Abstract
Introduction
Many people with rheumatoid arthritis (RA) do not receive care from a rheumatologist. We surveyed primary care physicians (PCPs) to better understand their attitudes, knowledge, and practices regarding the optimal treatment of RA.
Methods
Randomly selected PCPs practicing in the US were surveyed. The survey encompassed their experience with RA, use of disease modifying anti-rheumatic drugs (DMARDs), and experience with rheumatology referrals. Logistic regression analyses described the responses and examined the correlation between physician variables and use of DMARDs.
Results
E-mail invitations were opened by 1, 103 PCPs and completed by 267 (25%). Most respondents were men (68%) in practice for over 10 years (64%) who reported 6 or more RA patients under their care in the last year (71%). The majority reported some RA training after medical school (59%), but only one-third felt very confident managing this condition. Most (81%) reported prescribing DMARDs, but 37% do not initiate them, with only 9% reporting being very confident starting a DMARD. In unadjusted analyses, several respondent characteristics were strongly associated with not prescribing DMARDs, but none was significant after adjustment. Almost half (44%) of PCPs noted that patients report difficulty getting appointments with rheumatologists.
Conclusions
We found many PCPs are uncomfortable managing RA with DMARDs, despite common beliefs that their patients lack access to a rheumatologist. Lack of accessibility to rheumatologists and discomfort in prescribing DMARDs for patients with RA are potential barriers to optimal treatment.
Introduction
Rheumatoid arthritis (RA) is the most common type of systemic inflammatory arthritis and causes substantial pain and disability [1]. The standard of care for RA has been well established by all professional organizations of rheumatology and includes the early initiation of disease-modifying anti-rheumatic drugs (DMARDs) [2,3]. Strong evidence supports such recommendations; early DMARD use demonstrated better long-term functional outcomes [4]. However, multiple studies from various settings show that many patients with RA do not use these drugs [5-8].
The strongest correlate of DMARD use across multiple studies is the involvement of a rheumatologist in the care of patients with RA [5,7]. Investigators have estimated that patients who see a rheumatologist are four to five times more likely to receive a DMARD than those who receive care from an internist or family practitioner [5,6]. Furthermore, at least two studies found a more favorable outcome for patients treated regularly by rheumatologists [9,10]. However, many patients with RA do not see rheumatologists.
Many factors play a role in the management of RA, but primary care physicians (PCPs) may play the biggest role since they are often the first and only point of contact for the patient. Thus, it is primarily up to the PCPs to use their own clinical knowledge to determine the 'course of action' to meet each patient's needs [11]. Studies on the management of RA have shown that, despite their abilities to diagnose RA, PCPs frequently choose not to refer patients with RA to a rheumatic disease expert; furthermore, it is rare for PCPs to initiate DMARD therapy [12,13]. Moreover, even when PCPs refer patients with RA to rheumatologists, there are too few rheumatologists to adequately care for the growing population in need of rheumatic disease expertise [9,14]. The shortage of rheumatologists manifests as long wait times for rheumatology visits or lack of rheumatology referral altogether or both [13]. Despite this evidence of a rheumatologist shortage, relatively little is known about PCPs' interactions with patients with RA [14]. Therefore, it is important to explore the factors that are related to PCPs' management of RA and that may present barriers to recommended RA care. Our aim was to study PCPs' attitudes, knowledge, and practices regarding optimal treatment of RA, including DMARD use and rheumatology referral.
Materials and methods
Survey participants
Participant recruitment was achieved through collaboration with a survey vendor that has access to the American Medical Association physician directory. Through this vendor, we sent e-mails to 5, 331 randomly selected physicians who had e-mail addresses in the American Medical Association physician directory and who had self-designated as 'family medicine', 'general practice', or 'internal medicine' providers. Each physician was sent the e-mail on two occasions approximately 1 month apart. The e-mail invitation included a link to the web-based survey. A $75 honorarium gift card was offered for completion of the survey. The study protocol and survey were approved by the institutional review board of Partners' Healthcare. Informed consent was implied by PCPs' completion of the survey.
