Abstract
Neurodegenerative diseases have been intensively studied, but a comprehensive understanding of their pathogenesis remains elusive. An increasing body of evidence suggests that non-cell autonomous processes play critical roles during the initiation, and spatiotemporal progression or propagation of the dominant pathology. Here we review findings highlighting the importance of pathological cell-cell communication in neurodegenerative disease. We focus primarily on the accumulating evidence suggesting dysfunctional cross-talk between neurons and astroglia, neurons and innate immune system cells, as well as cellular processes leading to transmission of pathogenic proteins between cells. Insights into the complex intercellular perturbations underlying neurodegeneration will enhance our efforts to develop effective therapeutic approaches for preventing or reversing symptomatic progression in this devastating class of human diseases.
Introduction
Despite intensive study over the past three decades, neurodegenerative diseases remain insufficiently understood, precluding rational design of therapeutic interventions that can reverse or even arrest the progressive loss of neurological function. The identification and study of genetic mutations responsible for numerous neurodegenerative syndromes have led to several compelling theories on disease pathogenesis. Some of these theories, such as those involving a central role for protein misfolding, mitochondrial dysfunction, oxidative stress, excitotoxicity, and transcriptional dysregulation, have been proposed for a wide variety of neurodegenerative disorders. Data supporting a role for each of these pathogenic processes in a variety of clinical syndromes has been generated from primary patient material (usually post mortem), in vitro cell culture and primary neuron models, invertebrate and vertebrate model systems, and most recently, induced pluripotent stem cell modeling. These wide-ranging disease studies, coupled with powerful model systems, have yielded a wealth of information regarding pathways of neuronal demise in the face of mutant gene expression, and have revealed neuroprotective mechanisms that effectively counter pathogenic cellular processes.
However, even with this wealth of information, effective interventions for these diseases remain agonizingly elusive. Why? One potential answer to this question is that experimentation into the basis of neuronal degeneration and death has generated hypotheses of pathogenesis that are overly focused on: 1) late stage events, and 2) events that are best modeled in isolated neurons. For example, many groups have promoted the idea that protein misfolding is a critical step in neurodegeneration. This theory posits that once the capacity of a neuron to handle misfolded proteins is exceeded, mitochondrial dysfunction results, thereby promoting increased oxidative stress – which in turn promotes the further accumulation of damaged proteins that must be handled by an already overwhelmed protein degradation system (Saxena and Caroni, 2011; Williams and Paulson, 2008). Under this “proteinopathy” theory, interventions that would enhance the capacity of neurons to handle misfolded proteins, increase mitochondrial reserves, or reduce oxidative stress should prevent neurodegeneration. Unfortunately, the proposed “proteinopathy” cascade of events cannot explain a number of important factors critical for understanding neurodegeneration. For example, mutant proteins are ubiquitously expressed by neurons (and typically non-neuronal cells in the CNS), yet for each disorder, neurodegeneration occurs in selectively vulnerable cell populations. If a common molecular cascade can explain pathogenesis, why then are certain types of neurons more vulnerable than others? Furthermore, most neurodegenerative disease, associated with protein misfolding, develops in middle or late adulthood, but the responsible proteins are expressed throughout the patient’s lifetime. How does age or time influence the pathogenic potential of a mutant or misfolded protein that characterizes a specific disease? Some speculate that the proteinopathy cascade may manifest in vivo only during the final stages of the degenerative process. Thus, the anatomic, functional or age-dependent features that drive the proteinopathy cascade in subsets of neurons at a specific time remain undefined.
One hypothesis potentially explaining how neurodegenerative diseases are initiated in their characteristic patterns was adopted from the study of cancer. The “multi-hit” theory of carcinogenesis addresses a number of key features of this disease, including the increased incidence of cancer with age, and the clear influence of both genetic background and environmental exposures. That neurodegenerative disorders are similarly initiated by a combination of acquired and inherited cellular/molecular abnormalities has been proposed to explain the epidemiology of sporadic disease (Mahley et al., 2007; Sulzer, 2007). We hypothesize that a multi-hit paradigm involving the impact of synergistic forms of cellular dysfunction via cell-cell interaction may account for both age dependence and regional specificity of neurodegeneration for a specific disorder. A corollary to this hypothesis is that disease-causing mutations result in cell type specific dysfunctions, which individually do not cause the full spectrum of disease symptoms, but in concert and over time will result in the distinct patterns of neurological dysfunction and/or neurodegeneration that characterize a given disorder.
Support for this hypothesis is found in numerous studies suggesting that disease pathogenesis in neurodegenerative syndromes involves communication between different cell types. Interacting cell types in different diseases are one unit of organization, defined by certain populations of neurons, surrounding glia, elements of the neurovascular interface, and CNS innate immune system. This hypothesis is consistent with recent, intriguing evidence for the prion-like spread of pathogenic misfolded proteins from cell to cell (Aguzzi and Rajendran, 2009). Given the wide range of non-cell-autonomous mechanisms potentially at play, and the inherent challenge of describing each in great depth, our goal with this review is to rather present a broad overview of select types of cell-cell communication that are disrupted, altered, or co-opted to promote disease pathogenesis in neurodegenerative disease.
Non-cell autonomous degeneration
The majority of human neurodegenerative diseases initially involve a discrete set of selectively vulnerable neurons. Identification of the genetic mutations responsible for familial forms of a variety of neurodegenerative disorders – such as amyotrophic lateral sclerosis (ALS), Parkinson’s Disease (PD), or Alzheimer’s Disease (AD) – has provided keen insights into molecular mechanisms of neuronal injury. However, identifying the toxic gain or loss of function imparted by disease-causing mutations often fails to explain disease phenotypes, because expression of the mutant protein is seldom restricted to the affected neuronal populations. Indeed, when the causal mutant gene product of several inherited neurodegenerative diseases is selectively expressed in the vulnerable neuron populations, some mouse models do not yield the complete disease phenotype (Boillee et al., 2006; Brown et al., 2008; Gu et al., 2007; Yvert et al., 2000). Conversely, widespread expression of disease genes in multiple CNS cell types can recapitulate disease patterns akin to the human disease being modeled, sometimes even when the disease gene is not expressed in the selectively vulnerable population (Garden et al., 2002). Thus selective neuronal vulnerability in neurodegenerative disease likely arises from the complex interactions between interconnected cell types. When the net effect of dysfunction in one CNS cell type is the degeneration of a second neighboring or interconnected cell type, the process is known as “non-cell autonomous” neurodegeneration.
There is strong evidence for non-cell autonomous neurodegeneration in a number of neurological diseases. For example, human transplantation studies in Parkinson’s disease patients have shown that cellular and molecular pathology will develop in healthy neurons grafted into the brains of affected patients (Dawson, 2008). This finding suggests that replacement of selectively vulnerable neuronal populations may not be sufficient to alleviate disease. Several experimental models of inherited neurodegenerative disease provide direct evidence for non-cell autonomous degeneration. These include examples of neurodegeneration induced in one cell type, when the disease gene is restricted to a surrounding or connecting cell, or when selective removal of a disease-causing gene from one cell population prevents toxicity in a second cell population despite continued expression of the mutant protein (Clement et al., 2003; Gu et al., 2005). That selective expression of mutant protiens in surrounding non-neuronal cells (e.g. glia) can induce neurodegeneration has also provided strong experimental evidence supporting the hypothesis of non-cell autonomous pathogenesis (Custer et al., 2006; Lioy et al., 2011). In the following sections, we will discuss evidence supporting the involvement of aberrant cell-cell communication between neurons, neurons and glia, as well as prion-like spreading of aggregate proteins between cells, in neurodegeneration.
Dysfunctional communication between neurons
How does dysfunction in one type of neuron lead to degeneration in a distinct population of neurons? This question can be parsed according to the different types of interactions known to occur between neurons. In general, neural circuits involve pre-synaptic (afferent) input from one population of neurons to a separate population of neurons that comprise the post-synaptic target cells. During nervous system development, some neurons require the formation of synaptic circuitry for sustained survival (Linden, 1994). In a variety of sensory systems, not only are appropriate physical connections a prerequisite for neuronal survival, but the synapses must also be activated by sufficient sensory input (Aamodt and Constantine-Paton, 1999; Harris and Rubel, 2006). This exquisite sensitivity for appropriate synaptic circuitry typically has a brief developmental time course, known as the “critical period” during which loss of normal synaptic input can lead to neurodegeneration (Harris and Rubel, 2006). Neurons of the adult mammalian brain appear more robust in the face of lost sensory experience, suggesting that once neural circuits are well established, the individual components of the circuit become less interdependent. However, studies performed using a range of approaches demonstrate that certain adult neurons are susceptible to second order neurodegeneration (Al-Abdulla and Martin, 1998; Al-Abdulla et al., 1998; Baquet et al., 2004; Martin et al., 2003; Marty and Peschanski, 1995; Rossi and Strata, 1995). For example, Huntington’s disease (HD), an inherited neurodegenerative disease caused by a CAG – polyglutamine repeat expansion in the huntingtin gene, results in the atrophy and degeneration of GABAergic medium spiny neurons (MSNs) in the caudate and putamen. However, cortical neuron degeneration is also an important feature of HD pathology (Sapp et al., 2001; Vonsattel et al., 1985). Interestingly, when the Cre/lox system was employed to express mutant huntingtin protein in a cell type specific manner, cortical neurodegeneration could not be achieved in a cell autonomous manner (Gu et al., 2005). This study suggested that additional neuronal cell types must concurrently express mutant huntingtin to induce degeneration of cortical excitatory neurons. However, these experiments did not specifically reveal which afferent inputs to, or synaptic targets of, cortical neurons are involved in mediating their eventual degeneration.
The observation that inherited forms of neurodegeneration may require expression of the disease-causing protein more broadly than in the selectively vulnerable population of neurons, suggests that patterns of regional or cell type specific neurodegeneration result from dysfunction in one small population of the initially affected neurons, leading to waves of secondary changes in other neuron populations (Figure 1). This second order neuronal dysfunction can develop in a bidirectional manner, resulting either from loss of afferent input or post-synaptic targets, and/or defects in the molecular machinery at the synapse that disrupt neurotransmission and plasticity, which frequently precede structural-anatomic alterations. In the following section, we will focus primarily on the role of afferent inputs in the evolution of neurodegenerative pathology.
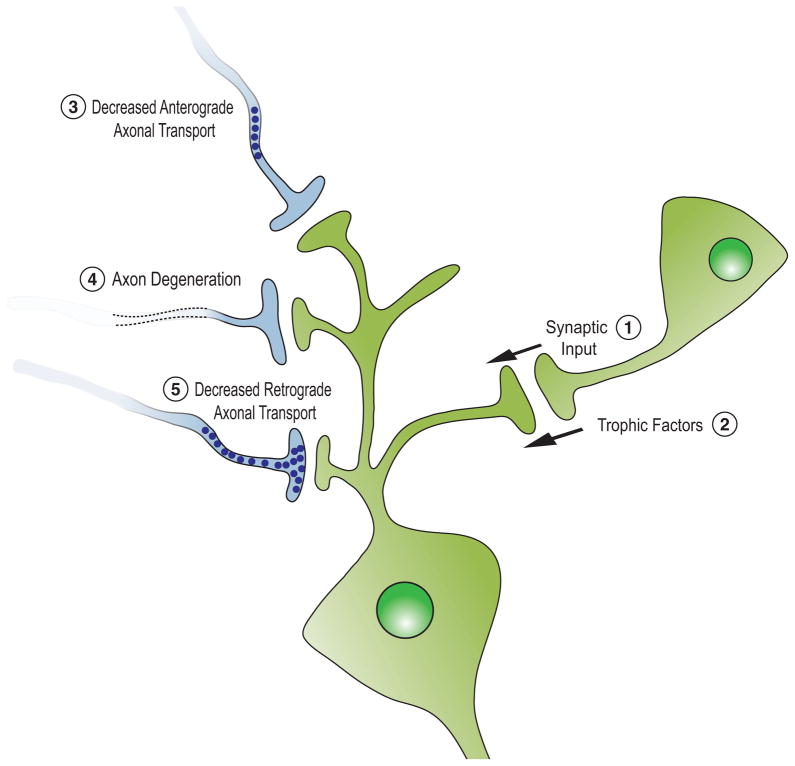
Figure 1. A variety of non-cell autonomous factors influence neuronal survival.
