Abstract
Nesprins are a family of nuclear transmembrane proteins anchored via Sun proteins to the nuclear membrane. Analysis of nesprins during human muscle development revealed an increase in nesprin-1-giant during early myogenesis in vitro. During the transition from immature to mature muscle fibres in vivo, nesprin-2 partly replaced nesprin-1 at the nuclear envelope and short nesprin isoforms became dominant. Sun1 and Sun2 proteins remained unchanged during this fibre maturation. In emerin-negative skin fibroblasts, nesprin-2-giant was relocated from the nuclear envelope to the cytoplasm, not to the endoplasmic reticulum, while nesprin-1 remained at the nuclear envelope. In emerin-negative keratinocytes lacking nesprin-1, nesprin-2 remained at the nuclear envelope. HeLa cell nuclear envelopes lacked nesprin-1, which was the dominant form in myoblasts, while a novel 130kD nesprin-2 isoform dominated Ntera-2 cells. The results suggest the possibility of isoform-specific and tissue-specific roles for nesprins in nuclear positioning.
Keywords: nesprin, SYNE, MYNE, NUANCE, enaptin, emerin, lamin A/C, nuclear lamina, nuclear envelope, nuclear membrane, myogenesis, muscle development, NT-2, Golgi, nuclear positioning, monoclonal antibody, epitope mapping, phage display
INTRODUCTION
Nesprins are a family of spectrin repeat proteins (Zhang et al, 2001, 2002; Wilhelmsen et al, 2005; Roux et al, 2009) and missense mutations in these proteins have been described in Emery-Dreifuss muscular dystrophy (Zhang et al, 2007a), a disease that is also caused by mutations in the nuclear envelope proteins, emerin and lamin A/C (Morris, 2001). Alternate initiation and splicing of the genes encoding nesprins 1 and 2 generate multiple isoforms of different sizes but mostly with a common C-terminal region (Apel et al, 2000; Zhen et al, 2002, Mislow et al, 2002a; Padmakumar et al, 2004). In this respect, they resemble another pair of structurally-related spectrin repeat proteins, dystrophin and utrophin, which also show tissue-specific expression of isoform patterns and functions (Jin et al, 2007). The largest nesprin isoforms have a spectrin-repeat rod domain separating a C-terminal transmembrane KASH (Klarsicht-ANC-Syne-homology) domain from N-terminal calponin-homology (CH) domains that bind actin. These “nesprin-giant” molecules (also known as enaptin and NUANCE, as well as synes and mynes) attach to the outer nuclear membrane through their KASH domain which is bound by SUN proteins in the lumen between inner and outer nuclear membranes, and they are thus able to form a link to the actin cytoskeleton through their CH domains (Padmakumar et al, 2005; Haque et al, 2006). Nesprin-3, a product of the SYNE3 gene, is anchored in a similar way but interacts with the cytoskeleton by binding to plectin (Wilhelmsen et al, 2005). In striated muscle, in a somewhat analogous manner, dystrophin is anchored to the plasma membrane by a C-terminal interaction with the transmembrane dystroglycan complex and also binds to the actin cytoskeleton through its own CH domains. This suggests a possible link both between nuclear and plasma membranes via the cytoskeleton (the LINC complex: Starr and Han, 2003; Crisp et al, 2006) and between muscular dystrophies caused by defects in nuclear and plasma membrane proteins (Morris, 2001). The importance of the LINC complex is illustrated by the Emery-Dreifuss-like features of transgenic mice lacking only the nesprin-1 KASH domain (Puckelwartz et al, 2009).
Nesprin isoforms with a C-terminal region are also present at the inner nuclear membrane (INM), but it is not yet clear which particular isoforms are involved or whether they are anchored by the same KASH-SUN interaction. One current hypothesis is that the smallest isoforms occupy the INM, whereas the giant forms link the outer nuclear membrane (ONM) with the actin cytoskeleton (Warren et al, 2005; Worman and Gundersson, 2006). This view is partly based on the observation that only membrane proteins smaller than about 60kD, such as emerin (29kD) and nesprin-2-α (49-64kD) can enter the nucleus by diffusion through lateral channels of the nuclear pores, without the need for a nuclear localization signal (NLS) sequence (Worman and Gundersson, 2006). One group has presented experimental evidence for the presence of nesprin-2-giant at both INM and ONM, using both a digitonin permeabilization method (Zhen et al, 2002) and immunogold electron microscopy (Libotte et al, 2005), but others have reported it at the ONM only (Crisp et al, 2006), so further evidence is needed to resolve this issue, which is discussed in greater detail elsewhere (Morris and Randles, 2009). Nesprins are also found at various cytoplasmic locations away from the nucleus (Gough et al, 2003; Zhang et al, 2005), but, although nesprin-2-giant is involved, lack of specific antibodies has made it difficult to determine whether other isoforms are also present. The function of short nesprin forms that lack an actin-binding domain is unclear, although most of them do contain the sequences required for direct interaction with emerin and lamin A/C and a role in linking the INM to the nuclear lamina has been proposed (Mislow et al, 2002b).
It is also clear that nesprins may have cell-specific or tissue-specific functions. Thus, one very short C-terminal isoform of nesprin-2 is found only in striated muscle (Zhang et al, 2005) and the location of nesprin-1 in the epidermis is quite different from the nuclear rim location found in most other cell types (Luke, 2008). In the present study, we have used novel panels of monoclonal antibodies against the C-terminal regions of nesprins 1 and 2 as a first step in addressing these issues.
RESULTS
Human skin fibroblasts express giant nesprin isoforms, while short isoforms predominate in adult human skeletal muscle
We produced new mAb panels against C-terminal regions of nesprin-1 and nesprin-2 (Table 1) that should recognize most known nesprin isoforms and all isoforms that have the KASH/transmembrane domain for insertion into nuclear membranes. At least four nesprin-1 epitopes and at least seven nesprin-2 epitopes are within the mAb panels (see Methods). For this reason, they will clearly distinguish authentic nesprin gene products from cross-reacting proteins, but cannot be used to distinguish short isoforms from degradation products of larger isoforms.
TABLE 1.
Panels of monoclonal antibodies against nesprin-1 and nesprin-2.
All mAbs, except MANNES2A, were specific for human nesprin-1 or nesprin-2. Cross-reaction in IMF with nesprins from other species is shown (mo = mouse, rb = rabbit, hu = human). IMF = immunofluorescence microscopy; NHM = normal human muscle. Epitope groups identified by specificity for nesprin-1, or by tryptic digestion and phage-display (if applicable) for nesprin-2, are shown in square brackets.
| Name of mAb | Clone No. |
mAb subclass |
Cross-reactivity in IMF (all hu+) [epitope, if known] |
Western blot (NHM extract) |
IMF (NHM sections) |
|---|---|---|---|---|---|
| MANNES1A | 7A12 | IgG1 | Mo, rat, rb, centrosome |
++ | ++++ |
| MANNES1B | 7B2 | IgG1 | ++ | +++ | |
| MANNES1C | 6C10 | IgG1 | Mo, rat, rb | w | +++ |
| MANNES1D | 8B8 | IgG1 | + | +++ | |
| MANNES1E | 8C3 | IgG1 | Mo, rat | +++ | +++ |
| MANNES1F | 9G10 | IgG1 | Mo, rat, rb | w (high bgd) | +++ |
| MANNES1G | 6G2 | IgG1 | Mo, rat, rb | w | +++ |
| MANNES2A | 11A3 | IgG1 | [aa6653-6656] | ++++ | ++++ |
| MANNES2B | 6A8 | IgG2a | Nesprin-1 | ++++ | +++ |
| MANNES2C | 12A5 | IgG1 | w | +++ | |
| MANNES2D | 6H5 | IgG1 | w | ++ | |
| MANNES2E | 18F7 | IgG1 | [aa6674-6679] | w | +++ |
| MANNES2F | 11C5 | IgG 1 | [aa6653-6656] | ++ | +++ |
| MANNES2G | 4B5 | IgG2b | [aa6694-6701] | +++ | +++ |
| MANNES2H | 3C4 | IgG1 | w | +++ | |
| MANNES2I | 4A11 | IgG1 | ++++ | +++ | |
| MANNES2J | 3H4 | IgG1 | ++ | +++ | |
| MANNES2K | 1A11 | IgG1 | ++ | +++ | |
| MANNES2L | 17F5 | IgG1 | +++ | +++ | |
| MANNES2M | 16H2 | IgG1 | w | +++ |
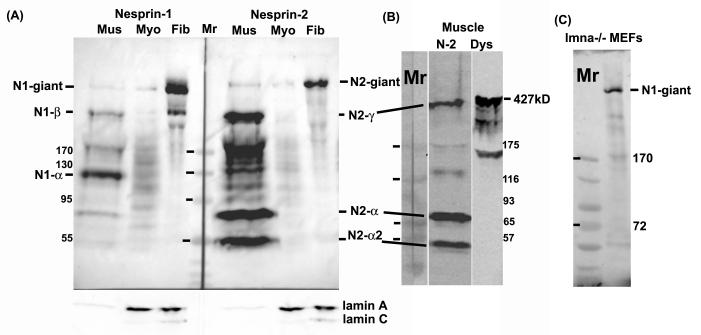
Human skin fibroblasts were found to express almost exclusively the largest known isoforms, nesprin-1-giant and nesprin-2-giant, on western blots (Fig.1A). The bands recognized by all mAbs are authentic nesprin (Fig. 2A), while some lower Mr bands are clearly cross-reacting proteins recognized by a single mAb (e.g. lane 5 of nesprin-2 has a 300kD cross-reacting band). Nesprin-2 migrated as a single band consistent with the 792kD Mr of nesprin-2-giant. Nesprin-1 (predicted Mr 1,008kD) migrated as a double band in the 800-1000kD region of the blot. Human myoblast cultures contained much lower amounts of both nesprins, when similar amounts of extract were loaded (Fig. 1A).
