Abstract
Although down-regulation of GNG7 in cancer was reported before, its role in carcinogenesis is poorly understood. It belongs to a family of large G-proteins that may be involved in cell-contact-induced growth arrest and function in tumor suppression. In the present study, we stained immunohistochemically 188 tumors derived from larynx or floor of the mouth for GNG7 protein and confronted it with clinicopathologic data. Moreover, we performed bisulfite pyrosequencing to analyze GNG7 promoter methylation. We identified recurrent loss of GNG7 protein expression in 68/188 (36%) cases and promoter hypermethylation in (42/98; 43%) primary tumors, predominantly in young patients (p < 0.001). Loss of GNG7 expression correlated with hypermethylation of GNG7 promoter region (p < 0.001). Moreover, loss of GNG7 protein expression correlated with tumor size (p = 0.012) and lack of cervical metastasis (p = 0.02) whereas sustained expression correlated with keratinization (p = 0.008). Taken together, loss of GNG7 protein expression is a frequent event in head and neck cancer. Moreover, our data suggest that hypermethylation of the promoter region of GNG7 is probably the mechanism of the observed inactivation.
Keywords: Bisulfite pyrosequencing, GNG7, Head and neck cancer, Keratinization, Young adults
Introduction
The guanine nucleotide binding protein 7 belongs to the large G protein gamma family with GTPase activity that is involved in transmembrane signalling pathways. The involvement of small nucleotide-binding proteins like RAS or RHO in malignant transformation is well established (Bos 1989; Symons 1995). In contrast, the role of large G proteins in carcinogenesis is poorly understood. However, down-regulation of GNG7 in pancreatic and gastrointestinal cancers was reported more than a decade ago (Shibata et al. 1998; 1999). Additional evidence supporting the tumor suppressor functionality of this gene was obtained recently (Ohta et al. 2008). Whether the observed downregulation of GNG7 is restricted only to esophageal cancer as reported by Ohta et al. or extends also to head and neck tumors of squamous epithelial origin remains so far unknown (Ohta et al. 2008).
It has been speculated that GNG7 might be involved in cell contact induced growth arrest and thus block uncontrolled cell proliferation in multicellular organisms (Shibata et al. 1999). In light of this hypothesis, cells encountering other cells would stop proliferating and start a differentiation process that is mediated via G protein signalling. A similar process has been described in invertebrates (Saccharomyces cerevisiae) where it has been shown that G proteins mediate cell contact induced growth arrest in a mating pheromone-response pathway (Fujimura 1989; Nomoto et al. 1990).
We recently performed RNA expression profiling in ten laryngeal cancer cell lines and three non-cancerous controls using U133 plus 2.0 microarrays (Giefing et al. 2011). Although, GNG7 was not among the candidate genes investigated in that study we used the available expression profiles to analyze its expression on mRNA level. In this analysis, transcriptional downregulation of GNG7 (p < 0.01) in tumor cell lines as compared to controls (normal squamous epithelium) was observed. This downregulation was further confirmed by quantitative reverse transcription PCR on RNA from cell lines as compared to non-cancerous controls (unpublished data).
Hence, we addressed the question if the loss of GNG7 protein expression and its prognostic significance as suggested by Ohta et al. (2008) for esophageal cancer could be extended to the anatomically and histologically similar tumors of the head and neck. Therefore we investigated GNG7 protein expression by immunohistochemistry in 188 primary tumor samples from larynx and floor of the mouth. In addition, we investigated whether hypermethylation of GNG7 promoter region might be responsible for the observed expression silencing.
Materials & methods
Paraffin sections evaluated for protein expression (group A)
Altogether 116 primary squamous cell carcinoma of the larynx and 72 squamous cell carcinomas of the floor of the mouth sections were collected with corresponding clinicopathologic data of the patients. The sections were obtained from the Head and Neck Department of the University Hospital Eppendorf Hamburg, Germany; the Department of Clinical Pathomorphology, Collegium Medicum in Bydgoszcz, Poland and the Senckenberg Institute of Pathology, University of Frankfurt, Germany and stained toward GNG7 protein expression. DNA from 15 of the mentioned paraffin embedded primary laryngeal samples were isolated and evaluated toward GNG7 promoter hypermethylation as described below.