Survey
The survey consisted of sociodemographic information as well as RA-specific items. The RA-specific items focused on the respondents' experience with RA as well as their attitudes and knowledge of RA, DMARDs, and rheumatology referrals. Experience with RA was gauged through the respondents' years in practice and current number of patients with RA. Other questions ascertained physicians' attitudes and level of confidence toward referring patients with RA to rheumatologists, diagnosing RA, and prescribing DMARDs to patients with RA. Knowledge questions dealt with the appropriateness of DMARDs in RA as well as with RA training that respondents may have received beyond medical school. The survey ended with an open-ended item asking subjects whether they had any ideas for improving care for patients with RA.
Statistical analysis
The statistical analyses described the survey responses by calculating means and frequencies. We stratified survey responses according to whether respondents reported DMARD prescribing or not. Also, survey responses were stratified on the basis of the likelihood of referral to rheumatologists. Correlates of DMARD prescribing were examined in logistic regression models that calculated the odds ratio for prescribing and 95% confidence intervals. We assessed the odds ratios for all variables and then advanced selected variables with P values of less than 0.10 to a multivariable logistic regression model. All analyses were conducted with SAS 9.1 (SAS Institute Inc., Cary, NC, USA).
Results
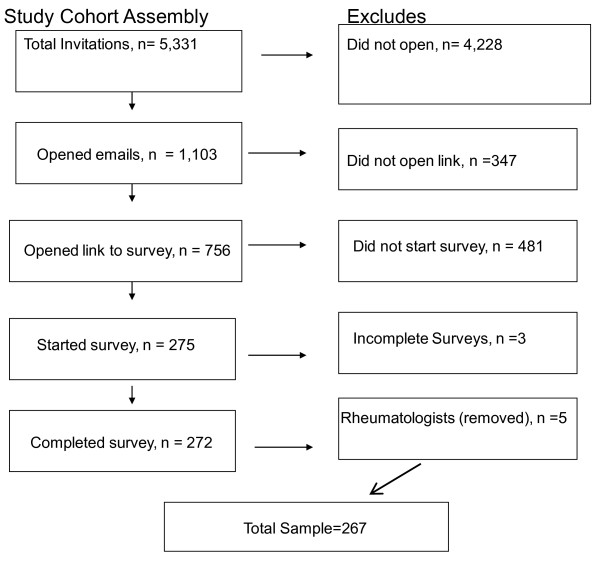
Invitations were sent by e-mail to 5, 331 physicians; 1, 103 (20.7%) opened the e-mail and 756 opened the survey. Of those who opened the survey, 275 (36%) started the survey and 272 completed the survey. Five were removed from our analysis because we discovered, from their answer to an open-ended question regarding RA education beyond medical school, that they had training in rheumatology. Our total sample consisted of 267 subjects (Figure 1).
Figure 1.
describes the assembly of the cohort.
Characteristics of our sample are shown in Table 1. The majority of our sample reported being in medical practice for more than 10 years. The sample consisted of physicians representing all regions of the US. Almost two thirds of the physicians in our sample see 10 or fewer patients with RA per year. The majority of our sample stated that they were 'somewhat confident' in their ability to diagnose RA, and approximately one third described themselves as 'very confident'. Over half of the sample stated that they had received additional RA education beyond medical school; 'journals or textbooks' (39%) followed by 'residency' (37%) were the most cited educational sources. Other popular sources of RA education included 'grand rounds', 'other continuing medical education', and 'online continuing medical education'.
Table 1.
Characteristics of survey respondents (n = 267)
| Number (percentage) | |
|---|---|
| Female gendera | 86 (32%) |
| Years practicing medicine | |
| 0-1 | 4 (1%) |
| 2-5 | 43 (16%) |
| 6-10 | 50 (19%) |
| 11-15 | 40 (15%) |
| 16+ | 130 (49%) |
| Region of practice | |
| West | 125 (47%) |
| Midwest | 41 (15%) |
| Northeast | 14 (5%) |
| South | 86 (32%) |
| RA patients seen in last year | |
| 0-5 | 77 (29%) |
| 6-10 | 91 (34%) |
| 11+ | 99 (37%) |
| Confidence in ability to diagnose RA | |
| Very confident | 83 (31%) |
| Somewhat confident | 161 (61%) |
| Less than confident | 19 (7%) |
| No confidence | 3 (1%) |
| Additional RA education beyond medical school | |
| Yes | 156 (59%) |
| Source of training regarding RA | |
| Residency | 100 (37%) |
| Fellowship | 4 (2%) |
| Grand rounds | 59 (22%) |
| Online continuing medical education | 54 (20%) |
| Other continuing medical education | 59 (22%) |
| Journals or textbooks or both | 104 (39%) |
| Other conferences | 48 (18%) |
| Other | 3 (1%) |
aSix missing. RA, rheumatoid arthritis.