Neurons receive synaptic input (1), delivering both neurotransmitters and neurotrophic factors (2) that sustain neuronal survival. Neurodegenerative diseases can alter synaptic input by inhibiting anterograde axonal transport (3) and/or axon degeneration (4), resulting in decreased release of transmitters and neurotrophic peptides. (5) Failed retrograde transport as well as molecular dysfunction in target neurons or in non-neural target organs (e.g. muscle and blood vessels) can also damage presynaptic neurons, mimicking events that occur during development, when pathways of target-dependent neuronal survival are active.
Dysfunctional afferent input
Presynaptic inputs influence the function and health of their target neurons in a variety of ways. One of the most extensively studied means by which afferent input can influence the health and survival of a target cell population is through the delivery of neurotrophic factors. Loss of pro-survival neurotrophic factors has been hypothesized to contribute to the pathogenesis of several neurodegenerative disorders. Examples include nerve grown factor (NGF) in AD, glial-derived neurotrophic factor (GDNF) in PD, and both insulin-like growth factor-1 (IGF-1) and vascular endothelial growth factor (VEGF) in ALS (Mickiewicz and Kordower; Rangasamy et al.; Sakowski et al., 2009; Wyatt and Keirstead; Zuccato and Cattaneo, 2009). The role of trophic support has perhaps been best studied in HD, where several lines of evidence suggest that a key factor contributing to the selective vulnerability of MSNs in HD is reduced delivery of brain-derived neurotrophic factor (BDNF) from cortical afferent input. Reduced cortical BDNF expression is sufficient to induce age-related degeneration of MSNs (Baquet et al., 2004), suggesting that this mechanism can account for several aspects of selective vulnerability in these neurons. HD patient samples and mouse models both demonstrate reduced BDNF in the caudate and putamen (Canals et al., 2004; Ferrer et al., 2000), resulting from the effect of mutant huntingtin on BDNF gene transcription and/or the anterograde transport of BDNF to the presynaptic terminal (Gauthier et al., 2004; Zuccato et al., 2001).
Another mechanism by which communication from afferents may negatively impact the survival or function of a selectively vulnerable population is through a change in the actual pattern of synaptic activity onto the target cells. In this scenario, degeneration in second order neurons can develop either from decreased synaptic input, or when afferents become dysfunctional – leading to overstimulation/excitotoxicity of the target population. Decreased synaptic input has been described in various animal models of neurodegeneration (Barnes et al., 2011; Mentis et al., 2011). One such example was recently reported using an animal model of Spinal Muscular Atrophy (SMA). Patients with SMA inherit a motor neuron degenerative disorder caused by loss of function of the survival motor neuron (SMN) protein. In a mouse model of SMA, loss of motor function precedes loss of motor neurons (Le et al., 2005). To explore what influences motor neuron function prior to neurodegeneration, Mentis et al. examined the primary afferent input and found that loss of synaptic input from sensory spindle afferents followed the temporal and topographic pattern of later motor neuron loss (Mentis et al., 2011). Treatment with a histone deacetylase inhibitor, an intervention that improves motor function in this mouse model, also improved synaptic input from muscle spindle afferents (Mentis et al., 2011). Since a treatment that prevents reduction of the muscle spindle afferents also ameliorated motor neuron loss, this paper suggests that that loss of afferent input may contribute to eventual motor neuron degeneration in SMA.
A second example of decreased synaptic input contributing to neurodegeneration involves the spinocerebellar ataxias (SCAs), a group of neurodegenerative disorders that predominantly affect neurons in the brainstem and cerebellum involved in motor coordination and balance. Spinocerebellar ataxia type 1 (SCA1) is a CAG repeat disorder characterized by a selective degeneration of cerebellar Purkinje cells (PCs). PCs receive excitatory synaptic input from two cell types, cerebellar granule neurons and inferior olive (IO) neurons, whose climbing fibers (CFs) synapse on PC dendrites in the cerebellar molecular layer. It has recently been reported that CF input is reduced well before PC degeneration occurs in several mouse models of SCA1 (Barnes et al., 2011; Duvick et al.). Furthermore, using a conditional expression system, one study found that the effect of the disease gene on CF input occurs during the first 5 postnatal weeks (Barnes et al., 2011). When disease gene expression was prevented during this early period, loss in CF input was partially reversed and PC degeneration was completely prevented (Barnes et al., 2011). Since expression of mutant ataxin-1 in this model is selectively restricted to PCs, the interaction between CF and PC neurons must be occurring in a bidirectional fashion. Thus, PCs expressing mutant protein prevent normal synaptic structure and function of CFs, through an unknown signaling mechanism, and subsequent CF dysfunction early in the course of disease contributes to the eventual PC degeneration.
Additional evidence for the importance of CF input to PC survival comes from the study of spinocerebellar ataxia type 7 (SCA7), another polyglutamine expansion disorder (Garden and La Spada, 2008). Both cerebellar PCs and IO neurons are among the selectively vulnerable populations in SCA7. When SCA7 was modeled in mice via transgenic expression of human mutant ataxin-7 protein, evidence for non-cell autonomous degeneration of cerebellar PCs was noted early on. One group directed expression of the mutant gene specifically to PCs and did not observe significant pathology (Yvert et al., 2000). We employed an approach where mutant gene expression was expressed ubiquitously in the CNS, with the exception of PCs, and observed early onset ataxia and PC degeneration (Garden et al., 2002). Taken together, these studies indicate that in SCA7, PCs degenerate in response to signals from their surrounding environment. IO neurons also undergo degeneration in SCA7 models (Wang et al., 2010). Thus, reduced CF input could be a driving factor in PC degeneration. Eliminating mutant protein expression concurrently in both IO neurons and PCs concomitantly ameliorated the behavioral phenotype in the SCA7 mice (Furrer et al., 2011). Since prior studies had shown that expression of the mutant protein was neither necessary (Garden et al., 2002) nor sufficient (Yvert et al., 2000) to induce PC degeneration in a SCA7 model, our results support the hypothesis that degeneration of IO neurons and resulting loss of CF input contribute to PC degeneration in SCA7.
Abnormal neuron – glia interactions
Astrocytes perform a variety of important roles
Although the vast majority of the neuroscience research performed in the 20th century took a neuron-centric view, a growing appreciation of the importance of non-neural cells in nervous system function sparked a paradigm shift in our understanding of how the CNS is organized and operates, by the close of the 20th century. This revolution was driven by seminal studies that increasingly recognized astrocytes as not only support cells for neurons, but also as partners in fundamental neural processes (Bezzi et al., 1998; Parpura et al., 1994; Pasti et al., 1997). It is now well established that astrocytes can sense and respond to neuronal activity, as they possess receptors for neurotransmitters (Jourdain et al., 2007). The discovery of neurotransmitter release from astrocytes led to further characterization of these so-called ‘gliotransmitters’, and has revealed a potentially robust mechanism of neuron – astrocyte cross-talk during glutamatergic neurotransmission (Rossi and Volterra, 2009). Careful histological and ultrastructural studies have documented an exquisitely refined organization of neuronal synaptic networks and astrocyte support networks. Astrocytes make up as much as 50% of the brain’s volume, and they are organized into discrete subdivisions at the anatomical level, within which as many as 100,000 synapses can be located (Benarroch, 2005). In such regions, astrocytes extend their cell membranes into and among neuronal synapses, forming intermingled and closely interdigitating areas of direct apposition, which facilitates rapid and efficient removal of neurotransmitters from synaptic clefts. Astrocytes also extend their cell membranes along capillaries, and form ‘end-foot’ processes, which create the blood-brain barrier (Benarroch, 2005). The positioning of astrocytes in this way enables them to regulate vascular blood flow and nutrient delivery based upon neuronal activity – processes referred to respectively as ‘neurovascular coupling’ and ‘metabolic coupling’. By being adjacent to capillaries, astrocytes are poised to respond to extracellular signals sent over long distances and thus participate in the elaboration of inflammatory responses initiated upon activation of microglia – the resident immune cells of the CNS (Glass et al., 2010). Hence, astrocytes appear to be central players in the pathological cell – cell communication that is now emerging as a defining feature of many neurodegenerative disorders (Figure 2).
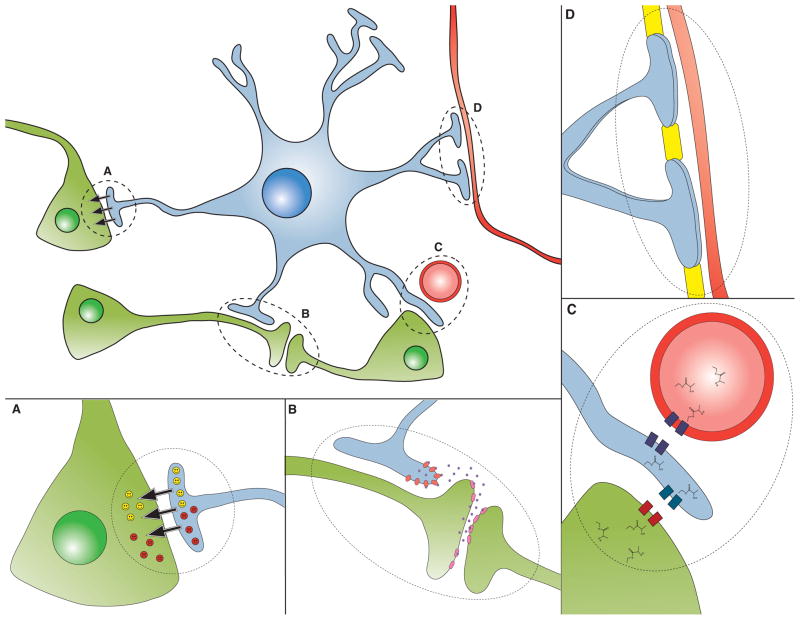
Figure 2. Astrocyte dysfunction in neurodegenerative disease.
Astrocytes perform a variety of functions reflecting their communication and close interactions with neurons. Here we see a representative astrocyte (blue) engaging in various activities, which when disrupted or altered, can lead to dysfunction and degeneration of neurons (green):
A) Astrocytes (blue) release neurotrophic factors (yellow smiley faces), and loss of neurotrophic factor release has been implicated in a number of neurological disorders. Alternatively, astrocytes can release toxic factors (red frown faces), which are believed to be a feature of motor neuron disease and possibly other disorders, as delineated in the section on ‘Astrocyte-derived toxicity’.
B) Neurons (green) release neurotransmitters to propagate signals between each other, and these neurotransmitters must be removed from the synaptic cleft in order to prevent persistent activation, a pathological process known as “excitotoxicity”. Astrocytes (blue) have transporters on their cell membranes to mediate neurotransmitter uptake, and impairment in neurotransmitter uptake by astrocytes can contribute to neuron dysfunction and degeneration.
C) Astrocytes (blue) shuttle metabolites (e.g. lactate) between blood vessels (red) and neurons (green), as shown here. Disturbances in this metabolic coupling can result in neuron dysfunction.
D) Astrocytes form the blood brain barrier in cooperation with cells lining blood vessels. The breakdown of the blood brain barrier at the level of the astrocyte may also occur in certain neurodegenerative diseases.
Astroglial loss-of-function: Perturbations in glutamate handling
Crucial to the process of synaptic neurotransmission is the timely removal of glutamate from the synaptic cleft upon release and activation of postsynaptic glutamatergic receptors. Failure to eliminate glutamate from the synapse leads to continued activation of postsynaptic and extrasynaptic glutamate receptors. This persistent activation drives excess Ca++ into neurons, resulting in “excitotoxicity” culminating in neuron cell death, once the capacity of the neuron to safely sequester Ca++ is exceeded. To achieve rapid and efficient glutamate removal at the synapse, higher organisms have evolved a family of five glutamate transporters known as the excitatory amino acid transporters (EAATs). Of the five EAATs, EAAT1 (formerly known as GLAST) and EAAT2 (formerly known as GLT-1) are predominantly expressed on astroglia.
