Abstract
Orchestration of the growth and remodeling of tissues and responses of cells to their extracellular environment is mediated by metalloproteinases of the Metzincin clan. This group of proteins comprises several families of endopeptidases in which a zinc atom is liganded at the catalytic site to three histidine residues and an invariant methionine residue. Tissue inhibitors of metalloproteinases (TIMPs) are endogenous protein regulators of the matrix metalloproteinase (MMPs) family, and also of families such as the disintegrin metalloproteinases (ADAM and ADAMTS). TIMPs therefore have a pivotal role in determining the influence of the extracellular matrix, of cell adhesion molecules, and of many cytokines, chemokines and growth factors on cell phenotype. The TIMP family is an ancient one, with a single representative in lower eukaryotes and four members in mammals. Although much is known about their mechanism of action in proteinase regulation in mammalian cells, less is known about their functions in lower organisms. Recently, non-inhibitory functions of TIMPs have been identified in mammalian cells, including signaling roles downstream of specific receptors. There are clearly still questions to be answered with regard to their overall roles in biology.
Gene organization and evolutionary history
The naturally occurring inhibitory activities of the matrix metalloproteinases (MMPs) were initially identified in many cell and tissue culture studies, carried out over several decades. Between 1985 and 1996, however, four members of the tissue inhibitor of metalloproteinases (TIMP) family were definitively identified at the gene level in mammals. In fact, orthologs of the TIMPs are widely distributed across the animal kingdom and have now been identified in species as widely separated as Trichoplax, Hydra, molluscs, worms and insects, as well as in vertebrates such as fish and birds. Plants do have metzincins, but no plant TIMP ortholog has been identified.
TIMP1 was originally cloned in 1985 when it was found to have an erythroid potentiating activity [1] and to be an inhibitor of metalloproteinases [2]. TIMP2 was cloned in 1990 by Stetler-Stevenson et al. [3], TIMP3 by Pavloff and colleagues in 1992 [4], and TIMP4 in 1996 [5]. These proteins act as significant regulators of the activities of MMPs and, in some instances, of other metalloendopeptidases of the metzincin clan, namely the disintegrin metalloproteinases (ADAM) and the disintegrin metalloproteinases with thrombospondin motifs (ADAMTS). TIMPs inhibit with a 1:1 molar stoichiometry. Their importance in modulating the ability of a cell to control its extracellular environment, from the remodeling of the extracellular matrix to the interaction of cells via adhesion and signaling molecules such as growth factors has long been appreciated [6], but the significance of TIMPs as both proteinase inhibitors and signaling molecules in their own right is only just beginning to be documented [7].