We explored DMARD prescribing patterns in the survey (Table 2). Approximately one fifth of subjects stated that they did not prescribe DMARDs. Of the 217 who reported prescribing DMARDs, 45% stated that they had only continued a prescription (initially written by another physician), 9% stated that they had only initiated DMARDs, and the remainder reported writing first prescriptions and continued prescriptions. The most frequently prescribed DMARDs were non-biologics such as methotrexate, hydroxychloroquine, and sulfasalazine. However, 44% of prescribers reported prescribing biologic DMARDs.
Table 2.
Information on prescribing disease-modifying anti-rheumatic drugs
| Total | DMARD prescribers a | DMARD non-prescribers | |
|---|---|---|---|
| n = 266 b | n = 217 | n = 49 | |
| Initial prescription, continuation, or both | |||
| First only | 20 (9%) | ||
| Continuation only | 98 (45%) | ||
| Both | 99 (46%) | ||
| Prescribed DMARDs | |||
| Methotrexate | 195 (90%) | ||
| Hydroxychloroquine | 171 (79%) | ||
| Sulfasalazine | 135 (62%) | ||
| Leflunomide | 43 (20%) | ||
| Gold | 35 (16%) | ||
| Azathioprine | 54 (25%) | ||
| Cyclosporine | 32 (15%) | ||
| Abatacept | 8 (4%) | ||
| Etanercept | 64 (29%) | ||
| Infliximab | 56 (26%) | ||
| Rituximab | 20 (9%) | ||
| Adalimumab | 43 (20%) | ||
| Certolizumab pegol | 2 (1%) | ||
| Golimumab | 1 (0%) | ||
| Type of DMARDs prescribeda | |||
| Biologic and non-biologic DMARDs | 96 (44%) | ||
| Non-biologic DMARDs only | 118 (54%) | ||
| Biologic DMARDs only | 2 (1%) | ||
| Factors that make patients inappropriate candidates for DMARDs | |||
| No need (well controlled without DMARDs) | 131 (49%) | 110 (51%) | 21 (43%) |
| Too sick to take a DMARD | 92 (35%) | 78 (36%) | 14 (29%) |
| Side effects of DMARDs too problematic | 148 (56%) | 128 (59%) | 20 (41%) |
| Drug interactions | 83 (31%) | 74 (34%) | 9 (18%) |
| Drug cost and monitoring are too high | 142 (55%) | 115 (53%) | 27 (55%) |
| Cannot get laboratory monitoring | 20 (8%) | 17 (8%) | 3 (6%) |
| Best time to initiate DMARDs | |||
| After a trial of NSAIDs or steroids | 92 (35%) | 75 (35%) | 17 (35%) |
| Within the first 6 months of diagnosis | 159 (60%) | 132 (61%) | 27 (55%) |
| At least 6 months after diagnosis | 13 (5%) | 9 (4%) | 4 (8%) |
| Comfort level starting a DMARDc | |||
| Very comfortable | 23 (9%) | 23 (11%) | 0 (0%) |
| Somewhat comfortable | 79 (30%) | 73 (34%) | 6 (12%) |
| Somewhat uncomfortable | 112 (42%) | 89 (41%) | 23 (47%) |
| Very uncomfortable | 50 (19%) | 30 (14%) | 20 (41%) |
| Comfort level continuing a DMARDd | |||
| Very comfortable | 75 (28%) | 71 (33%) | 4 (8%) |
| Somewhat comfortable | 130 (49%) | 107 (50%) | 23 (47%) |
| Somewhat uncomfortable | 54 (20%) | 37 (17%) | 17 (35%) |
| Very uncomfortable | 6 (2%) | 1 (0%) | 5 (10%) |
aOne prescriber did not fill in types of disease-modifying anti-rheumatic drugs (DMARDs). bOne missing response. cThree missing responses. dTwo missing responses. NSAID, non-steroidal anti-inflammatory drug.