Ethical approvals have been obtained from the Ethical Commissions of the Medical Council in Hamburg, Germany and the L. Rydygiers’ Collegium Medicum in Bydgoszcz, Poland. All clinicopathologic data are summarized in Table 1.
Table 1.
Clinicopathologic data of the analyzed head and neck tumors
| GNG7 protein expression analysis (group A) | GNG7 promoter methylation analysis (group B) | |||
|---|---|---|---|---|
| (Number of patients and the % in the analysis) | (Number of patients and the % in the analysis) | |||
| Total No. of patients | 188 | 98 | ||
| Localization | Larynx 116 (62%) | Floor of mouth 72 (38%) | Larynx 98 (100%) | |
| Age (years) | age ≤45; 44 (45%) | age >45; 54 (55%) | ||
| median | 61 | n.a. | 43 | 66 |
| range | (38–87) | n.a. | 34-45 | 46-79 |
| Gender | ||||
| male | 82 (85%) | 49 (69%) | 38 (86%) | 52 (96%) |
| female | 14 (15%) | 22 (31%) | 6 (14%) | 2 (4%) |
| not available | 20 | 1 | 0 | 0 |
| T classification (size) | ||||
| pT1 | 4 (7%) | 21 (30%) | 5 (11%) | 0 (0%) |
| pT2 | 4 (7%) | 22 (31%) | 9 (21%) | 5 (9%) |
| pT3 | 32 (53%) | 15 (21%) | 19 (43%) | 32 (59%) |
| pT4 | 20 (33%) | 13 (18%) | 11 (25%) | 17 (32%) |
| not available | 56 | 1 | 0 | 0 |
| N classification (cervical metastases) | ||||
| N = 0 | 73 (78%) | 32 (45%) | 30 (68%) | 36 (67%) |
| N = 1 | 12 (13%) | 15 (21%) | 5 (11%) | 11 (20%) |
| N = 2 | 5 (5%) | 22 (31%) | 9 (21%) | 7 (13%) |
| N = 3 | 4 (4%) | 2 (3%) | 0 | 0 |
| not available | 22 | 1 | 0 | 0 |
| G classification (grading) | ||||
| G1 | 9 (10%) | 3 (4%) | 11 (28%) | 19 (39%) |
| G2 | 67 (71%) | 52 (73%) | 28 (70%) | 28 (57%) |
| G3 | 18 (19%) | 15 (21%) | 1 (2%) | 2 (4%) |
| G4 | 0 (0%) | 1 (1%) | 0 (0%) | 0 (0%) |
| not available | 22 | 1 | 4 | 5 |
| Overall survival, months | ||||
| mean | 45 | 41 | 52 | 46 |
| median | 44 | 19 | 56 | 37 |
| range | (1–121) | (1–179) | (3–129) | (0–93) |
| Smoking habit | ||||
| smoker | 32 (94%) | n.a. | 41 (93%) | 43 (83%) |
| non smoker | 2 (6%) | n.a. | 3 (7%) | 9 (17%) |
| not available | 82 | n.a. | 0 | 2 |
n.a. - not available
Controls, primary samples and cell lines evaluated for GNG7 promoter hypermethylation (group B)
Eight buccal swabs (epithelial cells) samples were collected from inner oral cavity using medical wooden sticks from eight healthy volunteers for control purposes. Ninety-eight primary laryngeal squamous cell carcinoma samples were obtained from the Department of Otolaryngology, University of Medical Sciences in Poznan, Poland. Within this group 44 samples derived from young adult laryngeal cancer patients defined as age ≤45 years. Approvals from the Ethical Commission at the Medical University in Poznan were obtained. The tumor tissues were assessed histopathologically to confirm the presence of at least 80% tumor cell content. Clinicopathologic data of the patients is summarized in Table 1. Moreover, DNA from 13 laryngeal squamous cell carcinoma cell lines (LSCC) (UT-SCC-6A, UT-SCC-11, UT-SCC-19B, UT-SCC-22, UT-SCC-29, UT-SCC-34, UT-SCC-35, UT-SCC-38, UT-SCC-42B, UT-SCC-57, UT-SCC-106A, UT-SCC-107, UT-SCC-116) characterized elsewhere was used (Giefing et al. 2011).