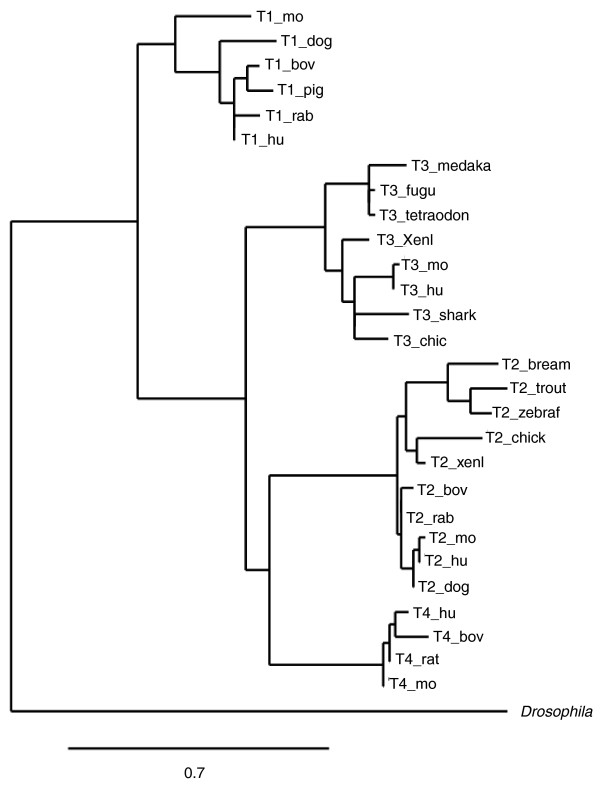
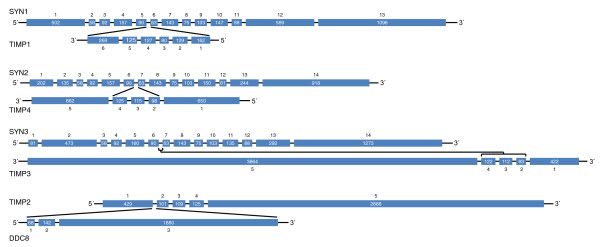
The four mammalian TIMPs are thought to be products of gene duplication because there is a single gene in insects, but orthologs of all four proteins are not found in all vertebrates. The TIMP proteins share a similar domain structure, composed of an amino-terminal domain and a carboxy-terminal sub-domain. TIMP1 and TIMP3 seem to have originated earlier than TIMP2 and TIMP4, with TIMP1 having undergone the least evolutionary change (Figure 1). The amino-terminal domain of human TIMP3 is more closely related to that of the Drosophila melanogaster TIMP in terms of sequence, isoelectric point, and functional properties, such as the inhibition of MT1-MMP and ADAM17. Hence, it has been suggested that TIMP3 might have more of the preserved functions of the ancestral protein than do the other human TIMPs. Invertebrate TIMPs differ more markedly in sequence than vertebrate TIMPs, having differing disulfide bond arrangements [6], but they maintain the N- and C-domain structure of vertebrate TIMPs. Nematode TIMPs, exceptionally, are single-domain proteins with similarity to the (inhibitory) N-domain of mammalian TIMPs. The human genes TIMP1 (chromosome Xp11.3-11.23), TIMP3 (chromosome 22q12.3) and TIMP4 (chromosome 3p25) are each nested within an intron of human synapsin genes, but with reverse directionality (that is, reading 3' and 5') (Figure 2). Synapsins comprise a multigene family of phosphoproteins that are neuron specific and are the most abundant protein of the synaptic vesicle. They are proposed to tether synaptic vesicles and regulate neurotransmitter release. Phylogenetic analysis shows that the Synapsin-TIMP gene nesting relationship began as far back as Drosophila, suggesting a strong linkage between these two gene families, although the significance of this association is not known. By contrast, the human TIMP2 gene (chromosome 17q25) hosts the gene Differential display clone 8 (DDC8) within its first intron (Figure 2) and there is evidence of alternative splicing between the two genes.
Figure 1.
A phylogenetic tree showing the inferred evolutionary relationships between the vertebrate TIMPs, rooted by the TIMP from Drosophila melanogaster. Reproduced from [6] with permission from Elsevier. TIMP, tissue inhibitors of metalloproteinases.
Figure 2.
Genomic structures of human TIMP genes. The TIMP1, TIMP4 and TIMP3 genes are embedded in reverse orientation (3' to 5') within the synapsin genes SYN1, SYN2 and SYN3, respectively. The TIMP2 gene has the Differential display clone 8 (DDC8) gene nested in its first intron. Exon sizes in number of bases are as indicated [15,22]. Ensembl IDs are: SYN I-ENSG00000008056; SYN II-ENSG00000157152; SYN III-ENSG00000185666; and TIMP2-ENSG00000035862. TIMP, tissue inhibitors of metalloproteinases.