When asked what the best time to initiate DMARD therapy was, a little over one half of subjects chose 'within the first 6 months of diagnosis', whereas 35% chose 'after a trial of NSAIDs [non-steroidal anti-inflammatory drugs] or steroids', and 5% chose 'at least 6 months after diagnosis'. Less than 10% of subjects reported being very comfortable initiating DMARDs. The majority (61%) of subjects stated that they were 'somewhat uncomfortable' or 'very uncomfortable' starting a DMARD; however, almost half (49%) stated that they were 'somewhat comfortable' continuing DMARD therapy. Common reasons cited for discomfort using DMARDs included 'toxicities', 'infections', and 'intravenous therapy'.
To better understand physician correlates of reporting not prescribing DMARDs, we examined the probabilities of DMARD use in logistic regression models (Table 3). In unadjusted analyses, we found several strong correlates of not prescribing DMARDs, such as fewer years in practice, fewer patients with RA, lower confidence diagnosing RA, belief that less than half of patients with RA are good candidates for DMARDs, and lacking sufficient knowledge of DMARDs. However, none of these variables was statistically significant in adjusted analyses.
Table 3.
Probability of not prescribing a disease-modifying anti-rheumatic drug
|
Univariate, OR (95% CI) |
Multivariatea, OR (95% CI) |
|
|---|---|---|
| Years practicing medicine | ||
| 0-1 | 5.07 (0.69-37) | 1.44 (0.14-14.83) |
| 2-10 | 1.30 (0.68-2.49) | 0.98 (0.48-2.01) |
| 11+ | 1 | 1 |
| RA patients seen in last year | ||
| 0-5 | 2.46 (1.30-4.67) | 1.74 (0.85-3.58) |
| 6+ | 1 | 1 |
| Confidence in ability to diagnose RA | ||
| Very of somewhat confident | 1 | 1 |
| Less than or no confidence | 3.53 (1.41-8.82) | 2.07 (0.72-5.95) |
| Additional RA education beyond medical school | ||
| Yes | 1 | 1 |
| No | 1.46 (0.79-2.73) | 0.92 (0.45-1.89) |
| Proportion of RA patients who are good candidates for DMARDs | ||
| 0%-50% | 2.88 (1.22-6.82) | 2.41 (0.96-6.08) |
| 51%-75% | 1.01 (0.39-2.59) | 0.85 (0.31-2.32) |
| 76%-100% | 1 | 1 |
| Physicians' knowledge level of DMARDs | ||
| Very knowledgeable | 1 | 1 |
| Somewhat knowledgeable | 2.13 (0.27-16) | 1.22 (0.15-10.2) |
| Lacking sufficient or any knowledge | 9.87 (1.23-79) | 5.20 (0.60-44) |
aMultivariate model included all variables shown in the table. CI, confidence interval; DMARD, disease-modifying anti-rheumatic drug; OR, odds ratio; RA, rheumatoid arthritis.
The majority (71%) of our sample stated that they were very likely to refer patients with RA to a specialist (Table 4). When the question 'Under what situations would you refer your patients to a rheumatologist?' was asked, 'advanced disease' was the most cited reason, followed by 'patient desire' and 'uncomfortable prescribing DMARDs'. However, approximately one quarter reported that patients had difficulty getting appointments with rheumatologists. All of the physicians who were unlikely to refer gave 'no need' as their reason for not referring. However, they stated that they would refer if the patient desired. Some answers respondents gave when asked to describe 'other' reasons for referring their patients to rheumatologists included 'if they have insurance', 'for biologics or for medications requiring intravenous infusion', 'if not responding to DMARDs or failing NSAIDs', and 'compliance'. Some of the main reasons the PCPs in our sample did not refer included 'insurance problems', 'too difficult to get a rheumatology appointment', and 'no need'. Seven percent of the total sample stated their reason for not referring as 'do not know a practicing rheumatologist'.
Table 4.
