Abstract
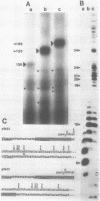
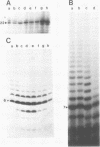
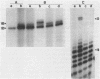
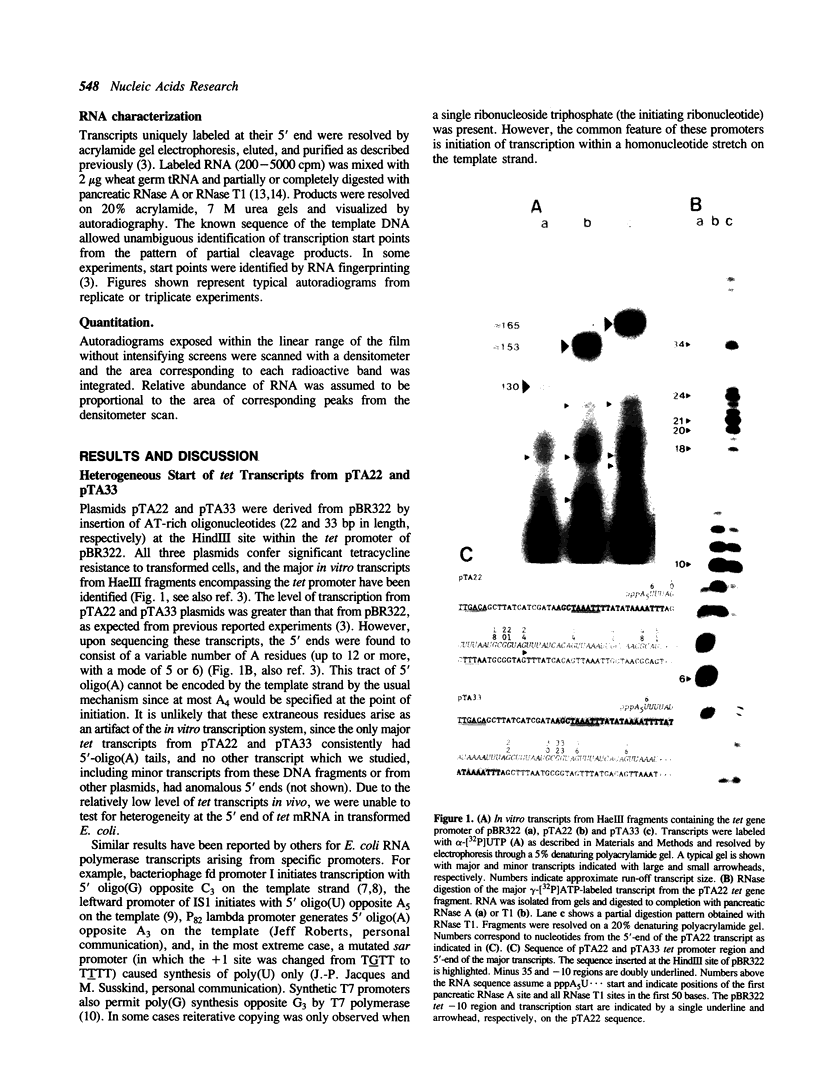
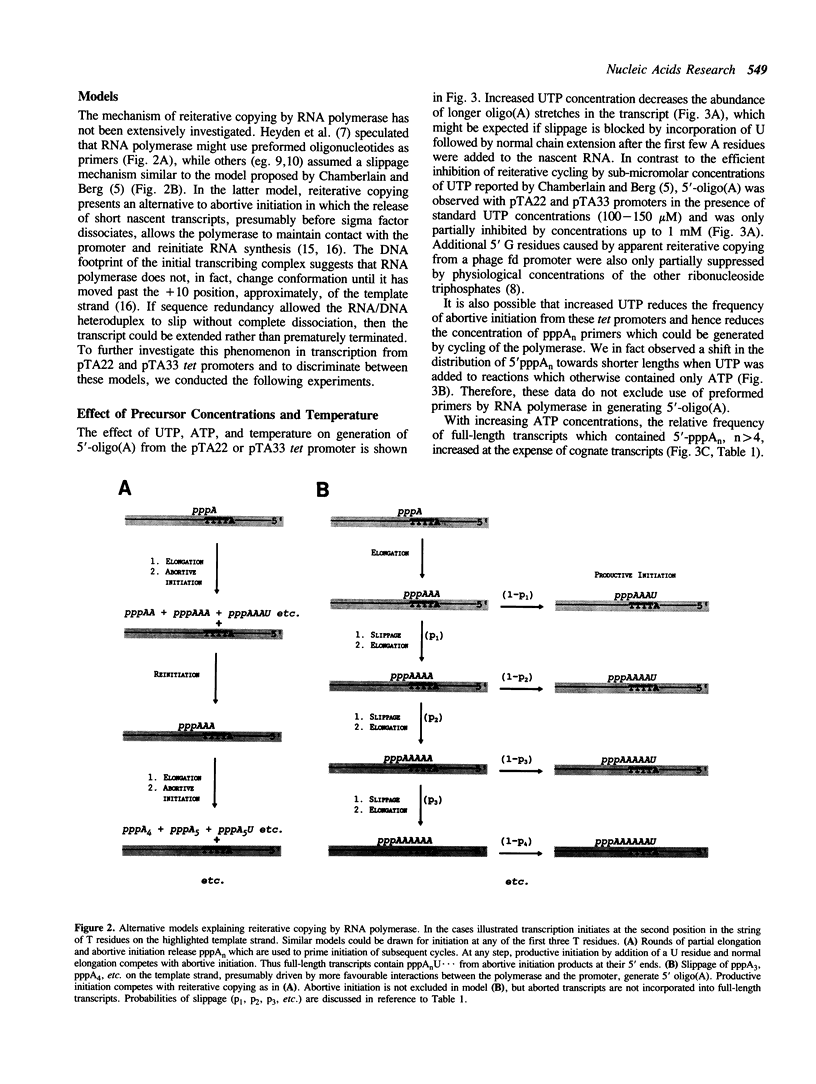
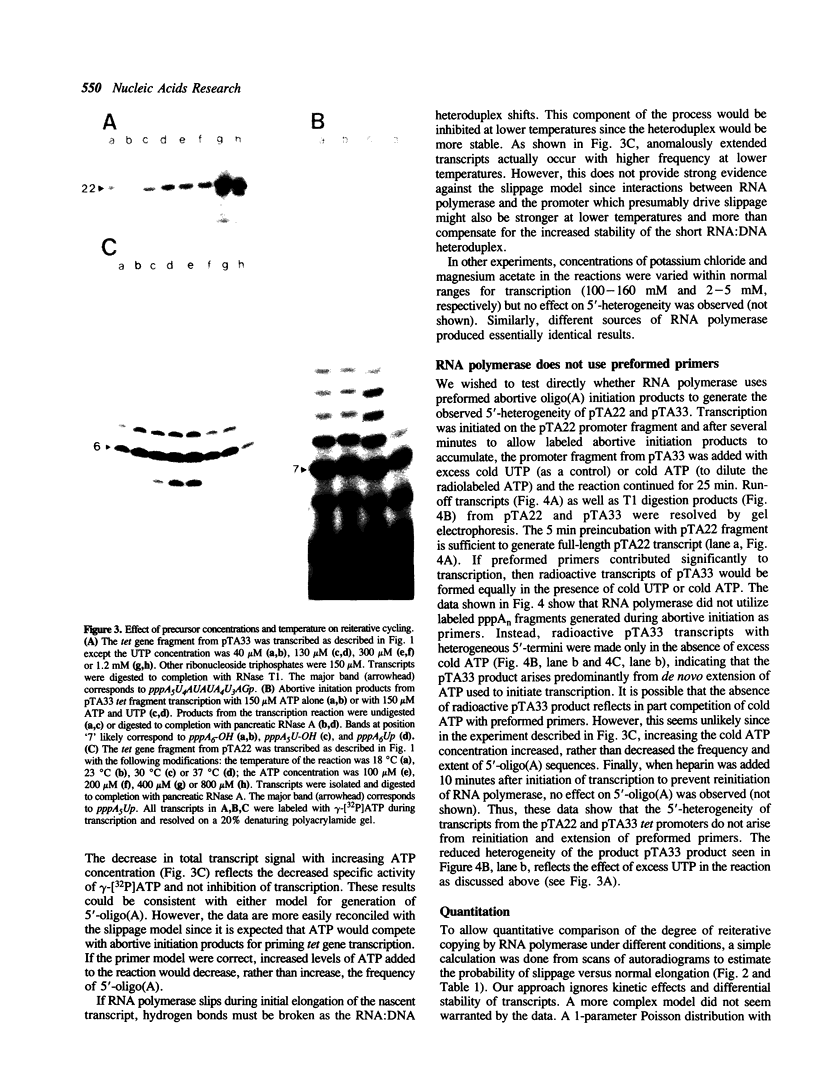
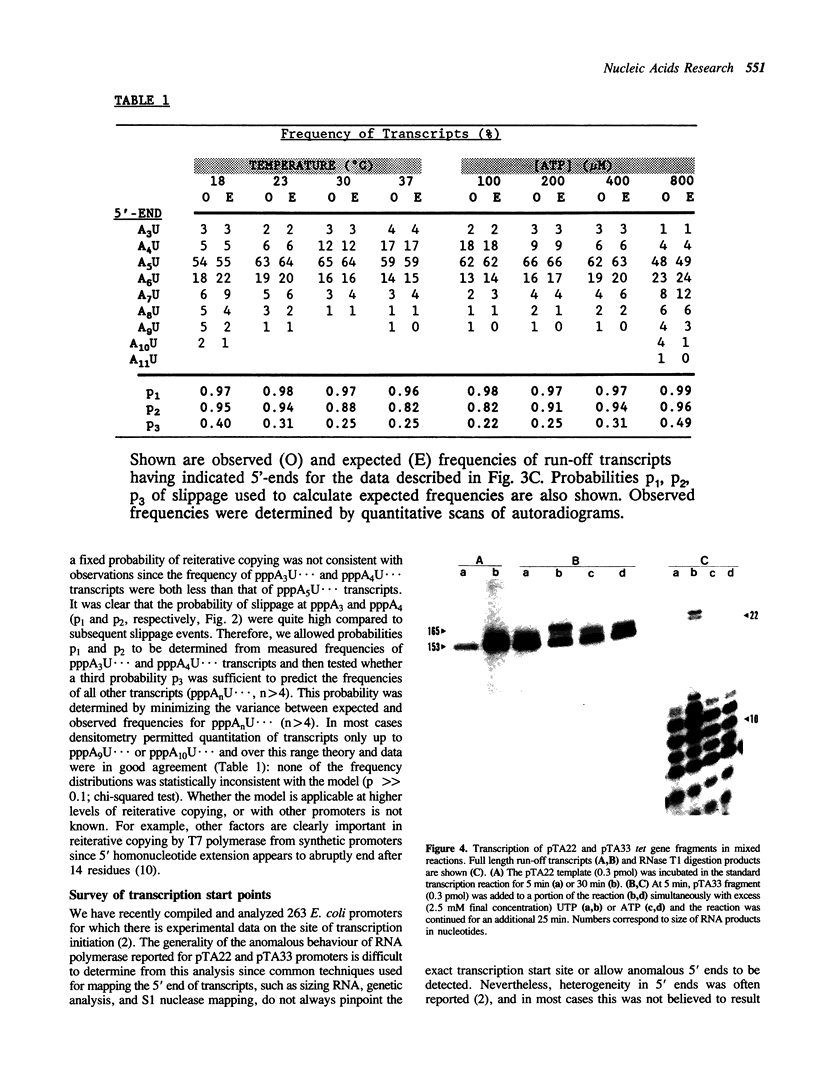
The major in vitro transcripts from the tet promoter of pBR322 derivatives pTA22 and pTA33 have heterogeneous 5' ends consisting of variable lengths of oligo(A). Their structure is 5'pppAnU..., where n ranges from 1 to greater than 12, but the template strand can encode at most four A residues at the site of transcription initiation. The abundance of additional A residues at the 5' end of the pTA22 and pTA33 tet transcripts could be reduced by elevating the concentration of UTP, but even at high concentrations (greater than 1 mM) non-cognate A residues were still observed. Aberrant initiation was not artifactual since the major and minor transcripts of the pBR322 tet promoter region, and other transcripts arising from minor promoters on pTA22 or pTA33 DNA all had unique 5' termini. Mixing experiments showed that RNA polymerase did not utilize pppA2-4-OH produced by abortive initiation as primers. The data suggest that the initial nascent RNA chain 'slips' in the 5' direction during elongation opposite T4 on the template strand causing RNA polymerase to reiteratively add A residues to the 5' end of