Abstract
Over forty-five complex free oligosaccharides (of which several are novel) have been isolated and chemically characterized by gas chromatography and high resolution and high mass accuracy matrix-assisted laser desorption/ionization mass spectrometry (MALDI-FTICR MS) in red and white wines, Grignolino and Chardonnay, respectively. Oligosaccharides with a degree of polymerization between 3 and 14 were separated from simple monosaccharides and disaccharides by solid-phase extraction. The concentrations free oligosaccharides were over 100 mg/L in both red and white wines. The free oligosaccharides—characterized for the first time in the present study include hexose-oligosaccharides, xyloglucans and arabinogalactans, and may be the natural by-products of the degradation of cell wall polysaccharides. The coupled gas chromatography and accurate mass spectrometry approach revealed an effective method to characterize and quantify complex functional oligosaccharides in both red and white wine.
Keywords: Oligosaccharides, wine, MALDI-FTICR, hexose oligosaccharides, xyloglucans, arabinogalactans
Introduction
The macromolecules of wines include polyphenols, proteins, and polysaccharides. Polysaccharides have been thoroughly studied because of their important technological and sensory properties in wines. Polysaccharides have the ability to interact and aggregate with tannins (1), to decrease astringency in wine-like model solutions (2), to inhibit hydrogen tartrate crystallization (3), to interact with wine aroma compounds (4), and to form specific coordination complexes with Pb2+ ions (5, 6). The structures and amounts of polysaccharides released into wines depend on the specific wine-making process and can be modified by enzymatic treatment (7, 8). Unlike wine polysaccharides, which have been the subject of many studies, oligosaccharides from wines have not been isolated and characterized, with the exception of a recent work focused on Carignan and Merlot red wines (9).
Oligosaccharides are strictly defined as non-digestible carbohydrates that contain between three and fifteen monosaccharide residues covalently linked through glycosidic bonds. Oligosaccharides are neither digested nor absorbed in the upper intestinal tract of humans and are delivered intact into the colon, where they can act as nutrients for colonic microflora (10, 11). Oligosaccharides are divided into two broad classes, neutral and acidic. Neutral oligosaccharides do not contain charged anionic residues, whereas acidic oligosaccharides contain one or more negatively charged residues such as sialic acids (12, 13).
The study of oligosaccharides has so far been focused almost exclusively on animal milks, with hundreds of articles published just on the topic of human milk oligosaccharides (14-16). Oligosaccharides and polysaccharides exhibit both high structural specificity, much as do proteins and polynucleotides, and complexity of structure and function. The structure-function properties of oligosaccharides are being studied—much the same as are those of proteins—as bioactive components that aid intestinal functions in humans (17-19). One of the most studied and well-demonstrated actions of oligosaccharides is the prebiotic activity (20). It is thus necessary to determine the exact composition of oligosaccharides in wine and to analyze their molecular structures in order to understand their organoleptic and bioactive properties.
Classical structural characterization of oligosaccharides has been obtained by combining enzymatic digestions based on glycosyl hydrolases with techniques such as nuclear magnetic resonance (NMR) or mass spectrometry (MS) in order to provide insight into their structural complexity (17, 18). The analysis of oligosaccharides by MS in complex matrices has been made possible by the development of soft ionization techniques such as the matrix-assisted laser desorption ionization (MALDI). The matrix-assisted laser desorption/ionization Fourier transform ion cyclotron resonance mass spectrometry (MALDI-FTICR MS) method is a sensitive and robust analytical method with high performance capability, and it allows rapid and unambiguous assignment of oligosaccharide signals (21).
The aim of the present study was to demonstrate the presence in significant amounts of complex free oligosaccharides in red and white wine using a combination of gas chromatography (GC) and MALDI-FTICR MS.
Materials and Methods
Samples
Grignolino (vintage 2008) and Chardonnay (vintage 2009) wines were purchased, respectively, from retail stores in Italy and California, USA. All monosaccharide standards (glucose, xylose, arabinose, galactose, galacturonic acid, mannose, rhamnose, glucuronic acid, and fucose) were purchased from Sigma–Aldrich (Milan, Italy).
Sample preparation for MS analysis
Wine samples were first concentrated (from 3 L to 0.5 L) using a rotatory-evaporator and then purified by solid-phase extraction (SPE). Wine contains only trace amounts of lipids and protein, and therefore, does not require delipidation and deproteinization processes usually necessary for oligosaccharide characterization by MS in other food matrices (22). However, to ensure proper oligosaccharide identification by MS, a two-step, solid-phase extraction was performed using a SPE C-18 cartridge to eliminate interfering substances such as proanthocyanidins and anthocyanins. Then a SPE carbograph was applied to remove residual salts and monosaccharides that would interfere with MS analysis. C-18 cartridges (3-mL SupelCleanTM LC-18 SPE tubes, Supelco, PA, USA) were conditioned with three volumes of acetonitrile (ACN) and three volumes of water.