Characteristic structural features and mechanism
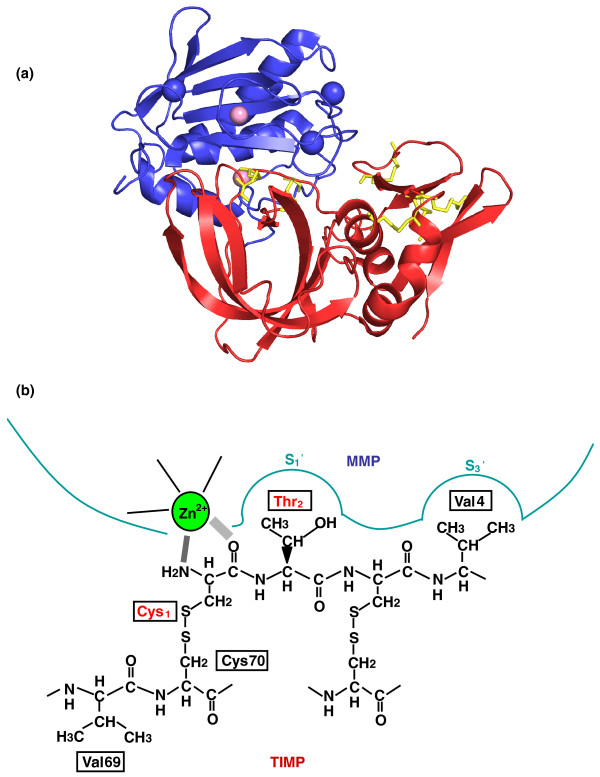
The human TIMPs comprise 184 to 194 amino acids that form an N-domain and a C-sub-domain that are stabilized by six disulfide bonds. The TIMPs are about 40% identical and have overlapping abilities to inhibit individual MMPs (Table 1). Their amnio-terminal domain is the inhibitory domain and it binds to the active site of the MMPs. MMPs do not form covalent bonds with TIMPs, neither do they cleave TIMPs, but they do form tight 1:1 complexes with TIMPs with inhibition constants in the sub-nanomolar range. ProMMP2 and proMMP9 zymogens of MMPs are secreted latent forms of the MMP in which a cleavable propeptide is inserted into the catalytic cleft; this propeptide must be removed proteolytically to allow activity. These pro-enzymes bind to TIMPs in an interaction between the carboxy-terminal non-inhibitory domain of the TIMP and the carboxy-terminal hemopexin domain of the proMMP. These complexes act as MMP inhibitors because the amino-terminal inhibitory domain of TIMPs is not blocked. The N-domain has a characteristic 'oligonucleotide and oligosaccharide (OB)' fold, composed of five beta strands arranged in a twisted β barrel and three α helices (Figure 3a). The ability of these proteins to inhibit the MMPs is largely due to the interaction of a wedge-shaped ridge on the N-domain, which binds within the active-site cleft of the target MMP (Figure 3b). The C-domain has two parallel β strands that are connected by an α helix to two anti-parallel β strands. This structure provides the ability of TIMPs to interact with the hemopexin domain of some MMPs, including the pro-forms of some MMPs, and could have a stabilizing role. Human TIMP1 and TIMP3 are glycoproteins that have complex sugar chains. These do not appear to play a role in the proteins' inhibitory activities but are important for interactions with the cellular environment. Notably, TIMP3 is incorporated into the extracellular matrix of tissues by charge interactions with sulfated glycosaminoglycans and could be classified as a 'matricellular' protein.
Table 1.
Properties of human TIMPs, summarizing their activities in inhibiting metzincin metalloproteinases and in modulating phenotypic cell behavior [6]
| Property | TIMP1 | TIMP2 | TIMP3 | TIMP4 |
|---|---|---|---|---|
| Amino acid residues | 184 | 194 | 188 | 194 |
| pI | 8.5 | 6.5 | 9.1 | 7.2 |
| Protein kDa | 28 | 22 | 24 or 27 | 22 |
| N-glycosylation sites | 2 | 0 | 1 | 0 |
| Synapsin gene | SYN1 | n/a | SYN3 | SYN2 |
| Protein localization | Soluble/cell surface | Soluble/cell surface | Extracellular matrix/cell surface | Soluble/cell surface |
| Pro-MMP binding | proMMP9 | proMMP2 | proMMP2, 9 | proMMP2 |
| MMPs poorly inhibited | MT1-MMP, MT3-MMP, MT5-MMP, MMP19 | None | None | None |
| ADAM inhibition | ADAM10 | ADAM12 | ADAM10, ADAM12, ADAM17, ADAM19, ADAM33, ADAMTS1, ADAMTS4, ADAMTS5, ADAMTS2 (weak) | ADAM17, ADAM28 |
| Binding partners | CD63, LRP1/MMP9 | α3β1integrin, LRP1 | EFEMP1, AngIIR, VEGFR | |
| Cell proliferation | ↑Erythroid precursors | ↑Erythroid precursors | ↑Smooth muscle cells and cancer cells | ↑Mammary tumor cells |
| ↑Tumor cells | ↑Tumor cells | ↓Wilm's tumor cells | ||
| ↑Fibroblasts | ||||
| ↑Smooth muscle cells | ||||
| ↓Endothelial cells | ||||
| Apoptosis | ↓Burkitt's lymphoma cells | ↑Colorectal cancer cells ↓Melanoma |
↑Smooth muscle ↑Tumor cells ↑Retinal pigment epithelial cells |
|
| Tumor angiogenesis | ↑Mammary ↓Liver |
↓Melanoma ↓Mammary |
↓Melanoma | |
| Angiogenesis in 3D collagen or fibrin gels | No effect | Inhibits | Inhibits | Inhibits |
| Tumorigenesis effects | Inhibits | Inhibits | Inhibits | Inhibits |
| Metastasis effects | Stimulates | Stimulates | ||
| Genetic defects | Idiopathic scoliosis | Sorsby's fundus dystrophy | Kawasaki disease |
AngIIR, angiotensin II receptor; EFEMP1, EGF-containing fibulin-like extracellular matrix protein 1; VEGFR, vascular endothelial growth factor receptor
Figure 3.