the transcript. The generality and possible significance of this mechanism is discussed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burgess R. R., Jendrisak J. J. A procedure for the rapid, large-scall purification of Escherichia coli DNA-dependent RNA polymerase involving Polymin P precipitation and DNA-cellulose chromatography. Biochemistry. 1975 Oct 21;14(21):4634–4638. doi: 10.1021/bi00692a011. [DOI] [PubMed] [Google Scholar]
- CHAMBERLIN M., BERG P. MECHANISM OF RNA POLYMERASE ACTION: CHARACTERIZATION OF THE DNA-DEPENDENT SYNTHESIS OF POLYADENYLIC ACID. J Mol Biol. 1964 May;8:708–726. doi: 10.1016/s0022-2836(64)80120-0. [DOI] [PubMed] [Google Scholar]
- Carpousis A. J., Gralla J. D. Cycling of ribonucleic acid polymerase to produce oligonucleotides during initiation in vitro at the lac UV5 promoter. Biochemistry. 1980 Jul 8;19(14):3245–3253. doi: 10.1021/bi00555a023. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FALASCHI A., ADLER J., KHORANA H. G. CHEMICALLY SYNTHESIZED DEOXYPOLYNUCLEOTIDES AS TEMPLATES FOR RIBONUCLEIC ACID POLYMERASE. J Biol Chem. 1963 Sep;238:3080–3085. [PubMed] [Google Scholar]
- Gilmartin G. M., McDevitt M. A., Nevins J. R. Multiple factors are required for specific RNA cleavage at a poly(A) addition site. Genes Dev. 1988 May;2(5):578–587. doi: 10.1101/gad.2.5.578. [DOI] [PubMed] [Google Scholar]
