Abstract
Previous studies indicate that peripheral nerve conditioning lesions significantly enhance central axonal regeneration via modulation of cAMP-mediated mechanisms. To gain insight into the nature and temporal dependence of neural mechanisms underlying conditioning lesion effects on central axonal regeneration, we compared the efficacy of peripheral sciatic nerve crush lesions to cAMP elevations (in lumbar dorsal root ganglia) on central sensory axonal regeneration when administered either before or after cervical spinal cord lesions. We found significantly greater effects of conditioning lesions compared to cAMP elevations on central axonal regeneration when combined with cellular grafts at the lesion site and viral neurotrophin delivery; further, these effects persisted whether conditioning lesions were applied prior to or shortly after spinal cord injury. Indeed, conditioning lesions recruited extensively greater sets of genetic mechanisms of possible relevance to axonal regeneration compared to cAMP administration, and sustained these changes for significantly greater time periods through the post-lesion period. We conclude that cAMP-mediated mechanisms account for only a portion of the potency of conditioning lesions on central axonal regeneration, and that recruitment of broader genetic mechanisms can extend the effect and duration of cellular events that support axonal growth.
Keywords: neurotrophin-3, CTB, regeneration, spinal cord injury, microarray, dorsal column sensory, lentivirus
INTRODUCTION
Regeneration in the injured CNS is limited by several mechanisms including the inhibitory central environment (Filbin, 2003, Schwab, 2004, Silver and Miller, 2004), a lack of growth-promoting substrates and diffusible proteins in the lesion site (Hendriks, et al., 2004, Oudega and Xu, 2006), inflammatory responses (Bethea and Dietrich, 2002, Popovich and McTigue, 2009), and insufficient recruitment of intrinsic neuronal growth mechanisms (Costigan, et al., 2002, Goldberg, et al., 2002, Plunet, et al., 2002).
One means of stimulating central axonal regeneration is the application of conditioning lesions to the peripheral branch of sensory axons, before central spinal cord lesions are placed (Neumann, et al., 2005, Neumann and Woolf, 1999, Richardson and Issa, 1984). Mechanisms underlying the pre-conditioning effect on axonal regeneration have been the subject of extensive study and involve Il-6 and stat-3 signaling (Cafferty, et al., 2004, Cao, et al., 2006, Qiu, et al., 2005), activation of transcription factors including CREB (Gao, et al., 2004) and ATF3 (Seijffers, et al., 2006, Seijffers, et al., 2007), and cAMP-related pathways to induce protein kinase A signaling (Cai, et al., 2002, Cai, et al., 2001, Lu, et al., 2004, Neumann, et al., 2002, Nikulina, et al., 2004, Qiu, et al., 2002). Indeed, injection of cAMP alone has been reported to replicate the effects of peripheral conditioning lesions on central axonal regeneration (Lu, et al., 2004, Neumann, et al., 2002). However, it remains unclear what portion of the conditioning effect is mediated by cAMP (Gao, et al., 2004, Neumann, et al., 2002, Qiu, et al., 2002), and whether other mechanisms are necessary or sufficient for peripheral conditioning lesions (Andersen, et al., 2000, Han, et al., 2004). Further, the temporal dependence of central axonal regeneration on peripheral conditioning remains incompletely understood. It has been reported that conditioning lesions or cAMP injections into dorsal root ganglia support central axonal regeneration when applied prior to, but not after, a central lesion (Neumann, et al., 2005, Neumann and Woolf, 1999) but recent studies have suggested that conditioning lesions remain effective when applied up to 16 months post spinal cord injury (Kadoya, et al., 2009). As peripheral pre-conditioning lesions or “post-conditioning” at stages of chronic spinal cord injury appear to recruit similar genetic mechanisms (Kadoya, et al., 2009), one would, a priori, expect similar regenerative responses when conditioning lesions are applied prior to or following central lesions.
Given the importance of further understanding the specific nature and temporal dependence of the conditioning lesion effect on central axonal regeneration, we compared the relative potency of cAMP and sciatic nerve crush (conditioning lesion) on sensory axonal growth in vitro, and following cervical spinal cord injury in vivo. Further, we examined the relative potency of these approaches when conditioning lesions preceded, or followed, spinal cord lesions. The ability of cAMP increases or conditioning lesions to influence genetic mechanisms was assessed by Affymetrix whole-genome arrays and confirmed by PCR. We now report significantly greater efficacy of conditioning lesions on neuritic growth in vitro and in vivo compared to cAMP-mediated effects, retention of this efficacy whether applied before or shortly after central injury, and recruitment of extensively greater genetic mechanisms related to transcriptional activation and candidate regeneration-associated gene expression. These findings have important implications for the targeting of intraneuronal mechanisms to enhance regeneration in a time frame of practical relevance.
EXPERIMENTAL PROCEDURES
Experimental Design
Effects of conditioning lesions versus cAMP were examined in explant assays of adult and postnatal dorsal root ganglion (DRG) neurons and, separately, postnatal day 7 cerebellar granule neuron cultures. In addition, we examined effects of systemic cAMP augmentation on neurite outgrowth by systemic infusions of the phosphodiesterase-IV (PDE-IV) inhibitor mesopram (Schering AG, Berlin) (Dinter, et al., 2000). Neurons in both DRG and cerebellar granule cell assays were cultured either on poly-L-lysine substrates or myelin substrates. Conditioning lesions, cAMP injections or mesopram administration were further examined in in vivo models of spinal cord injury, when applied prior to, or following, placement of C3 dorsal column lesions. Some data were replicated using infusions of rolipram, a PDE IV inhibitor similar to mesopram (Nikulina, et al., 2004, Pearse, et al., 2004)(presented in supplementary figures). Finally, to understand recruitment of genetic mechanisms related to conditioning lesions or mesopram administration, Affymetrix whole-genome arrays were used to measure gene expression changes in DRG neurons in a total of 138 rats at time points of 1, 3, 7 and 14 days following these treatments.
For in vivo models of axonal regeneration, lesion sites that would normally become cystic and cannot support axonal growth were filled with autologous bone marrow stromal cells to provide a cellular matrix, as previously reported (Alto, et al., 2009, Lu, et al., 2007, Lu, et al., 2004, Taylor, et al., 2006). In addition, a number of previous reports indicate that axonal bridging beyond a site of spinal cord injury requires growth factor gradients beyond the lesion site; provision of cAMP or a conditioning lesion with a MSC graft without growth factor do not support axonal bridging (Alto, et al., 2009, Lu, et al., 2004, Taylor, et al., 2006). For this reason, studies of axonal regeneration in vivo kept constant the provision of marrow stromal cell grafts in the lesion cavity and injection of lentiviral vectors expressing NT-3 (Lenti-NT-3) or GFP (Lenti-GFP) beyond the lesion site, and varied only the method of either conditioning lesion, cAMP injection into the DRG, or systemic mesopram treatment.
DRG In Vitro Assay
Adult L4–L6 DRGs for neurite outgrowth assays were harvested from animals without spinal cord lesions at 3 and 7 days after mesopram pump implantation or conditioning lesions (n=7 and n=8, respectively, see below for description of surgery). Naïve animals (n=8) served as controls. Adult animals (>3 months old) were deeply anesthetized with isofluorane, decapitated, and the spinal column containing the L4–6 DRGs was transferred into ice-cold DMEM/Ham’s F12. DRGs were dissected, washed twice with DMEM/Ham’s F12, digested for 1 h at 37°C in 0.25% collagenase type XI (Sigma, St. Louis) in L15 medium, spun down, and washed with 1 ml DMEM/F-12 with 10% FBS. Cells were resuspended in DMEM/F-12 (without serum) with B27 supplement and antibiotics (Penicillin/Streptomycin/Glutamine mix) and triturated with a 1 ml pipette tip. Large tissue chunks were allowed to sink and the supernatant containing cell suspension (3–4 × 104 cells/ in 2 ml) was plated on 35 mm cell culture dishes coated for 1 h with poly-D-lysine (16.6 µg/ml) and, if indicated with myelin (18 µg/ml/ per well) overnight. Myelin was isolated from rat spinal cord as previously described (Norton and Poduslo, 1973). 2 mM db-cAMP f.c. (Sigma) was immediately added to the culture medium where indicated. Cells were fixed 72h later with 4% paraformaldehyde and labeled for neurofilament heavy chain (NF200; Chemicon, Temecula; 1:2000) followed by a Alexa-594 secondary antibody (1:300, Molecular Probes, Eugene, OR). A minimum of 60 labeled cells/animal/well were photographed using a 10× objective, and the length of the longest neurite per cell was measured using NIH image and NeuroJ plugin to determine mean neurite length per animal and condition. Data are presented as mean neurite length (in µm) of all animals in each group. In addition, DRGs were isolated from animals at postnatal day 5, 8 and 28 (n=4 each), cells were dissociated and seeded on PLL and myelin coated plates and cultivated with or without db-cAMP (2 mM f.c.) and neurite length was quantified as described above.
Cerebellar Granule Neuron (CGN) In Vitro Assay
Cerebellar neurons were isolated from postnatal day 7 rats. Animals were anesthetized, decapitated and the cerebella were dissected. After mincing, tissue was digested in trypsin/EDTA and DNase. Cells were washed 2× with DMEM +10% FBS, triturated, and cell suspensions were underlayed with 2ml of 35% Percoll and 2ml of 60% Percoll. Cells were centrifuged at 3000rpm (~1600Gs) for 10min. CGNs were collected, washed, and resuspended in Neurobasal Medium with B27 Supplement and Penicillin/Streptomycin/Glutamine (Gibco) and 25 mM (f.c.) glucose. CGNs were plated in 4-well plates (40,000 cells/well) onto confluent layers of CHO-MAG or CHO-R2 cells (gift of Marie Filbin, New York). 2 mM db-cAMP f.c. was added as indicated. After overnight growth (~18hrs), cells were fixed with 4% Paraformaldehyde and neurons were labeled with Anti-βIII Tubulin (1:1000 dilution; Promega, Madison, WI) overnight at 4°C followed by incubation with Alexa Fluor 594 secondary antibody (1:1000) for 2.5h at room temperature. Neurite length was determined as described above.
In Vivo Spinal Cord Injury Assay
Adult female Fischer 344 animals (3–4 months old) were used in all in vivo experiments. Animals were anesthetized using a combination (2ml/kg) of ketamine (25mg/ml), rompun (1.8mg/ml) and acepromazine (0.25mg/ml). The dorsal funiculi were completely transected bilaterally at C3 using a tungsten wire knife (Kopf Instruments, Tujunga, CA) combined with dorsal tract compression to completely transect axons, as previously described (Lu, et al., 2004, Taylor, et al., 2006). Primary adult syngenic marrow stromal cells (MSCs) were isolated and cultivated as described (Azizi, et al., 1998, Taylor, et al., 2006). Immediately following the dorsal column lesion, 2 µl (75,000 cells/µl) of MSCs mixed with NT-3 protein (1 µg/µl) were injected into the spinal cord lesion site through a pulled glass micropipette using a PicoSpritzer II (General Valve, Fairfield, NJ). Lenti-NT-3 and Lenti-GFP were generated as previously described (Taylor, et al., 2006). Lentiviral vectors (2.5µl total; titer 100 µg/ml p24, ~1 × 108 infectious units/ml) was injected through pulled glass micropipettes, 2.5 mm rostral to the lesion site, into the spinal cord midline at a depth of 0.5 and 1 mm. Pipettes were left in place for 1 min before withdrawal. All groups and animal numbers are summarized in Table 1.