Factors influencing referral to rheumatologist for rheumatoid arthritis
| Likelihood of referring | ||||
|---|---|---|---|---|
| Total cohort | Very likely | Possible | Unlikely | |
| (n = 266a) | (n = 189) | (n = 73) | (n = 4) | |
| Situations leading up to referral | ||||
| Advanced disease | 213 (80%) | 153 (81%) | 60 (82%) | 0 (0%) |
| Patient desire for a referral | 211 (79%) | 153 (81%) | 54 (74%) | 4 (100%) |
| Uncomfortable prescribing DMARDs | 186 (70%) | 152 (80%) | 33 (45%) | 1 (25%) |
| Patient comorbidities | 107 (40%) | 85 (45%) | 20 (27%) | 2 (50%) |
| Other | 14 (5%) | 11 (6%) | 3 (4%) | 0 (0%) |
| Patients report of difficulty getting rheumatology appointmentb | ||||
| Yes, most of the time | 74 (44%) | 49 (40%) | 25 (57%) | 0 (0%) |
| No, never | 95 (56%) | 74 (60%) | 19 (43%) | 2 (50%) |
| Main reason for not referring | ||||
| Do not know a rheumatologist | 19 (7%) | 10 (5%) | 9 (12%) | 0 (0%) |
| Rheumatology appointment too difficult to get | 73 (27%) | 46 (24%) | 27 (37%) | 0 (0%) |
| No need | 63 (24%) | 32 (17%) | 27 (37%) | 4 (100%) |
| Insurance problems | 107 (40%) | 74 (39%) | 33 (45%) | 0 (0%) |
| Other | 48 (18%) | 37 (20%) | 11 (15%) | 0 (0%) |
aOne missing response. bOne hundred three missing responses because of an error in the electronic survey. DMARD, disease-modifying anti-rheumatic drug.
Discussion
We studied the prescribing and referring habits of a national sample of PCPs who took part in an e-mailed survey. Most of our subjects had more than 10 years of practice and saw six or more patients with RA in the last year. Nearly all subjects felt either somewhat confident or very confident in their ability to diagnose RA, and most had received additional RA education beyond medical school. Approximately one fifth of respondents reported not prescribing DMARDs, and although 46% of those who did claim to prescribe DMARDs stated that they would both initiate and continue DMARD therapy, 45% stated that they would only continue DMARD therapy and 9% stated they would only initiate DMARDs. Of the 81% of our sample who reported prescribing a DMARD, just over half stated that they were either 'somewhat' or 'very' uncomfortable starting a DMARD. Most of our sample stated that they would refer their patients with RA to rheumatologists, but 44% noted that patients report difficulty getting appointments with a rheumatologist.
These results suggest that PCPs commonly continue DMARDs but that a minority initiate them. A high level of discomfort prescribing DMARDs with difficulty accessing rheumatology referrals may underpin reports of suboptimal prescribing of DMARDs. There is also evidence of early use of DMARDs; however, 40% of those PCPs who prescribe DMARDs report that delayed initiation is appropriate (Table 2). This 'wait and see' approach implies a lack of urgency toward aggressive treatment and may contribute to the 'no need' attitude, felt by some, for referral to a rheumatologist (Table 4).
Furthermore, when asked what factors make patients inappropriate candidates for DMARD therapy, approximately half of the total respondents reported that there was no need for a DMARD, one third noted patients were too sick to get a DMARD, and more than half felt that side effects of DMARDs were too problematic (Table 2). Other concerns raised were drug interactions and the cost of medications and their monitoring. We asked our sample to give ideas for improving patient care in regard to RA. The most popular idea was further training and education to improve comfort level diagnosing RA and prescribing treatment. Much of our sample saw a need for more continuing medical education, and several requested a treatment algorithm to follow. Another frequently cited idea by respondents for improving RA care was increased access to rheumatologists for consultation and patient appointments as well as more patient education regarding RA and DMARD therapy.
The literature exploring PCPs' referring and prescribing habits in regard to RA management is limited. One study in Ontario, Canada, conducted a survey using a case scenario to examine PCPs' management of RA and found that most physicians would diagnose RA properly; however, rates of referral to specialists tended to be low [13]. Another Canadian study surveyed family physicians in Quebec and found that the vast majority of physicians would make proper RA diagnoses in a vignette case and that about three fourths said that they would refer patients to a rheumatic disease specialist; however, only 4% mentioned initiating DMARD therapy [12].