Figure 1.
Expression of nesprin isoforms in muscle and fibroblasts.
(A) Western blots of normal adult human muscle (Mus), human primary skeletal muscle cell culture (Myo) and human primary dermal fibroblasts (Fib) were probed with MANNES1A or MANNES2A antibodies (1:100) as described in Methods. The blot was reprobed with MANLAC1 mAb against lamin A/C (Manilal et al, 2004) as a loading control. Mr (relative molecular mass) markers were EZ-Run (Fisher Scientific, Loughborough, UK).
(B) A second muscle biopsy developed with MANNES2A had less degradation products, though the nesprin-2-giant band was less clear. Minor bands at 180kD and 140kD may be degradation products. The gel was run as a single lane and the blot was cut into strips. MANDYS1 (1:500) to dystrophin (Nguyen thi Man et al, 1990) was used as a high Mr marker (427kD) and as an indicator of the extent of degradation of “spectrin-like” proteins in the samples. Sigma prestained Mr markers were used.
(C) Mouse embryonic fibroblasts (MEFs) from lmna -/- knockout mice also express mainly nesprin-1-giant. The other bands of lower Mr do not correspond in size to any known shorter isoform. Mr markers were EZ-Run (Fisher).
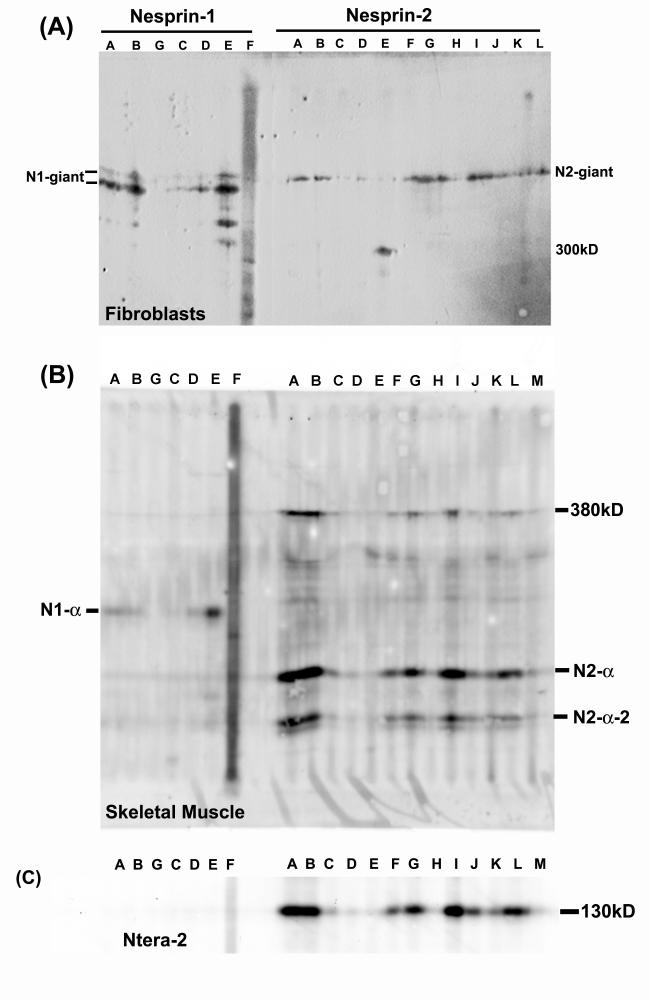
Figure 2.
Multi-epitope mAb panels confirm the identity of authentic nesprin proteins.
Extracts of (A) human dermal fibroblasts, (B) adult human skeletal muscle or (C) human Ntera-2 cells, were subjected to SDS-PAGE as a strip and the western blots were developed with multiple nesprin mAbs (1:100 dilution) using a miniblotter. The mAbs vary in strength (see also Table 1), but the strongest are strong for all nesprin isoforms. In (A), MANNES2E recognizes a 300kD cross-reacting protein, similar only in size to the 300kD band in (B), which is recognized by many mAbs.
In human skeletal muscle extracts, the short isoforms, nesprin-1-α at 113kD and nesprin-2-α and nesprin-2-α-2 at 64kD and 49kD respectively, were very prominent, with only weak bands of the giant isoforms present (Fig.1A). Bands migrating slightly faster than dystrophin in muscle (Fig. 1B; 427kD) were tentatively identified as nesprin-1-β (380kD) and nesprin-2-γ (376kD). Although some proteolysis has occurred in the human muscle samples (see dystrophin blot, Fig. 1B), it is probably not enough to account for such prominent lower Mr bands. There was no prominent band corresponding to nesprin-2-β (88kD) in muscle or any other cell type studied. All mAbs that recognized the giant isoforms strongly (Fig. 2A) also recognized the short forms strongly (Fig. 2B), confirming that all are nesprin gene products and not cross-reacting proteins.
Emerin anchors nesprin-2-giant, but not nesprin-1, at the nuclear rim of skin fibroblasts
Immunostaining with antibodies to nesprin 1 and 2 in human fibroblasts produced a signal mainly at the nuclear envelope (Fig. 3B, D), co-localizing with emerin (Fig. 3A, C), and the western blots (Fig, 1A) suggest that this is mainly due to the giant nesprin isoforms. Nuclear envelope staining was observed with all nesprin-1 and nesprin-2 mAbs in Table 1 (Randles, 2008). The absence of lamin A/C in cells from lmna -/- mice resulted in the redistribution of nesprin-1 from the nuclear rim into the cytoplasmic ER (Fig. 3J). Our nesprin-2 mAbs do not react with mouse nesprins (Table 1), but nesprin-2 is known to redistribute in a similar way (Libotte et al, 2005). Emerin co-distributes with nesprins in lmna -/- cells (Fig. 3I), consistent with the hypothesis that, in normal cells, both proteins are trapped from the ER at the nuclear membrane by their interactions with lamin A/C and with each other (Sullivan et al, 1999). Western blots show that, nesprin-1 in lmna -/- mouse embryonic fibroblasts is also almost exclusively the giant isoform (Fig. 1C).
Figure 3.
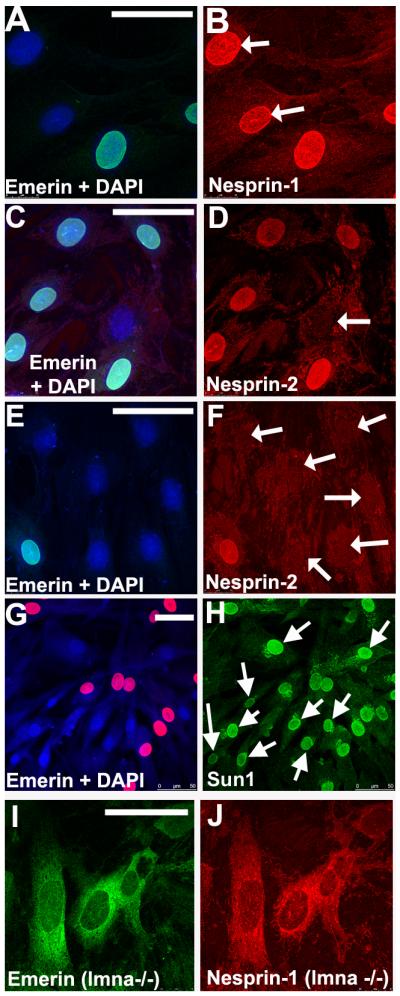
Human skin fibroblasts without emerin show normal nuclear nesprin-1 and Sun protein staining, but nuclear nesprin-2 is absent.