The mode of interaction of TIMP1 and the catalytic domain of MMP3. (a) Ribbon diagram of the complex involving TIMP1 (red) and MMP3 (blue). Zinc and calcium ions are shown as pink and blue spheres, respectively. The disulfide bonds of TIMP1 are shown in yellow. This image was prepared from the Brookhaven Protein Data bank entry (1UEA) using PyMol [24]. (b) Schematic representation of the amino-terminal ridge of TIMP1, which interacts with the active site of the MMP. Reproduced from [23] with permission from Springer Science+Business Media.
The α-amino and carbonyl groups of the amino-terminal Cys1 of the human TIMPs have been shown in crystallographic analyses to chelate the Zn2+ of the enzyme active site; the OH group of Ser/Thr2 interacts with the nucleophilic Glu of the MMP catalytic cleft (Figure 3b). The latter interaction displaces a water molecule that is essential for peptide hydrolysis from the active site. TIMP1, TIMP2, TIMP3 and TIMP4 all inhibit all of the MMPs tested, but TIMP1 is a poor inhibitor of MT1-MMP, MT3-MMP, MT5-MMP and MMP19 [6,8] (Table 1). TIMP2 is unique in that, in addition to inhibiting MMP activity, it selectively interacts with MT1-MMP to facilitate the cell-surface activation of proMMP2. Recent studies have shown that TIMPs can inhibit some of the disintegrin metalloproteinases (ADAM and ADAMTS). TIMP1 inhibits ADAM10 [9], whereas TIMP2 inhibits ADAM12 [10]. TIMP3 has a much broader inhibition profile, including ADAM10 [9], ADAM12 [10], ADAM17 [11] and several ADAMTSs (ADAMTS1, ADAMTS2, ADAMTS4 and ADAMTS5) [12-14]. The crystal structures of a number of TIMP-metalloproteinase complexes have shown that the mode of inhibition described above does not vary. The properties of the human TIMPs are summarized in Table 1.
Localization and function
The TIMPs show tissue-specific, constitutive, or inducible expression, which is regulated at the transcriptional level by various cytokines and growth factors. TIMP1 is widely expressed in many mammalian tissues, notably in the reproductive organs. In the central nervous system, TIMP1 expression is restricted to regions of persistent neuronal plasticity, such as the hippocampus, olfactory bulb, and cerebellum [15]. TIMP2 is constitutively expressed in most tissues but is not inducible by common growth factors. TIMP3 is expressed in many tissues and is often found as a significant matrix protein in the basement membranes of the eye and kidney. The relatively restricted tissue distribution of TIMP4 (in the heart, kidney, ovary, pancreas, colon, testes, brain and adipose tissue) suggests that it plays a role in tissue-specific physiological functions [5], but the role of this protein is relatively understudied.
The roles of TIMPs in both normal physiology and in pathological processes have been investigated by gene ablation studies in mice, and these have indicated that there is some functional redundancy. Timp1-null mice do not exhibit many inherent abnormalities, apart from alterations in the reproductive cycle and impaired learning and memory, unless the animals are challenged pathologically. Timp2-null mice have neurological and motor-function abnormalities, but again they only manifest phenotypes that are associated with matrix remodeling upon challenge (reviewed in [6,15]). Timp3 ablation causes lung emphysema-like alveolar damage [16] and faster apoptosis of mammary epithelial cells after weaning [17]. This suggests that TIMP3 is a major regulator of MMP activities in vivo. Furthermore, TIMP3 plays a key role in innate immunity by regulating the processing of tumor necrosis factor α by ADAM17 [18].