Table 1. Experimental groups for in vivo studies on regeneration of CTB labeled axons.
All animals received C3 dorsal column wire knife lesions and BMSC grafts mixed with NT-3 protein. Lentivirus for expression of NT-3 or GFP were injected at the same time rostral to the lesion site. Rolipram and mesopram infusions (2.6 mg/kg/day) were started 1 week prior to C3 lesions, pumps were exchanged at the time lesions were made, and infusions continued for one additional week. Conditioning lesions were done 1 week prior to C3 dorsal column lesions (preconditioning) or 1 or 7 days after C3 lesions (post-conditioning). Injections of cAMP or PBS into L4, 5 DRGs was done 1 day after C3 lesions.
| Groups | Conditioning/Infusion/Injection | Rostral vector | Number of animals for CTB labeling |
|---|---|---|---|
| 1 | No treatment | GFP | n=6 |
| 2 | No treatment | NT-3 | n=6 |
| 3 | Pre-Conditioning lesion | GFP | n=6 |
| 4 | Pre-Conditioning lesion | NT-3 | n=6 |
| 5 | Rolipram infusion | GFP | n=6 |
| 6 | Rolipram infusion | NT-3 | n=6 |
| 7 | Mesopram infusion | No vector | n=6 |
| 8 | Mesopram infusion | NT-3 | n=6 |
| 9 | 1 day Post-Conditioning lesion | NT-3 | n=7 |
| 10 | 7 day Post-Conditioning lesion | NT-3 | n=7 |
| 11 | cAMP injection | NT-3 | n=6 |
| 12 | PBS injections | NT-3 | n=6 |
Seven days before, one day after, or seven days after spinal cord lesions, rats were subjected to peripheral conditioning lesions (Table 1). Sciatic nerves were exposed bilaterally at mid-thigh level and firmly compressed with fine jeweler’s forceps for 15 seconds. Animals that received pump infusions were anesthetized and Alzet minipumps (2ML1 delivering 10 µl/hr) were implanted subcutaneously under the skin of the back. Pumps were filled with 1.79 mg/ml rolipram (Sigma, St. Louis, MO; 4-(3-cyclopentyloxy-4-methoxy-phenyl)-2-pyrrolidone) or mesopram (Schering AG, Berlin, R-(−)-5-(4-methoxy-3-propoxyphenyl)-5-methyl-2-oxazolidinone) in 16% DMSO/PBS, a dose of 2.6 mg/kg/day. In animals that received pump implantations and spinal cord lesions, spinal cord lesions were made 7 days after starting the infusion, analogous to pre-conditioning lesions. In animals that received infusions for more than 7 days, pumps were exchanged at the one-week time point. Control animals received infusions of vehicle (16% DMSO in PBS).
Injections of cAMP or PBS in controls were performed as previously described (Lu, et al., 2004). Animals were deeply anesthetized and L4–5 DRG were exposed bilaterally. 2.5 µl dibutyryl-cAMP (50 mM in PBS), a dose used successfully in earlier studies (Lu, et al., 2004, Qiu, et al., 2002), or PBS were injected 1 day following C3 lesions, cell grafting and Lenti-NT-3 injections (Table 1) through pulled glass pipettes using a Picospritzer II. L6 DRGs were not injected because of their small size.
Anatomical Analysis
Dorsal-column sensory axons were labeled transganglionically by CTB injection into the sciatic nerve proximal to the conditioning lesion site (2µl of 1% solution per sciatic nerve) three days before perfusion, as described previously (Alto, et al., 2009, Bradbury, et al., 1999, Lu, et al., 2004, Taylor, et al., 2006). 4 weeks after spinal cord lesions, animals were transcardially perfused with 4% paraformaldehyde, post-fixed overnight, and cryoprotected in 30% sucrose at 4°C. Spinal cords were sectioned sagittally at 35 µm intervals on a cryostat. All sections were processed free-floating. For visualization of CTB-labeled sensory axons, endogenous peroxidase activity was blocked with 0.6% hydrogen peroxide and non-specific antibody reactions were blocked with 5% horse serum for 1 hr at room temperature. Sections were incubated for 72 hours at 4°C with the primary CTB antibody (goat polyclonal 1:80,000 dilution, List Biological Labs) followed by incubation with a biotinylated horse anti-goat IgG secondary antibody (1:200 dilution, Vector Laboratory Inc.) for 1 hr at room temperature. After 1 hr incubation in avidin-biotin peroxidase complex (1:100 dilution, Elite kit, Vector Laboratories Inc.) at room temperature, diaminobenzidine (0.05%) with nickel chloride (0.04%) were used as chromagens. GFP and GFAP were detected subsequently in the same sections by fluorescence labeling using antibodies against GFP (1:1500, Invitrogen, Carlsbad, CA) and GFAP (1:1000 Chemicon, Temecula, CA) incubated overnight at 4°C. After washes, sections were incubated with Alexa 488 and Alexa 594 fluorophore-conjugated secondary antibodies for 2.5 hr at room temperature (1:300, Invitrogen).
To quantify the number and distance of dorsal column sensory axons bridging beyond the lesion site, serial 35µm-thick sections (1 out of 7) double-labeled for CTB and GFAP were examined. The number of CTB-labeled axons encountered at a virtual line along the rostral host/graft interface identified by GFAP labeling was counted at 400× magnification. Using a 10× ocular with a calibrated grid and a 40× objective, axons crossing a line perpendicular to the dorsal spinal cord at distances of 50, 100, 200, 400, 800 and 1600 µm rostral to the rostral lesion border were also counted. In addition, the distance between the rostral host/graft interface and the longest CTB-labeled axon identified rostral to the lesion site was noted for each animal. Only axons located in areas that contained GFAP-labeled cell bodies were counted. All quantifications were done by an observer blinded to the nature of the experimental manipulation.
cAMP ELISA of Dorsal Root Ganglia
One day, 3 days or 7 days after conditioning lesions or implantation of rolipram and mesopram infusion pumps (Table 2), experimental animals (n=38) and naïve control animals (n=11) were deeply anesthetized with isofluorane, decapitated, and L4–6 DRGs were dissected and homogenized in 0.1M HCl. Direct measurement of cAMP in DRG was performed using cAMP ELISA, according to the manufacturer’s instruction (Assay Designs, MI).
Table 2. Experimental groups for cAMP ELISA and in vitro growth assays.
| Groups | Treatment | cAMP ELISA (day of isolation) | In vitro growth assay (day of isolation) |
|---|---|---|---|
| 1 | Naïve | n=11 | n=8 |
| 2 | Conditioning lesion | n=5 (day 1) | |
| n=5 (day 3) | n=4 (day 3) | ||
| n=5 (day 7) | n=4 (day 7) | ||
| 3 | Mesopram infusion | n=5 (day 1) | |
| n=4 (day 3) | n=3 (day 3) | ||
| n=4 (day 7) | n=4 (day 7) | ||
| 4 | Rolipram infusion | n=3 (day 1) | |
| n=3 (day 3) | |||
| n=4 (day 7) |
Microarray Analyses
A total of 210 animals were used for microarray analysis of gene expression. L4–L6 DRGs were harvested from naïve animals, and from animals 1, 3, 7, or 14 days after receiving either: a) conditioning lesions, or b) mesopram infusions. In addition, DRGs were harvested 7 days after control (DMSO) infusions, a time point when most changes in gene expression were expected from mesopram infusions (n = 9 subjects/group/time point, divided into 3 arrays/group/time point; Table 3). To examine changes after db-cAMP, L4–L5 DRGs were also harvested at 1, 3, 7 and 14 days after db-cAMP or PBS control injections into L4 and L5 DRGs (n = 9 subjects/group/time point, divided into 3 arrays/group/time point). Rats were anesthetized using isofluorane gas anesthesia, decapitated, the lumbar spinal column was excised and immediately transferred to ice-cold PBS. Using a surgical microscope L4, L5 and L6 DRGs were microdissected and transferred to RNAlater (Ambion) and frozen at −80°C until RNA was extracted using the RNeasy Mini Kit (Qiagen, Valencia, CA). DRGs from 3 animals were pooled for each microarray. Concentration and quality of RNA samples was examined using the NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Rockland, DE) and an Agilent 2100 Bioanalyzer (Agilent Technologies, Inc., Santa Clara, CA). RNA samples were reverse transcribed and labeled according to manufacturer’s instructions and hybridized to Affymetrix high-density oligonucleotide GeneChip Rat Genome 230 2.0 Arrays (Affymetrix, Santa Clara, CA). Array data analysis was done in the R computing environment (http://www.r-project.org) using Bioconductor (http://www.bioconductor.org (Gentleman, et al., 2004)). One sample (Group #4, conditioning lesion day 7) failed the quality control and was excluded from further analyses. Raw data were processed using robust multi-array average (RMA (Irizarry, et al., 2003), and normalization using the 'quantile' method (Bolstad, et al., 2003). All p values were corrected for multiple testing using the Benjamini-Hochberg false discovery rate (Benjamini, 1995). Additional analyses were done using Ingenuity pathway analysis software (Ingenuity Systems, Redwood City, CA). Comparisons were made between intact animals and animals with conditioning lesions or mesopram infusions and between animals injected with PBS and db-cAMP. In addition to these analyses, and to further support the quality of our dataset, we compared in a separate study L4–6 DRG array data from naïve animals, animals that received control DMSO infusions for 1 day and animals that underwent anesthesia only, without infusion or lesion, 1 day prior to DRG dissection (Table 3, study III). This study was done to control for the possibility that anesthesia or vehicle infusion (DMSO) had a short-term influence on gene expression. Because these manipulations only resulted in minor changes in gene expression (see Results), comparisons of animals with conditioning lesions or mesopram infusions to naïve animals were deemed appropriate to identify changes in gene expression.
Table 3. Experimental groups for microarray analysis.