Primary skin fibroblast cultures from a female carrier of X-linked Emery-Dreifuss MD contain both emerin-positive and emerin-negative cells as a result of random X-inactivation. Acetone-methanol-fixed cells were double-labelled with rabbit anti-emerin serum (1:100: green with a DAPI counterstain; Holt et al, 2003) and either MANNES1A (A-B, nesprin-1: red) or MANNES2A (C-F, nesprin-2: red). Emerin-negative nuclei (white arrows) had normal nuclear nesprin-1, but nuclear nesprin-2 was absent. Sun1 (H: green) was present at the nuclear rim in all cells and was not significantly different in cells lacking emerin (G: red). MEFs (mouse embryonic fibroblasts) from lmna -/- mice illustrate the redistribution of both emerin (I) and nesprin-1 (J) into the ER in the absence of lamin A/C. The emerin gene mutation was a premature stop codon in exon 4. Bars (white) = 50μm
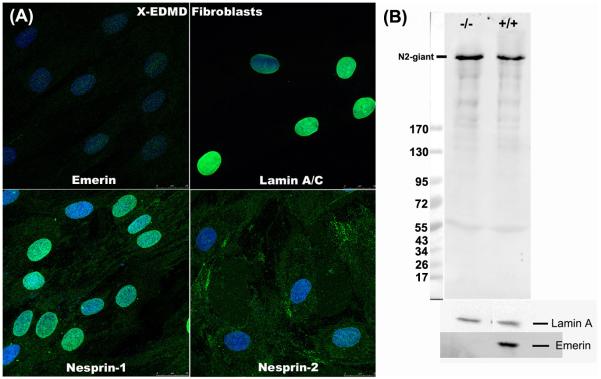
In skin fibroblasts from a carrier of Emery-Dreifuss muscular dystrophy, emerin-positive and emerin-negative cells with a similar genetic background exist side-by-side (Fig. 3A, C, E). Unexpectedly, nesprin-2 staining was always absent from emerin-negative nuclear rims (Fig. 3D, F), while nesprin-1 at the nuclear rim was never affected (Fig. 3B). Sun1 (Fig. 3H), Sun2 (data not shown) and lamin A/C (Manilal et al, 1996) were also unaffected by the absence of emerin. This means that lamin A/C and Sun proteins are insufficient to retain nesprin-2 at the nuclear rim, though they are evidently enough to retain nesprin-1. The emerin requirement for nesprin-2 retention was confirmed in emerin-null fibroblasts from an unrelated X-linked EDMD patient (Fig. 4A). Although nesprin-2 was absent from the nucleus of emerin-negative cells, western blotting showed that its total levels were unchanged (Fig. 4B). However, nesprin-2 did not redistribute to the ER, but appears to be more generally dispersed inside the cell (Fig. 4A). Emerin and nesprin-2 both relocate to the ER in lmna -/- cells (Fig. 3I and Libotte et al, 2005), but nesprin-2 appears unable to localise at either NE or ER in the absence of emerin. Libotte et al (2005) found that emerin also re-distributed to the cytoplasm when nesprin-2 was absent from the NE of COS7 cells, suggesting a mutual dependence.
Figure 4.
Nesprin-2 is absent from the nuclear rim of emerin-negative skin fibroblasts from an X-EDMD patient, but western blots show that nesprin-2 levels are unchanged.
(A) Staining as in Fig. 3 with single antibody labelling (green) for emerin, lamin A/C or nesprins and DAPI counterstain (blue). This patient had a frameshift deletion of bp 329-388 in the emerin gene leading to a stop after 19 amino-acids and was unrelated to the carrier in Fig. 3. Bar (white) = 20μm.
(B) Monolayer cultures of control (Coriell cell line No. 8333) and X-EDMD fibroblasts were harvested with trypsin-EDTA and extracted. Extracts were subjected to SDS-PAGE on 3-15% gradient gels and blots developed with MANNES2A for nesprin-2, MANLAC1 for lamin A and MANEM5 (Manilal et al, 1996) for emerin. The lamin A band was used as a loading control and Mr markers were EZ-Run.
This interdependency of emerin and nesprin-2 in fibroblasts is not universal, since emerin-negative keratinocytes in the epidermal layer of a skin biopsy section from an EDMD carrier did have nesprin-2 at their nuclear rim (Fig. 5). A striking feature of all keratinocytes, however, is the absence of nesprin-1 from the NE (Fig. 5), as previously reported (Luke et al, 2008).
Figure 5.
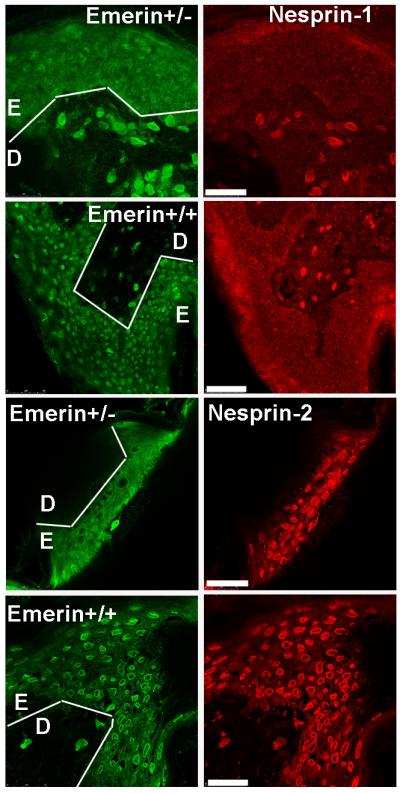
Nesprin-2 is present in emerin-negative keratinocytes in a carrier EDMD skin biopsy.
Frozen sections (5 μm) were fixed with acetone-methanol and double-labelled for emerin (green) and nesprin-1 or nesprin-2 (red). Nesprin-1 is positive at the nuclear rim in dermal fibroblasts and negative in epidermal keratinocytes, both with and without emerin. White lines show the boundary between dermis (D) and epidermis (E). Unlike cultured dermal fibroblasts, all epidermal keratinocytes, with or without emerin, are positive for nesprin-2 at the nuclear rim. Bars (white) = 50μm
Nesprin expression in human muscle: nesprin-1 increases at the nuclear rim during early myogenesis, but is partially replaced by nesprin-2 at later stages of muscle development
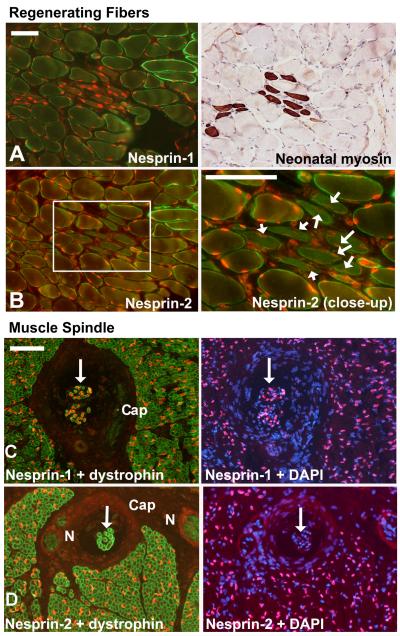
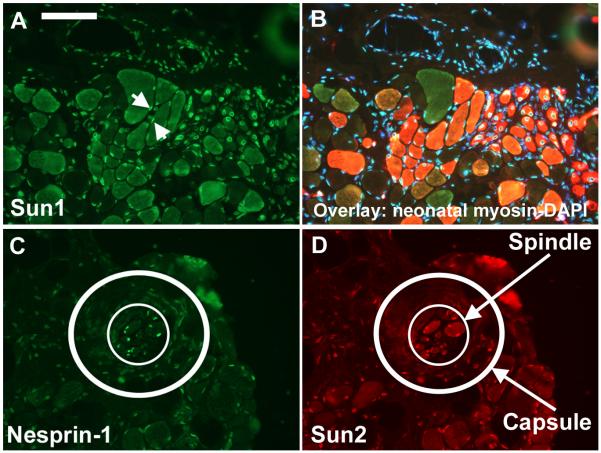
In adult human skeletal muscle biopsy sections, nuclear rim staining was very strong for both nesprins in myofibre nuclei (Fig. 6). Muscle biopsies from Becker muscular dystrophy patients had frequent groups of smaller, regenerating fibres and nesprin-1 staining was stronger in these than in surrounding mature fibres (Fig.6A). Nesprin-2 staining in regenerating fibre nuclei (white arrows in Fig. 6B close-up) was weaker than surrounding fibres. This suggests that immature muscle fibres preferentially express nesprin-1. Stronger staining of regenerating fibre nuclei by nesprin-1 antibodies has been reported previously (Apel, 2000). Sun1 levels appeared unchanged in regenerating fibres (Fig. 7A, B) and similar results were obtained with Sun2 (not shown). The structure known as the muscle spindle contains small specialized muscle fibres with an immature pattern of muscle gene expression and nuclei in these fibres expressed only nesprin-1 (Fig. 6C-D). Fibroblast nuclei in the external capsule (“Cap”) that surrounds the spindle were negative for both nesprins (Fig. 6C-D). Both Sun1 (not shown) and Sun2 (Fig. 7C, D) appeared to follow the overall nesprin level, being strongly expressed in the spindle muscle fibre nuclei and very weak in nuclei of the outer capsule.