Glutamate excitotoxicity due to impaired function of EAAT1 or EAAT2 has been proposed to contribute to neurodegeneration in ALS, HD, and SCA7 (Ilieva et al., 2009). Best studied in ALS, a rapidly progressive combined upper motor neuron – lower motor neuron disorder, attention has been focused upon EAAT2 as this glial glutamate transporter is estimated to remove ~90% of the neurotransmitter glutamate from the motor neuron synapse (Rothstein et al., 1996). Initial studies on transgenic mice generated to model a familial form of ALS (ALS1), due to mutations in superoxide dismutase 1 (SOD1), yielded evidence for reduced expression of EAAT2 (Bruijn et al., 1997), a process that may involve apoptotic pathway activation culminating in the production of activated caspase-3 (Boston-Howes et al., 2006). Elevated glutamate levels in the cerebrospinal fluid of sporadic ALS patients, however, independently suggest that excitotoxicity is contributing to the disease process in sporadic cases (Spreux-Varoquaux et al., 2002), consistent with studies documenting EAAT2 reductions in sporadic ALS spinal cord material (Rothstein et al., 1995). While the basis of the EAAT2 expression reduction in sporadic ALS may involve altered EAAT2 splicing in astrocytes (Lin et al., 1998), more recent work indicates that a loss of upper motor neuron – lower motor neuron connectivity might lead to reduced transcription of EAAT2 in astrocytes (Yang et al., 2009). Recent high throughput drug screens have further identified a compound, ceftriaxone, capable of inducing EAAT2 expression and modestly extending the lifespan of ALS1 SOD1 G93A transgenic mice (Rumbaugh et al., 2007). Although the importance of dysfunctional astrocyte glutamate handling for ALS disease progression is not entirely clear, clinical trials are now underway to assess whether boosting astrocyte function with ceftriaxone will be an effective therapy for this currently untreatable disorder.
Interestingly, the SCA7 disease protein – ataxin-7 – is widely expressed throughout the nervous system in astroglia as well as nerve cells (Custer et al., 2006). As previously discussed, cell-type specific expression studies of polyQ-expanded ataxin-7 revealed that non-cell-autonomous mechanisms may be involved in Purkinje cell degeneration characteristic of SCA7 (Garden et al, 2002). As cerebellar Purkinje cell neurons are intimately associated with specialized astroglial cells known as the Bergmann glia, we considered the role of Bergmann glia dysfunction in SCA7 disease pathogenesis (Custer et al., 2006). We found that Bergmann glia-specific transgenic expression of ataxin-7-92Q in mice was sufficient to produce ataxia and Purkinje cell degeneration, and that reduced EAAT1 expression precipitates the excitotoxic demise of cerebellar Purkinje cell neurons. This study did not however implicate astrocytes as the primary mediator of PC degeneration in SCA7 mice, since glial-driven expression resulted in a milder phenotype than animals with more widespread expression of polyQ-ataxin-7. Using a BAC transgenic and cell-type specific Cre-recombinase driver lines, we further determined that expression of mutant ataxin-7 protein is deleterious in multiple cell types to different extents (Furrer et al., 2011), underscoring the importance of neuron – glia communication for normal cerebellar function.
Astroglial gain-of-function: Astrocyte-derived toxicity
In ALS, it appears that astrocytes promote disease pathogenesis not only because of their impaired glutamate uptake, but also through a toxic gain-of-function. Such evidence for astrocyte-mediated toxic effects upon motor neurons comes from both in vitro and in vivo studies. When chimeric mice composed of cells expressing either normal SOD1 protein or mutant SOD1 protein were created, wild-type motor neurons encircled by mutant non-neuronal cells suffered degeneration as denoted by the formation of ubiquitin-positive protein aggregates (Clement et al., 2003). Building on this finding, two later studies directly modeled astrocyte – neuron interactions in co-culture systems. In one study, embryonic stem cells (ESCs) were derived from the blastocysts of transgenic mice expressing either normal SOD1 or mutant SOD1, and these ESCs were differentiated into motor neurons, and then plated onto monolayers of glia generated from either non-transgenic mice, WT SOD1 transgenic mice, or SOD1 G93A mutant transgenic mice (Di Giorgio et al., 2007). Motor neurons obtained from either WT SOD1 transgenic mice or mutant SOD1 transgenic mice exhibited signs of neurodegeneration and reduced survival only when co-cultured with glia from SOD1 transgenic mice that express the mutant SOD1 protein. Another study observed this same effect, and showed that culturing of primary motor neurons or ES-derived motor neurons in media obtained from cultures of mutant SOD1 transgenic astrocytes was sufficient to induce apoptosis of motor neurons (Nagai et al., 2007). Interestingly, conditioned media from ALS1 SOD1 mouse microglia, cortical neurons, myocytes, or fibroblasts was not toxic to motor neurons – only conditioned media from ALS1 SOD1 mutant astrocytes possessed this property. Although the specific molecule or protein responsible for mutant SOD1 astrocyte toxicity eluded identification in this study, SOD1 and glutamate were ruled out as the offending substance (Nagai et al., 2007). Defining the nature of this astrocyte-derived soluble toxin could yield crucial insights into ALS disease pathogenesis and may have therapeutic implications. The clinical relevance of astrocyte-mediated neurotoxicity for FALS and SALS was recently demonstrated by a provocative study in which neural progenitor cells derived from the spinal cords of FALS and SALS patients and differentiated into astrocytes were sufficient to kill co-cultured motor neurons (Haidet-Phillips et al., 2011). Interestingly, this study indicated that SOD1 appears to contribute to the neurotoxicity imparted by SALS and FALS astrocytes, as knock-down of SOD1in these astrocytes suppressed motor neuron toxicity.
Neurodegenerative Diseases and the Innate Immune Response
Innate immune responses include the initial cellular and molecular reaction to the detection of pathogens or tissue injury. Key components of the CNS innate immune response include the complement cascade and cells capable of performing phagocytosis, generating reactive oxygen species and signaling via cytokines, chemokines and additional immunomodulatory small molecules to other cells involved in the response to injury or pathogens. Evidence for the activation of the CNS innate immune response in neurodegenerative diseases have been extensively documented and recently reviewed (Prinz et al., 2011). However, the mechanisms by which neuronal injury is signaled to the immune system, and how this immune response may subsequently influence the progression of the disease, have only recently been elucidated. The principal mechanism through which an innate immune response is initiated, involves signaling through the TLR family of receptors (Crack and Bray, 2007; Kielian, 2006). TLR receptors were initially discovered for their role in binding a variety of pathogen associated molecular pattern (PAMP) ligands common to pathogenic organisms (Akira et al., 2001). More recently however, it has become clear that injured cells, including neurons (Sloane et al., 2009), release a class of molecules known as “danger associated molecular pattern” (DAMP) ligands that also bind to TLR receptors and initiate an innate immune response. The DAMP/TLR signaling pathway, in addition to release of the chemokine CX3CL1 (previously known as fractalkine) by injured neurons (Streit et al., 2005), explain how innate CNS immune response can produce a strong inflammatory reaction, in the absence of pathogens, that may impact disease onset and progression.
The CNS has a highly specialized innate immune system that uniquely involves astrocytes and CNS resident leukocytes known as microglia (Figure 3). Microglia typically express molecular tags associated with resting (non-inflammatory) macrophages, but can adopt novel morphological and molecular features associated with both pro- and anti-inflammatory states in the context of neurodegenerative disease (Colton, 2009). While inflammatory activation may typically be a secondary response to primary neuronal injury, there is a great deal of evidence suggesting that dysfunctional innate immune responses actively contribute to neurodegeneration in HIV associated neurodegeneration and autoimmune disorders of the CNS (Kaul et al., 2005; Lassmann and van Horssen, 2011). In addition, numerous examples of age-related alterations in the inflammatory response are thought to contribute to the pathogenesis of other disorders of ageing, such as atheroscelerosis and diabetes. Thus, it is possible that neurodegenerative diseases display age dependency due to the loss of an optimized inflammatory response in the CNS.
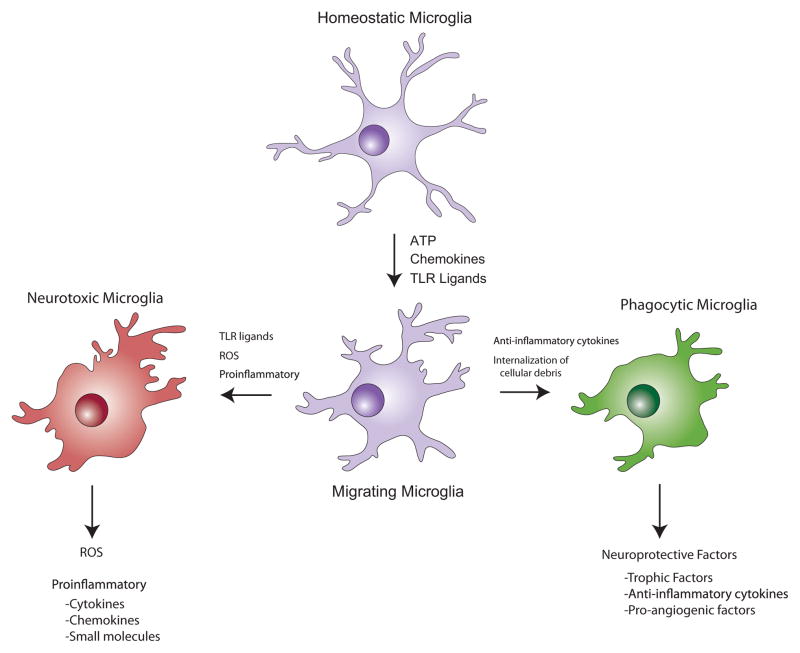
Figure 3. Microglia play divergent roles during neurodegeneration.
In the healthy CNS, microglia survey their immediate environment, and in this “resting state”, do not express inflammatory mediators. However, after exposure to a number of chemical signals from damaged neurons, microglia respond rapidly and physically migrate to the site of injury. Responding microglia may then adopt a pattern of behavior similar to pro-inflammatory macrophages (shown in red), as they release molecules intended to protect against pathogens, including neurotoxic cytokines, reactive oxygen species (ROS), and small molecules, such as quinolinic acid, that promote excitotoxicity. The release of cytokines and chemokines can lead to the recruitment of additional inflammatory cells from adjacent blood vessels, and may also engage astrocytes in the pro-inflammatory response. Alternatively, activated microglia may exhibit behaviors associated with anti-inflammatory macrophages (shown in green), secreting molecules that promote tissue repair, and internalizing cellular debris including aggregated, misfolded proteins such as β-amyloid, through phagocytosis. Whether two distinct populations of microglia exist that are committed to either of these response patterns, or all microglia can be induced to exhibit either response behavior when exposed to the correct combination of signals, remains to be determined.
Alzheimer’s Disease
In AD, there are many ways by which the innate immune system influences disease pathogenesis. For example, inflammatory phagocytic cells may modulate neurodegenerative pathology in AD as they have been speculated to be involved in the clearance of Aβ from the CNS. It was consequently reasoned that stimulating the inflammatory response to Aβ via immunization could increase Aβ clearance, decrease plaque formation, and ameliorate neurodegeneration (Hoozemans et al., 2001). Significant resources have been, and continue to be spent on evaluating a means to generate immunotherapy aimed at improving Aβ clearance from the CNS. Unfortunately, a clinical trial of Aβ immunization resulted in fatal autoimmune encephalitis (Schenk, 2002), suggesting that modulating the immune response to Aβ may be a “double-edged sword”. Indeed, it is difficult to discern whether the net effect of the innate immune response in AD is neurotoxic or neuroprotective. However, when a mouse model of AD was crossed onto a line deficit for the chemokine receptor CCR2, thus preventing chemokine-induced infiltration of monocytes across the blood brain barrier, the animals developed more rapid disease and increased Aβ deposition (El Khoury et al., 2007). Hence, monocyte infiltration into the CNS appears critical to ameliorate AD progression, at least in mice. However, subsequent studies suggest that resident microglia may have a much more complex role in AD pathogenesis. Microglia and neurons have a unique means for communication, with neurons expressing the chemokine CX3CL1 and microglia expressing its corresponding cognate receptor, CX3CR1. Injured neurons release CX3CL1, which signals microglia migration to the site of injury and initiation of an inflammatory response. When this communication was blocked by genetic deletion of CX3CR1 in a murine AD model, Aβ plaque pathology was reduced (Lee et al., 2010d). Taken together, these reports indicate that while infiltrating monocytes are important for Aβ clearance, the microglial response to neurodegeneration may actually exacerbate disease progression.
