vi polysaccharide is ineffective under two years of age. Investigational Vi conjugate vaccine demonstrated to be safe and efficacious in infants and young children [10, 51, 52], however, the licensed Vi-conjugate is only for local distribution [19]. As could be expected, our conjugate induces functional antibodies in mice, as evidenced by killing assays using genetically-engineered S. typhimurium strains. Although at the present time it is difficult to determine the cost of such a vaccine, it is reasonable to assume that a combination of Vi and CWPS conjugates would be significantly less expensive to produce than currently licensed 10- or 13-valent conjugate vaccines, with or without the addition of a Vi-conjugate.
In conclusion, we describe here a candidate vaccine consisting of a combination of antigens directed against S. typhi and S. pneumoniae. The modifications to the antigens include fusion of two conserved pneumococcal proteins to the pneumolysoid PdT and conjugation to polysaccharides, which assists in Th17 elicitation [36]. The inclusion of Vi as one of the two conjugated polysaccharides renders the combination an effective immunogen against S. typhi. The development of a vaccine that may confer protection against both pathogens offers the promise of an economical and effective vaccine directed against diseases that are associated with major mortality and morbidity in children in developing countries. Further preclinical studies to optimize the bivalent immunogen structurally and to confirm the findings presented here for additional pneumococcal strains are underway.
MATERIALS AND METHODS
Materials
Aluminum hydroxide (alum) was from Brenntag North America (2% Alhydrogel). Vi polysaccharide was purified as described previously [14]. Adipic acid dihydrazide (ADH), 1-Ethyl-3-[3-dimethylaminopropyl] carbodiimide Hydrochloride (EDC) and N-hydroxysulfosuccinimide (Sulfo-NHS) were purchased from Pierce. All other reagents were obtained from Sigma. The SPWCA was made by Dr. Luciana Leite’s group at Instituto Butantan (Sao Paulo, Brazil) as previously described [37].
Construct fusion protein expression vectors
pQE30 vector with His-tagged PdT inside was modified by inserting four restriction sites: SphI, SacI, SacII and ClaI between His-tag and GSGGGS polylinker, to generate vector pQE-PdT. SP1572 and SP2070 were amplified using S. pneumoniae TIGR4 genomic DNA as template. SphI and SacII sites were introduced at the beginning and end of PCR products by primers. Double digested PCR products were ligated into pQE-PdT and transformed with E. coli Xl/blue strain. The correct nucleotide sequences were confirmed at the Children’s Hospital Boston Molecular Genetics Core Facility.
Protein Purification
E. coli transformants containing the relevant cloned proteins were grown to OD600=0.6, and protein expression was induced with 0.2 mM IPTG at 16°C overnight. Cells were spun down and pellets were resuspended in lysis buffer (20 mM Tris-HCl, 500 mM NaCl, pH8.0) and then lysed by sonication. The proteins of interest were purified from supernatant over a Ni-NTA column; proteins were eluted in imidazole buffer. Protein-containing elutions were combined, purified over a gel-filtration column and eluted in PBS buffer.
Generation of the Vi and CWPS conjugates
Proteins were conjugated to Vi polysaccharide according to Szu et al. with some modifications. [15]. Briefly, Vi was resuspended to 5 mg/ml in buffer A (0.2 M MES, 150 mM NaCl, pH 5.9), and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) and NHS-Sulfo was added into solution as powder for 30 minutes at room temperature. Excess EDC was removed by dialysis against PBS three times. Proteins were then added to the reaction as 1mg/mg, protein/sugar. The reaction was carried out at 4°C overnight with rotation. Conjugates were separated by elution through a Superose-6 gel filtration column and collection of the void volume fractions. The CWPS conjugate was prepared as described previously [36]. Protein concentration was determined by the BCA method (Pierce), and Vi concentration was determined by measurement of O-acetyl groups using Vi as a standard[42]. CWPS concentration was determined using the Anthrone method [53].
Antigen preparations
One day prior to immunization, vaccines were prepared as follows. Frozen aliquots were thawed or lyophilized vials were reconstituted with sterile water, diluted to the appropriate concentration, and mixed with aluminum hydroxide (alum) at the indicated concentration in a 15 ml conical tube, which was then tumbled overnight at 4°C to allow for adsorption.