Figure 6.
A nesprin isoform switch between immature and mature muscle fibres.
Double-labels of nesprin mAbs with a polyclonal anti-dystrophin to show muscle fibre outlines were counterstained with DAPI to show nuclei. Serial sections. Bars (white) = 100μm
(A, B) In a Becker MD patient, dystrophin staining is weak except in a few revertant fibres. Groups of immature, regenerating fibres are common in Becker MD and their nuclei are more brightly stained for nesprin-1 than surrounding mature fibres (A), but they have less nesprin-2 than surrounding mature fibres (B: white arrows). Immature fibres were identified using a neonatal-specific anti-myosin mAb (NCL-MHCn, Leica, Milton Keynes, UK) on a serial section.
(C, D) In a child with congenital myotonic dystrophy, fibres are smaller with large central nuclei, but dystrophin staining at the sarcolemma is normal. The muscle spindle is a specialized sensory structure in muscle that contains a group of immature muscle fibres (white arrows). These are strongly positive for nesprin-1 (C) but virtually negative for nesprin-2 (D). “N” = nerve, “Cap” = external capsule of the spindle.
Figure 7.
Levels of both Sun proteins reflect total nesprin levels at the NE in muscle, but are not dependent on whether nesprin-1 or nesprin-2 is expressed.
In contrast to Fig 6AB, Sun protein levels at the NE were similar in mature and regenerating fibres (A, B). Small regenerating fibres have central nuclei, but two comparable peripheral nuclei are visible (white arrows). In a muscle spindle, the muscle fibre nuclei (inner white circle) are positive for both nesprin-1 and Sun proteins, while nuclei in the capsule (outer circle, DAPI not shown; cf Fig. 6CD) are weak or negative for both. These results were obtained with both Sun1 and Sun2 though only one is illustrated. Bar (white) = 100μm
Compared with dermal fibroblasts, expression of giant nesprins was much weaker on western blots of primary human skeletal myoblasts (Fig. 1A). The muscle-specific nesprin-2-α-1 isoform was not detected either, even in cultures containing differentiated myotubes (Fig. 8H). Post-mitotic myotubes are differentiated in so far as they express muscle-specific genes like creatine kinase and myosin, but, without innervation, they never achieve a fully mature pattern of muscle gene expression. In human myoblasts and myotubes in cell culture, nesprin-1 staining was concentrated in the nucleus (Fig. 8B, D). In multinucleated myotubes, nesprin-1 staining was always strong, as reported in C2C12 cells (Mislow et al, 2002a). Nesprin-2 staining of cultured muscle cell nuclei was much weaker than nesprin-1 (Fig. 8E, F), consistent with the in vivo situation, where nesprin-2 is weak in immature fibre nuclei (Fig. 6B). This difference between nesprin-1 and nesprin-2 is also seen on western blots of myotube cultures (Fig. 8H), where nesprin-1-giant is strongly expressed, while nesprin-2 levels remain as low as in the myoblast cultures shown in Fig. 1A.
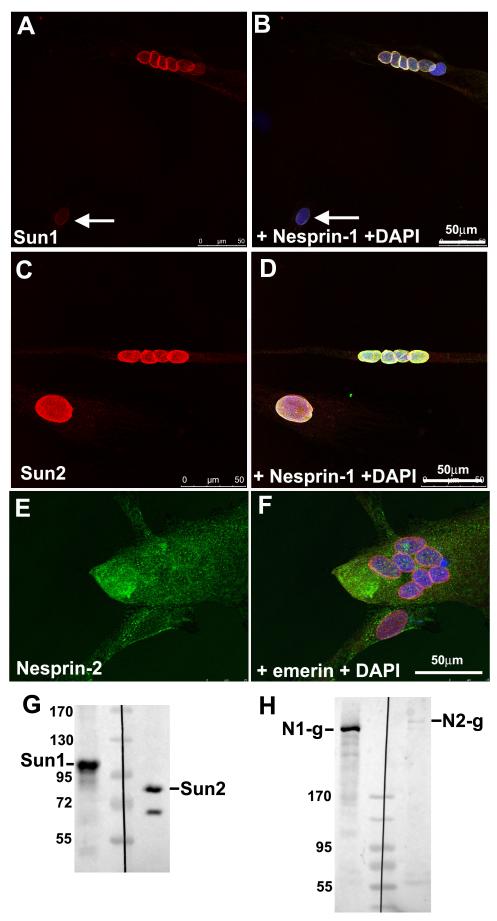
Figure 8.
Differentiating human myoblast cultures have nesprin-1 and both Sun proteins at the nuclear envelope, but very little nesprin-2.
Myoblast cultures undergoing differentiation were double-stained with anti-Sun1 serum (A: red) and MANNES1A (B: green with DAPI), or with anti-Sun2 serum (C: red) and MANNES2A (D: green with DAPI). Multinucleate myotube nuclear envelopes were always strongly positive for nesprin-1 and both Sun proteins, but mononucleated myoblasts were variable; sometimes very weak for both nesprin-1 and Sun protein (white arrows in A, B) and sometimes very strong for both (C, D). These results were obtained with both Sun1 and Sun2 though only one is illustrated. Nesprin-2 staining at the NE, however, was very weak, even in multinucleated myotubes (E: MANNES2A, green, F: anti-emerin serum, red with DAPI). Western blots confirm the presence of both Sun proteins (G) and confirm the increase in nesprin-1, but not nesprin-2, in myotubes (H), relative to myoblasts in Fig. 1A.
Nesprin-1 staining in mononucleate cells of myoblast cultures showed variable intensity at all culture times (cf B and D in Fig. 8) which might be due to the presence of myofibroblasts or of differentiated mononucleate cells. However, the variability occurred in both desmin-positive myoblasts and in desmin–negative “fibroblastic” cells and there was no direct correlation with cell cycle withdrawal (Ki-67 staining) associated with myogenic differentiation (data not shown). Sun protein levels at the NE of individual nuclei followed the levels of nesprin-1 staining (Fig. 8A, C), both Sun1 and Sun2 being strongly represented in western blots (Fig. 8G).
Non-nuclear nesprins
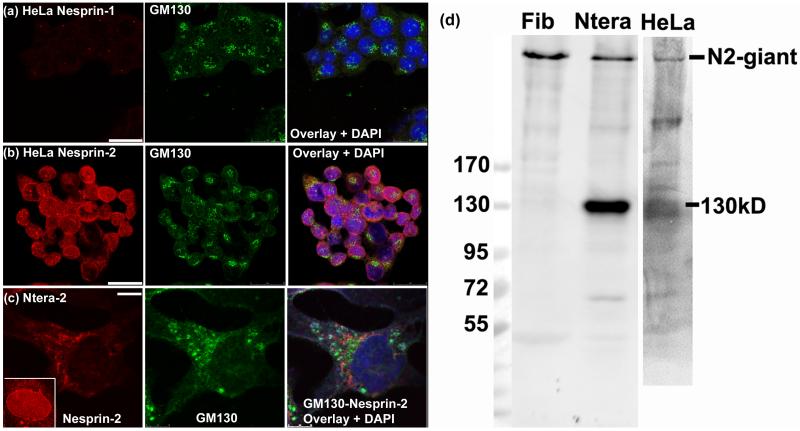
In HeLa cells, nesprin-1 immunostaining was strikingly absent (Fig. 9a) (only the centrosome cross-reaction is visible), while nesprin-2 staining was mainly cytoplasmic or perinuclear (Fig. 9b). In Ntera-2 cells, nesprin-2 staining were sometimes concentrated in a region of perinuclear cytoplasm occupied by the Golgi apparatus (Fig. 9c) with weaker nuclear rim staining, and this was interesting in view of earlier reports of nesprins in Golgi (Gough et al, 2000). Other cells in the same culture showed typical nesprin localization at the nuclear rim (Fig. 9c inset). Western blots confirmed the presence of nesprin-2-giant in Ntera-2 and HeLa (Fig. 9d), but Ntera-2 had a dominant additional band at 130kD, which is an authentic nesprin-2 because it was recognized by the full panel of mAbs (Fig. 2C).