Huntington’s Disease
As in AD, the role of inflammation in HD pathogenesis may similarly involve both peripheral and CNS-resident components of the innate immune system. In patients with HD, increased production of inflammatory cytokines can be detected many years prior to symptom onset, and plasma levels of pro-inflammatory cytokines correlate with symptom progression (Bjorkqvist et al., 2008). Circulating monocytes from HD patients are more responsive to a pro-inflammatory signal than monocytes from control patients, a finding that has been recapitulated in multiple HD mouse models (Bjorkqvist et al., 2008). This hyperreactivity of monocytes may reflect functional alterations triggered by the presence of mutant huntingtin protein. Whether such functional alterations directly contribute to neurodegeneration in HD remains to be determined. One mechanism whereby peripheral innate immune function could potentially influence neuron survival or degeneration in HD involves the tryptophan catabolism pathway, which has been shown to be altered by the expression of mutant huntingtin in yeast (Giorgini et al., 2005). One upstream metabolite in this pathway, L-kynurenine is neuroprotective, while downstream metabolites, 3-hydroxykynurenine and quinolinic acid, are neurotoxic (Zadori et al., 2009).
MSNs are preferentially susceptible to the toxicity of quinolinic acid (Roberts et al., 1993). A recent study reported that pharmacological inhibition of the rate-limiting enzyme in this pathway, kyneurenine 3-monooxygenase (KMO), markedly slowed disease progression in HD mice (Zwilling et al., 2011). Since the KMO inhibitor employed in this study does not cross the blood-brain-barrier, the authors suggest that inhibition of KMO in the peripheral innate immune system is sufficient to increase levels of neuroprotective metabolites from the tryptophan catabolism pathway in the CNS. Since KMO expression is promoted by pro-inflammatory stimuli (Connor et al., 2008), the increased inflammatory responses reported in HD peripheral monocytes may enhance KMO expression and/or activity and exacerbate neurodegeneration. Interestingly, KMO inhibition also ameliorated pathology in a murine AD model (Zwilling et al., 2011), suggesting that a similar metabolic mechanism may comprise another facet of CNS-innate immunity cross-talk involved in AD neurodegeneration.
Parkinson’s disease
Both pathological and positron emission tomography (PET) studies have shown that patients with PD exhibit a robust inflammatory response in brain regions undergoing neurodegeneration (Gerhard et al., 2006; McGeer et al., 1988; Ouchi et al., 2009; Wersinger and Sidhu, 2002). Furthermore, as in AD, epidemiological studies suggest that chronic users of non-steroidal anti-inflammatory drugs (NSAID’s) may have a decreased risk of PD (Samii et al., 2009). TLR agonist molecules can be employed to generate animal models of dopaminergic neuron degeneration that recapitulate PD, and rare PD mutations that exacerbate inflammatory responses also worsen the phenotype in certain models of PD (Saijo et al., 2009). Furthermore, numerous animal studies using toxin-induced models of PD have shown that modulating the inflammatory response can ameliorate neuronal loss (Wang et al., 2005). However, it remains unclear how these models relate to the slowly progressive neurodegeneration that occurs in patients with idiopathic or familial forms of PD. As PD is associated with an abnormal accumulation of α-synuclein into Lewy bodies, one hypothesis is that misfolded α-synuclein induces an inflammatory response. This could occur either through the release of α-synuclein into the extracellular space, or by direct engulfment of α-synuclein as microglia participate in the regulation of synaptic membranes (Zhang et al., 2005). Interestingly, histological studies in PD patients grafted with non-diseased fetal dopaminergic neurons reveal that Lewy bodies emerge in transplanted neurons (Kordower et al., 2008a; Li et al., 2008). Specifically, only patients with a robust peri-graft inflammatory response were observed to have Lewy bodies in grafted neurons, while grafted neurons survived without Lewy pathology in patients lacking evidence of peri-graft microglia activation (Mendez et al., 2008). One parsimonious explanation for these findings is that an immune response to the graft facilitates the spread of Lewy body pathology from the host to the graft (Dawson, 2008). If this conjecture is valid, it raises the intriguing possibility that disease-associated misfolded or aggregate proteins such as α-synuclein can acquire prion-like properties, and that prion-like propagation of diseased proteins from cell to cell may be facilitated by exposure to an inflammatory milieu.
Cell-to-cell spreading of misfolded proteins: rise of the propagation hypothesis
Of prions and prionoids
The prevailing view that neurodegenerative pathology is driven by protein misfolding and generation of toxic conformers originated in the late 1990’s with the observation that disease-causing proteins such as α-synuclein and polyglutamine share common amyloidogenic properties, and cascades characteristic of Alzheimer’s and prion diseases. A common theme of misfolded protein toxicity thus links prion diseases with Alzheimer’s disease, Parkinson’s disease, ALS, polyglutamine diseases, and tauopathies. However, prion diseases have been viewed as unique among the neurodegenerative proteinopathies, since prions have the capacity for cell-to-cell and organism-to-organism dissemination. The infectivity of prion protein is well established, so much so that in its prion-like state, designated as PrPSc, prion protein can induce non-pathogenic prion protein, PrPc, to undergo a conformational change into the pathogenic PrPsc state (Pan et al., 1993). In this conformational conversion, which can even occur across species barriers in certain cases (e.g. cow to human), pathogenic PrPsc seeds the transformation of PrPc to PrPsc through a mechanisms that remains to be fully elucidated. Within the last few years, studies of non-prion neurodegenerative proteinopathies have demonstrated in vitro cell-to-cell transmission of protein aggregates (Desplats et al., 2009; Frost et al., 2009; Magalhaes et al., 2005; Munch et al., 2011; Ren et al., 2009). Propagation of protein aggregates from one cell to another has now been documented for several neurodegenerative proteinopaties, and sometimes over large distances – even traversing the blood-brain barrier (Clavaguera et al., 2009; Desplats et al., 2009; Meyer-Luehmann et al., 2006), raising the intriguing, but far from proven notion that non-prion neurodegenerative disorders are more prion-like (prionoid) than we had imagined.
Alzheimer’s disease
According to the amyloid cascade hypothesis, the pathogenesis of AD begins with changes in Aβ metabolism that promote the production of the Aβ42 peptide, which is followed by the formation of Aβ aggregates from seeds of Aβ42 peptides that grow into fibrils and finally plaques (Hardy and Selkoe, 2002). The Aβ plaques then alter synaptic function and interfere with tau protein metabolism to ultimately yield a neurodegenerative process in cortical and hippocampal neurons. More than a decade ago, evidence emerged that misfolded Aβ42 peptide seeds from AD patient brains can greatly accelerate amyloid plaque formation in amyloid precursor protein (APP) transgenic mice (Kane et al., 2000). The removal of Aβ42 peptides from such brain extracts or protein denaturation could prevent the promotion of amyloid plaque formation in such mice (Meyer-Luehmann et al., 2006), however the mechanistic basis of this phenomenon remains unclear, though protein co-factors could be principally involved. When follow-up studies with Aβ42-containing brain lysates, using oral, intravenous, intraocular, and intranasal delivery schemes, failed to yield amyloidogenesis in the brains of genetically susceptible mice (Eisele et al., 2009), the prion-like properties of Aβ42 peptide were questioned. Contrastingly, more recent work has shown that intraperitoneal injection of Aβ42-containing brain lysate material dramatically promotes amyloid plaque formation in APP transgenic mice (Eisele et al., 2010). Why was intraperitoneal delivery successful, while all other delivery schemes failed? Although suggested explanations must remain speculative, it is possible that earlier studies utilizing diverse delivery schemes did not isolate propagation-competent conformational forms of the Aβ42 peptide. As the basis for the success of the intraperitoneal study is also unclear, future experimentation should investigate if the Aβ42 peptide seeds are taken up by macrophages and monocytes that then travel to the brain parenchyma via the cerebral vasculature, as has been proposed (Eisele et al., 2010), or if the Aβ42 peptide seeds exist extracellularly without entering membrane-bound structures or cells on their journey to the CNS. In vivo studies in which the Aβ42 peptide seeds are tagged or tracked using an amyloid-binding compound, such as Pittsburgh compound B (PIB) (Klunk et al., 2004), might yield insight into the nature of the propagation process.
Parkinson’s disease
As mentioned previously, cell transplantation studies in PD patients have implicated the possible prionoid propensity of α-synuclein, as autopsies revealed that Lewy body pathology was present not only in the patients’ own neurons, but also in the donor neurons (Kordower et al., 2008a; Kordower et al., 2008b; Li et al., 2008; Li et al., 2010). In a number of studies further assessing cell-cell transmission of α-synuclein, uptake of α-synuclein from the medium into cells grown in culture was documented and observed to result in Lewy body-like aggregates in recipient cells (Danzer et al., 2007; Danzer et al., 2009; Luk et al., 2009; Nonaka et al., 2010; Waxman and Giasson, 2010). These aggregates consisted of both the exogenous recombinant α-synuclein protein supplied in the media, and endogenous cellular α-synuclein protein. In addition to this in vitro work, one group has investigated propagation of α-synuclein proteotoxicity in vivo, and found that mouse cortical neuron stem cells engrafted into the hippocampus of Thy-1 α-synuclein transgenic mice exhibited uptake of transgenic human α-synuclein protein as soon as one week after transplant (Desplats et al., 2009). By four weeks after engraftment, 15% of the transplanted neurons displayed α-synuclein immunoreactivity, which resembled inclusion bodies in a subset of neurons revealing this propagation. Other studies have also found evidence for transfer of α-synuclein from neuron to astroglia or vice versa. In α-synuclein transgenic mice with a platelet-derived growth factor (PDGF) promoter, expression of α-synuclein is restricted to neurons, yet prominent accumulation of α-synuclein is present in glial cells, and transmission of α-synuclein from neurons to astroglia was confirmed in co-culture experiments (Lee et al., 2010a). In a multiple system atrophy model, transgenic mice exclusively expressing α-synuclein in oligodendrocytes develop α-synuclein -containing axonal inclusions as well as the classic glial cytoplasmic inclusions (Yazawa et al., 2005). Hence, numerous studies strongly support the conclusion that α-synuclein can move from cell-to-cell and this process can involve different glial cell types as well as neurons.
Tauopathy
Aggregation of the microtubule-associated protein tau is a neuropathological feature of roughly two dozen neurodegenerative disorders in humans. The process of tau protein aggregation is linked to post-translational modification, in particular phosphorylation, and it is the hyperphosphorylated form of tau that is most prone to aggregate and produce neurotoxicity (Haass, 2010). In AD, hyperphosphorylated tau isoforms are initially apparent in the enterorhinal cortex, and then become detectable in the hippocampus and neocortex (Braak and Braak, 1991), suggesting that the histopathology is focal and then propagates to adjacent regions of the brain. Similar to Aβ and α-synuclein peptides, exogenous extracellular mutant tau protein can be taken up by cells, and once internalized, will promote misfolding of endogenously expressed tau protein (Frost et al., 2009). The relevance of tau propagation is further supported in vivo by a provocative series of experiments performed in tau transgenic mice (Clavaguera et al., 2009). In this work, mice expressing WT tau develop prominent filamentous tau inclusions after receiving hippocamal and cortical injection of brain homogenates from transgenic mice harboring the pathological phospho-tau mutation (P301S) (Clavaguera et al., 2009). Indeed, this acquired filamentous tau pathology did not remain confined to the injected regions, but actually spread beyond the injection zone to neighboring brain regions (Clavaguera et al., 2009). Two very recent studies further support a role for cell-to-cell transmission of misfolded tau protein, as transgenic expression of mutant P301L tau restricted to the enterorhinal cortex spreads to synaptically connected neurons in the hippocampus, recapitulating the progressive neurofibrillary tangle histopathology characteristic of AD (de Calignon et al., 2012; Liu et al., 2012). These findings suggest that propagation of tau protein aggregation, which occurs intracellularly, could be operating in a variety of disorders featuring tau pathology.