Immunization and challenge of mice
C57BL/6J mice were used in all the experiments. The age at time of first immunization was between 4–6 weeks. Gently restrained, nonanesthetized mice received 2 or 3 subcutaneous injections of 200 μl containing adjuvant with or without antigen in the lower part of the back at 2-week intervals. Blood was drawn 2 weeks after the last immunization, and assayed for antibody and for IL-17A production in vitro upon stimulation with WCA. Nasopharyngeal colonization with the clinical pneumococcal isolate 0603 (serotype 6B) and aspiration challenge with strain WU-2 were carried out as previously described [37]. All animal studies were approved by our local animal ethics committees.
Enzyme-linked immunosorbent assay (ELISA) and IL-17A production in whole blood samples
Assays for murine antibodies for WCA, individual protein and CWPS and IL-17A production in whole blood were carried out as previously described [36]. Vi antibody was measured using the method described by Azze et al. [54].
Binding of Vi antibody to Vi-expressing Salmonella
Salmonella typhimurium carrying an empty vector (strain C5, Vi-negative) or the genes necessary for expression of Vi polysaccharide (strain C5.507, Vi-positive) were grown into late log phase and collected by centrifugation[13]. Bacteria were washed with PBS, and mouse plasma was then added with 1:50 dilution and incubated at room temperature for 30 minutes. Samples were then washed with PBS for 3 times and treated with SDS buffer. The gel was transferred to a nitrocellulose membrane and then blocked with 5% skim milk in PBS/0.05% Tween. Donkey anti-mouse IgG HRP conjugate (Sigma) was added 1:10,000 in 1% skim milk in PBS/Tween. The membrane was developed following addition of ECL substrate.
Bacterial killing by phagocytic cells
Killing assays were performed as described previously [13, 55]. Salmonella typhimurium carrying an empty vector (Strain C5) or expressing Vi polysaccharide on the surface (Strain C5.507) were grown into late log phase and frozen in LB/10% glycerol at −80°C. HL-60 cells were differentiated into phagocytic cells by the addition of 100 mM N,N-dimethylformamide for 5 days. On the day of the experiment, bacteria were thawed and diluted to 106 cfu/mL. Bacteria were incubated with a 1:10 dilution of plasma for 20 minutes at room temperature, and cells were added at a 100:1 ratio. Samples were incubated on a rocker plate at 20 rpm at 37 °C and numbers of viable Salmonella determined after 60, 120, and 180 min by serial dilution on Luria Bertani agar. Human neutrophils were purified from the peripheral blood of human volunteers using a Histopaque 1077, 1119 gradient (Sigma-Aldrich, St. Louis, MO) according to the manufacturers instructions and used immediately. Killing was performed similarly except the cell:bacteria ratio was 300:1.
Statistical analysis
Antibody and IL-17A concentrations and NP colonization densities were compared by the Mann-Whitney U test using PRISM (version 4.0a, GraphPad Software, Inc). Differences in survival were analyzed with the Kaplan-Meier test, using PRISM as well.
Highlights.
Fusion conjugates are protein-TLR agonist fusions coupled to polysaccharides (PS)
Salmonella typhi Vi PS and pneumococcal wall PS were coupled to pneumococcal fusions
High-titer Vi antibody opsonic to S. typhi was elicited in mice.