DNA from buccal swabs was isolated using High Pure PCR Template Preparation Kit (Roche, Mannheim, Germany) and eluted with 200 μl sterile water (instead of elution buffer delivered in the kit). Paraffin embedded samples were deparaffinized by xylene washing, then hydrated and homogenized prior to DNA isolation. Thereafter, DNA was isolated from all samples according to the standard procedure (proteinase K digestion, phenol/chloroform extraction and ethanol precipitation). For pyrosequencing, 1μg DNA from each sample was bisulfite converted using the EpiTect DNA Modification Kit supplied by Qiagen (Qiagen, Hilden, Germany).
Immunohistochemical staining of samples from group A
GNG7 immunohistochemical staining was performed using a polyclonal rabbit antibody (sc-377, Santa Cruz Biotechnology, Santa Cruz, USA, dilution 1:100). Tissue sections were heat pretreated and incubation of the primary antibody occurred at 4°C overnight. Antibody binding was detected using the Dako Envision Detection System (DAKO, Glostrup, Denmark). Normal squamous epithelium was analyzed for the physiological pattern of GNG7 protein expression. As control for specificity, sections of reactive tonsils incubated with the corresponding blocking peptide (sc-377P, Santa Cruz Biotechnology, Santa Cruz, USA) were used. All slides were evaluated by two pathologists on a multihead microscope.
The cases were assigned to three groups based on the intensity of GNG7 protein expression. Group one consisted of negative (−) cases where no specific staining for GNG7 protein was observed; group two were positive (+) cases with weak staining pattern (weak granular cytoplasmic staining) and group three were cases with an intense staining (++, strong homogeneous cytoplasmic staining) reflecting high expression of the protein. If several staining patterns occurred in one tumor, the dominating staining pattern was assessed.
Bisulfite pyrosequencing of samples from group B
Primers for GNG7 bisulfite pyrosequencing (Forward: GAGAGGTTTTTTAGGGTGATT; Reverse-5’biotin: TCTTCCCCAACAAATAAAC; Sequencing: GTTTTTTAGGGTGATTT) were designed with PyroMark Assay Design Software 1.0 (Biotage, Uppsala, Sweden). Primers were verified for potential SNPs occurrence within primer binding region.
PCR reactions were run using PyroMark kit (Qiagen, Germany) with the following program: 95°C for 15 min (HotStarTaq DNA Polymerase); 45x: 94°C for 30 s; 55°C for 30 s; 72°C for 30 and final 72°C for 10 min. PCR products were run on 2% agarose gel stained with ethidium bromide and visualized under UV light (Bioanalyzer, Biometra, Göttingen, Germany).
PCR products were purified prior to pyrosequencing using a water pump station and the following buffers: 70% EtOH, 0.2% NaOH and Washing buffer (according manufacturer’s instructions) (Qiagen, Hilden, Germany). After a final denaturation step at 85°C for 2 min sequencing primer was hybridized. Pyrosequencing was performed using the Pyrosequencer PyroMark ID and the DNA methylation analysis software Pyro Q-CpG 1.0.9 (Biotage, Uppsala, Sweden), which was also used to evaluate the ratio T:C (mC:C) at the CpG site analyzed.
For each pyrosequencing reaction two bisulfite treated controls were included: the totally methylated DNA (Millipore, Hilden, Germany) and the pooled DNA (mix of DNA from 20 peripheral blood from healthy donors, 10 male and 10 female).
Statistical analysis – protein expression in samples form group A
Statistical calculations have been performed using the SPSS software v.17.0 (SPSS Inc. Chicago, USA). To differentiate the cases depending on GNG7 protein expression we selected the GNG7(−) and the GNG7(++) groups for the calculations. Only in the case of keratinization - to assure a sufficient number of cases for the analysis - we compared the GNG7(−) against the merged GNG7(+/++) group. The Kaplan-Meier survival analysis has been used to analyze the difference in survival time between head and neck cancer patients depending on the localization of the tumor (larynx or floor of the mouth) and depending on GNG7 expression. The Chi-square crosstabulation test has been used to test for the correlation between GNG7 expression and the T, N, G classification parameters and keratinization. The number of samples used in various statistical calculations might differ for the numbers given above as complete clinicopathologic data was not available in all samples.