Figure 9.
Nesprin-2 is cytoplasmic or perinuclear in HeLa and in Ntera-2 cells, which express a novel 130kD form.
In HeLa cells, (a) nesprin-1 is absent and (b) nesprin-2 is perinuclear in many cells, though some show nuclear rim staining. (c) In the Ntera-2 human neurogenic cell line, nesprin-2 is sometimes perinuclear and close to the Golgi apparatus, but does not colocalize with it. The inset shows that many Ntera-2 nuclei have more typical nuclear rim staining for nesprin-2. Cells fixed with acetone-methanol were used for double-label with rabbit anti-GM130 antibody and either MANNES1A or MANNES2A mouse mAb (1:3 dilution). Bars (white) = 25μm
For the western blot, all cell pellets were extracted under conditions designed to minimize proteolysis. Dermal fibroblasts (Fib) have a single band at 800kD with only traces of possible degradation products. Ntera cells have a clear main band at 130kD that cannot be attributed to degradation, but corresponds to no known isoform. HeLa cells also produce lower Mr nesprins (a separate blot was aligned using the EZ-run Mr markers).
DISCUSSION
What isoforms of nesprins are expressed as proteins?
Table 3 summarises our findings on nesprin isoform expression. The giant isoforms of both nesprins are clearly the dominant proteins in dermal fibroblasts (Fig. 1A), though the 400kD band could be either nesprin-1-β (380kD) or a degradation product of nesprin-1-giant. In skeletal muscle, the dystrophin blot (Fig. 1B) indicates that some limited proteolysis has occurred. However, unless nesprins are much more sensitive to proteolysis than dystrophin, the lower Mr nesprin bands are unlikely to derive from giant isoforms expressed at very low levels. For the same reason, the 130kD nesprin-2 in Ntera-2 cells (Fig. 9d) is more likely to be a novel isoform than a degradation product. The principal nesprins in skeletal muscle are therefore nesprin-1-α and nesprin-1-β (Fig.1A) and nesprin-2-α1, nesprin-2-α2 and nesprin-2-γ (Fig. 1B). We have shown how multi-epitope panels of mAbs can be used to distinguish cross-reactions (e.g. the 300kD protein recognized by MANNES2E only in Fig. 2A) from authentic nesprin gene products. Distinguishing authentic short isoforms from degradation products of larger nesprins remains a problem, not only in the present study, but also in all studies on nesprin-2 western blots published so far (Zhang, 2005, 2007a, Kandert, 2007, Luke 2008). Zhang et al (2005) studied fibroblasts, vascular smooth muscle cells and C2C12 mouse skeletal myoblasts and only the 800kD nesprin-2-giant could be identified with complete certainty, a prominent 140kD band being also present. Kandert et al (2007) also detected bands at 250kD and 140kD in skin fibroblasts, while Luke et al (2008) detected bands of 140kD and 60kD in keratinocytes using a C-terminal nesprin-2 antibody. We did not detect a clear nesprin-2-β (88kD) band in any human cells or tissues studied here, although this isoform was prominent in mouse C2C12 myoblasts (Zhang et al, 2005) and we cannot exclude low level expression.
TABLE 3.
Differential expression of nesprin isoforms in different cell types.
This is based on western blots in Figs 1, 4 &10 (and absence of nesprin-1 immunostaining in HeLa). N1g and N2g are the giant isoforms of nesprin-1 and nesprin-2 respectively. Intensity is graded from + to ++++ and the dominant isoforms are shown in boldface. w = weak and (?) indicates a band that cannot be reliably distinguished from a degradation product of a higher Mr isoform. nd = present, but isoform not determined.
| N1g | N1β | N1α | N2g | N2γ | N130 | N2α1 | N2α2 | |
|---|---|---|---|---|---|---|---|---|
| Adult Muscle | + | ++(?) | +++ | + | +++ | − | ++++ | +++ |
| Myoblasts | w | − | w(?) | w | − | − | − | − |
| Myotubes | +++ | w | ||||||
| Fibroblasts | ++++ | ++(?) | − | ++++ | − | − | − | w(?) |
| Ntera-2 | nd | nd | nd | +++ | w(?) | ++++ | w(?) | w(?) |
| HeLa | − | − | − | ++ | +(?) | +(?) | w(?) | w(?) |
Developmental changes in skeletal muscle
Current evidence suggests that giant forms of nesprins are primarily ONM proteins involved in nuclear positioning within the cell by forming LINC complexes with the cytoskeleton (Starr and Han, 2003; Crisp et al, 2006). They may also be involved in the perinuclear positioning of the Golgi apparatus (Gough et al, 2003). We have found that primary human dermal fibroblasts express large amounts of both nesprins, almost exclusively as the giant isoforms, whereas primary human myoblasts have only low levels of nesprins, consistent with nuclear mobility during multinucleate myotube formation (Fig. 1). Indeed, nesprin-2 was undetectable at the NE in early myotubes and in most myoblasts, the small amounts being mainly cytoplasmic (Fig. 8). Nesprin-1 at the NE was initially variable in cultured myoblasts, but all myotube nuclei became positive after cell fusion. Comparison of mature and immature fibres in adult human muscle suggests that nesprin-1 dominates the NE at first but is partly replaced by nesprin-2 in fully mature fibre nuclei. This is reminiscent of the switch to mature patterns of gene expression that occurs in later fetal development but not in cell culture (Barbet et al, 1991). Nuclear positioning by giant nesprins at the ONM appears to be primarily a function of nesprin-1 in muscle, since it was affected by knockout of nesprin-1 but not by knockout of nesprin-2 (Zhang et al, 2007b); this is consistent with our evidence that nesprin-1 dominates the myonuclear envelope at early stages of myogenesis (Fig. 8). Myonuclear positioning is also affected when the nesprin-1 LINC complex is specifically disrupted in a transgenic mouse (Puckelwartz et al, 2009). Strikingly, the increased nesprin levels in adult muscle NEs are mainly attributable to shorter nesprin isoforms, which lack actin-binding domains. The increase in nesprin-2 in mature fibres could be due, in part, to the developmentally regulated expression of the muscle-specific nesprin-2-α-1 isoform (Zhang et al, 2005). The function of these shorter isoforms is less clear, though it is generally accepted that some of them occur, at least partly, at the INM where they interact with emerin and lamin A/C (Mislow et al. 2002b; Zhang et al, 2005). Emerin, lamin A/C and nesprin genes have all been linked to Emery-Dreifuss muscular dystrophy (Zhang et al, 2007a), a condition that affects skeletal and cardiac muscle specifically, so it will be of interest to determine whether the abundance of short nesprin isoforms is characteristic of striated muscle.
In the Sol8 mouse myoblast cell line, nesprin-1 at the NE in myotubes was much higher than in myoblasts (Apel et al, 2000), as in the present study. A shift from nucleus to cytoplasm, observed for both nesprin-1 (Zhang et al, 2001) and nesprin-2 (Zhang et al, 2005) when C2C12 myoblasts fused to form myotubes, may be specific for this cell line. Gene expression patterns in permanent myogenic cell lines may differ significantly from the primary human muscle cell cultures used in the present study.
Interdependency of nesprins and Sun proteins
Our data indicate a relationship between total nesprin levels at the NE and total Sun levels at the NE, consistent with anchorage of nesprins by KASH domain interaction with Sun proteins. When both nesprins were at low levels or absent, both Sun proteins were also low or absent (Figs. 7 and 8). When only nesprin-1 was present at the NE, both Sun proteins were present and their levels correlated with nesprin-1 levels (Fig. 7 and 8). From these data, at least, there is no need to invoke the theoretical possibility of Sun-independent anchorage, mediated, for example, by emerin or lamin A/C.