The individual TIMP genes appear to be regulated rather differently: TIMP1 and TIMP3 are upregulated in vitro in response to phorbol myrsistate acetate and transforming growth factor β, whereas TIMP2 is downregulated. All the TIMP promoters have sites for binding the transcription factor SP1. The TIMP1, TIMP2 and TIMP3 promoters have additional common elements, such as AP1 and PEA3, that are also present in several MMP promoters [19,20]. Otherwise, there is little organizational conservation of the common motifs in the TIMP promoters. Like many 'housekeeping genes' - that is, genes that are always expressed because they encode proteins that are constantly required - TIMP1, TIMP2 and TIMP4 lack obvious TATA boxes. TIMP1 has multiple transcription start sites and GC boxes. TIMP2, which has a TATA box and multiple transcription start sites, contains MEF2 and NF-IL6 binding sites in its promoter. The TIMP3 promoter uniquely has binding sites for the transcription factors NF-κB, c-MYC and p53. TIMP3 has a promoter-proximal GC-rich region that mediates its basal expression, and regions further upstream that mediate serum induction. TIMP3 is notably regulated by gene methylation, which can lead to its downregulation in many cancer cells. The TIMP4 promoter has no TATA box but does contain an initiator sequence and several transcription start points. TIMP4 also contains a myogenin-binding site, which is likely to be important for its skeletal muscle-specific expression, and an AP4 transcription factor binding site [20].
TIMP-mediated inhibition of MMP activity is an important determinant of cell function, but the concept of MMP-independent TIMP regulation of cell behavior is now supported by the identification of both specific cell-binding partners for TIMP family members and specific signaling events that involve these proteins [7]. Cell signaling mechanisms mediated by TIMP1 and TIMP2 have been shown to promote cell proliferation, although the receptor-mediated events that are involved are not well understood [7]. TIMP1 has anti-apoptotic effects on some cell types, possibly acting by enhancing the activities of survival and differentiation factors in a signaling network that is mediated by the tetraspanin CD63 and by focal adhesion kinase, phosphoinositide 3-kinase and ERK. TIMP2 binds to α3β1 integrin, leading to G1 phase growth arrest and enhanced de novo expression of the cyclin-dependent kinase inhibitor p27Kip1. TIMP3 has conflicting activities according to cell type. In some cells, it appears to promote the development of a transformed phenotype: TIMP3 promotes apoptosis in several tumor cell lines and in smooth muscle cells, but this appears to involve the modulation of metalloproteinase activities. Work with timp3-null mice suggests that TIMP3 can either promote or inhibit apoptosis depending on the model system examined. TIMP3 can be sequestered in the extracellular matrix in some tissues, but secreted TIMP3 can be rapidly endocytosed by cells such as chondrocytes through a mechanism that involves the LDL-receptor-related protein (LRP) [6,21].
Frontiers
Knowledge of the precise functions and spatio-temporal expression patterns of metalloproteinases are still somewhat limited. In vitro, the metalloproteinases have markedly overlapping substrate specificities and their in vivo functions will take some time to elucidate in detail (reviewed in [6]). Studies of function (for example, using transgenic mouse models) should be coupled with studies of the metalloproteinases' localization in tissues relative to the physiological or pathological status of those tissues. The importance of TIMPs in regulating metalloproteinases is consequently also less than clear. Nevertheless, gene-ablation studies in mice have indicated some key roles for TIMPs, with TIMP3 clearly being of some importance in both extracellular matrix remodeling and in inflammation. TIMP3 seems to function largely as a metalloproteinase inhibitor, but other TIMPs directly mediate signaling pathways that lead to the regulation of growth, apoptosis and other activities. TIMP1 concentrations are increased in cancer patients, particularly in those with breast or colorectal carcinomas, and this increase is negatively associated with patient outcome. Studies have shown the clinical utility of TIMP1 as a biomarker and independent prognostic factor in breast, colorectal, and several hematological cancers. There has been a growing awareness of the activity of TIMPs as modulators of nervous system physiology and pathology, and further elucidation of their roles alongside the activities of the metalloproteinases will be of interest in this context. Various polymorphisms of all the TIMP genes have been associated with predisposition to many different cancers. The function of TIMPs in lower organisms is not so well elucidated and might provide fascinating insights into the roles of metalloproteases in development.