Statistical analysis – GNG7 methylation in samples from group B
To calculate a cut-off value for methylation, bisulfite treated DNA from eight buccal swabs obtained from healthy volunteers were pyrosequenced. The mean methylation value for these samples was 2% and standard deviation (SD) was 1%. The cut-off was set by summing the mean methylation for the buccal swabs samples (2%) and three times the SD and was 5%. All samples for which the methylation value level was >5% were assigned as methylated. Statistical calculations have been performed using the SPSS software v.18.0 (SPSS Inc. Chicago, USA). The Chi-square crosstabulation test was used to analyze the correlation between GNG7 methylation and age.
For further statistical analyses the 44 samples of young adult patients with different clinical parameters were excluded. In the remaining samples from group B the Kaplan-Meier test has been used to analyze the difference in survival time depending on GNG7 methylation. Moreover, the Chi-square crosstabulation test has been used to analyze the correlation between GNG7 methylation and the T, N, G classification and smoking addiction.
For cell lines, with “significantly downregulated” we defined significant reduction of GNG7 expression using the Wilcoxon signed rank test versus at least 2/3 non-tumor laryngeal controls as described in detail in Giefing et al. (2011). To correlate GNG7 expression depending on its methylation status for cell lines where the GNG7 mRNA expression was known and the 15 samples analyzed both for GNG7 protein expression and promoter hypermethylation the Chi-square crosstabulation test was used. The number of samples used in various statistical calculations might differ for the numbers given above as complete clinicopathologic data was not available for each patient.
Results
Loss of GNG7 expression on protein level correlates with advanced tumor size, keratinization and low metastatic potential (group A)
First, we analyzed normal squamous epithelium for the physiological pattern of GNG7 expression. We observed a weak, granular cytoplasmic GNG7 expression in the spinous layer with an enhanced cytoplasmic expression in the superficial layer and granular layer, if present (Fig. 1a, arrow). In reactive tonsils germinal centers and the keratinizing parts of the squamous epithelium stained positive. To analyze the specificity of the GNG7 staining we incubated reactive tonsils with the corresponding blocking peptide. In consequently immunostained tonsils no staining was observed demonstrating the specificity of the reaction.
Fig. 1.
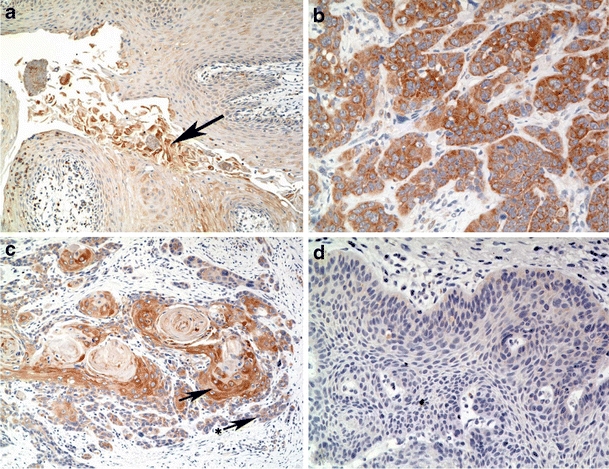
GNG7 immunostaining. a. Reactive squamous epithelium showing GNG7 expression in keratinizing cells (arrow). b. Well differentiated squamous cell carcinoma showing strong expression of GNG7. c. Keratinizing squamous cell carcinoma showing strong expression of GNG7 in keratinizing cells (arrow) and weak expression in the more basaloid differentiated cells (arrow with *). d. Poorly differentiated, non-keratininzing basaloid squamous cell carcinoma, GNG7-negative. (All images 200× magnification)
Thereafter, we stained immunohistochemically for GNG7 protein in 188 primary head and neck tumors. Thirty one cases showed an intense staining pattern (++), 89 cases presented weak GNG7 protein expression (+) and in 68 cases no expression was observed (−) (Fig. 1). Keratinizing tumors often showed an intense staining pattern around central keratin pearls (Fig. 1c). Reactive squamous epithelium adjacent to the tumors showed an inconsistent expression of GNG7 protein which was particularly high in cells showing keratinization.
No statistically significant difference between overall survival and the localization of the tumors in larynx or floor of the mouth was observed. As a consequence, the highly clinicopathologically similar groups were merged for further analyses. Moreover, no difference in the overall survival depending on GNG7 protein expression in laryngeal and floor of the mouth tumors together was observed.