Amyotophic lateral sclerosis
ALS is a highly heterogeneous disorder in terms of clinical presentation and neuropathology. Thus, there has been interest in deconstructing the natural history of ALS (Ravits and La Spada, 2009), which has revealed a number of themes, including focality of clinical onset, followed by contiguous spread of motor disease. Focality of clinical onset refers to the fact that most ALS patients initially present with motor deficits that are confined to a particular region of the neuraxis. Thereafter, motor deficits follow a relatively predictable pattern, as the next regions to become involved are typically adjacent ipsilaterally distributed motor units. ALS natural history is thus compatible with a propagating process that is orderly and moves locally, as opposed to distally. Recent work on familial ALS, due to mutations in SOD1, now suggests that cell-to-cell transmission of mutant SOD1 aggregates can occur (Chia et al., 2010; Munch et al., 2011). Much like in the AD and PD studies discussed above, in vitro aggregation of both wild-type and mutant SOD1 protein is observed upon exogenous addition of isolated spinal tissue from mice expressing mutant SOD1 (Chia et al., 2010). In another recent study, supplementation of the cell culture media with mutant SOD1 protein aggregates was sufficient for uptake of the SOD1 aggregates by Neuro2a cells, and internalization of mutant SOD1 protein aggregates drove aggregation of endogenously expressed soluble mutant SOD1 protein (Munch et al., 2011). Cell-to-cell spreading of mutant SOD1 protein aggregates then continued, despite the lack of contact between cells, through a process that was ATP-dependent, required actin filament formation, and involved lipid raft-mediated macropinocytosis. While these findings are provocative, further work is required to determine if this propagation of aggregates occurs in vivo or is relevant to TDP-43 protein aggregation, a proteotoxicity emerging as a cardinal feature of sporadic ALS (Mackenzie et al., 2010).
Polyglutamine-expansion diseases
While adoption of an alternative structure represents a key step in the pathogenic cascade for polyglutamine disease proteins, considerable work suggests that amyloid-like protein aggregates in these disorders are not the toxic species, but rather coincident with the production of toxic conformers whose exact nature remains uncertain (Arrasate et al., 2004; Chia et al., 2010; Poirier et al., 2002; Wacker et al., 2004). Nonetheless, production of protein aggregates always indicates that a process of polyglutamine proteotoxicity is underway; hence, if dynamic interconversion between toxic conformers and aggregates is ongoing, then cell-to-cell transmission of altered polyglutamine species could promote propagation of pathology. In an in vitro investigation, a highly amyloidogenic polyglutamine species was added to the culture media of HEK293 cells stably expressing a non-pathogenic huntingtin protein fragment (Ren et al., 2009). Cellular uptake of the toxic polyglutamine species unexpectedly led to aggregation of the non-pathogenic huntingtin protein, which normally does not form aggregates. Furthermore, the aggregation of huntingtin-Q25 persisted through multiple rounds of cell division, but such aggregation could not be induced with unrelated amyloidogenic proteins, such as yeast Sup35 or Aβ. In an independent study, huntingtin protein aggregation was monitored with a fluorescent signal, and cell-to-cell transmission of huntingtin protein oligomers was documented (Herrera et al., 2011). Further studies will be required to validate the significance and relevance of such cell-to-cell spreading in disease pathogenesis.
Mechanisms of cell-to-cell propagation
If it is true that misfolded proteins can spread from one cell to another, then the obvious question that we must address is how this occurs. One approach to this issue is to recognize that the process can operate in at least two different ways: 1) by extracellular release and uptake, or 2) by delivery within membrane-bound structures (Figure 4). In this section, we will briefly review potential pathways by which misfolded proteins could achieve intercellular transit.
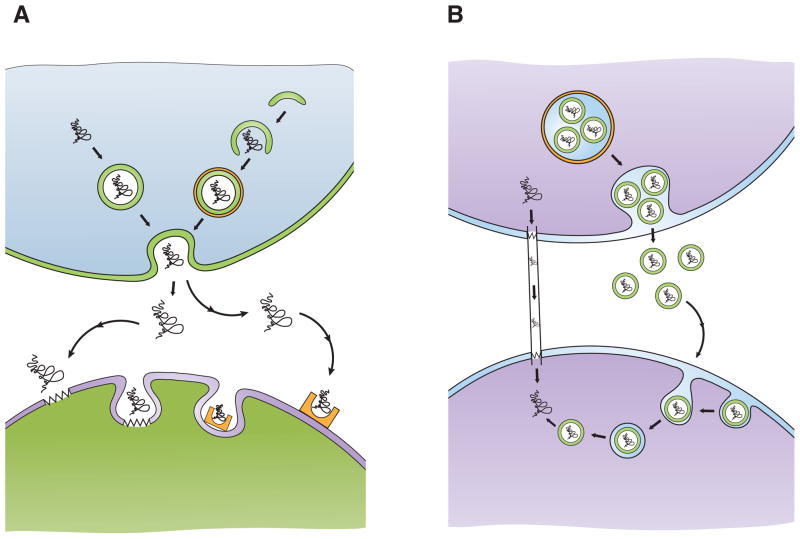
Figure 4. Proposed mechanisms to account for cell-to-cell transmission of misfolded proteins.
A) Exocytosis/endocytosis. Misfolded proteins can be packaged into exocytotic vesicles (top left; arrows), which fuse with the cell membrane and release their contents into the extracellular space. Misfolded proteins may be delivered to pre-autophagic vesicles that mature into double-membrane bound autophagosomes (top right; arrows). One possible fate of certain autophagosomes is to fuse with the cell membrane and release their contents into the extracellular space. Once in the extracellular space, misfolded proteins can be taken up by cells via lipid raft-mediated endocytosis (bottom left; zigzags represent lipid rafts), or more likely by receptor-mediated endocytosis (bottom right; half rectangle with oval indentation represents receptor).
B) Propagation without membrane-bound transport. Misfolded proteins may move from one cell to another via intercellular bridges, known as “tunneling nanotubes” (left; arrows). Another route for cell-to-cell transfer of misfolded proteins is within exosomes, small membrane-bound vesicles (green circles) that can form within multivesicular bodies (orange circle), which are released and endocytosed by a neighboring cell (right; arrows). Such exosomes can bud off of their original cell and travel large distances before fusing with the membrane of another cell. How misfolded protein conformers, sequestered in endosomes, gain access to the cytosolic compartment remains unclear.
Misfolded proteins that are aggregate-prone pose a continual challenge to degradative pathways, forcing neurons to heavily rely on autophagy for proteostasis. Hence, misfolded protein conformers are typically directed to membrane-bound structures, in particular autophagosomes that ultimately fuse with endosomes or lysosomes. Under conditions of suboptimal lysosome function, these membrane-bound structures will become exocytotic vesicles and release their contents into the extracellular space, as has been shown for α-synuclein (Jang et al., 2010). Although release of vesicle-bound materials into the extracellular space has been viewed as the consequence of impaired function, autophagosomes may normally become exocytotic vesicles and intentionally expel their contents into the extracellular space. The existence of two alternative destinies for autophagosomes may be restricted to a specialized version of the autophagy pathway, named “quality control autophagy” (Lee and Yao, 2010), which operates primarily in post-mitotic cells, and is tasked with maintaining protein and mitochondrial quality control. The ability of quality control autophagy to promote degradation of sequestered contents has been demonstrated for parkin-regulated mitophagy (Lee et al., 2010c). This pathway, which requires HDAC6 to promote fusion of autophagosomes with lysosomes (Lee et al., 2010b), may allow autophagosomes to achieve exocytotic secretion of protein aggregates, when the capacity for lysosomal degradation is exceeded – though this is yet to demonstrated. Thus, neurons may direct amyloidogenic proteins to the autophagy pathway not only to promote their intracellular degradation, but also to enable the cell to eliminate them by a process of secretion via exocytosis.
Once in the extracellular space, how do toxic protein conformers gain access to cells? Although lipophilic proteins such as monomeric α-synuclein could in theory passively diffuse across cellular membranes (Steiner et al., 2011), this method of entry is likely the exception. Another path of entry could be via lipid raft-mediated endocytosis, which has been proposed for both Aβ and α-synuclein (Park et al., 2009; Saavedra et al., 2007). However, α-synuclein has unique biophysical properties and even can associate with key proteins that regulate endocytosis (Desplats et al., 2009). Hence, most toxic peptides likely enter cells via receptor-mediated endocytosis. The rationale for the existence of such a pathway may be for cells to actively remove misfolded proteins from the extracellular space and achieve their destruction. According to this model, the burden of eliminating such toxic proteins would be shared between different cells and cell types.
Another mode for cell-to-cell transmission of misfolded proteins is within membrane-bound structures. One highly likely candidate for this process is the exosome, a small membrane-bound vesicle formed within almost all cell types in an intracellular membrane-bound structure known as a multivesicular body (Chaput and Thery, 2011). Exosomes bud off, and then either fuse with lysosomes, or fuse with the plasma membrane, where they are released as membrane-bound structures that can travel to nearby cells, or voyage to distant tissues via the circulation. Evidence for the role of exosomes in propagation has come from studies that have documented the packaging of α-synuclein protein into exosomes, prior to intercellular transfer (Alvarez-Erviti et al., 2011; Emmanouilidou et al., 2010). Similar investigations have also implicated exosomes in the intercellular delivery of prion proteins and Aβ aggregates (Steiner et al., 2011; Vella et al., 2007).
Yet another process of intercellular membrane-bound communication involves structures known as tunneling nanotubes, which are transient, long, actin-rich projections that directly connect cells to one another. At this time, the only evidence for tunneling nanotubes as a means of intercellular transmission of misfolded proteins comes from studies of prion disease. This work, performed exclusively in cell culture, revealed that PrPsc proteins can move from an infected neuron to an uninfected neuron, or from bone-marrow derived dendritic cells to an uninfected primary neuron (Gousset et al., 2009). The discovery of tunneling nanotubes underscores the diversity of pathways by which cell-to-cell communication is achieved in the nervous system, and suggests that other processes likely remain to be elucidated.
Unsolved Questions and Directions for the Future
The work reviewed here illustrates how intercellular communication may go awry within the CNS. Additional examples of altered cell-cell communication will likely be described over the coming years, strengthening the evidence for a multi-hit model for neurodegenerative disease. Three critical questions regarding this hypothesis, however, remain to be addressed: 1) What is the pathophysiological relationship between monogenic and sporadic forms of neurodegenerative disease?; 2) How does biological aging influence disease onset and progression?; and 3) How do diseases produced by ubiquitously expressed disease proteins cause selective patterns of neurodegeneration? The multi-hit hypothesis enables understanding of the first question by providing a context to consider how changes in response to a specific heritable mutation might be recapitulated by sporadic events. For example, somatic mutations, epigenetic modification, acquired mitochondrial dysfunction, or misfolded protein accumulation - in response to environmental exposures (e.g. toxins, stress etc) or normal aging, might sum to produce the same net effect as inheriting an extra copy of α-synuclein. Additionally, if CNS homeostasis requires that we maintain certain patterns of cell-cell communication, then the integration of aberrant sub-clinical events could yield a degenerative disease due to progressive loss of function in such cellular communication. For example, if communication between two neuronal populations, “A” and “B”, is required for their normal survival and function, while population B supports populations “C” and “D”, then loss of function in A type neurons could determine the survival of all four cell types (and then additional cells that depend upon the three affected groups). Such cellular network relationships may have several levels of complexity. One type of cell-cell communication could be involved in initiating a protein misfolding event, while interactions with a second cell type could influence the ability to remove or degrade a misfolded protein. We hypothesize that the mechanism for selective vulnerability involves specific alterations in cell-cell communication, and thus may consist of a unique series of events for each disease. For example, MSNs, the most vulnerable neuron population in HD, may have cell-autonomous vulnerabilities shared with other neuronal populations that degenerate later in the course of disease. But the MSNs may also depend upon signals from specific afferent or target neurons, making them exquisitely vulnerable to an altered balance between a certain molecule (e.g. kynurenine) and its neurotoxic metabolites. Thus, the mutation responsible for HD could alter the function of multiple cell types, and it would be the dysfunction of these other cell types that together make MSNs selectively vulnerable.
When one considers the complexity of the CNS, it should come as little surprise that the basis of nervous system disease would be similarly complicated. Neurons do not exist in isolation; hence, neurodegenerative diseases must be viewed as resulting from processes that ultimately target neurons – but are by no means restricted to them. In this review, we have attempted to delineate advances in our understanding of neurodegenerative disease pathogenesis, by focusing upon pathological processes occurring between different cells, some between identical cell types, but many involving cells of distinct lineage. These insights and discoveries, many quite recent, underscore the increasingly pivotal role for disrupted or altered cell-cell interactions in neurological disorders. While a “systems cell biology” approach to neurodegenerative disease may seem daunting, we have made great strides in developing methods and models that now permit us to evaluate a pathological process in finer detail and in a more physiological context than ever before. For example, approaches that enable both time and cell type specific gene expression in animal models will make it possible to determine if and when disease gene expression in specific cell populations contribute to the disease phenotype. In addition, novel imaging methods enable the study of specific cell populations in vivo over longer periods of time and will reveal how interacting populations influence each other’s survival. Another important line of research for the future will involve isolating specific cellular populations from CNS tissue in order to characterize the distinct genomic, proteomic and even epigenetic alterations that occur during disease onset or progression. Incorporating such strategies into our dissection of the mechanistic basis of neurodegenerative disease must be a goal for future studies, as it bodes well for greater success in deconstructing the cellular and the molecular pathophysiology of these devastating disorders. If we can achieve this goal, we increase the likelihood of developing effective therapies for numerous currently untreatable neurological disorders.