Lumbar DRGs (L4, 5, 6 for Experiments I, II, III; L4, 5 for Experiment IV) were dissected for RNA isolation.
| Experiment number | Group number | Treatment | Number of animals | Arrays for data analysis after quality control |
|---|---|---|---|---|
| I | 1 | Naïve | n=12 | n=4 |
| 2 | Conditioning lesion day 1 | n=9 | n=3 | |
| 3 | Conditioning lesion day 3 | n=9 | n=3 | |
| 4 | Conditioning lesion day 7 | n=9 | n=2 | |
| 5 | Conditioning lesion day 14 | n=9 | n=3 | |
| II | 6 | Mesopram infusion day 1 | n=9 | n=3 |
| 7 | Mesopram infusion day 3 | n=9 | n=3 | |
| 8 | Mesopram infusion day 7 | n=9 | n=3 | |
| 9 | Mesopram infusion day 14 | n=9 | n=3 | |
| 10 | Control infusion day 7 | n=9 | n=3 | |
| 1b | Naive | n=9 | n=3 | |
| 4b | Conditioning lesion day 7 | n=9 | n=3 | |
| III | 11 | Control (DMSO) infusion day | n=9 | n=3 |
| 12 | Naïve | n=9 | n=3 | |
| 13 | Anesthesia only day 1 | n=9 | n=3 | |
| IV | 14 | *PBS injection day 1 | n=9 | n=3 |
| 15 | *PBS injection day 3 | n=9 | n=3 | |
| 16 | *PBS injection day 7 | n=9 | n=3 | |
| 17 | *PBS injection day 14 | n=9 | n=3 | |
| 18 | db-cAMP injection day 1 | n=9 | n=3 | |
| 19 | db-cAMP injection day 3 | n=9 | n=3 | |
| 20 | db-cAMP injection day 7 | n=9 | n=3 | |
| 21 | db-cAMP injection day 14 | n=9 | n=3 |
Quantitative Real-Time PCR
RNA was isolated from L4–6 DRGs from animals that either: a) were unoperated, b) underwent bilateral conditioning lesions, c) received subcutaneous infusions of mesopram for 24hr, or d) received infusions of 16% DMSO (vehicle) for 24h. 2 animals were pooled for RNA isolation (n=6 animals/group) and 1 µg total RNA was reverse transcribed using Superscript III (Invitrogen, Carslbad, CA). 1µl 1st strand synthesis, 1 µl of each primer pair (10µM each), 12.5 µl SYBR-green mix (SABiosciences) and 10.5 µl water were mixed in a 25 µl reaction and amplified for 45 cycles at 95°C for 10 sec and 58°C for 30 sec using a BioRad MyIQ thermocycler. 1st strand synthesis without reverse transcriptase and no template samples served as control. Following completion of the program, melting curves were analyzed. Differences in gene expression between groups were determined using the ΔΔCt method using the Biorad software package using L37A and GAPDH as reference genes. Primer sequences for the chosen genes Cebp/d Egr1, Egr3, ATF3, Myc, Jun, Fos, Smad1, Sox11, Gadd45a, Gadd45g, ZFP367 and NFIL3 are presented in Supplementary Table 1.
Statistical Analysis
In all quantification procedures, multiple group comparisons were made by ANOVA with a significance criterion of p<0.05. Post hoc differences were tested by Fisher’s least square difference. Data are presented as mean ± standard error of the mean.
RESULTS
Conditioning Lesions Elicit Significantly Greater In Vitro Neurite Outgrowth from Adult DRG Neurons than cAMP
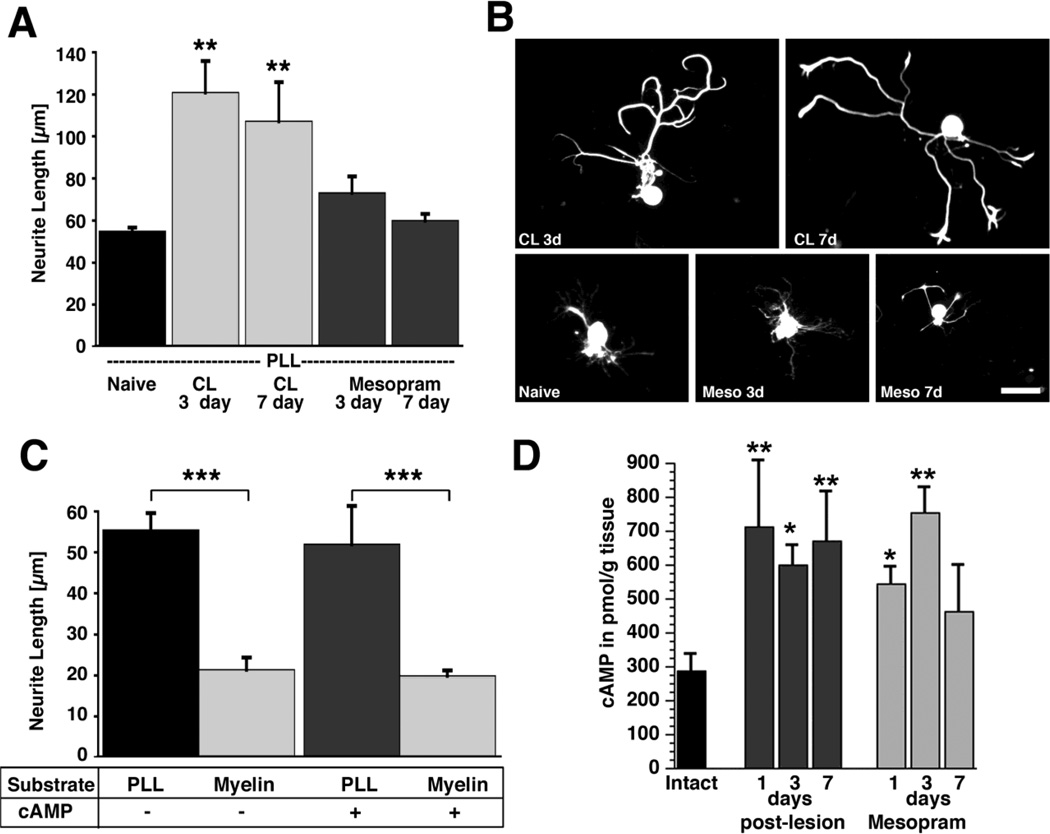
Adult DRG neurons cultivated on poly-L-lysine and isolated from animals 3 or 7 days after conditioning lesions exhibited a significant 2-fold increase in neurite length when compared to DRG neurons from naïve animals (Fig 1A,B). In contrast, infusion of the PDE-IV inhibitor mesopram for 3 or 7 days did not increase neurite length. Similarly, cultivation of adult DRGs in the presence of 2 mM db-cAMP did not significantly increase neurite extension on poly-L-lysine or myelin. (Fig. 1C). Thus, conditioning lesions are more effective than cAMP in enhancing in vitro outgrowth of adult DRG neurites. In contrast to adult DRG neurons, cAMP significantly increased the mean neurite length of postnatal DRGs and cerebellar granular neurons when cultivated on permissive or inhibitory substrates (Suppl. Fig. S1A–C) (comparison to conditioning lesions was not made due to the young age of the subjects). Taken together, these experiments suggest that increased cAMP levels at the time of cell plating (db-cAMP incubation) or prior to cell plating (in vivo mesopram infusion) are insufficient to replicate conditioning effects on neurite growth of adult DRG neurons.
Figure 1. Quantification of in vitro neurite growth and cAMP levels in adult DRG neurons after conditioning lesions and infusion of phosphodiesterase inhibitors.
(A) Adult lumbar DRGs (L4–6) were dissected from naïve animals, animals that underwent a sciatic nerve crush 3 or 7 prior to isolation, or animals that received subcutaneous infusions of mesopram for 3 or 7 days prior to isolation. Quantification of neurite length indicates a significant increase in neurite outgrowth on poly-L-lysine (PLL) 3 and 7 days after conditioning lesions (**p<0.01 compared to naive), whereas infusions of the PDE IV inhibitor mesopram have no effect. (B) Examples of NF-200 labeled neurons indicate enhanced growth 3 days (CL 3d) and 7 days (CL 7d) after conditioning lesions but not after mesopram infusion (Meso 3d; Meso 7d). (C) db-cAMP (2 mM) does not increase neurite outgrowth of adult neurons on PLL or myelin. Myelin is strongly inhibitory in the presence and absence of db-cAMP (*** p<0.001 comparing PLL to myelin). Cells were cultivated for 72h and labeled for NF-200 to identify large and medium sized neurons. Data are presented as the means ± SEM of the average neurite length obtained in at least 3 independent experiments. (D) Quantification of cAMP levels in DRGs by ELISA. L4–6 DRGs were dissected from naïve animals, animals that underwent sciatic nerve crush lesions, and animals that received subcutaneous infusions of mesopram for the time indicated. Sciatic nerve lesions result in significant increases in cAMP levels at 1, 3 and 7 days post-lesion. Infusions of mesopram only lead to transient increases in cAMP levels (ANOVA followed by Fischer’s posthoc testing **p<0.01, * p<0.05, compared to naïve controls). Scale bar= 79 µm in (B).
To determine whether infusion of the phosphodiesterase inhibitor mesopram results in cAMP increases comparable to conditioning lesions, we measured cAMP by ELISA in lumbar DRGs (L4–6). Conditioning lesions increase cAMP levels in DRGs approximately 2-fold as early as 1 day after sciatic nerve crush, consistent with previous reports (Qiu, et al., 2002) (Fig. 1D). Levels remain elevated 1 week later. Infusions of mesopram (or rolipram; Suppl. Fig S1D) result in cAMP increases of the same magnitude for at least 3 days, but cAMP levels are no longer significantly elevated compared to intact animals by day 7 despite continuous infusion. Thus, while mesopram increases cAMP levels at 3 days to the same extent as conditioning lesions, neurite outgrowth capacity is significantly enhanced only after conditioning lesions. Microarray data and in vivo assays (below) further support this result.
Conditioning lesions in combination with NT-3 enhance axonal bridging beyond the lesion site
To further clarify the role of cAMP in mediating conditioning lesion effects in vivo, we compared axon growth-promoting effects of conditioning lesions to infusion of phosphodiesterase inhibitors and direct injections of a cell permeable analog of cAMP (db-cAMP), at various time points after spinal cord injury.
First we determined whether pre-conditioning lesions alone or in combination with Lenti-NT-3 delivery are effective in increasing the number and distance of axons regenerating beyond a central lesion. Animals received bilateral sciatic nerve crush lesions; one week later, the dorsal funiculus was transected at cervical level (C3) (Lu, et al., 2004, Taylor, et al., 2006) and animals received grafts of bone marrow stromal cells (BMSC) mixed with NT-3 protein into the lesion site to provide a cellular substrate for regenerating axons (Fig. 2). Lenti-NT-3 or -GFP as control was injected 2.5 mm rostral to the lesion as previously described (Alto, et al., 2009, Taylor, et al., 2006). Additional control animals received the same graft and virus injection but no conditioning lesion (Suppl. Fig. S2; Table 1).