Interdependency of emerin and nesprin-2
The behaviour of nesprin-2 in emerin-negative cells appears to reflect significant differences in function both between nesprin-1 and nesprin-2 and between nesprin-2 in different cell types. Whereas lack of lamin A/C causes redistribution of emerin and both nesprins into the ER, lack of emerin caused dispersion of nesprin-2 from the NE, but into the cytoplasm rather than the ER, leaving both nesprin-1 and lamin A/C unaffected (Fig. 3). These results show that the two nesprins interact differently with emerin in dermal fibroblasts. These interactions are cell-type-dependent, since loss of nesprin-2 from the NE did not occur in emerin-negative skin keratinocytes (Fig. 5), a cell-type that lacks nesprin-1 at the NE. In a nesprin-2-giant knockout mouse, emerin was retained at keratinocyte NEs lacking both nesprin-1 and nesprin-2 (Luke, 2008). In the same study, emerin was lost from dermal fibroblast NEs lacking nesprin-2, even though nesprin-1 was present (Luke et al 2008). Combined with our current data, it thus seems that nesprin-2-giant and emerin are mutually dependent for both NE and ER localization in dermal fibroblasts, though not in keratinocytes. Nesprin-1 and emerin do not require each other for NE localization in either cell type. Loss of nesprin-2 is not mediated by loss of Sun proteins, which remained at their normal level at the NE (Fig. 3H). Although conventional wisdom would suggest that emerin-nesprin interactions must occur at the INM, a recent study suggesting that emerin can also function at the ONM should be kept in mind (Salpingidou et al, 2007). NE localization of shorter nesprin-2 isoforms requires nesprin-2-giant in skin cell nuclei, but not in skeletal muscle (Luke et al, 2008), possibly because the abundant expression of short isoforms in muscle (Fig. 1) is at least partly at the INM, where they can be retained by emerin or lamin A/C rather than nesprin-2-giant.
Localization and orientation of nesprins
The lateral diffusion model for localization of INM proteins would suggest that only the short nesprin 2-α forms can reach the INM, if a strict size limitation of around 60kD applies (Soullam and Worman, 1995). There is evidence that the last two spectrin repeats, present in all nesprin isoforms, can interact directly with lamin A/C (Mislow, 2002b; Libotte, 2005), though the sequence identified for emerin-binding (Wheeler et al, 2007) is absent from nesprin-2-α. The experimental evidence for large nesprins at the INM is still being evaluated (Libotte, 2005; Crisp, 2006). Even though transport of giant nesprins through the nuclear pores is possible in theory, with or without their own NLS, it is currently difficult to envisage how this could be achieved with proteins that are also firmly located in ER membranes. These problems of nesprin orientation have been reviewed in greater detail elsewhere (Morris and Randles, 2009).
Ntera-2 cells sometimes showed perinuclear staining for nesprin-2 in the cytoplasm, with a spatial relationship to the Golgi apparatus. Although it differs from the co-localization of nesprin-1 with Golgi reported in bovine kidney cells (Gough et al, 2003), this partial overlap with the Golgi looks very similar to that observed by Kobayashi et al (2006) using N-terminal antibodies that recognize only nesprin-1-giant and GSRP-56. Several non-nuclear membrane locations for nesprins in cultured cells have been reported by Zhang et al (2005) and the Ntera-2 example in the present study is one in which the possibility of a cross-reacting, non-nesprin protein has been ruled out.
MATERIALS AND METHODS
Antibody production
Human full-length nesprin-1α cDNA encoding 113kD of protein was cloned into pGEX-4T1, as previously described (Mislow, 2002a). Human nesprin-2 cDNA encoding 38kD of C-terminal protein sequence (nt 19831-20853) was amplified from a human muscle cDNA library (Clontech Matchmaker) using forward and reverse primers with restriction sites (underlined) added for cloning into pET21b: 5′-AGGATCCAGCACAGGAGCTTCACAATA -3′ and 5′-ATCTCGAGCATTGGTGTACCTCAGCAT -3. After transformation of E. coli BL21(DE3) and induction with IPTG, bacterial pellets were washed by sonication in TNE buffer and recombinant protein was extracted by sonication in 6M urea in PBS. Both proteins were purified by His-tag column chromatography in 6M urea and were used as immunogens for production of monoclonal antibodies using the hybridoma method (Nguyen thi Man and Morris, 2002). For nesprin-1 mAbs, Balb/c mice were immunized with a 139kD recombinant GST-fusion protein containing full-length nesprin-1-α sequence, plus an N-terminal extension into the nesprin-1-β sequence by 121 amino-acids. Seven mAbs were produced, none of which cross-reacted with the corresponding recombinant nesprin-2 sequence (Table 1). For nesprin-2 mAbs, a thioredoxin-fusion protein with a 38kD C-terminal fragment of nesprin-2 was produced using PCR-amplified cDNA from a human skeletal muscle library. All mAbs recognised the corresponding recombinant protein on western blots (data not shown). Variations from the published nesprin-2 sequence were two “third-base” polymorphisms, plus absence of two whole triplets that encoded glutamine and asparagine and occur as known variants in GenBank. Thirteen mAbs, MANNES2A-M, were produced, only one of which (MANNES2B) cross-reacted with the corresponding nesprin-1 protein (Table 1).
Epitope mapping
It is important to establish that mAbs recognize different epitopes on nesprin antigens, because this makes it possible to distinguish authentic nesprin from the non-specific cross-reactions that can occur with any single mAb. The seven nesprin-1 mAbs could be separated into three epitope groups by their species-specificity alone, since four reacted with human, mouse, rat and rabbit, one with human, mouse and rat, and two were human-specific (Table 1). MANNES1A is the only member of this mAb panel that immunostains the centrosome in cultured human cells, probably as a result of a cross-reaction. This increases the number of different epitopes to at least four. None of the nesprin-1 mAbs recognized phage-displayed peptides, possibly reflecting some conformation dependence. The 13 nesprin-2 mAbs gave three different patterns on western blots of a partial trypsin-digest of the recombinant fusion protein, suggesting three different epitope groups (data not shown). This result was confirmed by epitope mapping with phage-displayed peptide libraries, which enabled precise location of three epitopes for MANNES2A and F, MANNES2E and MANNES2G, all in the middle of the nesprin-2-α sequence (Table 2). Other mAbs did not bind these peptides and therefore recognize different epitopes. MANNES2B recognizes an epitope shared with nesprin-1, so at least seven different epitopes are represented in the nesprin-2 panel.
TABLE 2.
Identification of antibody binding sites on nesprin-2 using a phage-displayed peptide library.
The sequences of the phage-displayed peptides selected from a random 15-mer library (Scott and Smith, 1990) by each antibody are aligned with the corresponding sequence in nesprin-2 and the matched amino-acids are underlined in the nesprin sequence and shown in bold face in the phage sequence. The other 9 nesprin-2 mAbs did not bind any of these peptides and the nesprin-1 mAbs did not select any peptides from the library.
| Antibody Name | Phage-displayed peptide (matches in boldface) |
Nesprin-2 sequence (matches underlined) |
Epitope |
|---|---|---|---|
| MANNES2A | RSPFYVDLLSRGGLI | GAVDSWRGGLRQS | aa6653-56 |
| MANNES2F | RSPFYVDLLSRGGLI | GAVDSWRGGLRQS | aa6653-56 |
| MANNES2E | ESHGLLELASWLDL | SQNLLLWLASAKN | aa6674-79 |
| MANNES2G | (1) WYLCVSDPRCVLELT | TDPKADPRALLECR | aa6694-701 |
| (2)FWADPRSLLLVSPRV | |||
(note for printer: maintain alignment of last two lines in Courier font)
Epitope mapping using phage-displayed peptide libraries was performed as previously described in detail (Pereboev and Morris, 1996). Briefly, a mixture of all nesprin-1 or nesprin-2 mAbs, diluted 1:50 with Tris-buffered saline (TBS), was immobilized onto sterile 35mm Petri dishes coated with 1ml of 1:200 dilution of rabbit-anti-[mouse Ig] in TBS (Dako, Denmark). Biopanning was performed using a 15-mer peptide library in phage f88-4 maintained in the K91Kan strain of Escherichia coli, generously supplied by G. P. Smith (University of Missouri). Any remaining binding sites on the dishes were blocked using 4% BSA in sterile TBS. The phage library was pre-incubated in dishes coated with the rabbit-anti-mouse antibodies alone to ensure any binding was specific for nesprin mAbs. Following the first round of biopanning, the bound phage were eluted and amplified by infection of K91Kan E. coli cells. Two or three rounds of biopanning were performed. Colonies of the selected phage infected cells were then grown on nitrocellulose membranes and screened with mAbs to reveal positive clones. Phage DNA was purified and sequenced using a primer with 5′ – 3′ sequence: AGT AGC AGA AGC CTG AAG A.