The induced IL17 and antibody prevented murine pneumococcal colonization and sepsis
This bivalent combination may immunize infants vs typhoid and pneumococcal diseases
Acknowledgments
We thank Drs. Christine Hale and Gordon Dougan for providing Salmonella typhimurium strains C5 and C5.507. We thank Drs. Rachel Schneerson and John Robbins for helpful discussion and suggestions. This work was supported by The Bill & Melinda Gates Foundation through the Grand Challenge Explorations program and Children’s Hospital Technology Development grant. R.M. gratefully acknowledges support from the Translational Research Program at Children’s Hospital Boston and NIH grant R01 AI067737. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bull World Health Organ. 2004 May;82(5):346–53. [PMC free article] [PubMed] [Google Scholar]
- 2.Lin FY, Vo AH, Phan VB, Nguyen TT, Bryla D, Tran CT, et al. The epidemiology of typhoid fever in the Dong Thap Province, Mekong Delta region of Vietnam. Am J Trop Med Hyg. 2000 May;62(5):644–8. doi: 10.4269/ajtmh.2000.62.644. [DOI] [PubMed] [Google Scholar]
- 3.Sinha A, Sazawal S, Kumar R, Sood S, Reddaiah VP, Singh B, et al. Typhoid fever in children aged less than 5 years. Lancet. 1999 Aug 28;354(9180):734–7. doi: 10.1016/S0140-6736(98)09001-1. [DOI] [PubMed] [Google Scholar]
- 4.O’Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009 Sep 12;374(9693):893–902. doi: 10.1016/S0140-6736(09)61204-6. [DOI] [PubMed] [Google Scholar]
- 5.Robbins JD, Robbins JB. Reexamination of the protective role of the capsular polysaccharide (Vi antigen) of Salmonella typhi. J Infect Dis. 1984 Sep;150(3):436–49. doi: 10.1093/infdis/150.3.436. [DOI] [PubMed] [Google Scholar]
- 6.Raffatellu M, Chessa D, Wilson RP, Tukel C, Akcelik M, Baumler AJ. Capsule-mediated immune evasion: a new hypothesis explaining aspects of typhoid fever pathogenesis. Infect Immun. 2006 Jan;74(1):19–27. doi: 10.1128/IAI.74.1.19-27.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson RP, Winter SE, Spees AM, Winter MG, Nishimori JH, Sanchez JF, et al. The Vi capsular polysaccharide prevents complement receptor 3-mediated clearance of Salmonella enterica serotype Typhi. Infect Immun. 2010 Feb;79(2):830–7. doi: 10.1128/IAI.00961-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang HH, Wu CG, Xie GZ, Gu QW, Wang BR, Wang LY, et al. Efficacy trial of Vi polysaccharide vaccine against typhoid fever in south-western China. Bull World Health Organ. 2001;79(7):625–31. [PMC free article] [PubMed] [Google Scholar]
- 9.Thiem VD, Lin FY, Canh do G, Son NH, Anh DD, Mao ND, et al. The vi conjugate typhoid vaccine is safe, elicits protective levels of IgG anti-vi, and is compatible with routine infant vaccines. Clin Vaccine Immunol. 2011 May;18(5):730–5. doi: 10.1128/CVI.00532-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin FY, Ho VA, Khiem HB, Trach DD, Bay PV, Thanh TC, et al. The efficacy of a Salmonella typhi Vi conjugate vaccine in two-to-five-year-old children. N Engl J Med. 2001 Apr 26;344(17):1263–9. doi: 10.1056/NEJM200104263441701. [DOI] [PubMed] [Google Scholar]
- 11.Micoli F, Rondini S, Pisoni I, Proietti D, Berti F, Costantino P, et al. Vi-CRM 197 as a new conjugate vaccine against Salmonella Typhi. Vaccine. 2011 Jan 17;29(4):712–20. doi: 10.1016/j.vaccine.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haeuw JF, Libon C, Zanna L, Goetsch L, Champion T, Nguyen TN, et al. Physico-chemical characterization and immunogenicity studies of peptide and polysaccharide conjugate vaccines based on a promising new carrier protein, the recombinant Klebsiella pneumoniae OmpA. Dev Biol (Basel) 2000;103:245–50. [PubMed] [Google Scholar]