Acknowledgments
Due to space limitations, the authors regret not citing all original publications relevant to the topics covered. The authors’ work in this area is supported by grants from the N.I.H. (R01 NS041648 and R01 AG033082 to ARL, and R01 NS052535 to GAG). All figure illustrations were drawn by or with assistance from C. Butler.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aamodt SM, Constantine-Paton M. The role of neural activity in synaptic development and its implications for adult brain function. Adv Neurol. 1999;79:133–144. [PubMed] [Google Scholar]
- Aguzzi A, Rajendran L. The transcellular spread of cytosolic amyloids, prions, and prionoids. Neuron. 2009;64:783–790. doi: 10.1016/j.neuron.2009.12.016. [DOI] [PubMed] [Google Scholar]
- Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- Al-Abdulla NA, Martin LJ. Apoptosis of retrogradely degenerating neurons occurs in association with the accumulation of perikaryal mitochondria and oxidative damage to the nucleus. Am J Pathol. 1998;153:447–456. doi: 10.1016/S0002-9440(10)65588-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Abdulla NA, Portera-Cailliau C, Martin LJ. Occipital cortex ablation in adult rat causes retrograde neuronal death in the lateral geniculate nucleus that resembles apoptosis. Neuroscience. 1998;86:191–209. doi: 10.1016/s0306-4522(98)00014-1. [DOI] [PubMed] [Google Scholar]
- Alvarez-Erviti L, Seow Y, Schapira AH, Gardiner C, Sargent IL, Wood MJ, Cooper JM. Lysosomal dysfunction increases exosome-mediated alpha-synuclein release and transmission. Neurobiol Dis. 2011;42:360–367. doi: 10.1016/j.nbd.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrasate M, Mitra S, Schweitzer ES, Segal MR, Finkbeiner S. Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature. 2004;431:805–810. doi: 10.1038/nature02998. [DOI] [PubMed] [Google Scholar]
- Baquet ZC, Gorski JA, Jones KR. Early striatal dendrite deficits followed by neuron loss with advanced age in the absence of anterograde cortical brain-derived neurotrophic factor. J Neurosci. 2004;24:4250–4258. doi: 10.1523/JNEUROSCI.3920-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes JA, Ebner BA, Duvick LA, Gao W, Chen G, Orr HT, Ebner TJ. Abnormalities in the Climbing Fiber-Purkinje Cell Circuitry Contribute to Neuronal Dysfunction in ATXN1[82Q] Mice. J Neurosci. 2011;31:12778–12789. doi: 10.1523/JNEUROSCI.2579-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch EE. Neuron-astrocyte interactions: partnership for normal function and disease in the central nervous system. Mayo Clin Proc. 2005;80:1326–1338. doi: 10.4065/80.10.1326. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini BL, Pozzan T, Volterra A. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature. 1998;391:281–285. doi: 10.1038/34651. [DOI] [PubMed] [Google Scholar]
- Bjorkqvist M, Wild EJ, Thiele J, Silvestroni A, Andre R, Lahiri N, Raibon E, Lee RV, Benn CL, Soulet D, et al. A novel pathogenic pathway of immune activation detectable before clinical onset in Huntington’s disease. J Exp Med. 2008;205:1869–1877. doi: 10.1084/jem.20080178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boillee S, Yamanaka K, Lobsiger CS, Copeland NG, Jenkins NA, Kassiotis G, Kollias G, Cleveland DW. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312:1389–1392. doi: 10.1126/science.1123511. [DOI] [PubMed] [Google Scholar]
- Boston-Howes W, Gibb SL, Williams EO, Pasinelli P, Brown RH, Jr, Trotti D. Caspase-3 cleaves and inactivates the glutamate transporter EAAT2. J Biol Chem. 2006;281:14076–14084. doi: 10.1074/jbc.M600653200. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Brown TB, Bogush AI, Ehrlich ME. Neocortical expression of mutant huntingtin is not required for alterations in striatal gene expression or motor dysfunction in a transgenic mouse. Hum Mol Genet. 2008;17:3095–3104. doi: 10.1093/hmg/ddn206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijn LI, Becher MW, Lee MK, Anderson KL, Jenkins NA, Copeland NG, Sisodia SS, Rothstein JD, Borchelt DR, Price DL, et al. ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron. 1997;18:327–338. doi: 10.1016/s0896-6273(00)80272-x. [DOI] [PubMed] [Google Scholar]
- Canals JM, Pineda JR, Torres-Peraza JF, Bosch M, Martin-Ibanez R, Munoz MT, Mengod G, Ernfors P, Alberch J. Brain-derived neurotrophic factor regulates the onset and severity of motor dysfunction associated with enkephalinergic neuronal degeneration in Huntington’s disease. J Neurosci. 2004;24:7727–7739. doi: 10.1523/JNEUROSCI.1197-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaput N, Thery C. Exosomes: immune properties and potential clinical implementations. Semin Immunopathol. 2011;33:419–440. doi: 10.1007/s00281-010-0233-9. [DOI] [PubMed] [Google Scholar]
- Chia R, Tattum MH, Jones S, Collinge J, Fisher EM, Jackson GS. Superoxide dismutase 1 and tgSOD1 mouse spinal cord seed fibrils, suggesting a propagative cell death mechanism in amyotrophic lateral sclerosis. PLoS One. 2010;5:e10627. doi: 10.1371/journal.pone.0010627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavaguera F, Bolmont T, Crowther RA, Abramowski D, Frank S, Probst A, Fraser G, Stalder AK, Beibel M, Staufenbiel M, et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol. 2009;11:909–913. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement AM, Nguyen MD, Roberts EA, Garcia ML, Boillee S, Rule M, McMahon AP, Doucette W, Siwek D, Ferrante RJ, et al. Wild-type nonneuronal cells extend survival of SOD1 mutant motor neurons in ALS mice. Science. 2003;302:113–117. doi: 10.1126/science.1086071. [DOI] [PubMed] [Google Scholar]
- Colton CA. Heterogeneity of microglial activation in the innate immune response in the brain. J Neuroimmune Pharmacol. 2009;4:399–418. doi: 10.1007/s11481-009-9164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor TJ, Starr N, O’Sullivan JB, Harkin A. Induction of indolamine 2,3-dioxygenase and kynurenine 3-monooxygenase in rat brain following a systemic inflammatory challenge: a role for IFN-gamma? Neurosci Lett. 2008;441:29–34. doi: 10.1016/j.neulet.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Crack PJ, Bray PJ. Toll-like receptors in the brain and their potential roles in neuropathology. Immunol Cell Biol. 2007;85:476–480. doi: 10.1038/sj.icb.7100103. [DOI] [PubMed] [Google Scholar]
- Custer SK, Garden GA, Gill N, Rueb U, Libby RT, Schultz C, Guyenet SJ, Deller T, Westrum LE, Sopher BL, et al. Bergmann glia expression of polyglutamine-expanded ataxin-7 produces neurodegeneration by impairing glutamate transport. Nat Neurosci. 2006;9:1302–1311. doi: 10.1038/nn1750. [DOI] [PubMed] [Google Scholar]
- Danzer KM, Haasen D, Karow AR, Moussaud S, Habeck M, Giese A, Kretzschmar H, Hengerer B, Kostka M. Different species of alpha-synuclein oligomers induce calcium influx and seeding. J Neurosci. 2007;27:9220–9232. doi: 10.1523/JNEUROSCI.2617-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danzer KM, Krebs SK, Wolff M, Birk G, Hengerer B. Seeding induced by alphasynuclein oligomers provides evidence for spreading of alpha-synuclein pathology. J Neurochem. 2009;111:192–203. doi: 10.1111/j.1471-4159.2009.06324.x. [DOI] [PubMed] [Google Scholar]
- Dawson TM. Non-autonomous cell death in Parkinson’s disease. Lancet Neurol. 2008;7:474–475. doi: 10.1016/S1474-4422(08)70099-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Calignon A, Polydoro M, Suarez-Calvet M, William C, Adamowicz DH, Kopeikina KJ, RP, Sahara N, Ashe KH, Carlson GA, et al. Propagation of tau pathology in a model of early Alzheimer’s disease. Neuron. 2012 doi: 10.1016/j.neuron.2011.11.033. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desplats P, Lee HJ, Bae EJ, Patrick C, Rockenstein E, Crews L, Spencer B, Masliah E, Lee SJ. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci U S A. 2009;106:13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giorgio FP, Carrasco MA, Siao MC, Maniatis T, Eggan K. Non-cell autonomous effect of glia on motor neurons in an embryonic stem cell-based ALS model. Nat Neurosci. 2007;10:608–614. doi: 10.1038/nn1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvick L, Barnes J, Ebner B, Agrawal S, Andresen M, Lim J, Giesler GJ, Zoghbi HY, Orr HT. SCA1-like disease in mice expressing wild-type ataxin-1 with a serine to aspartic acid replacement at residue 776. Neuron. 67:929–935. doi: 10.1016/j.neuron.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisele YS, Bolmont T, Heikenwalder M, Langer F, Jacobson LH, Yan ZX, Roth K, Aguzzi A, Staufenbiel M, Walker LC, et al. Induction of cerebral beta-amyloidosis: intracerebral versus systemic Abeta inoculation. Proc Natl Acad Sci U S A. 2009;106:12926–12931. doi: 10.1073/pnas.0903200106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisele YS, Obermuller U, Heilbronner G, Baumann F, Kaeser SA, Wolburg H, Walker LC, Staufenbiel M, Heikenwalder M, Jucker M. Peripherally applied Abeta-containing inoculates induce cerebral beta-amyloidosis. Science. 2010;330:980–982. doi: 10.1126/science.1194516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Khoury J, Toft M, Hickman SE, Means TK, Terada K, Geula C, Luster AD. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat Med. 2007;13:432–438. doi: 10.1038/nm1555. [DOI] [PubMed] [Google Scholar]
- Emmanouilidou E, Melachroinou K, Roumeliotis T, Garbis SD, Ntzouni M, Margaritis LH, Stefanis L, Vekrellis K. Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J Neurosci. 2010;30:6838–6851. doi: 10.1523/JNEUROSCI.5699-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I, Goutan E, Marin C, Rey MJ, Ribalta T. Brain-derived neurotrophic factor in Huntington disease. Brain Res. 2000;866:257–261. doi: 10.1016/s0006-8993(00)02237-x. [DOI] [PubMed] [Google Scholar]
- Frost B, Jacks RL, Diamond MI. Propagation of tau misfolding from the outside to the inside of a cell. J Biol Chem. 2009;284:12845–12852. doi: 10.1074/jbc.M808759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furrer SA, Mohanachandran MS, Waldherr SM, Chang C, Damian VA, Sopher BL, Garden GA, La Spada AR. Spinocerebellar ataxia type 7 cerebellar disease requires the coordinated action of mutant ataxin-7 in neurons and glia, and displays non-cell-autonomous bergmann glia degeneration. J Neurosci. 2011;31:16269–16278. doi: 10.1523/JNEUROSCI.4000-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garden GA, La Spada AR. Molecular pathogenesis and cellular pathology of spinocerebellar ataxia type 7 neurodegeneration. Cerebellum. 2008;7:138–149. doi: 10.1007/s12311-008-0027-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garden GA, Libby RT, Fu YH, Kinoshita Y, Huang J, Possin DE, Smith AC, Martinez RA, Fine GC, Grote SK, et al. Polyglutamine-expanded ataxin-7 promotes non-cell-autonomous purkinje cell degeneration and displays proteolytic cleavage in ataxic transgenic mice. J Neurosci. 2002;22:4897–4905. doi: 10.1523/JNEUROSCI.22-12-04897.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier LR, Charrin BC, Borrell-Pages M, Dompierre JP, Rangone H, Cordelieres FP, De Mey J, MacDonald ME, Lessmann V, Humbert S, et al. Huntingtin controls neurotrophic support and survival of neurons by enhancing BDNF vesicular transport along microtubules. Cell. 2004;118:127–138. doi: 10.1016/j.cell.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Gerhard A, Pavese N, Hotton G, Turkheimer F, Es M, Hammers A, Eggert K, Oertel W, Banati RB, Brooks DJ. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson’s disease. Neurobiol Dis. 2006;21:404–412. doi: 10.1016/j.nbd.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Giorgini F, Guidetti P, Nguyen Q, Bennett SC, Muchowski PJ. A genomic screen in yeast implicates kynurenine 3-monooxygenase as a therapeutic target for Huntington disease. Nat Genet. 2005;37:526–531. doi: 10.1038/ng1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gousset K, Schiff E, Langevin C, Marijanovic Z, Caputo A, Browman DT, Chenouard N, de Chaumont F, Martino A, Enninga J, et al. Prions hijack tunnelling nanotubes for intercellular spread. Nat Cell Biol. 2009;11:328–336. doi: 10.1038/ncb1841. [DOI] [PubMed] [Google Scholar]