Nonporous graphitized carbon cartridges (150 mg carbon, 4-mL tube capacity, Alltech, Deerfield, IL, USA) were conditioned following the protocol described by Ninonuevo et al. (21). The oligosaccharides retained by the graphitized carbon were then eluted stepwise with two cartridge volumes of an 80:20 deionized water–ACN solution, and two cartridge volumes of a 60:40 deionized water–ACN solution containing 0.1% trifluoroacetic acid. Each fraction was dried in a vacuum centrifuge (automatic environmental Speedvac system AES 2010, Thermo Savant, Holbrook, NY, USA) and 20 μL of deionized water was added to re-suspend the dry oligosaccharide powder prior to MS analysis.
MALDI-FTICR MS analysis
MALDI-FTICR MS was used for chemical characterization. Mass spectra were recorded on an IonSpec Corporation ProMALDI FTICR MS instrument (Lake Forest, California, USA) equipped with a 7.0 T actively shielded superconducting magnet and an external MALDI source capable of hexapole ion accumulation and fitted with a pulsed Nd:YAG laser (355 nm). External accumulation of ions produced by 27 MALDI laser pulses was used to obtain optimum total ion intensity for each sample analyzed. Tandem MS was performed using a collision-induced dissociation (CID) method. Malto-oligosaccharides isolated from beer were used to calibrate the instrument and as a molecular reference standard for oligosaccharides consisting of hexose (Hex) residues. The instrumental conditions for oligosaccharide analysis were as previously described in detail (23). For MALDI, 0.5 μL of solution containing purified oligosaccharides was spotted onto a 100-well stainless steel sample plate (Applied Biosystems, Foster City, CA, USA), followed by 0.25 μL 10 mM NaCl as a dopant (for positive mode) and 0.5 μL 0.4 M 2,5-dihydroxybenzoic acid (in ACN-water [vol/vol]) as the matrix. The spots were then allowed to dry under vacuum prior to MS analysis. MALDI-FTICR MS analysis was performed in the m/z scan range from 220 to 4500. The ions were accumulated in the hexapole and then transferred to the ion cyclotron resonance cell via the ion guide for excitation and detection. Oligosaccharide compositions were assigned using the information obtained from tandem mass spectrometry and by using an in-home software, Glycan Finder written in Igor Pro version 5.04B software from WaveMetrics, Inc. (Portland, OR, USA) (21). The algorithm was designed to examine a list of experimentally measured masses and search for all possible monosaccharide combinations matching the experimental mass within a specified tolerance level (mass error). Oligosaccharide compositions were determined based on mass error as low as 10 ppm.
Sample preparation for GC analysis
Methanolysis and trimethylsilylation were performed following a procedure based on a protocol previously described (9). A 1 M anhydrous methanolic hydrochloric acid (MeOH:HCl) solution was prepared by adding acetyl chloride (140 μL) to anhydrous methanol (1 mL). The mixture of purified oligosaccharides (50–250 μg) and internal standard (200 μg of allose) were suspended in MeOH:HCl (0.5 mL) and kept for 16 h at 80 °C . Then the mixture was concentrated to dryness at room temperature under a stream of nitrogen. Twice, 250 μL of pure methanol were added and then dried under a nitrogen stream. An excess of silylating reagent (mixture of 10:2:1–pyridine, hexamethyldisilazane, chlorotrimethylsilan–(v/v)) (0.3 mL) was added and the solution kept for 20 min at 80 °C. The reagent was removed under a stream of nitrogen. The residue was then extracted with hexane (1 mL), centrifuged and the hexane solution containing silylated monosaccharides was concentrated to 200 μL, and 3 μL were used for GC-flame-ionization detector (FID) analysis. All analyses were performed in triplicate.
Gas chromatography analysis. GC was performed with a Hewlett Packard HP-6890 equipped with a capillary split/splitless inlet and a FID. A DB-1 fused-silica capillary column (30 m × 0.25 μm i.d., 0.25 μm film thickness, J&W Scientific, USA) was used. Hydrogen (flow rate of 2 mL/min and pressure 17 psi) was the carrier gas. Samples were injected in the pulsed split mode with a split ratio of 5:1. The injector and the FID were operated at 280 °C. The gas chromatograph was operated with temperature programming (120–200 °C at 1.5 °C/min, 200 °C held 5 min, and a post run of 2 min at 250 °C).
Standards and monosaccharides quantification
The following commercial monosaccharides D(+)glucose, D(+)galactose, D(+)mannose, D(-)arabinose, L(-)fucose, D(+)xylose, D(+)rhamnose, D(+)galacturonic acid, D-glucuronic acid, and D-allose were used as standards. These standards were used to build a calibration curve and detector response factor were calculated in order to obtain the amount of each monosaccharide present in the sample being analyzed.
Results and Discussion
One of the primary requirements for MS oligosaccharides analysis is elevated sample purity (22). Wine samples were purified by the sequential use of C-18 and nonporous graphitized carbon cartridges in SPE, to remove proanthocyanidins, anthocyanins, salts, monosaccharides, and residual contaminants (traces of proteins and lipids), thus minimizing potential suppression effects during the ionization process. Pure oligosaccharides were eluted from the solid phase in two fractions (20% ACN and 40% ACN in water) and the yield of the extracts was determined.