Our findings were similar to those of the aforementioned studies in regard to the ability and confidence of PCPs to make a proper diagnosis; however, the Ontario study showed that rates of referral were low whereas our sample's rate of referral was high. The Quebec study, like ours, showed that the majority of PCPs would refer patients with RA to specialists; however, only 4% mentioned initiating DMARD therapy whereas 80% of our sample stated that they would prescribe DMARDs. Our study is more recent, and this may explain the different attitudes of PCPs to the use of DMARDs. Additionally, differences may be due to different structures of the health-care systems. One additional difference that may be important to note between our study and these Canadian studies is the difference in response rates. Whereas studies by Bernatsky and colleagues [12] and Glazier and colleagues [13] yielded response rates of 31% and 68%, respectively, our study produced a response rate of only 25%. Possible reasons for this difference might be that the two Canadian studies mailed surveys via conventional mail whereas our surveys were internet-based and that the Canadian studies repeated their mailings at least two times whereas we did only one follow-up mailing. Additionally, we do not know what type of incentive the Canadian researchers offered their respondents for completing the survey and therefore these studies cannot be compared with ours.
Several limitations of this research are important to note. There are several issues regarding generalizability with internet surveys. One author [15] noted that respondents who answer internet-based surveys are those who are keyboard and internet literate - currently only a third of the adult population in the US. Second, internet based surveys frequently confuse respondents in their instructions. A third problem noted was a lack of time, leading to some respondents' preference to take the survey in locations other than online. It was suggested that respondents may then choose to print out the survey and take it in another location, and this would potentially exclude these respondents' answers, creating a bias [15]. There was also a relatively low response rate. Of those who opened the e-mail, 25% completed the survey. This response rate, while lower than one might hope, is similar to that of other e-mailed surveys [16]. Additionally, literature supporting the reliability of internet questionnaires as a surveying tool exists [17]. Our survey was not a validated questionnaire but a compilation of questions that regarded attitudes toward and knowledge about RA and that deserved further attention. Finally, we were unable to determine whether respondents were representative of the entire sample with regard to demographics.
Conclusions
We surveyed a US sample of PCPs in regard to the management of RA. We found that many PCPs are uncomfortable managing RA with DMARDs, despite common beliefs that their patients lack access to a rheumatologist. Lack of accessibility to rheumatologists and discomfort in prescribing DMARDs for patients with RA are potential barriers to optimal treatment. Future directions for research into improving RA care may include the design of educational programs for PCPs to gain greater comfort prescribing DMARDs. It might also be prudent to develop ways in which rheumatic disease specialists can work more closely with PCPs to treat RA by using a 'team' approach. Such models have been developed for diabetes and depression [18,19]. Other options for improving treatment would be to further involve non-physician providers, such as nurse practitioners or physician assistants, in managing DMARDs. If such approaches are found to be effective, RA management may be improved substantially.
Abbreviations
DMARD: disease-modifying anti-rheumatic drug; NSAID: non-steroidal anti-inflammatory drug; PCP: primary care physician; RA: rheumatoid arthritis.
Competing interests
DHS has received research support from Amgen (Thousand Oaks, CA, USA), Abbott (Abbott Park, IL, USA), and Lilly (Indianapolis, IN, USA) and directed an educational course funded by Bristol-Myers Squibb (Princeton, NJ, USA). He has also participated in an unpaid role in several trials funded by Pfizer Inc (New York, NY, USA) but these trials were not about rheumatoid arthritis. The other authors declare that they have no competing interests.
Authors' contributions
KLG participated in drafting the survey, data collection, and manuscript preparation. MDI participated in drafting the survey, data analysis, and manuscript preparation. HT participated in data analysis. DHS conceived of the study and participated in design of the study, drafting of the survey, data analysis, and manuscript preparation. All authors read and approved the final manuscript.
Contributor Information
Katie L Garneau, Email: kgarneau@bu.edu.
Maura D Iversen, Email: m.iversen@neu.edu.
Hsun Tsao, Email: htsao1@partners.org.
Daniel H Solomon, Email: dsolomon@partners.org.