- Greider C. W., Blackburn E. H. The telomere terminal transferase of Tetrahymena is a ribonucleoprotein enzyme with two kinds of primer specificity. Cell. 1987 Dec 24;51(6):887–898. doi: 10.1016/0092-8674(87)90576-9. [DOI] [PubMed] [Google Scholar]
- Harley C. B., Lawrie J., Betlach M., Crea R., Boyer H. W., Hedgpeth J. Transcription initiation at the tet promoter and effect of mutations. Nucleic Acids Res. 1988 Aug 11;16(15):7269–7285. doi: 10.1093/nar/16.15.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley C. B., Reynolds R. P. Analysis of E. coli promoter sequences. Nucleic Acids Res. 1987 Mar 11;15(5):2343–2361. doi: 10.1093/nar/15.5.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyden B., Nüsslein C., Schaller H. Initiation of transcription within an RNA-polymerase binding site. Eur J Biochem. 1975 Jun 16;55(1):147–155. doi: 10.1111/j.1432-1033.1975.tb02147.x. [DOI] [PubMed] [Google Scholar]
- Kreitman M. E., Aguadé M. Excess polymorphism at the Adh locus in Drosophila melanogaster. Genetics. 1986 Sep;114(1):93–110. doi: 10.1093/genetics/114.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machida C., Machida Y., Ohtsubo E. Both inverted repeat sequences located at the ends of IS1 provide promoter functions. J Mol Biol. 1984 Aug 5;177(2):247–267. doi: 10.1016/0022-2836(84)90455-8. [DOI] [PubMed] [Google Scholar]
- Martin C. T., Muller D. K., Coleman J. E. Processivity in early stages of transcription by T7 RNA polymerase. Biochemistry. 1988 May 31;27(11):3966–3974. doi: 10.1021/bi00411a012. [DOI] [PubMed] [Google Scholar]