The oligosaccharidic fractions were also analyzed by GC after hydrolyses to quantify these molecules, thus obtaining glycosyl residue composition of wine extracts. Figure 1 reports a typical monosaccharidic composition of wine oligosaccharides analyzed, including the response factors of each standard monosaccharide compared to allose (reference standard), and the amount relating to a liter of wine. The identified monosaccharides are known to be present in wine (24, 25). Commercial monosaccharide standards and purified wine fractions were subjected to a methanolytic cleavage step, then converted to their corresponding trimethylsilyl methyl glycoside derivatives (Figure 2).
Figure 1.
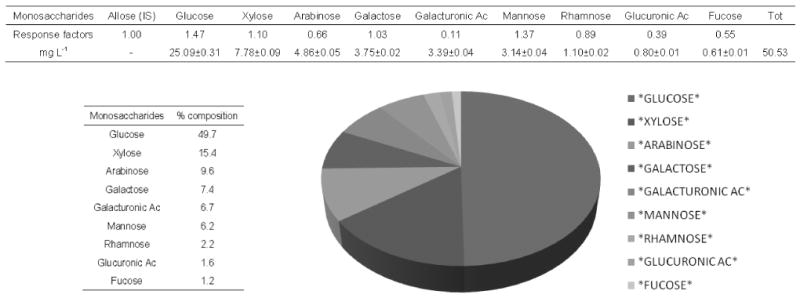
A typical glycosyl residue composition of wine oligosaccharides obtained by gas chromatographic analysis of their corresponding trimethylsilyl methyl derivatives after methanolic-HCl treatment of wine oligosaccharides (Chardonnay, fraction 20% ACN); Allose (reference standard).
Figure 2.
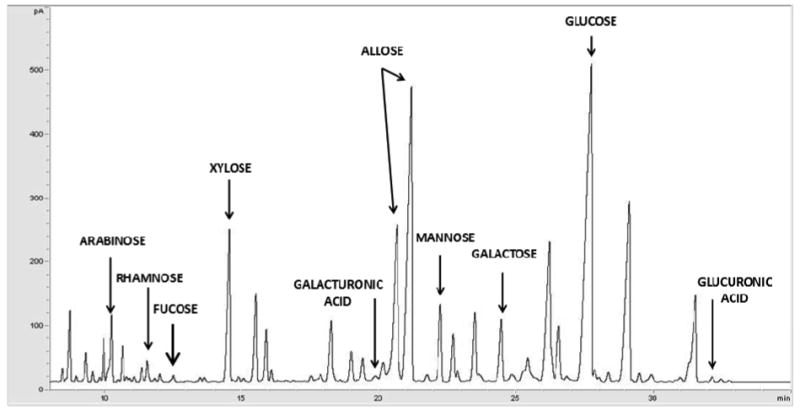
GC profile of the trimethylsilyl methyl glycoside derivatives generated after methanolic-HCl treatment of wine oligosaccharides (20% ACN fraction of Chardonnay wine).
The monosaccharides glucose (48–50%), xylose (14–15%), and arabinose (9– 10%) were the predominant constituents of the oligosaccharides in these wines. Galactose (6–7%), galacturonic acid (6–7%), mannose (5–6%), rhamnose (2%), and glucuronic acid (2%) were also detected, but with a lower abundances. Fucose was also detected in all the samples with even lower amounts (1%).
Grignolino and Chardonnay wines showed slight differences in total oligosaccharide concentration. The amount (calculated as the sum of individual monosaccharide amounts measured by GC) of isolated fractions indicated that total oligosaccharides were present at approximate concentrations of 127 and 102 mg/L, respectively, in the Grignolino and Chardonnay wines. The amounts of oligosaccharides detected in the 20% ACN and 40% ACN fractions were, respectively, 68 mg/L and 59 mg/L for Grignolino, and 50 mg/L and 47 mg/L for Chardonnay.
The differences in oligosaccharide concentration between the two wines could be related to differences in maturity stages between the cultivars at harvest, but also to the different wine making techniques used for red and white wine). The highest quantity of oligosaccharides detected in the fractions derived from Grignolino could be partly related to longer contact time between skins and must during the production of red wine than production of white one. It is known that the integrity of cell walls and their possible weakening modulates the extraction of various components, and in particular polysaccharides and oligosaccharides (26, 27), during wine making.
The oligosaccharide fractions were analyzed by MALDI-FTICR in order to gain compositional information (Figures 3 and 4). All of the fractions purified by solid-phase extraction produced mass spectra containing similar peak patterns, replicates varying only in relative peak intensity, thereby confirming the high suitability of this method for the chosen application. The MALDI-FTICR-MS coupled to CID (Collision-Induced Dissociation) provided high mass accuracy, high resolution, and the compositional information necessary for their identification. The oligosaccharide compositions reported in Table 1 were obtained by tandem MS and by using an in house software (Glycan Finder with high mass accuracy (less than 10 ppm).
Figure 3.