- 13.Hale C, Bowe F, Pickard D, Clare S, Haeuw JF, Powers U, et al. Evaluation of a novel Vi conjugate vaccine in a murine model of salmonellosis. Vaccine. 2006 May 15;24(20):4312–20. doi: 10.1016/j.vaccine.2006.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szu SC, Li XR, Schneerson R, Vickers JH, Bryla D, Robbins JB. Comparative immunogenicities of Vi polysaccharide-protein conjugates composed of cholera toxin or its B subunit as a carrier bound to high- or lower-molecular-weight Vi. Infect Immun. 1989 Dec;57(12):3823–7. doi: 10.1128/iai.57.12.3823-3827.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szu SC, Stone AL, Robbins JD, Schneerson R, Robbins JB. Vi capsular polysaccharide-protein conjugates for prevention of typhoid fever. Preparation, characterization, and immunogenicity in laboratory animals. J Exp Med. 1987 Nov 1;166(5):1510–24. doi: 10.1084/jem.166.5.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szu SC, Taylor DN, Trofa AC, Clements JD, Shiloach J, Sadoff JC, et al. Laboratory and preliminary clinical characterization of Vi capsular polysaccharide-protein conjugate vaccines. Infect Immun. 1994 Oct;62(10):4440–4. doi: 10.1128/iai.62.10.4440-4444.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rondini S, Micoli F, Lanzilao L, Hale C, Saul AJ, Martin LB. Evaluation of the immunogenicity and biological activity of the Citrobacter freundii Vi-CRM197 conjugate as a vaccine for Salmonella enterica serovar Typhi. Clin Vaccine Immunol. 2011 Mar;18(3):460–8. doi: 10.1128/CVI.00387-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cui C, Carbis R, An SJ, Jang H, Czerkinsky C, Szu SC, et al. Physical and chemical characterization and immunologic properties of Salmonella enterica serovar typhi capsular polysaccharide-diphtheria toxoid conjugates. Clin Vaccine Immunol. 2010 Jan;17(1):73–9. doi: 10.1128/CVI.00266-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shah NK. Indian conjugate Vi typhoid vaccine: do we have enough evidence? Indian Pediatr. 2009 Feb;46(2):181–2. [PubMed] [Google Scholar]
- 20.Levine MM, Ferreccio C, Abrego P, Martin OS, Ortiz E, Cryz S. Duration of efficacy of Ty21a, attenuated Salmonella typhi live oral vaccine. Vaccine. 1999 Oct 1;17( Suppl 2):S22–7. doi: 10.1016/s0264-410x(99)00231-5. [DOI] [PubMed] [Google Scholar]
- 21.Levine MM, Ferreccio C, Cryz S, Ortiz E. Comparison of enteric-coated capsules and liquid formulation of Ty21a typhoid vaccine in randomised controlled field trial. Lancet. 1990 Oct 13;336(8720):891–4. doi: 10.1016/0140-6736(90)92266-k. [DOI] [PubMed] [Google Scholar]
- 22.Prymula R, Schuerman L. 10-valent pneumococcal nontypeable Haemophilus influenzae PD conjugate vaccine: Synflorix. Expert Rev Vaccines. 2009 Nov;8(11):1479–500. doi: 10.1586/erv.09.113. [DOI] [PubMed] [Google Scholar]
- 23.Oosterhuis-Kafeja F, Beutels P, Van Damme P. Immunogenicity, efficacy, safety and effectiveness of pneumococcal conjugate vaccines (1998–2006) Vaccine. 2007 Mar 8;25(12):2194–212. doi: 10.1016/j.vaccine.2006.11.032. [DOI] [PubMed] [Google Scholar]
- 24.Chuck AW, Jacobs P, Tyrrell G, Kellner JD. Pharmacoeconomic evaluation of 10- and 13-valent pneumococcal conjugate vaccines. Vaccine. 2010 Jul 26;28(33):5485–90. doi: 10.1016/j.vaccine.2010.05.058. [DOI] [PubMed] [Google Scholar]
- 25.Johnson HL, Deloria-Knoll M, Levine OS, Stoszek SK, Freimanis Hance L, Reithinger R, et al. Systematic evaluation of serotypes causing invasive pneumococcal disease among children under five: the pneumococcal global serotype project. PLoS Med. 2010 Oct;7(1):e1000348. doi: 10.1371/journal.pmed.1000348. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weinberger DM, Malley R, Lipsitch M. Serotype replacement in disease after pneumococcal vaccination. Lancet. 2011 Apr 12; doi: 10.1016/S0140-6736(10)62225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tai SS. Streptococcus pneumoniae protein vaccine candidates: properties, activities and animal studies. Crit Rev Microbiol. 2006;32(3):139–53. doi: 10.1080/10408410600822942. [DOI] [PubMed] [Google Scholar]