- Gu X, Andre VM, Cepeda C, Li SH, Li XJ, Levine MS, Yang XW. Pathological cell-cell interactions are necessary for striatal pathogenesis in a conditional mouse model of Huntington’s disease. Mol Neurodegener. 2007;2:8. doi: 10.1186/1750-1326-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Li C, Wei W, Lo V, Gong S, Li SH, Iwasato T, Itohara S, Li XJ, Mody I, et al. Pathological cell-cell interactions elicited by a neuropathogenic form of mutant Huntingtin contribute to cortical pathogenesis in HD mice. Neuron. 2005;46:433–444. doi: 10.1016/j.neuron.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Haass C. Initiation and propagation of neurodegeneration. Nat Med. 2010;16:1201–1204. doi: 10.1038/nm.2223. [DOI] [PubMed] [Google Scholar]
- Haidet-Phillips AM, Hester ME, Miranda CJ, Meyer K, Braun L, Frakes A, Song S, Likhite S, Murtha MJ, Foust KD, et al. Astrocytes from familial and sporadic ALS patients are toxic to motor neurons. Nat Biotechnol. 2011;29:824–828. doi: 10.1038/nbt.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Harris JA, Rubel EW. Afferent regulation of neuron number in the cochlear nucleus: cellular and molecular analyses of a critical period. Hear Res. 2006;216–217:127–137. doi: 10.1016/j.heares.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Herrera F, Tenreiro S, Miller-Fleming L, Outeiro TF. Visualization of cell-to-cell transmission of mutant huntingtin oligomers. PLoS Curr. 2011;3:RRN1210. doi: 10.1371/currents.RRN1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoozemans JJ, Rozemuller AJ, Veerhuis R, Eikelenboom P. Immunological aspects of alzheimer’s disease: therapeutic implications. Bio Drugs. 2001;15:325–337. doi: 10.2165/00063030-200115050-00004. [DOI] [PubMed] [Google Scholar]
- Ilieva H, Polymenidou M, Cleveland DW. Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J Cell Biol. 2009;187:761–772. doi: 10.1083/jcb.200908164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang A, Lee HJ, Suk JE, Jung JW, Kim KP, Lee SJ. Non-classical exocytosis of alpha-synuclein is sensitive to folding states and promoted under stress conditions. J Neurochem. 2010;113:1263–1274. doi: 10.1111/j.1471-4159.2010.06695.x. [DOI] [PubMed] [Google Scholar]
- Jourdain P, Bergersen LH, Bhaukaurally K, Bezzi P, Santello M, Domercq M, Matute C, Tonello F, Gundersen V, Volterra A. Glutamate exocytosis from astrocytes controls synaptic strength. Nat Neurosci. 2007;10:331–339. doi: 10.1038/nn1849. [DOI] [PubMed] [Google Scholar]
- Kane MD, Lipinski WJ, Callahan MJ, Bian F, Durham RA, Schwarz RD, Roher AE, Walker LC. Evidence for seeding of beta -amyloid by intracerebral infusion of Alzheimer brain extracts in beta -amyloid precursor protein-transgenic mice. J Neurosci. 2000;20:3606–3611. doi: 10.1523/JNEUROSCI.20-10-03606.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M, Zheng J, Okamoto S, Gendelman HE, Lipton SA. HIV-1 infection and AIDS: consequences for the central nervous system. Cell Death Differ. 2005;12(Suppl 1):878–892. doi: 10.1038/sj.cdd.4401623. [DOI] [PubMed] [Google Scholar]
- Kielian T. Toll-like receptors in central nervous system glial inflammation and homeostasis. J Neurosci Res. 2006;83:711–730. doi: 10.1002/jnr.20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergstrom M, Savitcheva I, Huang GF, Estrada S, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med. 2008a;14:504–506. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Chu Y, Hauser RA, Olanow CW, Freeman TB. Transplanted dopaminergic neurons develop PD pathologic changes: a second case report. Mov Disord. 2008b;23:2303–2306. doi: 10.1002/mds.22369. [DOI] [PubMed] [Google Scholar]
- Lassmann H, van Horssen J. The molecular basis of neurodegeneration in multiple sclerosis. FEBS Lett. 2011;585:3715–3723. doi: 10.1016/j.febslet.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Le TT, Pham LT, Butchbach ME, Zhang HL, Monani UR, Coovert DD, Gavrilina TO, Xing L, Bassell GJ, Burghes AH. SMNDelta7, the major product of the centromeric survival motor neuron (SMN2) gene, extends survival in mice with spinal muscular atrophy and associates with full-length SMN. Hum Mol Genet. 2005;14:845–857. doi: 10.1093/hmg/ddi078. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Suk JE, Patrick C, Bae EJ, Cho JH, Rho S, Hwang D, Masliah E, Lee SJ. Direct transfer of alpha-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J Biol Chem. 2010a;285:9262–9272. doi: 10.1074/jbc.M109.081125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Koga H, Kawaguchi Y, Tang W, Wong E, Gao YS, Pandey UB, Kaushik S, Tresse E, Lu J, et al. HDAC6 controls autophagosome maturation essential for ubiquitin-selective quality-control autophagy. Embo J. 2010b;29:969–980. doi: 10.1038/emboj.2009.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Nagano Y, Taylor JP, Lim KL, Yao TP. Disease-causing mutations in parkin impair mitochondrial ubiquitination, aggregation, and HDAC6-dependent mitophagy. J Cell Biol. 2010c;189:671–679. doi: 10.1083/jcb.201001039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Yao TP. Quality control autophagy: A joint effort of ubiquitin, protein deacetylase and actin cytoskeleton. Autophagy. 2010:6. doi: 10.4161/auto.6.4.11812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Varvel NH, Konerth ME, Xu G, Cardona AE, Ransohoff RM, Lamb BT. CX3CR1 deficiency alters microglial activation and reduces beta-amyloid deposition in two Alzheimer’s disease mouse models. Am J Pathol. 2010d;177:2549–2562. doi: 10.2353/ajpath.2010.100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, Lashley T, Quinn NP, Rehncrona S, Bjorklund A, et al. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med. 2008;14:501–503. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- Li JY, Englund E, Widner H, Rehncrona S, Bjorklund A, Lindvall O, Brundin P. Characterization of Lewy body pathology in 12- and 16-year-old intrastriatal mesencephalic grafts surviving in a patient with Parkinson’s disease. Mov Disord. 2010;25:1091–1096. doi: 10.1002/mds.23012. [DOI] [PubMed] [Google Scholar]
- Lin CL, Bristol LA, Jin L, Dykes-Hoberg M, Crawford T, Clawson L, Rothstein JD. Aberrant RNA processing in a neurodegenerative disease: the cause for absent EAAT2, a glutamate transporter, in amyotrophic lateral sclerosis. Neuron. 1998;20:589–602. doi: 10.1016/s0896-6273(00)80997-6. [DOI] [PubMed] [Google Scholar]
- Linden R. The survival of developing neurons: a review of afferent control. Neuroscience. 1994;58:671–682. doi: 10.1016/0306-4522(94)90447-2. [DOI] [PubMed] [Google Scholar]
- Lioy DT, Garg SK, Monaghan CE, Raber J, Foust KD, Kaspar BK, Hirrlinger PG, Kirchhoff F, Bissonnette JM, Ballas N, et al. A role for glia in the progression of Rett’s syndrome. Nature. 2011;475:497–502. doi: 10.1038/nature10214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Drouet V, Wu JW, Witter MP, Small SA, Clelland C, Duff K. Trans-synaptic spread of tau pathology in vivo. PLoS One. 2012;7:e31302. doi: 10.1371/journal.pone.0031302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk KC, Song C, O’Brien P, Stieber A, Branch JR, Brunden KR, Trojanowski JQ, Lee VM. Exogenous alpha-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc Natl Acad Sci U S A. 2009;106:20051–20056. doi: 10.1073/pnas.0908005106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie IR, Rademakers R, Neumann M. TDP-43 and FUS in amyotrophic lateral sclerosis and frontotemporal dementia. Lancet Neurol. 2010;9:995–1007. doi: 10.1016/S1474-4422(10)70195-2. [DOI] [PubMed] [Google Scholar]
- Magalhaes AC, Baron GS, Lee KS, Steele-Mortimer O, Dorward D, Prado MA, Caughey B. Uptake and neuritic transport of scrapie prion protein coincident with infection of neuronal cells. J Neurosci. 2005;25:5207–5216. doi: 10.1523/JNEUROSCI.0653-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley RW, Huang Y, Weisgraber KH. Detrimental effects of apolipoprotein E4: potential therapeutic targets in Alzheimer’s disease. Curr Alzheimer Res. 2007;4:537–540. doi: 10.2174/156720507783018334. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Price AC, McClendon KB, Al-Abdulla NA, Subramaniam JR, Wong PC, Liu Z. Early events of target deprivation/axotomy-induced neuronal apoptosis in vivo: oxidative stress, DNA damage, p53 phosphorylation and subcellular redistribution of death proteins. J Neurochem. 2003;85:234–247. doi: 10.1046/j.1471-4159.2003.01659.x. [DOI] [PubMed] [Google Scholar]
- Marty S, Peschanski M. Effects of target deprivation on the morphology and survival of adult dorsal column nuclei neurons. J Comp Neurol. 1995;356:523–536. doi: 10.1002/cne.903560404. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology. 1988;38:1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- Mendez I, Vinuela A, Astradsson A, Mukhida K, Hallett P, Robertson H, Tierney T, Holness R, Dagher A, Trojanowski JQ, et al. Dopamine neurons implanted into people with Parkinson’s disease survive without pathology for 14 years. Nat Med. 2008;14:507–509. doi: 10.1038/nm1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentis GZ, Blivis D, Liu W, Drobac E, Crowder ME, Kong L, Alvarez FJ, Sumner CJ, O’Donovan MJ. Early functional impairment of sensory-motor connectivity in a mouse model of spinal muscular atrophy. Neuron. 2011;69:453–467. doi: 10.1016/j.neuron.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Luehmann M, Coomaraswamy J, Bolmont T, Kaeser S, Schaefer C, Kilger E, Neuenschwander A, Abramowski D, Frey P, Jaton AL, et al. Exogenous induction of cerebral beta-amyloidogenesis is governed by agent and host. Science. 2006;313:1781–1784. doi: 10.1126/science.1131864. [DOI] [PubMed] [Google Scholar]
- Mickiewicz AL, Kordower JH. GDNF family ligands: a potential future for Parkinson’s disease therapy. CNS Neurol Disord Drug Targets. 10:703–711. doi: 10.2174/187152711797247876. [DOI] [PubMed] [Google Scholar]