Although several structures of TIMP-proteinase complexes have been solved, there are outstanding questions as to the importance of the flexibilities of both inhibitor and enzyme during binding. This could be key to future engineering of more specific forms of TIMPs as potential therapeutic agents. The regulation of TIMP function by extracellular interaction sites and by endocytosis is still to be studied fully [22]. The role of TIMPs in the regulation of proteinase clearance (for example, by endocytosis) is also not well understood. The MMP-independent, cytokine-like functions of the TIMPs are now well recognized, and future work will involve the identification of cell surface receptors and the elucidation of downstream signaling [7]. The pleiotropic activities of the TIMP family members are complex and depend upon subtle interactions with other extracellular components, as well as on direct interactions with cell-binding partners [7]. Understanding these processes and how they are modulated during disease progression will be helpful to our understanding of disease pathology. TIMPs have rarely been considered as the basis for novel therapeutic interventions, but their use in model systems gives vital clues about the efficacy of metalloproteinase inhibitors in the abrogation of disease [9,18].
Abbreviations
ADAM: a disintegrin metalloproteinase; ADAMTS: a disintegrin metalloproteinase with thrombospondin motif; DDC8: Differential display clone 8; MMP: matrix metalloproteinase; TIMP: tissue inhibitor of metalloproteinases.
Acknowledgements
Thanks to Hideaki Nagase, Keith Brew and Ian Clark for invaluable advice on the manuscript, and to my 'TIMP' colleagues for their hard work over the years. TIMP studies in the author's laboratory have been supported by Arthritis Research UK, the Biotechnology and Biological Sciences Research Council (BBSRC), the Medical Research Council (MRC), The Wellcome Trust and Cancer Research UK.
References
- Gasson JC, Golde DW, Kaufman SE, Westbrook CA, Hewick RM, Kaufman RJ, Wong GG, Temple PA, Leary AC, Brown EL, Orr EC, Clark SC. Molecular characterization and expression of the gene encoding human erythroid-potentiating activity. Nature. 1985;315:768–771. doi: 10.1038/315768a0. [DOI] [PubMed] [Google Scholar]
- Docherty AJ, Lyons A, Smith BJ, Wright EM, Stephens PE, Harris TJ, Murphy G, Reynolds JJ. Sequence of human tissue inhibitor of metalloproteinases and its identity to erythroid-potentiating activity. Nature. 1985;318:66–69. doi: 10.1038/318066a0. [DOI] [PubMed] [Google Scholar]
- Stetler-Stevenson WG, Brown PD, Onisto M, Levy AT, Liotta LA. Tissue inhibitor of metalloproteinases-2 (TIMP-2) mRNA expression in tumor cell lines and human tumor tissues. J Biol Chem. 1990;265:13933–13938. [PubMed] [Google Scholar]
- Pavloff N, Staskus PW, Kishnani NS, Hawkes SP. A new inhibitor of metalloproteinases from chicken:ChIMP-3. A third member of the TIMP family. J Biol Chem. 1992;267:17321–17326. [PubMed] [Google Scholar]
- Greene J, Wang MS, Liu YLE, Raymond LA, Rosen C, Shi YNE. Molecular cloning and characterization of human tissue inhibitor of metalloproteinase 4. J Biol Chem. 1996;271:30375–30380. doi: 10.1074/jbc.271.48.30375. [DOI] [PubMed] [Google Scholar]
- Brew K, Nagase H. The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochim Biophys Acta. 2010;1803:55–71. doi: 10.1016/j.bbamcr.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetler-Stevenson WG. The tumor microenvironment: regulation by MMP-independent effects of tissue inhibitor of metalloproteinases-2. Cancer Metastasis Rev. 2008;27:57–66. doi: 10.1007/s10555-007-9105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker AH, Edwards DR, Murphy G. Metalloproteinase inhibitors: biological actions and therapeutic opportunities. J Cell Sci. 2002;115:3719–3727. doi: 10.1242/jcs.00063. [DOI] [PubMed] [Google Scholar]