SDS-polyacrylamide gel electrophoresis and western blotting
Cell pellets and muscle samples were left on ice with protease inhibitors (Sigma, P8340) in DMSO and PMSF in ethanol for 10 min. (to allow penetration) before adding SDS extraction buffer (2% sodium dodecyl sulphate-SDS, 5% 2-mercaptoethanol, 62.5 mM Tris-HCl, pH 6.8), sonicating to shear DNA and boiling for 2 min. Proteins (30 μg) were subjected to SDS-PAGE using 10% polyacrylamide or 3-15% gradient gels and transferred to nitrocellulose membranes. After blocking non-specific sites with powdered milk solution, membranes were incubated with primary monoclonal antibodies at a dilution of 1/100 of the culture supernatant in PBS. Antibody reacting bands were visualized by development with peroxidase-labelled goat anti-mouse Ig (1μg/ml in PBS, 1% FBS, 1%HS, 0.1% BSA) and a chemiluminescent detection system (West Pico or West Femto, Pierce).
Biopsies and eukaryotic cell culture
Myoblasts were isolated from the quadriceps muscle of a 5-day-old infant in accordance with the French legislation on ethical rules and established in culture as described previously. (Furling et al, 2003). Human myoblasts were grown in DMEM (Invitrogen, Paisley, UK) supplemented with 20% decomplemented fetal bovine serum (FBS; Invitrogen) and antibiotics. To promote differentiation, growth medium was changed to DMEM without serum. Human skin fibroblasts (Coriell Cell Bank Cat. No. 8333), HeLa, Ntera-2 and COS-7 (monkey) were also grown in DMEM with 10-20% FBS and antibiotics. All cultures were incubated at 37 °C in a humid air atmosphere containing 5 % CO2. After the incubation period, cells grown on untreated glass coverslips were fixed with 50:50 acetone-methanol for 5 minutes and air-dried.
Immunohistochemistry
For double labeling, fixed cells on coverslips were incubated with polyclonal antisera for 1h at 20°C, washed 4 times with PBS and incubated with mAb for 1h at 20°C. Coverslips were then washed four times and incubated for 1h with a mixture of 5μg ml−1 goat anti-rabbit ALEXA 488 and 5μg ml−1 goat anti-mouse ALEXA 546 (Molecular Probes, Eugene, Oregon, USA) diluted in PBS containing 1% horse serum, 1% fetal bovine serum and 0.1% BSA. DAPI (diamidinophenylindole 200 ng ml−1) was added for the final 5 mins of incubation to counterstain nuclei. After washing, coverslips were mounted on slides in Hydromount (Merck). High magnification images were obtained using a Leica SP5 confocal microscope with a 63x oil-immersion objective and low magnification images of muscle sections with an Olympus fluorescence photomicroscope with 20x or 40x objectives.
Rabbit antisera used were against emerin (Holt at al, 2003), GM130 (New England Biolabs #2296), Sun1 (anti-UNC84A, Sigma) and Sun2 (a generous gift from Dr. Sue Shackleton, University of Leicester, UK). Research protocols were approved by the RJAH Orthopaedic Hospital Research Committee. Human tissue sections and cell lines were obtained with the appropriate informed consent and ethical approval.
ACKNOWLEDGEMENTS
This work was supported by a Translational Infrastructure Grant from the Muscular Dystrophy Association (USA) to GEM. KNR thanks the Higher Education Funding Council (Wales) for a University of Wales studentship and Dr. Jane Wakeman (University of Bangor) for her support. MW was supported by a grant from the German Network of Muscular Dystrophies (MD-NET, 01GM0302) funded by the German ministry of education and research (BMBF). EAM was funded by NIH grant HL92433, Muscular Dystrophy Association (USA) and the Doris Duke Charitable Foundation. DF thanks the Human Cell Culture Platform of the Institut de Myologie, Paris. We thank Colin Stewart (NCI, Frederick, MD) for lmna -/- MEFs, George Smith (University of Missouri) for phage-displayed peptide libraries, and Sue Shackleton (University of Leicester) for anti-Sun2 and for recommending anti-UNC84A for Sun1.
Abbreviations
- X-EDMD
X-linked Emery-Dreifuss muscular dystrophy
- ONM
outer nuclear membrane
- INM
inner nuclear membrane
- NE
nuclear envelope
- ER
endoplasmic reticulum
- KASH
Klarsicht-ANC-Syne homology
- CH
calponin homology
REFERENCES
- Apel ED, Lewis RM, Grady RM, Sanes JR. Syne-1, a dystrophin- and Klarsicht-related protein associated with synaptic nuclei at the neuromuscular junction. J. Biol. Chem. 2000;275:31986–31995. doi: 10.1074/jbc.M004775200. [DOI] [PubMed] [Google Scholar]
- Barbet JP, Thornell LE, Butler-Browne GS. Immunocytochemical characterisation of two generations of fibers during the development of the human quadriceps muscle. Mech Dev. 1991;35:3–11. doi: 10.1016/0925-4773(91)90036-6. [DOI] [PubMed] [Google Scholar]
- Crisp M, Liu Q, Roux K, Rattner JB, Shanahan C, Burke B, Stahl PD, Hodzic D. Coupling of the nucleus and cytoplasm: role of the LINC complex. J. Cell Biol. 2006;172:41–53. doi: 10.1083/jcb.200509124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furling D, Lam LT, Agbulut O, Butler-Browne GS, Morris GE. Changes in myotonic dystrophy protein kinase levels and muscle development in congenital myotonic dystrophy. Am J Pathol. 2003;162:1001–1009. doi: 10.1016/s0002-9440(10)63894-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough LL, Fan J, Chu S, Winnick S, Beck KA. Golgi Localization of Syne-1. Mol. Biol. Cell. 2003;14:2410–2424. doi: 10.1091/mbc.E02-07-0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque F, Lloyd DJ, Smallwood DT, Dent CL, Shanahan CM, Fry AM, Trembath RC, Shackleton S. SUN1 interacts with nuclear lamin A and cytoplasmic nesprins to provide a physical connection between the nuclear lamina and the cytoskeleton. Mol. Cell. Biol. 2006;26:3738–3751. doi: 10.1128/MCB.26.10.3738-3751.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt I, Östlund C, Stewart CL, Nguyen thi Man, Worman HJ, Morris GE. The effect of pathogenic missense mutations in lamin A on its interaction with emerin in vivo. J. Cell Sci. 2003;116:3027–3035. doi: 10.1242/jcs.00599. [DOI] [PubMed] [Google Scholar]
- Hori S, Ohtani S, Nguyen thi Man, Morris GE. The N-terminal half of dystrophin is protected from proteolysis in situ. Biochem. Biophys. Res. Commun. 1995;209:1062–1067. doi: 10.1006/bbrc.1995.1605. [DOI] [PubMed] [Google Scholar]
- Jin H, Tan S, Hermanowski J, Böhm S, Pacheco S, McCauley JM, Greener MJ, Hinits Y, Hughes SM, Sharpe PT, Roberts RG. The dystrotelin, dystrophin and dystrobrevin superfamily: new paralogues and old isoforms. BMC Genomics. 2007;8:19. doi: 10.1186/1471-2164-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandert S, et al. Nesprin-2 giant safeguards nuclear envelope architecture in LMNA S143F progeria cells. Hum. Mol. Genet. 2007;16:2944–2959. doi: 10.1093/hmg/ddm255. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Katanosaka Y, Iwata Y, Matsuoka M, Shigekawa M, Wakabayashi S. Identification and characterization of GSRP-56, a novel Golgi-localized spectrin repeat-containing protein. Exp Cell Res. 2006;312:3152–3164. doi: 10.1016/j.yexcr.2006.06.026. [DOI] [PubMed] [Google Scholar]