Significant correlation between loss of GNG7 protein expression and tumor size (pT) was identified (p = 0.012). Loss of GNG7 protein expression was more frequent in the pT3/4 tumors. In detail, out of 40 GNG7(−) tumors, 31 (77%) were pT3/4. Similarly, the GNG7(−) cases were overrepresented in non cervically metastasing tumors, where 47/63 (75%) GNG7(−) cases showed no cervical metastases (p = 0.02). These findings suggest that the loss of GNG7 protein expression is characteristic to large tumors, not metastasing to cervical lymph nodes. No significant correlation was observed between GNG7 staining and tumor grade.
Finally, a highly significant correlation was identified between keratinization and GNG7 protein expression (p = 0.008) showing GNG7(+) and GNG7(++) expression together to be characteristic for keratinizing tumors. Therein, 21/29 (72%) GNG7 (+/++) positive tumors showed keratinization (p = 0.008). The results are summarized in Table 2.
Table 2.
Results of statistically significant tests
| Analysis | Results (number of patients and the % in the analysis) | p value | |||
|---|---|---|---|---|---|
| GNG7 protein expression analysis (group A) | GNG7 expression (−) vs (++) depending on the T stage (T = 1/2 vs T = 3/4) | T = 1/2 | T = 3/4 | 0.012* | |
| GNG7 (−) n = 40 | 9 (23%) | 31 (77%) | |||
| GNG7 (++) n = 22 | 12 (55%) | 10 (45%) | |||
| GNG7 expression (−) vs (++) depending on the N status (N = 0 vs N = 1/2/3) | N = 0 | N = 1/2/3 | 0.02* | ||
| GNG7 (−) n = 63 | 47 (75%) | 16 (25%) | |||
| GNG7 (++) n = 23 | 11 (48%) | 12 (52%) | |||
| GNG7 expression (−) vs (+) and (++) depending on keratinization (−) vs (+) | K (−) | K (+) | 0.008* | ||
| GNG7 (−) n = 27 | 17 (63%) | 10 (37%) | |||
| GNG7 (+/++) n = 29 | 8 (28%) | 21 (72%) | |||
| GNG7 promoter methylation analysis (group B) | GNG7 nonmethylated vs methylated depending on the age of patients (age ≤45 vs age >45) | age ≤45 n = 44 | age >45 n = 54 | < 0.001* | |
| GNG7 unmethylated | 15 (34%) | 41 (76%) | |||
| GNG7 methylated | 29 (66%) | 13 (24%) | |||
| GNG7 expression and promoter methylation | Loss of GNG7 expression vs methylation status | NO loss of expression | Loss of expression | < 0.001* (Yates' corrected) | |
| GNG7 unmethylated | 12 | 0 | |||
| GNG7 methylated | 3 | 10 | |||
T - size of the tumor
N = 0 - non nodular metastases
N = 1/2/3 - nodular metastases present
K(−) - no keratinization observed
K(+) - keratinization present
*statistically significant
GNG7 is frequently hypermethylated in cell lines and primary tumor samples (group B)
To analyze the methylation level in non-cancerous tissue we first pyrosequenced eight buccal swaps samples (oral squamous epithelial cells) collected from healthy volunteers. Based on these results we have established the cut-off for methylation to 5% as described in the materials and methods section. Applying this cut-off to the tumor samples pyrosequencing results we identified hypermethylation of the GNG7 promoter region in 8/13 (62%) laryngeal cancer cell lines and 42/98 (43%) primary tumors.
GNG7 promoter hypermethylation correlates with loss of GNG7 expression
To analyze if GNG7 mRNA expression in LSCC cell lines depends on promoter hypermethylation we used U133 plus 2.0 microarray expression profiles from our recent study Giefing et al. (2011). GNG7 expression and methylation levels were compared in the very same cancer cell lines. In detail, expression profiles were available for 10 laryngeal cancer cell lines (UT-SCC-6A, UTSCC-11, UT-SCC-19B, UT-SCC-22, UT-SCC-29, UT-SCC-34, UT-SCC-57, UT-SCC-106A, UT-SCC-107, UT-SCC-116) out of which significant downregulation of GNG7 (expression tag 206896_s_at) was observed in six cell lines (UTSCC-11, UT-SCC-19B, UT-SCC-22, UT-SCC-29, UT-SCC-106A, UT-SCC-116) compared to non-tumor laryngeal tissues. GNG7 promoter region hypermethylation was identified in all six cell lines and additionally in the UT-SCC-107 cell line. Moreover, DNA from 15 paraffin embedded samples stained for GNG7 protein expression in group A were analyzed toward GNG7 promoter methylation by pyrosequencing. All four samples negative for GNG7 protein expression were hypermethylated whereas out of the 11 GNG7 expressing cases we identified hypermethylation only in two. Taking together, significant correlation between loss of GNG7 expression and GNG7 promoter hypermethylation was observed (Yates’ corrected p < 0.001) (Table 2).