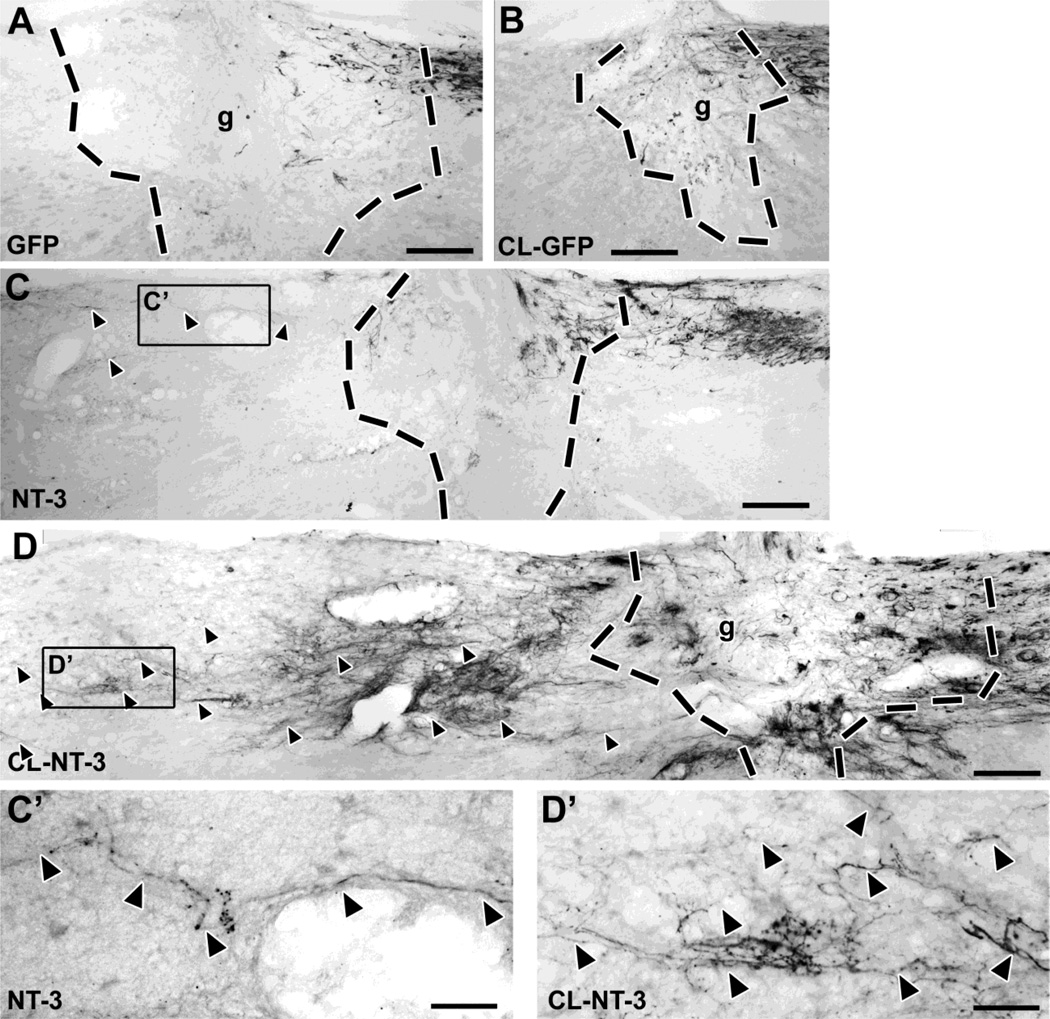
Figure 2. A combination of pre-conditioning lesions and lentiviral NT-3 gene transfer results in significant axonal bridging across the lesion site.
(A) CTB-labeled ascending sensory axons fail to bridge across a lesion site filled with BMSC in control animals that received injections of Lenti-GFP. (B) Conditioning lesions combined with control Lenti-GFP injections do not increase the number of bridging axons. (C) Animals that received Lenti-NT-3 rostral to a lesion (without conditioning lesions) show some bridging axons extending only for short distances (quantified in Fig. 4). (D) In contrast, a combination of Lenti-NT-3 and pre-conditioning lesions results in numerous axon bridging beyond the lesion and extension for longer distances. (C’, D‘) Higher magnification of boxed areas in (C) and (D), respectively, showing regenerating CTB-labeled axons (arrowheads) rostral to the lesion site. Rostral is to the left, dorsal to the top. Scale bar= 170 µm in (A–D), 42 µm in (C’, D’). Dashed lines indicate lesion/graft site determined by the absence of GFAP immunolabeling (see Fig. 3).
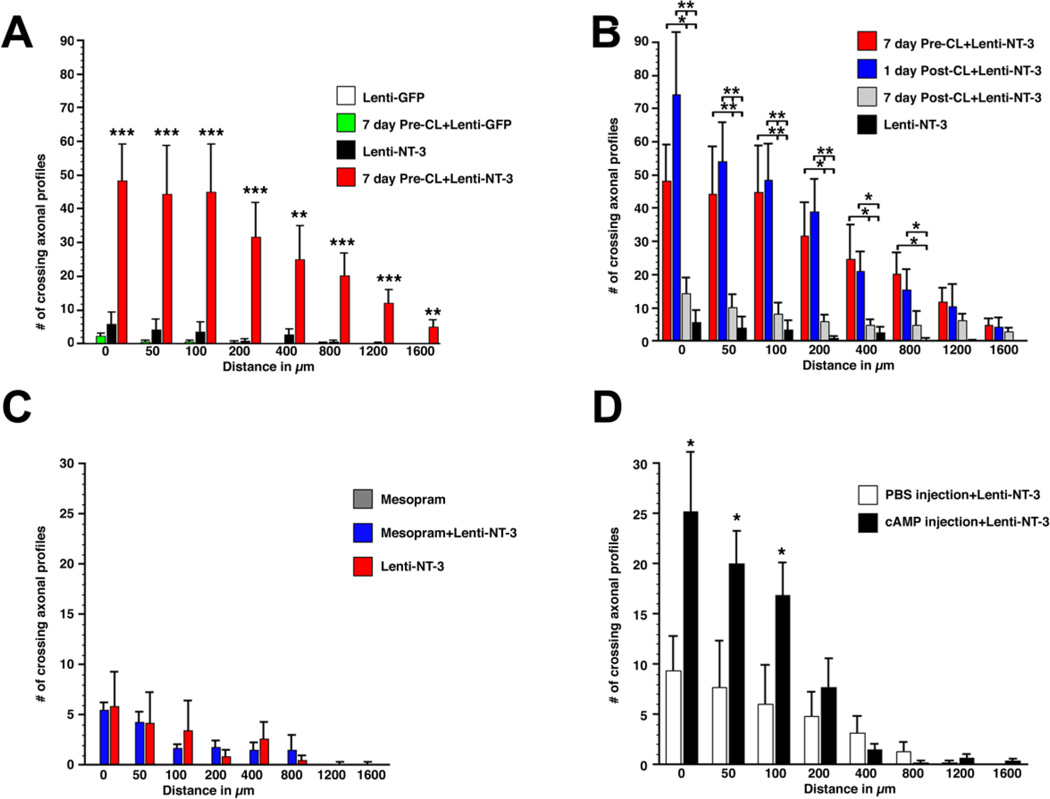
Tracing of ascending sensory axons with CTB showed significant axonal bridging across the graft into the rostral spinal cord only among animals that received Lenti-NT-3 (Figs. 2–4). In animals that received no conditioning lesions (CL) or CL with cellular grafts and Lenti-GFP injections (but not Lenti-NT-3), rare axons reached the rostral host/graft interface but virtually no axons extended further in the rostral white matter of the spinal cord (Fig. 2A,B). In animals with Lenti-NT-3 delivery without preconditioning lesions, a small number of axons extended across the rostral host/graft interface and for short distances beyond, as previously reported (Fig. 2C) (Taylor, et al., 2006). This number was increased approximately 8-fold in animals that received pre-conditioning lesions in addition to Lenti-NT-3 (Fig 2D). Indeed, subjects that underwent conditioning lesions and injections of Lenti-NT-3 beyond the lesion exhibited significantly more axons compared to all other groups over distances up to 1600 µm beyond the graft/lesion site (Fig. 4A; ANOVA p<0.001; Fischer’s posthoc p<0.01 comparing each group to conditioning lesion + Lenti-NT-3). Axons extended an average maximum distance of nearly 2.5 mm beyond the rostral host/graft interface, a distance six times greater than the distance of animals receiving only Lenti-NT-3 (Suppl. Fig. S3). As growth distance was measured from the rostral host/graft interface, the total length of axon growth (measured from the caudal aspect of the lesion site) was in fact 3–3.5 mm. Axons crossing from the graft into the host spinal cord beyond the lesion were present at all dorsoventral levels within the dorsal funiculus, and extended preferentially in white matter rostral to the graft along regions of NT-3 expression (Fig. 3). Axons were frequently found to orient along GFAP-labeled processes and were occasionally associated with blood vessels. Anatomical sectioning of the medulla confirmed lesion completeness (data not shown).
Figure 4. Quantification of axons bridging across a C3 lesion site filled with bone marrow stromal cells in animals that received pre- or post-conditioning lesions, infusions of mesopram or db-cAMP injections.
(A) Pre-conditioning lesions in combination with Lenti-NT-3 gene transfer (7 day Pre-CL+Lenti-NT-3) significantly increased the number of axons crossing a C3 dorsal funiculus lesion compared to animals that received only Lenti-NT-3, only Lenti-GFP, or a combination of Lenti-GFP/conditioning lesions (7 day Pre-CL-Lenti-GFP). The number of axons crossing the rostral host graft interface (0 µm) or a virtual line 50, 100, 200, 400, 800, 1200 and 1600 µm beyond the lesion site was quantified in a series of 1 out of 7 sections. (ANOVA followed by Fischer’s posthoc analysis *** p<0.001, ** p<0.01 comparing 7 day Pre-CL+Lenti-NT-3 to all other groups). (B) Axonal bridging after 1- or 7-day post-conditioning lesions in combination with Lenti-NT-3 gene transfer. The number of bridging axons in animals that received post-conditioning lesions 1 day after central lesions is not significantly different at any distance examined from animals that received pre-conditioning lesions 7 day before central lesions. Priming neurons by peripheral lesions 7 days after spinal cord lesions resulted in significantly fewer axons bridging beyond the lesion site. Data from pre-conditioned animals in combination with Lenti-NT-3 from (A) are included for clarity. (ANOVA followed by Fischer’s posthoc testing **p<0.01; * p<0.05). (C, D) Increases in cAMP levels result in only short-distance axon growth beyond the lesion. (C) Animals received infusions of the phosphodiesterase inhibitor mesopram starting one week prior to C3 dorsal column lesions, BMSC grafts, and Lenti-NT-3 or Lenti-GFP injections. Infusions of mesopram without NT-3 delivery failed to result in any axonal bridging; the combination of mesopram infusion with Lenti-NT-3 delivery did not further increase the number of bridging axons compared to Lenti-NT-3 delivery alone. Animals with Lenti-NT-3 injections alone, quantified in (A), are included for comparison. (D) Injections of dibutyryl-cAMP into L4 and L5 DRGs one day after spinal cord lesions and Lenti-NT-3 delivery increase axonal bridging only up to 100 µm beyond the rostral host/graft interface, when compared to animals injected with PBS and Lenti-NT-3 as controls (unpaired t-test *p<0.05). Data represent raw counts in one out of seven sections.