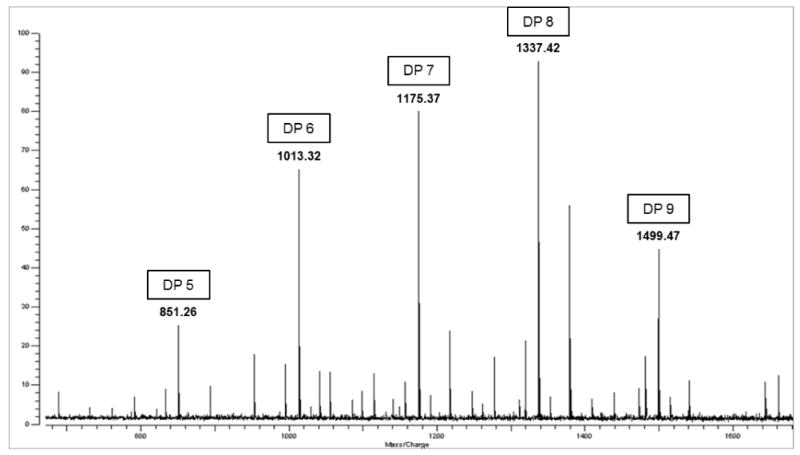
Positive-mode MALDI-FTICR spectra of the 20% ACN oligosaccharide fraction of Chardonnay wine. Major peaks at m/z 851, 1013, 1175, 1337, and 1499 represent sodium-coordinated ([M-Na]+) Hexose oligosaccharides with DP ranging from 5 to 9.
Figure 4.
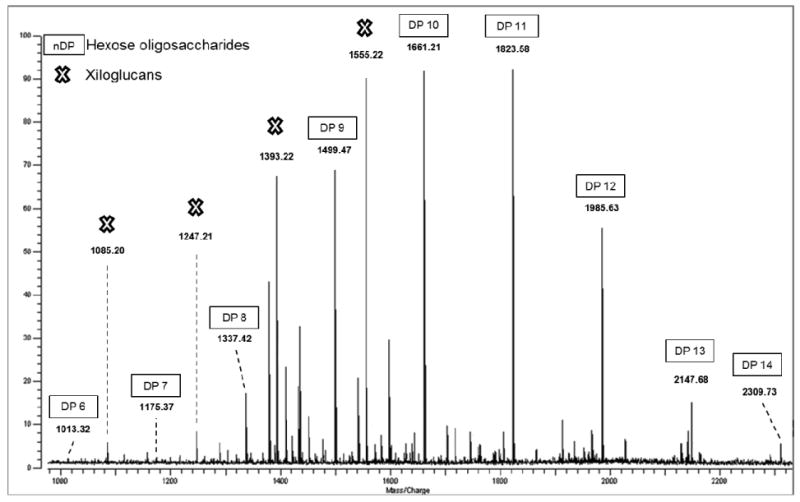
Positive-mode MALDI-FTICR spectra of the 40% ACN oligosaccharide fraction of Chardonnay wine. Major peaks at m/z 1337, 1499, 1661, 1823, 1985, 2147, and 2309 represent sodium-coordinated ([M-Na]+) with DP ranging from 8 to 14; 1393 and 1555 sodium-coordinated ([M-Na]+) XyGs with XXFG and XLFG/XFLG structure.
Table 1.
Oligosaccharides in the wine fractions eluted from the solid phase with 20% and 40% ACN solution, and their constituent monosaccharides. Data were obtained using MALDI-FTICR in positive ion-detection mode and tandem mass spectrometry by CID.
| Grignolino | Chardonnay | ||||||
|---|---|---|---|---|---|---|---|
| m/z [M+Na]+ | Hexose and deoxyhexose | Pentose | O-acetyl | G20 | G40 | C20 | C40 |
| Gal, Glu, Fuc | Ara, Xyl | Relative intensity | |||||
| 689.21 | 4 | (18) | - | (12) | - | ||
| 851.26 | 5 | (34) | - | (30) | - | ||
| 1013.32 | 6 | (65) | - | (70) | (6) | ||
| 1175.37 | 7 | (88) | (18) | (82) | (7) | ||
| 1337.42 | 8 | (100) | (24) | (100) | (21) | ||
| 1499.47 | 9 | (54) | (73) | (48) | (71) | ||
| 1661.21 | 10 | (36) | (100) | (12) | (100) | ||
| 1823.58 | 11 | (8) | (92) | (5) | (95) | ||
| 1985.63 | 12 | - | (55) | - | (60) | ||
| 2147.68 | 13 | - | (18) | - | (21) | ||
| 2309.73 | 14 | - | (11) | - | (12) | ||
| 893.18 | 5 | 1 | (14) | - | (11) | - | |
| 1055.21 | 6 | 1 | (9) | - | (13) | - | |
| 1097.23 | 6 | 2 | (6) | - | - | - | |
| 1217.31 | 7 | 1 | (5) | (7) | (22) | (12) | |
| 1379.34 | 8 | 1 | (32) | (47) | (52) | (41) | |
| 1421.35 | 8 | 2 | - | (24) | - | - | |
| 1541.33 | 9 | 1 | - | (15) | - | (19) | |
| 1583.35 | 9 | 2 | - | (11) | - | - | |
| 1703.38 | 10 | 1 | - | (22 | - | (5) | |
| 1745.39 | 10 | 2 | - | (16) | - | (7) | |
| 1865.41 | 11 | 1 | - | (14) | - | (8) | |
| 1907.42 | 11 | 2 | - | (6) | - | - | |
| 2027.44 | 12 | 1 | - | (9) | - | (4) | |
| 2069.46 | 12 | 2 | - | (3) | - | - | |
| Xyloglucans | |||||||
| 953.19 | 4 | 2 | (21) | - | (18) | - | |
| 1085.20 | 4 | 3 | (16) | - | (9) | - | |
| 1115.20 | 5 | 2 | (23) | - | (13) | - | |
| 1247.21 | 5 | 3 | (41) | (28) | (10) | - | |
| 1277.21 | 6 | 2 | (19) | - | (20) | (6) | |
| 1393.22 | 6 (1 Fucose) | 3 | (58) | (65) | - | (70) | |
| 1409.21 | 6 | 3 | - | (32) | (6) | (24) | |
| 1555.22 | 7 (1 Fucose) | 3 | - | (87) | - | (90) | |
| 1289.21 | 5 | 3 | 1 | - | - | - | (6) |
| 1435.22 | 6 (1 Fucose) | 3 | 1 | - | - | - | (35) |
| 1451.21 | 6 | 3 | 1 | - | (9) | - | (4) |
| 1477.22 | 6 (1 Fucose) | 3 | 2 | - | (12) | - | (4) |
| 1597.22 | 7 (1 Fucose) | 3 | 1 | - | (26) | - | (29) |
| 1639.21 | 7 (1 Fucose) | 3 | 2 | - | - | - | (5) |