- Munch C, O’Brien J, Bertolotti A. Prion-like propagation of mutant superoxide dismutase-1 misfolding in neuronal cells. Proc Natl Acad Sci U S A. 2011;108:3548–3553. doi: 10.1073/pnas.1017275108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai M, Re DB, Nagata T, Chalazonitis A, Jessell TM, Wichterle H, Przedborski S. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci. 2007;10:615–622. doi: 10.1038/nn1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka T, Watanabe ST, Iwatsubo T, Hasegawa M. Seeded aggregation and toxicity of {alpha}-synuclein and tau: cellular models of neurodegenerative diseases. J Biol Chem. 2010;285:34885–34898. doi: 10.1074/jbc.M110.148460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouchi Y, Yagi S, Yokokura M, Sakamoto M. Neuroinflammation in the living brain of Parkinson’s disease. Parkinsonism Relat Disord. 2009;15(Suppl 3):S200–204. doi: 10.1016/S1353-8020(09)70814-4. [DOI] [PubMed] [Google Scholar]
- Pan KM, Baldwin M, Nguyen J, Gasset M, Serban A, Groth D, Mehlhorn I, Huang Z, Fletterick RJ, Cohen FE, et al. Conversion of alpha-helices into beta-sheets features in the formation of the scrapie prion proteins. Proc Natl Acad Sci U S A. 1993;90:10962–10966. doi: 10.1073/pnas.90.23.10962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JY, Kim KS, Lee SB, Ryu JS, Chung KC, Choo YK, Jou I, Kim J, Park SM. On the mechanism of internalization of alpha-synuclein into microglia: roles of ganglioside GM1 and lipid raft. J Neurochem. 2009;110:400–411. doi: 10.1111/j.1471-4159.2009.06150.x. [DOI] [PubMed] [Google Scholar]
- Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signalling. Nature. 1994;369:744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- Pasti L, Volterra A, Pozzan T, Carmignoto G. Intracellular calcium oscillations in astrocytes: a highly plastic, bidirectional form of communication between neurons and astrocytes in situ. J Neurosci. 1997;17:7817–7830. doi: 10.1523/JNEUROSCI.17-20-07817.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier MA, Li H, Macosko J, Cai S, Amzel M, Ross CA. Huntingtin spheroids and protofibrils as precursors in polyglutamine fibrilization. J Biol Chem. 2002;277:41032–41037. doi: 10.1074/jbc.M205809200. [DOI] [PubMed] [Google Scholar]
- Prinz M, Priller J, Sisodia SS, Ransohoff RM. Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nat Neurosci. 2011;13:1227–1235. doi: 10.1038/nn.2923. [DOI] [PubMed] [Google Scholar]
- Rangasamy SB, Soderstrom K, Bakay RA, Kordower JH. Neurotrophic factor therapy for Parkinson’s disease. Prog Brain Res. 184:237–264. doi: 10.1016/S0079-6123(10)84013-0. [DOI] [PubMed] [Google Scholar]
- Ravits JM, La Spada AR. ALS motor phenotype heterogeneity, focality, and spread: deconstructing motor neuron degeneration. Neurology. 2009;73:805–811. doi: 10.1212/WNL.0b013e3181b6bbbd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren PH, Lauckner JE, Kachirskaia I, Heuser JE, Melki R, Kopito RR. Cytoplasmic penetration and persistent infection of mammalian cells by polyglutamine aggregates. Nat Cell Biol. 2009;11:219–225. doi: 10.1038/ncb1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RC, Ahn A, Swartz KJ, Beal MF, DiFiglia M. Intrastriatal injections of quinolinic acid or kainic acid: differential patterns of cell survival and the effects of data analysis on outcome. Exp Neurol. 1993;124:274–282. doi: 10.1006/exnr.1993.1197. [DOI] [PubMed] [Google Scholar]
- Rossi D, Volterra A. Astrocytic dysfunction: insights on the role in neurodegeneration. Brain Res Bull. 2009;80:224–232. doi: 10.1016/j.brainresbull.2009.07.012. [DOI] [PubMed] [Google Scholar]
- Rossi F, Strata P. Reciprocal trophic interactions in the adult climbing fibre-Purkinje cell system. Prog Neurobiol. 1995;47:341–369. [PubMed] [Google Scholar]
- Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, et al. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Van Kammen M, Levey AI, Martin LJ, Kuncl RW. Selective loss of glial glutamate transporter GLT-1 in amyotrophic lateral sclerosis. Ann Neurol. 1995;38:73–84. doi: 10.1002/ana.410380114. [DOI] [PubMed] [Google Scholar]
- Rumbaugh JA, Li G, Rothstein J, Nath A. Ceftriaxone protects against the neurotoxicity of human immunodeficiency virus proteins. J Neurovirol. 2007;13:168–172. doi: 10.1080/13550280601178218. [DOI] [PubMed] [Google Scholar]
- Saavedra L, Mohamed A, Ma V, Kar S, de Chaves EP. Internalization of beta-amyloid peptide by primary neurons in the absence of apolipoprotein E. J Biol Chem. 2007;282:35722–35732. doi: 10.1074/jbc.M701823200. [DOI] [PubMed] [Google Scholar]
- Saijo K, Winner B, Carson CT, Collier JG, Boyer L, Rosenfeld MG, Gage FH, Glass CK. A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell. 2009;137:47–59. doi: 10.1016/j.cell.2009.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakowski SA, Schuyler AD, Feldman EL. Insulin-like growth factor-I for the treatment of amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2009;10:63–73. doi: 10.1080/17482960802160370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samii A, Etminan M, Wiens MO, Jafari S. NSAID use and the risk of Parkinson’s disease: systematic review and meta-analysis of observational studies. Drugs Aging. 2009;26:769–779. doi: 10.2165/11316780-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Sapp E, Kegel KB, Aronin N, Hashikawa T, Uchiyama Y, Tohyama K, Bhide PG, Vonsattel JP, DiFiglia M. Early and progressive accumulation of reactive microglia in the Huntington disease brain. J Neuropathol Exp Neurol. 2001;60:161–172. doi: 10.1093/jnen/60.2.161. [DOI] [PubMed] [Google Scholar]
- Saxena S, Caroni P. Selective neuronal vulnerability in neurodegenerative diseases: from stressor thresholds to degeneration. Neuron. 2011;71:35–48. doi: 10.1016/j.neuron.2011.06.031. [DOI] [PubMed] [Google Scholar]
- Schenk D. Amyloid-beta immunotherapy for Alzheimer’s disease: the end of the beginning. Nat Rev Neurosci. 2002;3:824–828. doi: 10.1038/nrn938. [DOI] [PubMed] [Google Scholar]
- Sloane JA, Blitz D, Margolin Z, Vartanian T. A clear and present danger: endogenous ligands of Toll-like receptors. Neuromolecular Med. 2009;12:149–163. doi: 10.1007/s12017-009-8094-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreux-Varoquaux O, Bensimon G, Lacomblez L, Salachas F, Pradat PF, Le Forestier N, Marouan A, Dib M, Meininger V. Glutamate levels in cerebrospinal fluid in amyotrophic lateral sclerosis: a reappraisal using a new HPLC method with coulometric detection in a large cohort of patients. J Neurol Sci. 2002;193:73–78. doi: 10.1016/s0022-510x(01)00661-x. [DOI] [PubMed] [Google Scholar]
- Steiner JA, Angot E, Brundin P. A deadly spread: cellular mechanisms of alpha-synuclein transfer. Cell Death Differ. 2011;18:1425–1433. doi: 10.1038/cdd.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, Davis CN, Harrison JK. Role of fractalkine (CX3CL1) in regulating neuron-microglia interactions: development of viral-based CX3CR1 antagonists. Curr Alzheimer Res. 2005;2:187–189. doi: 10.2174/1567205053585765. [DOI] [PubMed] [Google Scholar]
- Sulzer D. Multiple hit hypotheses for dopamine neuron loss in Parkinson’s disease. Trends Neurosci. 2007;30:244–250. doi: 10.1016/j.tins.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Vella LJ, Sharples RA, Lawson VA, Masters CL, Cappai R, Hill AF. Packaging of prions into exosomes is associated with a novel pathway of PrP processing. J Pathol. 2007;211:582–590. doi: 10.1002/path.2145. [DOI] [PubMed] [Google Scholar]
- Vonsattel JP, Myers RH, Stevens TJ, Ferrante RJ, Bird ED, Richardson EP., Jr Neuropathological classification of Huntington’s disease. J Neuropathol Exp Neurol. 1985;44:559–577. doi: 10.1097/00005072-198511000-00003. [DOI] [PubMed] [Google Scholar]
- Wacker JL, Zareie MH, Fong H, Sarikaya M, Muchowski PJ. Hsp70 and Hsp40 attenuate formation of spherical and annular polyglutamine oligomers by partitioning monomer. Nat Struct Mol Biol. 2004;11:1215–1222. doi: 10.1038/nsmb860. [DOI] [PubMed] [Google Scholar]
- Wang HL, Chou AH, Lin AC, Chen SY, Weng YH, Yeh TH. Polyglutamineexpanded ataxin-7 upregulates Bax expression by activating p53 in cerebellar and inferior olivary neurons. Exp Neurol. 2010;224:486–494. doi: 10.1016/j.expneurol.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Wang T, Pei Z, Zhang W, Liu B, Langenbach R, Lee C, Wilson B, Reece JM, Miller DS, Hong JS. MPP+-induced COX-2 activation and subsequent dopaminergic neurodegeneration. FASEB J. 2005;19:1134–1136. doi: 10.1096/fj.04-2457fje. [DOI] [PubMed] [Google Scholar]
- Waxman EA, Giasson BI. A novel, high-efficiency cellular model of fibrillar alpha-synuclein inclusions and the examination of mutations that inhibit amyloid formation. J Neurochem. 2010;113:374–388. doi: 10.1111/j.1471-4159.2010.06592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wersinger C, Sidhu A. Inflammation and Parkinson’s disease. Curr Drug Targets Inflamm Allergy. 2002;1:221–242. doi: 10.2174/1568010023344580. [DOI] [PubMed] [Google Scholar]
- Williams AJ, Paulson HL. Polyglutamine neurodegeneration: protein misfolding revisited. Trends Neurosci. 2008;31:521–528. doi: 10.1016/j.tins.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt TJ, Keirstead HS. Stem cell-derived neurotrophic support for the neuromuscular junction in spinal muscular atrophy. Expert Opin Biol Ther. 10:1587–1594. doi: 10.1517/14712598.2010.529895. [DOI] [PubMed] [Google Scholar]
- Yang Y, Gozen O, Watkins A, Lorenzini I, Lepore A, Gao Y, Vidensky S, Brennan J, Poulsen D, Won Park J, et al. Presynaptic regulation of astroglial excitatory neurotransmitter transporter GLT1. Neuron. 2009;61:880–894. doi: 10.1016/j.neuron.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazawa I, Giasson BI, Sasaki R, Zhang B, Joyce S, Uryu K, Trojanowski JQ, Lee VM. Mouse model of multiple system atrophy alpha-synuclein expression in oligodendrocytes causes glial and neuronal degeneration. Neuron. 2005;45:847–859. doi: 10.1016/j.neuron.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Yvert G, Lindenberg KS, Picaud S, Landwehrmeyer GB, Sahel JA, Mandel JL. Expanded polyglutamines induce neurodegeneration and trans-neuronal alterations in cerebellum and retina of SCA7 transgenic mice. Hum Mol Genet. 2000;9:2491–2506. doi: 10.1093/hmg/9.17.2491. [DOI] [PubMed] [Google Scholar]
- Zadori D, Klivenyi P, Vamos E, Fulop F, Toldi J, Vecsei L. Kynurenines in chronic neurodegenerative disorders: future therapeutic strategies. J Neural Transm. 2009;116:1403–1409. doi: 10.1007/s00702-009-0263-4. [DOI] [PubMed] [Google Scholar]
- Zhang W, Wang T, Pei Z, Miller DS, Wu X, Block ML, Wilson B, Zhou Y, Hong JS, Zhang J. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. FASEB J. 2005;19:533–542. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]
- Zuccato C, Cattaneo E. Brain-derived neurotrophic factor in neurodegenerative diseases. Nat Rev Neurol. 2009;5:311–322. doi: 10.1038/nrneurol.2009.54. [DOI] [PubMed] [Google Scholar]
- Zuccato C, Ciammola A, Rigamonti D, Leavitt BR, Goffredo D, Conti L, MacDonald ME, Friedlander RM, Silani V, Hayden MR, et al. Loss of huntingtin-mediated BDNF gene transcription in Huntington’s disease. Science. 2001;293:493–498. doi: 10.1126/science.1059581. [DOI] [PubMed] [Google Scholar]
- Zwilling D, Huang SY, Sathyasaikumar KV, Notarangelo FM, Guidetti P, Wu HQ, Lee J, Truong J, Andrews-Zwilling Y, Hsieh EW, et al. Kynurenine 3-monooxygenase inhibition in blood ameliorates neurodegeneration. Cell. 2011;145:863–874. doi: 10.1016/j.cell.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]