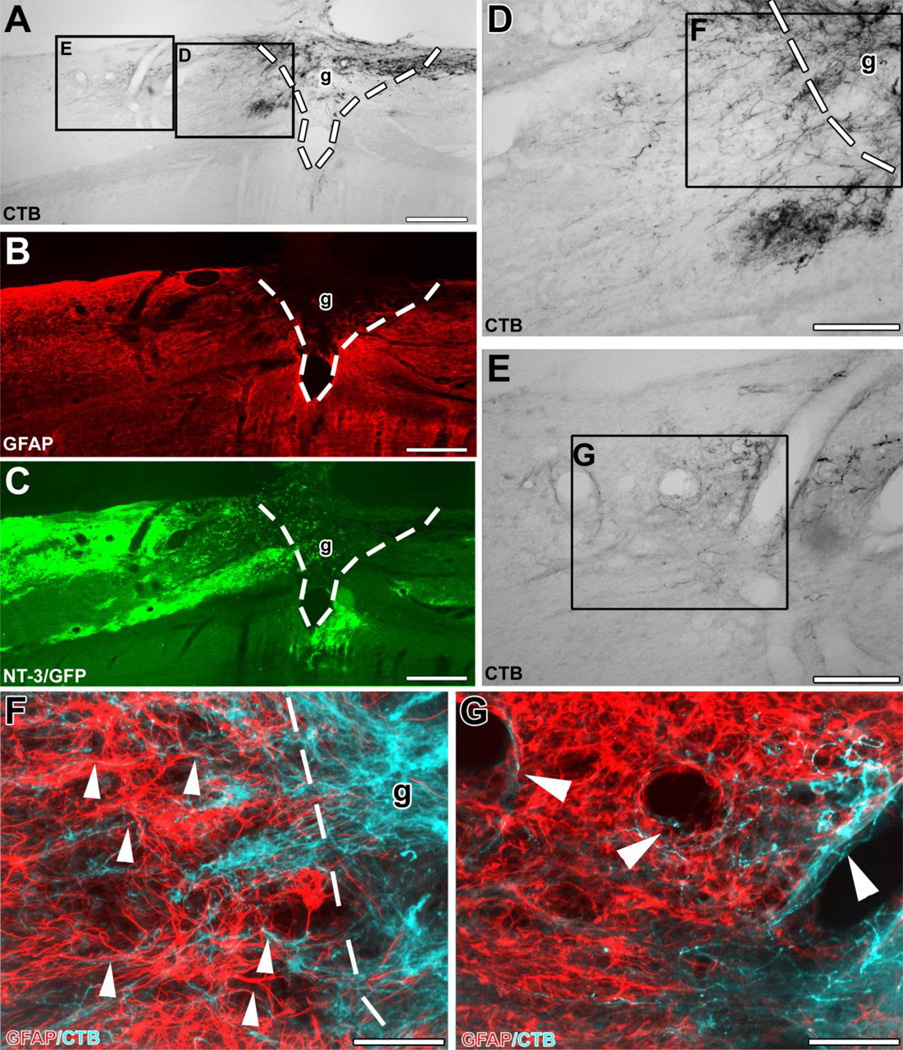
Figure 3. Ascending sensory axons extend beyond the graft/lesion site towards Lenti-NT-3-transduced cells in an animal that received pre-conditioning lesions and Lenti-NT-3 gene transfer.
Triple immunolabeling for (A) CTB to label ascending sensory axons, (B) GFAP to indicate the extent of the lesion/graft, and (C) GFP to label Lenti-NT-3-transduced cells in sagittal spinal cord sections. (D, E) Higher magnification of insets in (A) shows (D) axons growing across the rostral graft (g)/lesion site border (indicated by dashed lines). (E) Numerous axons are present further rostral to the lesion site, shown at higher magnification in (G). (F, G) Double immunolabeling for CTB-labeled axons (pseudocolored blue) and GFAP (red) at the (F) rostral host/graft interface (indicated by dashed lines) and (G) in the host spinal cord beyond the lesion. Bridging axons were often found to (F) orient along GFAP-labeled processes (arrowheads) and (G) were occasionally associated with blood vessels beyond the lesion site. Rostral is to the left, dorsal to the top. Scale bars 424 µm in (A–C), 170 µm in (D, E), 85 µm in (F, G).
Thus, initiation of an axonal growth program by peripheral pre-conditioning lesions before spinal cord lesions allows axonal extension across a growth substrate into an otherwise inhospitable environment of degenerating white matter, but only if an additional NT-3 trophic stimulus is provided.
Conditioning lesions are effective when administered shortly after central lesions
Next we explored the temporal requirements for neuronal conditioning to allow axonal bridging beyond a spinal cord lesion. Previous studies have indicated that pre-conditioning lesions are most effective when applied 5–7 days before central lesions. Analogous to the in vivo experiments described above, cell grafting and Lenti-NT-3 injections were made immediately following the C3 lesion, but conditioning lesions were placed at a delay of 1 or 7 days after spinal cord lesions. Quantification of axonal growth beyond the lesion site indicated that conditioning lesions one day after central lesions were at least as effective as pre-conditioning lesions in promoting axonal bridging beyond the C3 lesion site (Fig. 4B). A significant increase in the number of bridging axons was found up to 800 µm beyond the lesion site when compared to animals that received only Lenti-NT-3 and cell graft but no conditioning lesion (Fig. 4B). In contrast, when conditioning lesions were delayed by one week after the central lesion, a substantial reduction in axonal bridging was observed. Despite the lack of a significant post-conditioning effect at this time point, axons extended for longer distances in animals with a one week conditioning delay compared to animals without conditioning lesions (Suppl. Fig. S3; p<0.01) and axon numbers quantified at each distance beyond the lesion were slightly higher in animals with a 7 day “post-conditioning” lesion compared to animals without peripheral lesions.
Thus, signaling mechanisms underlying a conditioning lesion are still fully effective one day following a CNS lesion, but the effect is reduced when applied one week after spinal cord injury.
Conditioning lesions significantly enhance axonal regeneration compared to cAMP modulation
To determine whether direct cAMP augmentation exhibits equipotency compared to conditioning lesions in eliciting axonal regeneration, we examined central sensory axon regeneration after C3 spinal cord lesions. Both direct cAMP injections and infusions of the PDE-IV inhibitor mesopram (Dinter, et al., 2000) were compared to conditioning lesions, in the same lesion/treatment paradigm described in the preceding paragraph. Subcutaneous infusions of mesopram were initiated 7 days prior to C3 dorsal funiculus lesions. Pumps were exchanged at the time of spinal cord lesions, cell grafting and viral NT-3 delivery, and infusions continued for one more week. Consistent with our in vitro data, quantification of axons beyond the lesion site 4 weeks after spinal cord lesions did not indicate significant increases in the number of axons beyond the lesion site/cellular graft when comparing animals that received a combination of mesopram infusion with Lenti-NT-3 injections to animals that received only Lenti-NT-3 injections (Fig. 4C). Mesopram infusions alone (without rostral Lenti-NT-3) did not induce axonal growth beyond the rostral lesion border. Similar results were obtained with rolipram infusion (data not shown).
Next, we investigated whether a combination of Lenti-NT-3 with injections of a cell permeable analog of cAMP (db-cAMP) into L4–5 DRGs would enhance axonal bridging. As data reported above indicate that conditioning lesion one day after spinal cord lesions are equally effective as 7-day pre-conditioning lesions, db-cAMP or PBS (control) were injected directly into L4–5 DRGs 1 day after spinal cord lesions. Consistent with previous findings using db-cAMP injections 5 days before spinal cord lesions (Lu, et al., 2004), a 2-fold increase in the number of bridging axons was detected comparing animals with db-cAMP injections and Lent-NT-3 to animals with PBS injections and Lenti-NT-3 (Fig. 4D). This difference was significant up to 100 µm distal to the rostral lesion border. PBS injections in control animals also resulted in an increase in the number of bridging axons compared to Lenti-NT-3 alone (compare to Fig. 4A), likely a result of a partial conditioning effect from PBS injections into DRGs.
Taken together, cAMP injections with NT-3 delivery resulted in a modest number of axons (2-fold increase) bridging for short distances compared to conditioning lesions with NT-3 delivery (10-fold increase) indicating that effects of conditioning lesions on axonal regeneration exceed targeted cAMP modulation.
Genetic programs induced by conditioning lesions exceed cAMP effects
To investigate genetic mechanisms underlying conditioning lesion effects compared to cAMP, we examined changes in gene expression in lumbar DRGs (L4–6) by microarray analysis at 1, 3, 7 and 14 days after either conditioning lesions, mesopram or vehicle infusions, and after injection of db-cAMP or PBS into DRGs. Three animals/group were pooled for RNA isolation and for each microarray, and 3–4 microarrays were hybridized at each time point using a total of 210 animals and 70 microarrays (Table 3).
Analysis of changes in gene expression in DRGs that underwent peripheral nerve conditioning lesions demonstrated early and long-lasting changes in gene expression compared to intact DRGs, consistent with previous reports (Costigan, et al., 2002, Kubo, et al., 2002, Stam, et al., 2007, Tanabe, et al., 2003, Yang, et al., 2004). Using a false discovery rate of 5% (pFDR<0.05), a significance level of p<0.05, and a fold-change in expression of at least 20%, a total of 4883 differentially expressed probe sets were detected (Fig. 5, Suppl. Table 2). A large number of changes in gene expression occurred one day after conditioning lesions: 773 probe sets were upregulated and 497 downregulated. This number increased to 1721 upregulated probes and 1198 downregulated probe sets at 3 days, 1083 and 997 at 7 days, and 1867 and 1400 significantly changed probe sets at 14 days (Table 4). Several genes previously identified to exert an important role in peripheral nerve regeneration and to contribute to the priming effect of conditioning lesions were upregulated, including arginase-1, interleukin-6, ATF-3 and jun (see Suppl. Table 2). Between 79% and 89% of significantly changed probes were altered by more than 20% (depending on the post-injury day of analysis), and 5–8% of probe sets changed by more than 2-fold over the 14 day period after conditioning lesions. 60% (881/1479) of probe sets that exhibited significant changes on day 1 were still significantly changed 14 days after peripheral lesions. Thus, rapid and sustained activation of genes occurs after peripheral conditioning lesions. The sustained activation pattern correlates with the in vivo observation that central projections of DRG neurons exhibit significantly increased growth when spinal cord injury is accompanied by a peripheral conditioning lesion.
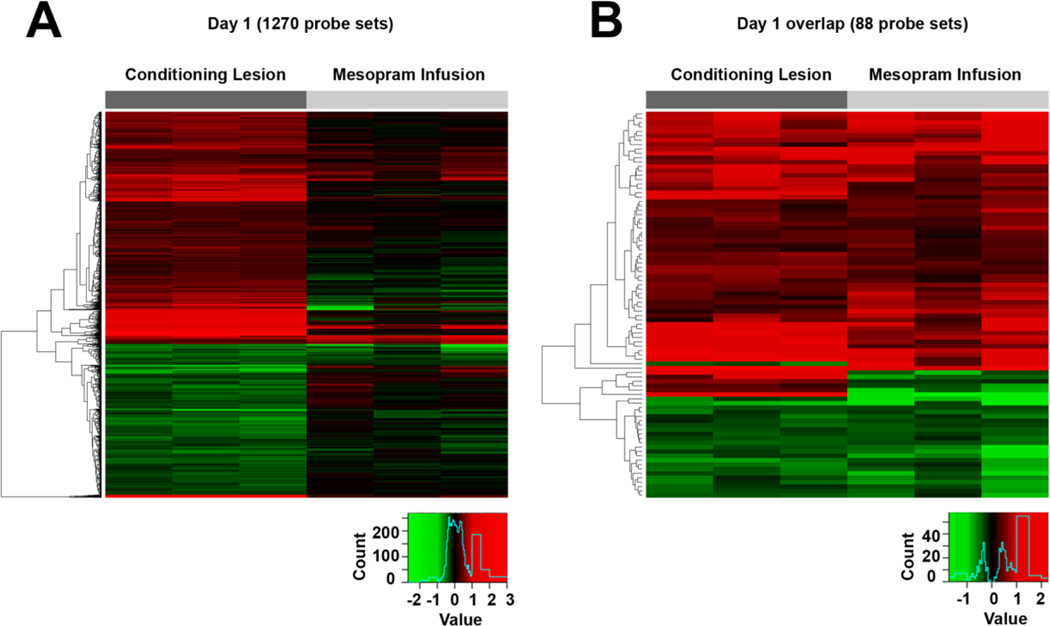
Figure 5. Heat maps depicting fold changes in gene expression one day following conditioning lesions and infusions of the phosphodiesterase inhibitor mesopram.