| Arabinogalactans | |||||||
| 761.16 | 2 | 3 | - | - | (4) | - | |
| 791.17 | 3 | 2 | - | - | (9) | - | |
| 821.17 | 4 | 1 | (7) | - | (5) | - | |
| 953.18 | 4 | 2 | (21) | - | (18) | - | |
| 1115.20 | 5 | 2 | (23) | - | (13) | - | |
| 1277.21 | 6 | 2 | (19) | - | (20) | (6) | |
| 1439.23 | 7 | 2 | - | (8) | - | (12) | |
The oligosaccharides observed in this study mainly belonged to three different classes: hexose oligosaccharides (potentially galacto-oligosaccharides GOS), xyloglucans (XyGs), and arabinogalactans. The most represented class, hexose oligosaccharides, are carbohydrates of galactose and glucose monomers and are in principle non-digestible.
To determine the degree of polymerization (DP) of the hexose (glucose-galactose) and the other free oligosaccharides in wine fractions, samples were diluted and analyzed by tandem MS. Exact molecular mass measurement was used and the quasi-molecular ions were assigned with < 5 ppm difference between the theoretical and calculated molecular masses. However, both glucose and galactose, the constituents of Hexose oligosaccharides, have an exact molecular mass of 162.0528 Da, which makes it impossible to discriminate between them. The positive-ion-mode MALDI-FTICR spectrum obtained contained peaks at m/z 689.21, 851.26, 1013.32, 1175.37, 1337.42, and 1499.47, and the tandem MS and the software analysis indicated that the 20% ACN wine fraction included oligosaccharides with DP ranging from 4 to 9 (Figure 3). The ion guide of the MALDI-FTICR, which works like a molecular mass filter, was increased in order to detect oligosaccharides with higher masses. As a result, signals corresponding to hexose oligosaccharides with DP of 10 and 11 (m/z 1661.21 and 1823.58) were also observed. MALDI-FTICR analysis of wine samples obtained with 40% ACN showed even larger oligosaccharides with DP up to 14 (m/z 1985.63, 2147.68, and 2309.73) (Figure 4). Further, wine fractions showed the presence of some hexose oligosaccharides further decorated by one of two O-acetyl groups (Table 1). Tandem MS analysis of selected oligosaccharide peaks was carried out using the CID method. An example of a typical CID mass spectrum of the hexose oligosaccharides identified is shown in Figure 5. In this spectrum, the fragmentation of the ion m/z 1337.42 is observed. Tandem MS analysis yielded a mixture of fragment ions corresponding to glycosidic bond cleavages (losses of hexoses at 162.05 Da). Fragment ions corresponding to cross-ring cleavages shifted in 60, 90, and 120 Da from all parent ions were also abundant. The analysis of all fragments allowed identification of the oligosaccharide composition, i.e, 8 Hex (DP8).
Figure 5.
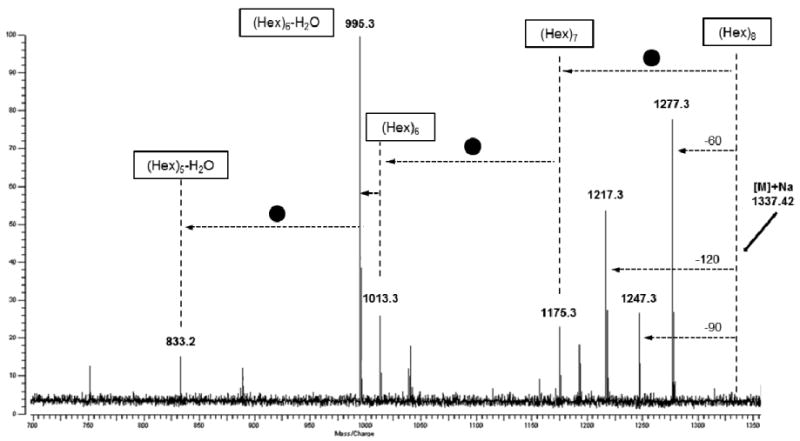
CID MALDI-FTICR spectra of Hexose oligosaccharides with a DP of 8 (m/z = 1337). Fragment ions corresponding to glycosidic bond cleavages (Hex) and cross-ring cleavages (60, 90, and 120) were obtained.