- Libotte T, et al. Lamin A/C-dependent localization of Nesprin-2, a giant scaffolder at the nuclear envelope. Mol. Biol. Cell. 2005;16:3411–3424. doi: 10.1091/mbc.E04-11-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüke Y, et al. Nesprin-2 Giant (NUANCE) maintains nuclear envelope architecture and composition in skin. J Cell Sci. 2008;121:1887–1898. doi: 10.1242/jcs.019075. [DOI] [PubMed] [Google Scholar]
- Manilal S, Randles KN, Aunac C, Nguyen thi Man, Morris GE. A lamin A/C beta-strand containing the site of lipodystrophy mutations is a major surface epitope for a new panel of monoclonal antibodies. Biochim. Biophys. Acta. 2004;1671:87–92. doi: 10.1016/j.bbagen.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Manilal S, Nguyen thi Man, Sewry CA, Morris GE. The Emery-Dreifuss muscular dystrophy protein, emerin, is a nuclear membrane protein. Hum. Mol. Genet. 1996;5:801–808. doi: 10.1093/hmg/5.6.801. [DOI] [PubMed] [Google Scholar]
- Mislow JM, Kim MS, Davis DB, McNally EM. Myne-1, a spectrin repeat transmembrane protein of the myocyte inner nuclear membrane, interacts with lamin A/C. J. Cell Sci. 2002a;115:61–70. doi: 10.1242/jcs.115.1.61. [DOI] [PubMed] [Google Scholar]
- Mislow JM, Holaska JM, Kim MS, Lee KK, Segura-Totten M, Wilson KL, McNally EM. Nesprin-1alpha self-associates and binds directly to emerin and lamin A in vitro. FEBS Lett. 2002b;525:135–140. doi: 10.1016/s0014-5793(02)03105-8. [DOI] [PubMed] [Google Scholar]
- Morris GE. The role of the nuclear envelope in Emery-Dreifuss muscular dystrophy. Trends Mol. Med. 2001;7:572–577. doi: 10.1016/s1471-4914(01)02128-1. [DOI] [PubMed] [Google Scholar]
- Morris GE, Randles KN. Nesprin isoforms: are they inside or outside the nucleus? Biochem. Soc. Trans. 2009 doi: 10.1042/BST0380278. in press. [DOI] [PubMed] [Google Scholar]
- Nguyen thi Man, Cartwright AJ, Morris GE, Love DR, Bloomfield JF, Davies KE. Monoclonal antibodies against defined regions of the muscular dystrophy protein, dystrophin. FEBS Lett. 1990;262:237–240. doi: 10.1016/0014-5793(90)80199-s. [DOI] [PubMed] [Google Scholar]
- Nguyen thi Man, Morris GE. A rapid method for generating large numbers of high-affinity monoclonal antibodies from a single mouse. In: Walker JM, editor. The Protein Protocols Handbook. 2nd Edn Humana Press; Totowa, NJ: 2002. pp. 1129–1138. [Google Scholar]
- Padmakumar VC, Abraham S, Braune S, Noegel AA, Tunggal B, Karakesisoglou I, Korenbaum E. Enaptin, a giant actin-binding protein, is an element of the nuclear membrane and the actin cytoskeleton. Exp. Cell Res. 2004;295:330–339. doi: 10.1016/j.yexcr.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Padmakumar VC, Libotte T, Lu W, Zaim H, Abraham S, Noegel AA, Gotzmann J, Foisner R, Karakesisoglou I. The inner nuclear membrane protein Sun1 mediates the anchorage of Nesprin-2 to the nuclear envelope. J. Cell Sci. 2005;118:3419–3430. doi: 10.1242/jcs.02471. [DOI] [PubMed] [Google Scholar]
- Pereboev A, Morris GE. Reiterative screening of phage display peptide libraries for epitope mapping. In: Morris GE, editor. Epitope Mapping Protocols. Humana Press; Totowa, NJ: 1996. pp. 195–206. [DOI] [PubMed] [Google Scholar]
- Puckelwartz MJ, et al. Disruption of nesprin-1 produces an Emery Dreifuss muscular dystrophy-like phenotype in mice. Hum Mol Genet. 2009;18:607–620. doi: 10.1093/hmg/ddn386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randles KN. Ph.D. thesis. University of Wales; UK: 2008. Nesprins in nuclear organisation and disease. [Google Scholar]
- Roux KJ, Crisp ML, Liu Q, Kim D, Kozlov S, Stewart CL, Burke B. Nesprin-4 is an outer nuclear membrane protein that can induce kinesin-mediated cell polarization. Proc Natl Acad Sci U S A. 2009;106:2194–2199. doi: 10.1073/pnas.0808602106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salpingidou G, Smertenko A, Hausmanowa-Petrucewicz I, Hussey PJ, Hutchison CJ. A novel role for the nuclear membrane protein emerin in association of the centrosome to the outer nuclear membrane. J Cell Biol. 2007;178:897–904. doi: 10.1083/jcb.200702026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JK, Smith GP. Searching for peptide ligands with an epitope library. Science. 1990;249:386–390. doi: 10.1126/science.1696028. [DOI] [PubMed] [Google Scholar]
- Soullam B, Worman HJ. Signals and structural features involved in integral membrane protein targeting to the inner nuclear membrane. J Cell Biol. 1995;130:15–27. doi: 10.1083/jcb.130.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr DA, Han M. ANChors away: an actin based mechanism of nuclear positioning. J. Cell Sci. 2003;116:211–216. doi: 10.1242/jcs.00248. [DOI] [PubMed] [Google Scholar]
- Sullivan T, Escalante-Alcalde D, Bhatt H, Anver M, Bhat N, Nagashima K, Stewart CL, Burke B. Loss of A-type lamin expression compromises nuclear envelope integrity leading to muscular dystrophy. J. Cell Biol. 1999;147:913–920. doi: 10.1083/jcb.147.5.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren DT, Zhang Q, Weissberg PL, Shanahan CM. Nesprins: intracellular scaffolds that maintain cell architecture and coordinate cell function? Expert Rev. Mol. Med. 2005;7:1–15. doi: 10.1017/S1462399405009294. [DOI] [PubMed] [Google Scholar]
- Wilhelmsen K, Litjens SH, Kuikman I, Tshimbalanga N, Janssen H, van den Bout I, Raymond K, Sonnenberg A. Nesprin-3, a novel outer nuclear membrane protein, associates with the cytoskeletal linker protein plectin. J. Cell Biol. 2005;171:799–810. doi: 10.1083/jcb.200506083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worman HJ, Gundersson GG. Here come the SUNs: a nucleocytoskeletal missing link. Trends Cell Biol. 2006;16:67–69. doi: 10.1016/j.tcb.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Skepper JN, Yang F, Davies J, Hegyi L, Roberts RG, Weissberg PL, Ellis JA, Shanahan CM. Nesprins: a novel family of spectrin-repeat-containing proteins that localize to the nuclear membrane in multiple tissues. J. Cell Sci. 2001;114:4485–4498. doi: 10.1242/jcs.114.24.4485. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Ragnauth C, Greener MJ, Shanahan CM, Roberts RG. The nesprins are giant actin-binding proteins, orthologous to Drosophila melanogaster muscle protein MSP-300. Genomics. 2002;80:473–481. [PubMed] [Google Scholar]
- Zhang Q, Ragnauth CD, Skepper JN, Worth NF, Warren DT, Roberts RG, Weissberg PL, Ellis JA, Shanahan CM. Nesprin-2 is a multi-isomeric protein that binds lamin and emerin at the nuclear envelope and forms a subcellular network in skeletal muscle. J. Cell Sci. 2005;118:673–687. doi: 10.1242/jcs.01642. [DOI] [PubMed] [Google Scholar]
- Zhang Q, et al. Nesprin-1 and -2 are involved in the pathogenesis of Emery Dreifuss muscular dystrophy and are critical for nuclear envelope integrity. Hum Mol Genet. 2007a;16:2816–2833. doi: 10.1093/hmg/ddm238. [DOI] [PubMed] [Google Scholar]
- Zhang X, Xu R, Zhu B, Yang X, Ding X, Duan S, Xu T, Zhuang Y, Han M. Syne-1 and Syne-2 play crucial roles in myonuclear anchorage and motor neuron innervation. Development. 2007b;134:901–908. doi: 10.1242/dev.02783. [DOI] [PubMed] [Google Scholar]
- Zhen YY, Libotte T, Munck M, Noegel AA, Korenbaum E. NUANCE, a giant protein connecting the nucleus and actin cytoskeleton. J. Cell Sci. 2002;115:3207–3222. doi: 10.1242/jcs.115.15.3207. [DOI] [PubMed] [Google Scholar]