(A) A total of 898 probe sets are differentially expressed in DRGs after conditioning lesions (pFDR<0.05; change of > 20%) with limited overlap to the genes differentially expressed after mesopram infusion. (B) Gene expression is altered in the same direction for nearly all significantly changed probe sets overlapping between both groups. All comparisons are to DRGs of naïve control animals (see also Supplementary Data). Each column represents data from one array.
Table 4. Changes in gene expression after conditioning lesions compared to naïve control animals.
Numbers indicate probe sets changing at the criteria indicated. Numbers in parentheses indicate the number of significantly upregulated (+) and down regulated (−) probe sets. Percentage numbers in column 3 and 4 represent the percentage of probe sets changed at the criteria indicated (>1.2-fold and >2-fold). Detailed data for each probe set can be found in Supplementary Table 2. Significant changes were determined at p<0.05 at a false discovery rate of 5%. As many genes are represented by more than one probe set, the number of significantly changed genes is lower than the number of significantly changed probe sets.
| Group | # significantly changed probe sets | # of probe sets with 20% change | # of probe sets with > 100% change |
|---|---|---|---|
| Any Day | 5979 | 4838 | 340 |
| Conditioning Lesion Day 1 | 1479 (+861; −618) | 1270 (+773; −497) (85.9%) | 118 (+114; −4) (7.9%) |
| Conditioning Lesion Day 3 | 3709 (+ 2045; −1664) | 2919 (+ 1721; −1198) (78.7%) | 171 (+162; −9) (4.6%) |
| Conditioning Lesion Day 7 | 2336 (+ 1159; −1177) | 2080 (+ 1083; −997) (89.0%) | 176 (+167; −9) (7.5%) |
| Conditioning Lesion Day 14 | 4120 (+ 2262; −1858) | 3267 (+ 1867; −1400) (79.3%) | 238 (+230; −8) (5.8%) |
In contrast, infusion of the PDE IV inhibitor mesopram induced changes in a much smaller number of genes over time (544 probe sets using the same significance criterion of the preceding paragraph, Table 5 and Suppl. Table 3). The highest number of probe sets showing significant changes in expression compared to naïve controls occurred one day following the start of the infusion (524; pFDR<0.05): 275 genes were significantly upregulated and 249 probe sets showed reduced expression levels compared to intact animals. The number of differentially expressed probe sets rapidly declined at all subsequent time points, even before transient increases in cAMP levels returned toward baseline levels (see Fig. 1). These changes in gene expression were due to mesopram infusion, and were not a result of the vehicle infusion or induction of anesthesia, because only 1 and 3 differentially expressed genes were identified in the latter groups, respectively (pFDR<0.05; Suppl. Table 4).
Table 5. Changes in gene expression after mesopram and control infusions compared to naïve control animals (pFDR<0.05).
Numbers indicate probe sets changing at the criteria indicated. Numbers in parentheses indicate the number of significantly upregulated (+) and down regulated (−) probe sets. Percentage numbers in column 3 and 4 represent the percentage of probe sets changed at the criteria indicated (>1.2-fold and >2-fold). Detailed data for each probe set can be found in Supplementary Table 3. Significant changes were determined at p<0.05 at a false discovery rate of 5%.
| Group | # significantly changed probe sets | # of probe sets with > 20% change | # of probe sets with > 100% change |
|---|---|---|---|
| Any Day | 544 | 520 | 36 |
| Mesopram infusion Day 1 | 524 (+ 275 ; −249) | 500 (+ 261; −239) (95.4%) | 35 (+ 21; −14) (6.7%) |
| Mesopram infusion Day 3 | 14 (+ 6; −8) | 14 (+6; −8) (100%) | 2 (+ 1; −1) (14.2%) |
| Mesopram infusion Day 7 | 9 (+ 5; −4) | 9 (+5; −4) (100%) | 3 (+ 2; −1) (33.3%) |
| Mesopram infusion Day 14 | 18 (+7; −11) | 18 (+ 7; −11) (100%) | 1 (+ 0; −1) (5.6%) |
| Control infusion Day 7 | 8 (+2; −6) | 8 (+ 2; −6) (100%) | 1 (+ 0; −1) 12.5% |
Comparison of probe sets modulated by conditioning lesions versus mesopram (1 day after infusion) identified a limited but significant (p<0.0001) overlap in transcriptional responses (Figure 5, Suppl. Table 5). Of all probe sets significantly changed 1 day after mesopram infusion, 17% (88 probe sets) were also changed 1 day after conditioning lesions (at pFDR<0.05, more than 20% change). The 88 overlapping probe sets contained a large number of genes known to be regulated by cAMP such as cAMP responsive element modulator (crem) (Mioduszewska, et al., 2003), CCAAT/enhancer binding protein beta (C/EBPbeta) and delta (C/EBPdelta) (Yukawa, et al., 1998), c-fos and early growth response 1 (egr1, NGFI-A, zif268) (Vaccarino, et al., 1993). In contrast, many classical regeneration associated genes such as GAP-43, jun and β-III-tubulin were increased by conditioning lesions but not mesopram infusion. Taken together, more than 90% of early transcriptional responses induced by conditioning lesions were not induced by mesopram, despite equal increases in cAMP levels by ELISA. Virtually no persistent transcriptional modulation was achieved by mesopram infusion. Array data from animals that received cAMP injections into DRGs showed numerous changes compared to naïve control animals but similar changes were also observed after control PBS injections. Indeed, comparison of genes modulated by PBS and cAMP injections into DRGs did not indicate any significant differences using the same statistical criteria used for all other microarray studies (data not shown).
Network analysis using Ingenuity software confirmed that conditioning lesions influenced several broad networks of gene expression, including jun, p53 (Suppl. Fig. S4) and Il-6/stat3 (not shown), whereas mesopram recruited far more narrow activation (Fig. 5). Indeed, mesopram infusions influenced only 6.9% of genes that changed after conditioning lesions at the same time point, reflecting the broader pattern of genomic alteration induced by conditioning lesions. Taken together, fundamental differences in transcriptional responses to conditioning lesions versus cAMP modifying compounds were detected in our analysis, likely contributing to differences observed in potency of neurite outgrowth in our in vitro and in vivo models.
Microarray data were confirmed for several genes by quantitative real time PCR (qRT-PCR), including Cebp/d, Egr1, Egr3, ATF3, Myc, Jun, Fos, Smad1, Sox11, Gadd45a, Gadd45g, ZFP367and NFIL3. In no case did results of PCR considerably differ from array findings. These data confirmed changes in gene expression identified by microarray methods in subjects with conditioning lesions, and further indicated that mesopram infusions resulted in more modest effects (Table 6).
Table 6. qRT-PCR data confirming significant changes of selected genes in microarrays in DRGs of animals after mesopram infusions and conditioning lesions at day 1.
Values are log2 changes (% of control in parentheses) of gene expression compared to controls (naïve animals and animals with DMSO infusions, respectively).
| Conditioning Lesion | Mesopram Infusion | ||||
|---|---|---|---|---|---|
| Gene | Identifier | Microarray | qRT-PCR | Microarray | qRT-PCR |
| Cebp/d | 1368813_at 1387343_at |
1.82 (353%)**** 1.52 (286%)**** |
2.06 (417%)* | 0.63 (155%)NS 0.61 (153%)* |
0.59 (151%)# |
| Egr1 | 1368321_at | 1.00 (200%)**** | 1.31 (247%)** | 0.93 (191%)** | 0.91 (187%)** |
| Egr3 | 1392791_at | 0.95 (193%)**** | 1.79 (346%)** | 1.07 (210%)* | 0.43 (134%)NS |
| ATF3 | 1369268_at | 5.07 (3359%)**** | 6.17 (7201%)*** | 0.46 (138%)NS | 1.55 (295%)NS |
| Myc | 1368308_at | 1.26 (239%)**** | 1.96 (388%)* | −0.01 (99%)NS | 0.15 (111%)NS |
| Jun | 1389528_s_at 1374404_at 1369788_s_at |
1.47 (277%)**** 1.59 (301%)**** 1.40 (264%)**** |
1.05 (207%)* | −0.4 (76%)NS −0.54 (69%)NS −0.14 (91%)NS |
−0.45 (73%)NS |
| Fos | 1375043_at | 1.50 (283%)*** | 2.99 (792%)**** | 1.03 (204%)*** | 1.01 (201%)* |
| Smad1 | 1369174_at 1389373_at 1396061_at |
0.97 (196%)*** 0.93 (191%)**** 0.02 (101%)NS |
1.56 (294%)** | −0.1 (93%)NS −0.12 (92%)NS 0.13 (109%)NS |
−0.26 (83%)NS |
| Sox11 | 1371450_at 1387275_at |
2.21 (463%)**** 2.55(586%)**** |
3.54 (1164%)*** | 0.23 (117%)NS 0.33 (126%)NS |
0.01 (101%)NS |
| GADD45A | 1368947_at | 2.60(606%)**** | 4.0 (1589%)** | 0.08 (106%)NS | nd |
| GADD45g | 1388792_at | 1.93(381%)**** | 3.2 (926%)*** | 0 (100%)NS | nd |
| ZFP367 | 1379967_at | 1.22 (233%)**** | 3.06 (836%)*** | −0.12 (92%)NS | nd |
| NFIL3 | 1368488_at | 1.39 (262%)**** | 1.59 (300%)** | 0.25 (119%)NS | nd |
Some genes are represented by more than a single probe set;
not significantly different from control;
p<0.05;
p<0.01,
p<0.001;
p<0.0001,
p=0.07,
nd: not determined
DISCUSSION
Conditioning lesions of the peripheral branch of sensory axons have long been recognized to enhance the growth capacity of central sensory neuron projections when injury of the peripheral process precedes injury in the CNS (Neumann and Woolf, 1999, Richardson and Issa, 1984). Our studies show that a single post-conditioning lesion is also effective in enhancing central axonal bridging across a spinal cord lesion site when combined with NT-3 delivery. Consistent with previous studies, NT-3 delivery and stimulation of regenerative cell body responses were synergistic (Lu, et al., 2004). Compared to cAMP modulation, conditioning lesions initiate far more extensive regenerative responses in DRG neurons, activating a large number of cAMP-dependent and -independent intracellular signaling pathways leading to long lasting changes in gene expression and superior axonal regeneration in vivo.