Xyloglucan is the major hemicellulose in the type I primary cell wall of most higher plants. It is a polymer consisting of repetitive segments of four residues of a β1-4 glucan backbone substituted on the first three positions with α1-6 xylose. The xyloses at positions 2 and 3 can have galactose attached in a β1-2 linkage, and a fucose is usually found in an α1-2 linkage to the galactose at C-2. The wine oligosaccharide fractions showed the presence of seven major ions at, respectively, m/z = 953.19, 1085.20, 1115.20, 1247.21, 1393.22, 1409.21, and 1555.22 (Figure 4). The dominant XyG structure in these fractions, following the nomenclature previously described (28), was XLFG/XFLG (ion at m/z = 1555.92), followed by two structures, namely XXFG and XXLG/XLXG (ions at m/z = 1393.70 and 1247.81, respectively) corresponding to both non-fucosylated and fucosylated types of XyG (Figure 4). Important biological functions have been attributed to fucose in oligosaccharides (anti-pathogenic, anti-adherence, and antimicrobial) (29, 30). Arabinogalactans are a soluble dietary fiber, commonly consumed in such foods as carrots, tomatoes, radishes, pears, maize, wheat, and red wine (31). Arabinogalactans are fermented by human intestinal bacteria and can induce the enzymes necessary for their degradation (32). Fermentation is evidenced by the ability of human intestinal microflora to degrade arabinogalactans and produce short-chain fatty acids (33). The wine oligosaccharide fractions showed the presence of major ions at, respectively, m/z = 761.16, 791.17, 821.17, 953.18, 1115.20, 1277.21, and 1439.23. These oligosaccharides have the following predicted compositions: Gal2Ara3 (761), Gal3Ara2 (791), Gal4Ara (821), Gal4Ara2 (953), Gal5Ara2 (1115), Gal6Ara2 (1277), and Gal7Ara2 (1439).
The structures suggested for the oligosaccharides (Figure 6) identified in Grignolino and Chardonnay wines are predicted based on the fragmentation pattern. The results indicated that these oligosaccharides might be generally present in all fractions from various origins. The presence of these oligosaccharides from hemicellulosidic cell wall structures shows that these polysaccharides are modified and/or hydrolyzed either during the maturation of the grape berry or/and during wine making.
Figure 6.
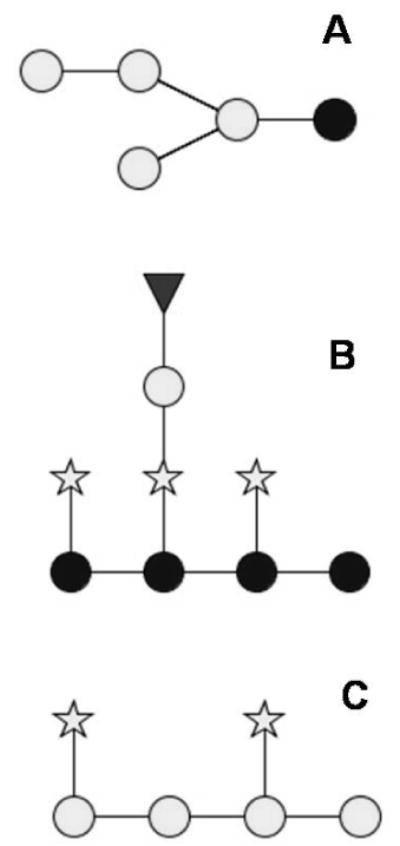
Suggested structures of main oligosaccharides present in Grignolino and Chardonnay. (A)Hexose oligosaccharides; (B) xyloglucans (XyGs); and (C) arabinogalactans. Glucose (dark circle), galactose (light circle), fucose (triangle), and xylose/arabinose (star).
In addition to the main classes of compounds here identified, it should be noted that other oligosaccharides are likely to be present in these complex extracts. As a matter of fact, the data obtained after complete hydrolysis of all carbohydrates retained by SPE with porous graphitized carbon indicate the presence of galacturonic acid, rhamnose, and glucuronic acid (Figure 1). However, they may be, in their intact (non-hydrolized form) too large to be detected and isolated, even by an instrument with a high mass range such as the MALDI FTICR. These issues and the complexity shown for these wine extracts will certainly be the benchmark for the future development of new analytical protocols focused on improving oligosaccharide extraction and fractionation with the aim to better characterize an important class of bioactive substances.
For the first time, a cocktail of complex oligosaccharides of up to 14 monomers has been isolated and characterized in two wines (Grignolino and Chardonnay) by two complementary methods: GC and MS (MALDI-FTICR MS). These molecules and other oligosaccharides in wine represent the degraded structures of polysaccharides originating from the grape berry cell wall, mainly as a result of malolactic/contaminant enzyme activities present during the various stages of the wine making process.