No correlation observed between GNG7 methylation and overall survival, TNM classification or smoking habit (group B excluding 44 young patients)
To look for correlation between GNG7 methylation and the overall survival of patients, we performed a Kaplan-Meier test but no correlation was observed. Similarly no correlation between the TNM classification or the smoking habit and methylation was observed.
GNG7 is hypermethylated predominantly in young patients
Last, we addressed the question if there are differences in the frequency and level of GNG7 methylation between the 44 samples derived from young adult laryngeal cancer patients and the 54 samples from patients age >45. We observed in the cohort of young patients a significantly enriched number of cases with GNG7 hypermethylation (29/44 (66%) hypermethylated cases) (chi-square tests p < 0.001) compared to the group of older patients (13/54 (24%) hypermethylated cases) (Table2). In addition, samples derived from young patients were not only more frequently methylated but also the mean methylation level of 27% was higher as compared to 18% in the samples from older patients (Fig. 2).
Fig. 2.
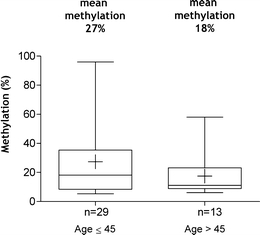
Young adult patients show higher mean methylation of GNG7 promoter region than older patients. “+” – mean methylation
Discussion
In this study we immunohistochemically stained 188 tumors from head and neck cancer derived from larynx and floor of the mouth for the GNG7 protein. Overall, we identified loss of GNG7 expression in 68/188 cases (36%) and showed that it is a recurrent event in head and neck cancer. Moreover, in an independent cohort of laryngeal tumor samples we detected recurrent hypermethylation of the GNG7 promoter region in 42/98 (43%) primary tumors. It is noteworthy that in a cohort of laryngeal cancer cell lines and 15 primary samples the downregulation of GNG7 gene expression significantly correlates with hypermethylation (p < 0.001). This suggests that at least for a subset of cases hypermethylation is the mechanism behind the observed loss of GNG7 expression. This is further stressed by the fact that no mutations in the coding exons of GNG7 were identified in a mutation screen in ten laryngeal cancer cell lines (data not shown).
In contrast to the recent findings of Ohta et al. concerning esophageal cancer, we did not observe correlation neither of GNG7 protein expression with overall survival nor for GNG7 methylation with overall survival of the patients diagnosed with head and neck cancer (Ohta et al. 2008). However, since no data on the treatment of the patients were available the discrepancy might reflect different treatment modalities of the head and neck cancer patients as compared to esophageal cancer patients in the two studies.
Regarding the clinicopathologic parameters, we found a statistically significant correlation between loss of GNG7 protein expression and the size of the tumor. Large tumors (pT3/4) showed more frequent loss of expression. Likewise, Ohta et al. (2008) reported that loss of GNG7 expression correlates with the depth of tumor invasion and aggressiveness.
It is tempting to speculate that loss of GNG7 protein could be a marker for tumor progression as we detected also a highly significant correlation between GNG7 protein expression and keratinization. Keratinization is usually present in well differentiated tumors and lost during progression which could be a predictor of early recurrence as presented recently by Pinto et al. (2010). On the other hand, we observed no correlation between GNG7 protein expression and the grading parameter G.
Besides, we found a correlation between loss of GNG7 protein expression and the absence of cervical metastases. In parallel, we show that loss of GNG7 protein was associated with large tumors of pT3/4 stage. This is surprising, as in the investigated cohort the pT3/4 tumors show more frequently cervical metastases than the pT1/2 tumors. Thus, loss of GNG7 defines probably a subgroup of locally advanced tumors with low metastatic potential. One should of course note that a different mechanism might be responsible for local metastasis with no relation to GNG7.