Conditioning lesions are effective after central lesions
In the current experiments the timing of conditioning lesions ranged from a seven day pre-conditioning to seven day “post-conditioning” stimulus to the sciatic nerve, while cell grafting and Lenti-NT-3 gene transfer into a site of spinal cord injury always occurred immediately postlesioning. In contrast to a previous study, which reported that: 1) priming concomitant with a lesion is insufficient to induce growth beyond a lesion site (Neumann and Woolf, 1999) and 2) that repeated priming is required if the conditioning lesion follows a central spinal cord lesion (Neumann, et al., 2005), our results show that peripheral conditioning is effective when applied shortly after a central lesion. The provision of a cellular substrate and NT-3 beyond the lesion site in the current experiment might underlie the different outcomes between these studies. However, conditioning effects are reduced significantly when applied one week after injury resulting in a 7–10 fold reduction in regenerating axons (current study) to a value that is similar to previously reported effects of conditioning lesions at a chronic time point of 3 months post-injury (Kadoya, et al., 2009). A similar decline can also be observed in the average maximum distance of axon growth from 2200 µm and 2000 µm with pre-conditioning and 1 day delay, respectively, to 1300 µm at a delay of 7 days and 3 months. As our previous studies indicate that transcriptional changes occurring in DRGs after pre-conditioning and conditioning at chronic time points are virtually identical (Kadoya, et al., 2009), a decline in regenerating axons after conditioning delays of more than 1 day is likely due to changes in the environment of the injured spinal cord or post-transcriptional neuronal responses occurring very early post-lesion. The distance of axonal growth (2–2.5 mm) beyond the lesion site was insufficient for regenerating axons to reach their brainstem target nuclei and is therefore unlikely to have functional effects. More extended growth might require the inactivation or neutralization of inhibitory cues present in the injured spinal cord and/or a decrease in NT-3 expression, which is necessary for significant axonal bridging to occur but might also present a terminal stop signal at high concentrations. Means to decrease neurotrophin expression as axons reach one spatial location and to establish additional growth factor gradients in more distal locations might allow for continued growth over more extended distances. Future experiments will address these possibilities.
Conditioning lesions are superior to cAMP in enhancing axonal growth
db-cAMP increased neurite outgrowth from early postnatal DRGs and postnatal cerebellar granular neurons, but had no effect on more mature neurons (4 –16 weeks). These findings suggest a postnatal developmental switch in the responsiveness to cAMP or changes of other intrinsic growth modulators necessary for cAMP effects on neurite outgrowth. Changes in neurite growth responses to extracellular stimuli in early postnatal development have been previously described. For example, until around P4, DRG neurons are attracted by myelin-associated glycoprotein (MAG) and inhibited by it thereafter (Mukhopadhyay, et al., 1994). While previous studies report a neurite growth stimulatory role of cAMP, early postnatal DRGs and CGNs were used (Cai, et al., 1999), the exact age of animals used for the isolation of DRG neurons was either not specified, or DRG were injected with cAMP in vivo before isolation resulting in a partial conditioning (Neumann, et al., 2002, Qiu, et al., 2002). In one study, cAMP effects on adult neurons were reported, although this study added serum, which itself is stimulatory (Andersen, et al., 2000). Another study recently confirmed that cAMP analogs increase neurite outgrowth in neonatal but not adult DRGs (Murray and Shewan, 2008). Similarly, under the experimental conditions used in this study, cAMP-signaling alone was insufficient to enhance neurite growth in adult DRG neurons suggesting that additional stimuli are required to achieve cAMP-mediated increases in neurite outgrowth that are comparable in extent to the growth promoting effects of conditioning lesions.
In vivo, NT-3 delivery was essential for axons to extend within white matter beyond the lesion irrespective of the timing of the conditioning lesion, and axon growth distal to the graft in response to NT-3 was only enhanced by conditioning lesions and db-cAMP injection, and not by infusions of phosphodiesterase inhibitors. Effects of db-cAMP injections are at least partially attributable to the injury caused by the injection procedure, indicated by the enhanced growth observed in PBS-injected control animals (Fig. 4D). In addition, microarrays from db-cAMP-injected DRGs indicated numerous changes in gene expression that were also observed in PBS control animals (data not shown). Compared to db-cAMP injections and phosphodiesterase inhibitor infusions, conditioning lesions in combination with NT-3 were more effective in increasing the distance and the number of axons extending across the lesion site. These observations might indicate that cAMP-mediated mechanisms only partially enhance neuronal regenerative programs. Alternatively, differences in the magnitude, timing and duration of cAMP elevation may have limited axonal growth responses in our experiments. ELISA of DRGs demonstrated that phosphodiesterase inhibitors and conditioning lesions both resulted in 2-fold increases in cAMP levels, thus amounts of cAMP elevation likely did not account for observed differences in axonal growth at early time points post-injury. However, differences in the duration of cAMP elevation were detected by ELISA when comparing conditioning lesions and phosphodiesterase inhibitors: conditioning lesions elevated cAMP in DRG neurons for at least 7 days, whereas phosphodiesterase inhibitors sustained cAMP elevation for only 3 days. While this difference could account for the lack of in vivo regeneration after infusion of PDE IV inhibitors, other data do not support this conclusion. First, neurite outgrowth assays demonstrated increased neurite extension only after conditioning lesions and not after phosphodiesterease inhibitor infusions, even though the assay was performed at a time point when cAMP levels were equally increased by conditioning lesions and phosphodiesterease inhibitor infusions. Second, gene array studies indicated that conditioning lesions initiated a far more extensive set of transcriptional programs compared to cAMP modulation. Conditioning lesions induced and sustained expression of many classical regeneration-associated genes including GAP43, c-jun and beta-III-tubulin, effects that were not observed with phosphodiesterase administration. Even one day after treatment with either a conditioning lesion or phosphodiesterase inhibitor, when cAMP levels are equally increased, conditioning lesions upregulated vastly greater numbers of transcriptional mechanisms (Fig. 5, Suppl. Fig. 4). Indeed, conditioning lesions profoundly upregulated ATF3, Stat3/Socs3 and other transcriptional regulators early and persistently after injury; these signaling pathways act independently of cAMP and have been implicated in neuritic growth (Cafferty, et al., 2004, Cao, et al., 2006, Liu and Snider, 2001, Qiu, et al., 2005, Seijffers, et al., 2006, Seijffers, et al., 2007). Supporting our findings, cAMP increases have been shown to lead to a decrease in GAP43 expression in adult DRG neurons in vitro, and rapid, reversible growth responses that are independent of transcriptional changes (Andersen, et al., 2000). In contrast, transcriptional responses are necessary for enhanced axonal growth after conditioning lesions (Smith and Skene, 1997). Pathway analysis of gene changes after conditioning lesions implicated p53 and jun signaling as two additional sets of gene networks implicated in regeneration (Di Giovanni, et al., 2006, Lindwall, et al., 2004, Raivich, et al., 2004) that are upregulated with conditioning lesions but not phosphodiesterase administration.
Taken together, these data suggest that conditioning lesions recruit a broader array of cellular mechanisms than cAMP-dependent signaling, resulting in greater neurite growth in vitro and axon growth in vivo.
In conclusion, the concerted activation of several integrated pathways might be necessary to initiate a complex program that enhances the intrinsic growth capacity of adult DRG neurons for regeneration after injury. While our experiments support a role for cAMP in axonal growth, they also indicate that conditioning lesions recruit mechanisms beyond cAMP increases that lead to superior axon regeneration, both in vitro and in vivo.
Highlights.
-
-
Conditioning lesions are effective after spinal cord injury in combination with NT-3 gene transfer
-
-
conditioning lesions have significant greater effects on axonal regeneration than increases in cAMP
-
-
Conditioning lesions activate more extensive transcriptional mechanisms than increases in cAMP
Supplementary Material
ACKNOWLEDGEMENTS
Supported by grants from the Veterans Administration, International Spinal Research Trust, the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation, the Bernard and Anne Spitzer Charitable Trust, Craig H. Neilson Foundation and the NIH (NS054833, NS049881, NS047101). We thank Fuying Gao for assistance with data analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Alto LT, Havton LA, Conner JM, Hollis Ii ER, Blesch A, Tuszynski MH. Chemotropic guidance facilitates axonal regeneration and synapse formation after spinal cord injury. Nat Neurosci. 2009;12:1106–1113. doi: 10.1038/nn.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen PL, Webber CA, Kimura KA, Schreyer DJ. Cyclic AMP prevents an increase in GAP-43 but promotes neurite growth in cultured adult rat dorsal root ganglion neurons. Exp Neurol. 2000;166:153–165. doi: 10.1006/exnr.2000.7485. [DOI] [PubMed] [Google Scholar]
- 3.Azizi SA, Stokes D, Augelli BJ, DiGirolamo C, Prockop DJ. Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats--similarities to astrocyte grafts. Proc Natl Acad Sci U S A. 1998;95:3908–3913. doi: 10.1073/pnas.95.7.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. 1995;57:289–300. [Google Scholar]
- 5.Bethea JR, Dietrich WD. Targeting the host inflammatory response in traumatic spinal cord injury. Curr Opin Neurol. 2002;15:355–360. doi: 10.1097/00019052-200206000-00021. [DOI] [PubMed] [Google Scholar]
- 6.Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19:185–193. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- 7.Bradbury EJ, Khemani S, Von R, King, Priestley JV, McMahon SB. NT-3 promotes growth of lesioned adult rat sensory axons ascending in the dorsal columns of the spinal cord. Eur J Neurosci. 1999;11:3873–3883. doi: 10.1046/j.1460-9568.1999.00809.x. [DOI] [PubMed] [Google Scholar]
- 8.Cafferty WB, Gardiner NJ, Das P, Qiu J, McMahon SB, Thompson SW. Conditioning injury-induced spinal axon regeneration fails in interleukin-6 knock-out mice. J Neurosci. 2004;24:4432–4443. doi: 10.1523/JNEUROSCI.2245-02.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai D, Deng K, Mellado W, Lee J, Ratan RR, Filbin MT. Arginase I and polyamines act downstream from cyclic AMP in overcoming inhibition of axonal growth MAG and myelin in vitro. Neuron. 2002;35:711–719. doi: 10.1016/s0896-6273(02)00826-7. [DOI] [PubMed] [Google Scholar]