In this context, whereas the average amount of oligosaccharides present in wine was approximately 100 mg/L, the MALDI-FTICR MS system coupled with the method developed may prove to be a valuable analytical support to address future research focusing on biological activities induced by these molecules. At the same time, it will be an important aspect to evaluate the influence of various wine making techniques on the final amount and composition of these molecules in wines.
Acknowledgments
Dr. Matteo Bordiga was the recipient of a fellowship from the Italian Politiche Agricole, Alimentari e Forestali Ministry (Food-Link project) and his stay at UC Davis was sponsored by Cariplo Foundation (Nutrial Network 2010 project; cod. 2009-2961). The authors acknowledge the National Institute of Environmental Health Sciences (P42ES004699) and Dr. Laura A. Gillies for technical assistance with the GC analysis and C.J. Dillard for editorial assistance.
Literature Cited
- 1.Riou V, Vernhet A, Doco T, Moutounet M. Aggregation of grape seed tannins in model wine - effect of wine polysaccharides. Food Hydrocolloid. 2002;16(1):17–23. [Google Scholar]
- 2.Vidal S, Courcoux P, Francis L, Kwiatkowski M, Gawel R, Williams P, Waters E, Cheynier V. Use of an experimental design approach for evaluation of key wine components on mouth-feel perception. Food Qual Prefer. 2004;15(3):209–217. [Google Scholar]
- 3.Gerbaud V, Gabas N, Laguerie C, Blouin J, Vidal S, Moutounet M, Pellerin P. Effect of wine polysaccharides on the nucleation of potassium hydrogen tartrate in model solutions. Chem Eng Res Des. 1996;74(A7):782–790. [Google Scholar]
- 4.Chalier P, Angot B, Delteil D, Doco T, Gunata Z. Interactions between aroma compounds and whole mannoprotein isolated from Saccharomyces cerevisiae strains. Food Chem. 2007;100(1):22–30. [Google Scholar]
- 5.O'Neill MA, Warrenfeltz D, Kates K, Pellerin P, Doco T, Darvill AG, Albersheim P. Rhamnogalacturonan-II, a pectic polysaccharide in the walls of growing plant cell, forms a dimer that is covalently cross-linked by a borate ester. J Biol Chem. 1996;271(37):22923–930. doi: 10.1074/jbc.271.37.22923. [DOI] [PubMed] [Google Scholar]
- 6.Pellerin P, O'Neill M, Pierre C, Cabanis M, Darvill A, Albersheim P, Moutounet M. Lead complexation in wines with the dimers of the grape pectic polysaccharide rhamnogalacturonan 2. J Int Sci Vigne Vin (France) 1997;31:33–41. [Google Scholar]
- 7.Ayestaran B, Guadalupe Z, León D. Quantification of major grape polysaccharides (Tempranillo v.) released by maceration enzymes during the fermentation process. Anal Chim acta. 2004;513(1):29–39. [Google Scholar]
- 8.Doco T, Williams P, Cheynier V. Effect of flash release and pectinolytic enzyme treatments on wine polysaccharide composition. J Agric Food Chem. 2007;55(16):6643–6649. doi: 10.1021/jf071427t. [DOI] [PubMed] [Google Scholar]
- 9.Ducasse MA, Williams P, Meudec E, Cheynier V, Doco T. Isolation of Carignan and Merlot red wine oligosaccharides and their characterization by ESI-MS. Carbohyd Polym. 2010;79(3):747–754. [Google Scholar]
- 10.Boehm G, Stahl B. Oligosaccharides. In: Mattila T, Saarela M, editors. Functional Dairy Products. Cambridge, England: Woodhead Pub. Ltd.; 2003. pp. 203–243. [Google Scholar]
- 11.British-Nutrition-Foundation. Complex Carbohydrates in Foods: Report of the British Nutrition's Task Force. London: Chapman and Hall; 1990. [Google Scholar]
- 12.Guggenbichler JP, De Bettignies-Dutz A, Meissner P, Schellmoser S, Jurenitsch J. Acidic oligosaccharides from natural sources block adherence of Escherichia coli on uroepithelial cells. Pharm Pharmacol Lett. 1997;7:35–38. [Google Scholar]
- 13.Harmsen HJM, Wildeboer-Veloo A, Raangs GC, Wagendorp AA, Klijn N, Bindels JG, Welling GW. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J Pediatr Gastr Nutr. 2000;30(1):61–67. doi: 10.1097/00005176-200001000-00019. [DOI] [PubMed] [Google Scholar]
- 14.Kunz C, Rudloff S, Baier W, Klein N, Strobel S. Oligosaccharides in human milk: Structural, functional, and metabolic aspects. Annual Rev Nutr. 2000;20:699–722. doi: 10.1146/annurev.nutr.20.1.699. [DOI] [PubMed] [Google Scholar]
- 15.Newburg DS. Innate immunity and human milk. J Nutr. 2005;135(5):1308–1312. doi: 10.1093/jn/135.5.1308. [DOI] [PubMed] [Google Scholar]