- 28.Swiatlo E, Ware D. Novel vaccine strategies with protein antigens of Streptococcus pneumoniae. FEMS Immunol Med Microbiol. 2003 Aug 18;38(1):1–7. doi: 10.1016/S0928-8244(03)00146-9. [DOI] [PubMed] [Google Scholar]
- 29.Briles DE, Hollingshead S, Brooks-Walter A, Nabors GS, Ferguson L, Schilling M, et al. The potential to use PspA and other pneumococcal proteins to elicit protection against pneumococcal infection. Vaccine. 2000 Feb 25;18(16):1707–11. doi: 10.1016/s0264-410x(99)00511-3. [DOI] [PubMed] [Google Scholar]
- 30.Moffitt KL, Malley R. Next generation pneumococcal vaccines. Curr Opin Immunol. 2011 Jun;23(3):407–13. doi: 10.1016/j.coi.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu YJ, Gross J, Bogaert D, Finn A, Bagrade L, Zhang Q, et al. Interleukin-17A mediates acquired immunity to pneumococcal colonization. PLoS Pathog. 2008;4(9):e1000159. doi: 10.1371/journal.ppat.1000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malley R, Trzcinski K, Srivastava A, Thompson CM, Anderson PW, Lipsitch M. CD4+ T cells mediate antibody-independent acquired immunity to pneumococcal colonization. Proc Natl Acad Sci U S A. 2005 Mar 29;102(13):4848–53. doi: 10.1073/pnas.0501254102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Rossum AM, Lysenko ES, Weiser JN. Host and bacterial factors contributing to the clearance of colonization by Streptococcus pneumoniae in a murine model. Infect Immun. 2005 Nov;73(11):7718–26. doi: 10.1128/IAI.73.11.7718-7726.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moffitt KL, Gierahn TM, Lu YJ, Gouveia P, Alderson M, Flechtner JB, et al. T(H)17-based vaccine design for prevention of Streptococcus pneumoniae colonization. Cell Host Microbe. 2011 Feb 17;9(2):158–65. doi: 10.1016/j.chom.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malley R. Antibody and cell-mediated immunity to Streptococcus pneumoniae: implications for vaccine development. J Mol Med. 2010 Feb;88(2):135–42. doi: 10.1007/s00109-009-0579-4. [DOI] [PubMed] [Google Scholar]
- 36.Lu YJ, Forte S, Thompson CM, Anderson PW, Malley R. Protection against Pneumococcal colonization and fatal pneumonia by a trivalent conjugate of a fusion protein with the cell wall polysaccharide. Infect Immun. 2009 May;77(5):2076–83. doi: 10.1128/IAI.01554-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu YJ, Leite L, Goncalves VM, Dias Wde O, Liberman C, Fratelli F, et al. GMP-grade pneumococcal whole-cell vaccine injected subcutaneously protects mice from nasopharyngeal colonization and fatal aspiration-sepsis. Vaccine. 2010 Nov 3;28(47):7468–75. doi: 10.1016/j.vaccine.2010.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu YJ, Yadav P, Clements JD, Forte S, Srivastava A, Thompson CM, et al. Options for inactivation, adjuvant, and route of topical administration of a killed, unencapsulated pneumococcal whole-cell vaccine. Clin Vaccine Immunol. 2010 Jun;17(6):1005–12. doi: 10.1128/CVI.00036-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vintini E, Villena J, Alvarez S, Medina M. Administration of a probiotic associated with nasal vaccination with inactivated Lactococcus lactis-PppA induces effective protection against pneumoccocal infection in young mice. Clin Exp Immunol. 2009 Mar;159(3):351–62. doi: 10.1111/j.1365-2249.2009.04056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Green BA, Zhang Y, Masi AW, Barniak V, Wetherell M, Smith RP, et al. PppA, a surface-exposed protein of Streptococcus pneumoniae, elicits cross-reactive antibodies that reduce colonization in a murine intranasal immunization and challenge model. Infect Immun. 2005 Feb;73(2):981–9. doi: 10.1128/IAI.73.2.981-989.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ling E, Feldman G, Portnoi M, Dagan R, Overweg K, Mulholland F, et al. Glycolytic enzymes associated with the cell surface of Streptococcus pneumoniae are antigenic in humans and elicit protective immune responses in the mouse. Clin Exp Immunol. 2004 Nov;138(2):290–8. doi: 10.1111/j.1365-2249.2004.02628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hestrin S. The reaction of acetylcholine and other carboxylic acid derivatives with hydroxylamine, and its analytical application. J Biol Chem. 1949 Aug;180(1):249–61. [PubMed] [Google Scholar]