- 10.Cai D, Qiu J, Cao Z, McAtee M, Bregman BS, Filbin MT. Neuronal cyclic AMP controls the developmental loss in ability of axons to regenerate. J Neurosci. 2001;21:4731–4739. doi: 10.1523/JNEUROSCI.21-13-04731.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai D, Shen Y, De Bellard M, Tang S, Filbin MT. Prior exposure to neurotrophins blocks inhibition of axonal regeneration by MAG and myelin via a cAMP-dependent mechanism. Neuron. 1999;22:89–101. doi: 10.1016/s0896-6273(00)80681-9. [DOI] [PubMed] [Google Scholar]
- 12.Cao Z, Gao Y, Bryson JB, Hou J, Chaudhry N, Siddiq M, Martinez J, Spencer T, Carmel J, Hart RB, Filbin MT. The cytokine interleukin-6 is sufficient but not necessary to mimic the peripheral conditioning lesion effect on axonal growth. J Neurosci. 2006;26:5565–5573. doi: 10.1523/JNEUROSCI.0815-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costigan M, Befort K, Karchewski L, Griffin RS, D'Urso D, Allchorne A, Sitarski J, Mannion JW, Pratt RE, Woolf CJ. Replicate high-density rat genome oligonucleotide microarrays reveal hundreds of regulated genes in the dorsal root ganglion after peripheral nerve injury. BMC Neurosci. 2002;3:16. doi: 10.1186/1471-2202-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Giovanni S, Knights CD, Rao M, Yakovlev A, Beers J, Catania J, Avantaggiati ML, Faden AI. The tumor suppressor protein p53 is required for neurite outgrowth and axon regeneration. Embo J. 2006;25:4084–4096. doi: 10.1038/sj.emboj.7601292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dinter H, Tse J, Halks-Miller M, Asarnow D, Onuffer J, Faulds D, Mitrovic B, Kirsch G, Laurent H, Esperling P, Seidelmann D, Ottow E, Schneider H, Tuohy VK, Wachtel H, Perez HD. The type IV phosphodiesterase specific inhibitor mesopram inhibits experimental autoimmune encephalomyelitis in rodents. J Neuroimmunol. 2000;108:136–146. doi: 10.1016/s0165-5728(00)00265-4. [DOI] [PubMed] [Google Scholar]
- 16.Filbin MT. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat Rev Neurosci. 2003;4:703–713. doi: 10.1038/nrn1195. [DOI] [PubMed] [Google Scholar]
- 17.Gao Y, Deng K, Hou J, Bryson JB, Barco A, Nikulina E, Spencer T, Mellado W, Kandel ER, Filbin MT. Activated CREB is sufficient to overcome inhibitors in myelin and promote spinal axon regeneration in vivo. Neuron. 2004;44:609–621. doi: 10.1016/j.neuron.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 18.Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, Hornik K, Hothorn T, Huber W, Iacus S, Irizarry R, Leisch F, Li C, Maechler M, Rossini AJ, Sawitzki G, Smith C, Smyth G, Tierney L, Yang JY, Zhang J. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 2004;5:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldberg JL, Klassen MP, Hua Y, Barres BA. Amacrine-signaled loss of intrinsic axon growth ability by retinal ganglion cells. Science. 2002;296:1860–1864. doi: 10.1126/science.1068428. [DOI] [PubMed] [Google Scholar]
- 20.Han PJ, Shukla S, Subramanian PS, Hoffman PN. Cyclic AMP elevates tubulin expression without increasing intrinsic axon growth capacity. Exp Neurol. 2004;189:293–302. doi: 10.1016/j.expneurol.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 21.Hendriks WT, Ruitenberg MJ, Blits B, Boer GJ, Verhaagen J. Viral vector-mediated gene transfer of neurotrophins to promote regeneration of the injured spinal cord. Prog Brain Res. 2004;146:451–476. doi: 10.1016/S0079-6123(03)46029-9. [DOI] [PubMed] [Google Scholar]
- 22.Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 23.Kadoya K, Tsukada S, Lu P, Coppola G, Geschwind D, Filbin M, Blesch A, Tuszynski MH. Combined intrinsic and extrinsic neuronal mechanisms facilitate bridging axonal regeneration one year after spinal cord injury. Neuron. 2009;64:165–172. doi: 10.1016/j.neuron.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kubo T, Yamashita T, Yamaguchi A, Hosokawa K, Tohyama M. Analysis of genes induced in peripheral nerve after axotomy using cDNA microarrays. J Neurochem. 2002;82:1129–1136. doi: 10.1046/j.1471-4159.2002.01060.x. [DOI] [PubMed] [Google Scholar]
- 25.Lindwall C, Dahlin L, Lundborg G, Kanje M. Inhibition of c-Jun phosphorylation reduces axonal outgrowth of adult rat nodose ganglia and dorsal root ganglia sensory neurons. Mol Cell Neurosci. 2004;27:267–279. doi: 10.1016/j.mcn.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Liu RY, Snider WD. Different signaling pathways mediate regenerative versus developmental sensory axon growth. J Neurosci. 2001;21:RC164. doi: 10.1523/JNEUROSCI.21-17-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu P, Jones LL, Tuszynski MH. Axon regeneration through scars and into sites of chronic spinal cord injury. Exp Neurol. 2007;203:8–21. doi: 10.1016/j.expneurol.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 28.Lu P, Yang H, Jones LL, Filbin MT, Tuszynski MH. Combinatorial therapy with neurotrophins and cAMP promotes axonal regeneration beyond sites of spinal cord injury. J Neurosci. 2004;24:6402–6409. doi: 10.1523/JNEUROSCI.1492-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mioduszewska B, Jaworski J, Kaczmarek L. Inducible cAMP early repressor (ICER) in the nervous system--a transcriptional regulator of neuronal plasticity and programmed cell death. J Neurochem. 2003;87:1313–1320. doi: 10.1046/j.1471-4159.2003.02116.x. [DOI] [PubMed] [Google Scholar]
- 30.Mukhopadhyay G, Doherty P, Walsh FS, Crocker PR, Filbin MT. A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron. 1994;13:757–767. doi: 10.1016/0896-6273(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 31.Murray AJ, Shewan DA. Epac mediates cyclic AMP-dependent axon growth, guidance and regeneration. Molecular and Cellular Neuroscience. 2008;38:578–588. doi: 10.1016/j.mcn.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 32.Neumann S, Bradke F, Tessier-Lavigne M, Basbaum AI. Regeneration of sensory axons within the injured spinal cord induced by intraganglionic cAMP elevation. Neuron. 2002;34:885–893. doi: 10.1016/s0896-6273(02)00702-x. [DOI] [PubMed] [Google Scholar]
- 33.Neumann S, Skinner K, Basbaum AI. Sustaining intrinsic growth capacity of adult neurons promotes spinal cord regeneration. Proc Natl Acad Sci U S A. 2005;102:16848–16852. doi: 10.1073/pnas.0508538102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neumann S, Woolf CJ. Regeneration of dorsal column fibers into and beyond the lesion site following adult spinal cord injury. Neuron. 1999;23:83–91. doi: 10.1016/s0896-6273(00)80755-2. [DOI] [PubMed] [Google Scholar]
- 35.Nikulina E, Tidwell JL, Dai HN, Bregman BS, Filbin MT. The phosphodiesterase inhibitor rolipram delivered after a spinal cord lesion promotes axonal regeneration and functional recovery. Proc Natl Acad Sci U S A. 2004;101:8786–8790. doi: 10.1073/pnas.0402595101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Norton WT, Poduslo SE. Myelination in rat brain: method of myelin isolation. J Neurochem. 1973;21:749–757. doi: 10.1111/j.1471-4159.1973.tb07519.x. [DOI] [PubMed] [Google Scholar]
- 37.Oudega M, Xu XM. Schwann cell transplantation for repair of the adult spinal cord. J Neurotrauma. 2006;23:453–467. doi: 10.1089/neu.2006.23.453. [DOI] [PubMed] [Google Scholar]
- 38.Pearse DD, Pereira FC, Marcillo AE, Bates ML, Berrocal YA, Filbin MT, Bunge MB. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat Med. 2004;10:610–616. doi: 10.1038/nm1056. [DOI] [PubMed] [Google Scholar]
- 39.Plunet W, Kwon BK, Tetzlaff W. Promoting axonal regeneration in the central nervous system by enhancing the cell body response to axotomy. J Neurosci Res. 2002;68:1–6. doi: 10.1002/jnr.10176. [DOI] [PubMed] [Google Scholar]
- 40.Popovich P, McTigue D. Damage control in the nervous system: beware the immune system in spinal cord injury. Nat Med. 2009;15:736–737. doi: 10.1038/nm0709-736. [DOI] [PubMed] [Google Scholar]
- 41.Qiu J, Cafferty WB, McMahon SB, Thompson SW. Conditioning injury-induced spinal axon regeneration requires signal transducer and activator of transcription 3 activation. J Neurosci. 2005;25:1645–1653. doi: 10.1523/JNEUROSCI.3269-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qiu J, Cai D, Dai H, McAtee M, Hoffman PN, Bregman BS, Filbin MT. Spinal axon regeneration induced by elevation of cyclic AMP. Neuron. 2002;34:895–903. doi: 10.1016/s0896-6273(02)00730-4. [DOI] [PubMed] [Google Scholar]
- 43.Raivich G, Bohatschek M, Da Costa C, Iwata O, Galiano M, Hristova M, Nateri AS, Makwana M, Riera-Sans L, Wolfer DP, Lipp HP, Aguzzi A, Wagner EF, Behrens A. The AP-1 transcription factor c-Jun is required for efficient axonal regeneration. Neuron. 2004;43:57–67. doi: 10.1016/j.neuron.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 44.Richardson PM, Issa VM. Peripheral injury enhances central regeneration of primary sensory neurones. Nature. 1984;309:791–793. doi: 10.1038/309791a0. [DOI] [PubMed] [Google Scholar]
- 45.Schwab ME. Nogo and axon regeneration. Curr Opin Neurobiol. 2004;14:118–124. doi: 10.1016/j.conb.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 46.Seijffers R, Allchorne AJ, Woolf CJ. The transcription factor ATF-3 promotes neurite outgrowth. Mol Cell Neurosci. 2006;32:143–154. doi: 10.1016/j.mcn.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 47.Seijffers R, Mills CD, Woolf CJ. ATF3 increases the intrinsic growth state of DRG neurons to enhance peripheral nerve regeneration. J Neurosci. 2007;27:7911–7920. doi: 10.1523/JNEUROSCI.5313-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 49.Smith DS, Skene JH. A transcription-dependent switch controls competence of adult neurons for distinct modes of axon growth. J Neurosci. 1997;17:646–658. doi: 10.1523/JNEUROSCI.17-02-00646.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stam FJ, MacGillavry HD, Armstrong NJ, de Gunst MC, Zhang Y, van Kesteren RE, Smit AB, Verhaagen J. Identification of candidate transcriptional modulators involved in successful regeneration after nerve injury. Eur J Neurosci. 2007;25:3629–3637. doi: 10.1111/j.1460-9568.2007.05597.x. [DOI] [PubMed] [Google Scholar]
- 51.Tanabe K, Bonilla I, Winkles JA, Strittmatter SM. Fibroblast growth factor-inducible-14 is induced in axotomized neurons and promotes neurite outgrowth. J Neurosci. 2003;23:9675–9686. doi: 10.1523/JNEUROSCI.23-29-09675.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taylor L, Jones L, Tuszynski MH, Blesch A. Neurotrophin-3 gradients established by lentiviral gene delivery promote short-distance axonal bridging beyond cellular grafts in the injured spinal cord. J Neurosci. 2006;26:9713–9721. doi: 10.1523/JNEUROSCI.0734-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vaccarino FM, Hayward MD, Le HN, Hartigan DJ, Duman RS, Nestler EJ. Induction of immediate early genes by cyclic AMP in primary cultures of neurons from rat cerebral cortex. Brain Res Mol Brain Res. 1993;19:76–82. doi: 10.1016/0169-328x(93)90151-e. [DOI] [PubMed] [Google Scholar]
- 54.Yang L, Zhang FX, Huang F, Lu YJ, Li GD, Bao L, Xiao HS, Zhang X. Peripheral nerve injury induces trans-synaptic modification of channels, receptors and signal pathways in rat dorsal spinal cord. Eur J Neurosci. 2004;19:871–883. doi: 10.1111/j.0953-816x.2004.03121.x. [DOI] [PubMed] [Google Scholar]
- 55.Yukawa K, Tanaka T, Tsuji S, Akira S. Expressions of CCAAT/Enhancer-binding proteins beta and delta and their activities are intensified by cAMP signaling as well as Ca2+/calmodulin kinases activation in hippocampal neurons. J Biol Chem. 1998;273:31345–31351. doi: 10.1074/jbc.273.47.31345. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.