- 16.Urashima T, Saito T, Nakamura T, Messer M. Oligosaccharides of milk and colostrum in non-human mammals. Glycoconjugate J. 2001;18(5):357–371. doi: 10.1023/a:1014881913541. [DOI] [PubMed] [Google Scholar]
- 17.Hopkins MJ, Macfarlane GT. Nondigestible oligosaccharides enhance bacterial colonization resistance against Clostridium difficile in vitro. Appl Environ Microbiol. 2003;69(4):1920–1927. doi: 10.1128/AEM.69.4.1920-1927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Macfarlane GT, Steed H, Macfarlane S. Bacterial metabolism and health-related effects of galacto-oligosaccharides and other prebiotics. J Appl Microbiol. 2008;104(2):305–344. doi: 10.1111/j.1365-2672.2007.03520.x. [DOI] [PubMed] [Google Scholar]
- 19.Shoaf K, Mulvey GL, Armstrong GD, Hutkins RW. Prebiotic galactooligosaccharides reduce adherence of enteropathogenic Escherichia coli to tissue culture cells. Infect Immun. 2006;74(12):6920–6928. doi: 10.1128/IAI.01030-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gibson GR, Roberfroid MB. Dietary Modulation of the Human Colonic Microbiota - Introducing the Concept of Prebiotics. J Nutr. 1995;125(6):1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 21.Ninonuevo MR, Park Y, Yin H, Zhang J, Ward RE, Clowers BH, German JB, Freeman SL, Killeen K, Grimm R. A strategy for annotating the human milk glycome. J Agric Food Chem. 2006;54(20):7471–7480. doi: 10.1021/jf0615810. [DOI] [PubMed] [Google Scholar]
- 22.Barile D, Tao N, Lebrilla CB, Coisson JD, Arlorio M, German JB. Permeate from cheese whey ultrafiltration is a source of milk oligosaccharides. Int Dairy J. 2009;19(9):524–530. doi: 10.1016/j.idairyj.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Penn SG, Cancilla MT, Green MK, Lebrilla CB. Direct comparison of matrix-assisted laser desorption/ionisation and electrospray ionisation in the analysis of gangliosides by Fourier transform mass spectrometry. Eur Mass Spectrom. 1997;3(1):67–79. [Google Scholar]
- 24.Belleville MP, Williams P, Brillouet JM. A Linear Arabinan from a Red Wine. Phytochemistry. 1993;33(1):227–229. [Google Scholar]
- 25.Doco T, Brillouet JM. Isolation and Characterization of a Rhamnogalacturonan-Ii from Red Wine. Carbohyd Res. 1993;243(2):333–343. doi: 10.1016/0008-6215(96)00139-5. [DOI] [PubMed] [Google Scholar]
- 26.Nunan KJ, Sims IM, Bacic A, Robinson SP, Fincher GB. Changes in cell wall composition during ripening of grape berries. Plant Physiol. 1998;118(3):783–792. doi: 10.1104/pp.118.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vicens A, Fournand D, Williams P, Sidhoum L, Moutounet M, Doco T. Changes in Polysaccharide and Protein Composition of Cell Walls in Grape Berry Skin (Cv. Shiraz) during Ripening and Over-Ripening. J Agric Food Chem. 2009;57(7):2955–2960. doi: 10.1021/jf803416w. [DOI] [PubMed] [Google Scholar]
- 28.Fry SC, York WS, Albersheim P, Darvill A, Hayashi T, Joseleau JP, et al. An unambiguous nomenclature for xyloglucan-derived oligosaccharides. Physiol Plant. 1993;89:1–3. [Google Scholar]
- 29.Ruiz-Palacios GM, Cervantes LE, Ramos P, Chavez-Munguia B, Newburg DS. Campylobacter jejuni binds intestinal H(O) antigen (Fuc alpha 1, 2Gal beta 1, 4GlcNAc), and fucosyloligosaccharides of human milk inhibit its binding and infection. J Biol Chem. 2003;278(16):14112–14120. doi: 10.1074/jbc.M207744200. [DOI] [PubMed] [Google Scholar]
- 30.Newburg DS, Ruiz-Palacios GM, Altaye M, Chaturvedi P, Guerrero ML, Meinzen-Derr JK, Morrow AL. Human milk alpha 1,2-linked fucosylated oligosaccharides decrease risk of diarrhea due to stable toxin of E-coli in breastfed infants. Adv Exp Med Biol. 2004;554:457–461. doi: 10.1007/978-1-4757-4242-8_64. [DOI] [PubMed] [Google Scholar]
- 31.Egert D, Beuscher N. Studies on Antigen-Specificity of Immunoreactive Arabinogalactan Proteins Extracted from Baptisia-Tinctoria and Echinacea-Purpurea. Planta Med. 1992;58(2):163–165. doi: 10.1055/s-2006-961420. [DOI] [PubMed] [Google Scholar]
- 32.Englyst H, Hay S, Macfarlane G. Polysaccharide breakdown by mixed populations of human faecal bacteria. FEMS Microbiol Lett. 1987;45(3):163–171. [Google Scholar]
- 33.Bradburn DM, Mathers JC, Gunn A, Burn J, Chapman PD, Johnston ID. Colonic fermentation of complex carbohydrates in patients with familial adenomatous polyposis. Gut. 1993;34(5):630–636. doi: 10.1136/gut.34.5.630. [DOI] [PMC free article] [PubMed] [Google Scholar]