Last, we show that GNG7 promoter methylation is significantly more frequent in young adult patients age ≤45 as compared to patients age >45 (p < 0.001). Interestingly, O'Regan et al. showed that promoter hypermethylation of the most frequently inactivated tumor suppressor gene in head and neck cancer namely CDKN2A is a more common event in patients younger than 40 years than in older patients where genomic deletion was more common (O'Regan et al. 2008). Taken together, this might suggest that epigenetic silencing of tumor suppressor genes is more prevalent in young patients and with age shifts toward genomic loss in line with the general observation that genomic instability increases with age.
In summary we show here that loss of GNG7 protein expression is a frequent event in head and neck cancer. Our results indicate moreover, that GNG7 protein expression is lost predominantly in advanced T stage tumors with low metastatic potential. Besides, we suggest epigenetic silencing as the mechanism of GNG7 gene inactivation which might be especially true for young adult patients.
Acknowledgements
Supported by the Polish Ministry of Science and Higher Education grant N301 238736 and Förderung Nachwuchsforscher der Goethe Universität Frankfurt (S.H.). M.G. was supported by the FEBS Long-term fellowship and the “Support for International Mobility of Scientists” fellowship of the Polish Ministry of Science and Higher Education. M. Sz. was supported by the EMBO short-term fellowship. I.S was supported by the Alexander von Humboldt Foundation fellowship.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Bos JL. Ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682–4689. [PubMed] [Google Scholar]
- Fujimura HA. The yeast G-protein homolog is involved in the mating pheromone signal transduction system. Mol Cell Biol. 1989;9:152–158. doi: 10.1128/mcb.9.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giefing M, Zemke N, Brauze D, Kostrzewska-Poczekaj M, Luczak M, Szaumkessel M, Pelinska K, Kiwerska K, Tönnies H, Grenman R, Figlerowicz M, Siebert R, Szyfter K, Jarmuz M. High resolution ArrayCGH and expression profiling identifies PTPRD and PCDH17/PCH68 as tumor suppressor gene candidates in laryngeal squamous cell carcinoma. Genes Chromosomes Cancer. 2011;50:154–166. doi: 10.1002/gcc.20840. [DOI] [PubMed] [Google Scholar]
- Nomoto S, Nakayama N, Arai K, Matsumoto K. Regulation of the yeast pheromone response pathway by G protein subunits. EMBO J. 1990;9:691–696. doi: 10.1002/j.1460-2075.1990.tb08161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta M, Mimori K, Fukuyoshi Y, Kita Y, Motoyama K, Yamashita K, Ishii H, Inoue H, Mori M. Clinical significance of the reduced expression of G protein gamma 7 (GNG7) in oesophageal cancer. Br J Cancer. 2008;98:410–417. doi: 10.1038/sj.bjc.6604124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Regan EM, Toner ME, Finn SP, Fan CY, Ring M, Hagmar B, Timon C, Smyth P, Cahill S, Flavin R, Sheils OM, O'Leary JJ. p16(INK4A) genetic and epigenetic profiles differ in relation to age and site in head and neck squamous cell carcinomas. Hum Pathol. 2008;39:452–458. doi: 10.1016/j.humpath.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Pinto LS, de Aguiar FC, Jr KLP, Graner E, Lopes MA. FAS and ErbB2 expression in early local recurrent oral cancer. J Oral Pathol Med. 2010;39:176–181. doi: 10.1111/j.1600-0714.2009.00822.x. [DOI] [PubMed] [Google Scholar]
- Shibata K, Mori M, Tanaka S, Kitano S, Akiyoshi T. Identification and cloning of human G-protein gamma 7, down-regulated in pancreatic cancer. Biochem Biophys Res Commun. 1998;246:205–209. doi: 10.1006/bbrc.1998.8581. [DOI] [PubMed] [Google Scholar]
- Shibata K, Tanaka S, Shiraishi T, Kitano S, Mori M. G-protein gamma 7 is down-regulated in cancers and associated with p 27kip1-induced growth arrest. Cancer Res. 1999;59:1096–1101. [PubMed] [Google Scholar]
- Symons M. The Rac and Rho pathways as a source of drug targets for Ras-mediated malignancies. Curr Opin Biotechnol. 1995;6:668–674. doi: 10.1016/0958-1669(95)80110-3. [DOI] [PubMed] [Google Scholar]