- Nüsslein C., Schaller H. Stabilization of promoter complexes with a single ribonucleoside triphosphate. Eur J Biochem. 1975 Aug 15;56(2):563–569. doi: 10.1111/j.1432-1033.1975.tb02263.x. [DOI] [PubMed] [Google Scholar]
- Rajbhandary U. L. Recent developments in methods for RNA sequencing using in vitro 32P-labeling. Fed Proc. 1980 Aug;39(10):2815–2821. [PubMed] [Google Scholar]
- SCHACHMAN H. K., ADLER J., RADDING C. M., LEHMAN I. R., KORNBERG A. Enzymatic synthesis of deoxyribonucleic acid. VII. Synthesis of a polymer of deoxyadenylate and deoxythymidylate. J Biol Chem. 1960 Nov;235:3242–3249. [PubMed] [Google Scholar]
- Straney D. C., Crothers D. M. Comparison of the open complexes formed by RNA polymerase at the Escherichia coli lac UV5 promoter. J Mol Biol. 1987 Jan 20;193(2):279–292. doi: 10.1016/0022-2836(87)90219-1. [DOI] [PubMed] [Google Scholar]
- Tautz D., Trick M., Dover G. A. Cryptic simplicity in DNA is a major source of genetic variation. Nature. 1986 Aug 14;322(6080):652–656. doi: 10.1038/322652a0. [DOI] [PubMed] [Google Scholar]
- von Hippel P. H., Bear D. G., Morgan W. D., McSwiggen J. A. Protein-nucleic acid interactions in transcription: a molecular analysis. Annu Rev Biochem. 1984;53:389–446. doi: 10.1146/annurev.bi.53.070184.002133. [DOI] [PubMed] [Google Scholar]