- Amour A, Knight CG, Webster A, Slocombe PM, Stephens PE, Knäuper V, Docherty AJP, Murphy G. The in vitro activity of ADAM-10 is inhibited by TIMP-1 and TIMP-340. FEBS Lett. 2000;473:275–279. doi: 10.1016/S0014-5793(00)01528-3. [DOI] [PubMed] [Google Scholar]
- Jacobsen J, Visse R, Sorensen HP, Enghild JJ, Brew K, Wewer UM, Nagase H. Catalytic properties of ADAM12 and its domain deletion mutants. Biochemistry. 2008;47:537–547. doi: 10.1021/bi701629c. [DOI] [PubMed] [Google Scholar]
- Amour A, Hutton M, Knauper V, Slocombe PM, Webster A, Butler M, Knight CG, Smith BJ, Docherty AJ, Murphy G. Inhibition of the metalloproteinase domain of mouse TACE. Ann NY Acad Sci. 1999;878:728–731. doi: 10.1111/j.1749-6632.1999.tb07774.x. [DOI] [PubMed] [Google Scholar]
- Kashiwagi M, Tortorella M, Nagase H, Brew K. Timp-3 is a potent inhibitor of aggrecanase 1 (adam-ts4) and aggrecanase 2 (adam-ts5). J Biol Chem. 2001;276:12501–12504. doi: 10.1074/jbc.C000848200. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Manzaneque JC, Westling J, Thai SN, Luque A, Knauper V, Murphy G, Sandy JD, Iruela-Arispe ML. ADAMTS1 cleaves aggrecan at multiple sites and is differentially inhibited by metalloproteinase inhibitors. Biochem Biophys Res Commun. 2002;293:501–508. doi: 10.1016/S0006-291X(02)00254-1. [DOI] [PubMed] [Google Scholar]
- Wang WM, Ge G, Lim NH, Nagase H, Greenspan DS. TIMP-3 inhibits the procollagen N-proteinase ADAMTS-2. Biochem J. 2006;398:515–519. doi: 10.1042/BJ20060630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera S, Khrestchatisky M, Kaczmarek L, Rosenberg GA, Jaworski DM. Metzincin proteases and their inhibitors: foes or friends in nervous system physiology?. J Neurosci. 2010;30:15337–15357. doi: 10.1523/JNEUROSCI.3467-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leco KJ, Waterhouse P, Sanchez OH, Gowing KL, Poole AR, Wakeham A, Mak TW, Khokha R. Spontaneous air space enlargement in the lungs of mice lacking tissue inhibitor of metalloproteinases-3 (TIMP-3). J Clin Invest. 2001;108:817–829. doi: 10.1172/JCI12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fata JE, Leco KJ, Voura EB, Yu HY, Waterhouse P, Murphy G, Moorehead RA, Khokha R. Accelerated apoptosis in the Timp-3-deficient mammary gland. J Clin Invest. 2001;108:831–841. doi: 10.1172/JCI13171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smookler DS, Mohammed FF, Kassiri Z, Duncan GS, Mak TW, Khokha R. Cutting edge: tissue inhibitor of metalloproteinase 3 regulates TNF-dependent systemic inflammation. J Immunol. 2006;176:721–725. doi: 10.4049/jimmunol.176.2.721. [DOI] [PubMed] [Google Scholar]
- Cruz-Munoz W, Khokha R. The role of tissue inhibitors of metalloproteinases in tumorigenesis and metastasis. Crit Rev Clin Lab Sci. 2008;45:291–338. doi: 10.1080/10408360801973244. [DOI] [PubMed] [Google Scholar]
- Clark IM, Swingler TE, Sampieri CL, Edwards DR. The regulation of matrix metalloproteinases and their inhibitors. Int J Biochem Cell Biol. 2008;40:1362–1378. doi: 10.1016/j.biocel.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Troeberg L, Fushimi K, Khokha R, Emonard H, Ghosh P, Nagase H. Calcium pentosan polysulfate is a multifaceted exosite inhibitor of aggrecanases. FASEB J. 2008;22:3515–3524. doi: 10.1096/fj.08-112680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candiani S, Moronti L, Pennati R, De Bernardi F, Benfenati F, Pestarino M. The synapsin gene family in basal chordates: evolutionary perspectives in metazoans. BMC Evol Biol. 2010;10:32. doi: 10.1186/1471-2148-10-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagase H, Murphy G. In: The Cancer Degradome. Edwards D, Hoyer-Hansen G, Blasi F, Sloane BF, editor. Dordrecht, The Netherlands: Springer; 2009. Tailoring TIMPs for selective metalloproteinase inhibition. pp. 787–810. [Google Scholar]
- PyMOL. http://www.pymol.org