- 43.Malley R, Lipsitch M, Stack A, Saladino R, Fleisher G, Pelton S, et al. Intranasal immunization with killed unencapsulated whole cells prevents colonization and invasive disease by capsulated pneumococci. Infect Immun. 2001 Aug;69(8):4870–3. doi: 10.1128/IAI.69.8.4870-4873.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Malley R, Srivastava A, Lipsitch M, Thompson CM, Watkins C, Tzianabos A, et al. Antibody-independent, interleukin-17A-mediated, cross-serotype immunity to pneumococci in mice immunized intranasally with the cell wall polysaccharide. Infect Immun. 2006 Apr;74(4):2187–95. doi: 10.1128/IAI.74.4.2187-2195.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.CDC. Direct and indirect effects of routine vaccination of children with 7-valent pneumococcal conjugate vaccine on incidence of invasive pneumococcal disease--United States, 1998–2003. MMWR Morb Mortal Wkly Rep. 2005 Sep 16;54(36):893–7. [PubMed] [Google Scholar]
- 46.Zhang Z, Clarke TB, Weiser JN. Cellular effectors mediating Th17-dependent clearance of pneumococcal colonization in mice. J Clin Invest. 2009 Jul;119(7):1899–909. doi: 10.1172/JCI36731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karlsson C, Jansson PE, Skov Sorensen UB. The pneumococcal common antigen C-polysaccharide occurs in different forms. Mono-substituted or di-substituted with phosphocholine. Eur J Biochem. 1999 Nov;265(3):1091–7. doi: 10.1046/j.1432-1327.1999.00835.x. [DOI] [PubMed] [Google Scholar]
- 48.Wallick S, Claflin JL, Briles DE. Resistance to Streptococcus pneumoniae is induced by a phosphocholine-protein conjugate. J Immunol. 1983 Jun;130(6):2871–5. [PubMed] [Google Scholar]
- 49.Yother J, Forman C, Gray BM, Briles DE. Protection of mice from infection with Streptococcus pneumoniae by anti-phosphocholine antibody. Infection & Immunity. 1982;36(1):184–8. doi: 10.1128/iai.36.1.184-188.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Briles DE, Nahm M, Schroer K, Davie J, Baker P, Kearney J, et al. Antiphosphocholine antibodies found in normal mouse serum are protective against intravenous infection with type 3 streptococcus pneumoniae. J Exp Med. 1981;153(3):694–705. doi: 10.1084/jem.153.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mai NL, Phan VB, Vo AH, Tran CT, Lin FY, Bryla DA, et al. Persistent efficacy of Vi conjugate vaccine against typhoid fever in young children. N Engl J Med. 2003 Oct 2;349(14):1390–1. doi: 10.1056/NEJM200310023491423. [DOI] [PubMed] [Google Scholar]
- 52.Kossaczka Z, Lin FY, Ho VA, Thuy NT, Van Bay P, Thanh TC, et al. Safety and immunogenicity of Vi conjugate vaccines for typhoid fever in adults, teenagers, and 2- to 4-year-old children in Vietnam. Infect Immun. 1999 Nov;67(11):5806–10. doi: 10.1128/iai.67.11.5806-5810.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roe JH. The determination of sugar in blood and spinal fluid with anthrone reagent. J Biol Chem. 1955 Jan;212(1):335–43. [PubMed] [Google Scholar]
- 54.Azze RF, Rodriguez JC, Iniesta MG, Marchena XR, Alfonso VM, Padron FT. Immunogenicity of a new Salmonella Typhi Vi polysaccharide vaccine--vax-TyVi--in Cuban school children and teenagers. Vaccine. 2003 Jun 20;21(21–22):2758–60. doi: 10.1016/s0264-410x(03)00177-4. [DOI] [PubMed] [Google Scholar]
- 55.Gondwe EN, Molyneux ME, Goodall M, Graham SM, Mastroeni P, Drayson MT, et al. Importance of antibody and complement for oxidative burst and killing of invasive nontyphoidal Salmonella by blood cells in Africans. Proc Natl Acad Sci U S A. 2010 Feb 16;107(7):3070–5. doi: 10.1073/pnas.0910497107. [DOI] [PMC free article] [PubMed] [Google Scholar]