References
- Pincus T, Callahan LF, Sale WG, Brooks AL, Payne LE, Vaughn WK. Severe functional declines, work disability, and increased mortality in seventy-five rheumatoid arthritis patients studied over nine years. Arthritis Rheum. 1984;27:864–872. doi: 10.1002/art.1780270805. [DOI] [PubMed] [Google Scholar]
- Saag KG, Teng GG, Patkar NM, Anuntiyo J, Finney C, Curtis JR, Paulus HE, Mudano A, Pisu M, Elkins-Melton M, Outman R, Allison JJ, Suarez Almazor M, Bridges SL Jr, Chatham WW, Hochberg M, MacLean C, Mikuls T, Moreland LW, O'Dell J, Turkiewicz AM, Furst DE. American College of Rheumatology. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59:762–784. doi: 10.1002/art.23721. [DOI] [PubMed] [Google Scholar]
- Smolen JS, Landewé R, Breedveld FC, Dougados M, Emery P, Gaujoux-Viala C, Gorter S, Knevel R, Nam J, Schoels M, Aletaha D, Buch M, Gossec L, Huizinga T, Bijlsma JW, Burmester G, Combe B, Cutolo M, Gabay C, Gomez-Reino J, Kouloumas M, Kvien TK, Martin-Mola E, McInnes I, Pavelka K, van Riel P, Scholte M, Scott DL, Sokka T, Valesini G, van Vollenhoven R, Winthrop KL, Wong J, Zink A, van der Heijde D. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann Rheum Dis. 2010;69:964–975. doi: 10.1136/ard.2009.126532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finckh A, Liang MH, van Herckenrode CM, de Pablo P. Long-term impact of early treatment on radiographic progression in rheumatoid arthritis: a meta-analysis. Arthritis Rheum. 2006;55:864–872. doi: 10.1002/art.22353. [DOI] [PubMed] [Google Scholar]
- Schmajuk G, Schneeweiss S, Katz JN, Weinblatt ME, Setoguchi S, Avorn J, Levin R, Solomon DH. Treatment of older adult patients diagnosed with rheumatoid arthritis: improved but not optimal. Arthritis Rheum. 2007;57:928–934. doi: 10.1002/art.22890. [DOI] [PubMed] [Google Scholar]
- Lacaille D, Anis AH, Guh DP, Esdaile JM. Gaps in care for rheumatoid arthritis: a population study. Arthritis Rheum. 2005;53:241–248. doi: 10.1002/art.21077. [DOI] [PubMed] [Google Scholar]
- Widdifield J, Bernatsky S, Paterson JM, Thorne JC, Cividino A, Pope J, Gunraj N, Bombardier C. Quality care in seniors with new-onset rheumatoid arthritis: a Canadian perspective. Arthritis Care Res (Hoboken) 2011;63:53–57. doi: 10.1002/acr.20304. [DOI] [PubMed] [Google Scholar]
- Schmajuk G, Trivedi AN, Solomon DH, Yelin E, Trupin L, Chakravarty EF, Yazdany J. Receipt of disease-modifying antirheumatic drugs among patients with rheumatoid arthritis in Medicare managed care plans. JAMA. 2011;305:480–486. doi: 10.1001/jama.2011.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipton D, Glazier RH, Guan J, Badley EM. Effects of use of specialty services on disease-modifying antirheumatic drug use in the treatment of rheumatoid arthritis in an insured elderly population. Med Care. 2004;42:907–913. doi: 10.1097/01.mlr.0000135810.39691.f6. [DOI] [PubMed] [Google Scholar]
- Yelin EH, Such CL, Criswell LA, Epstein WV. Outcomes for persons with rheumatoid arthritis with a rheumatologist versus a non-rheumatologist as the main physician for this condition. Med Care. 1998;36:513–522. doi: 10.1097/00005650-199804000-00007. [DOI] [PubMed] [Google Scholar]
- Foley KA, Denke MA, Kamal-Bahl S, Simpson R Jr, Berra K, Sajjan S, Alexander CM. The impact of physician attitudes and beliefs on treatment decisions: lipid therapy in high-risk patients. Med Care. 2006;44:421–428. doi: 10.1097/01.mlr.0000208017.18278.1a. [DOI] [PubMed] [Google Scholar]
- Bernatsky S, Feldman D, Shrier I, Toupin K, Haggerty J, Tousignant P, Zummer M. Care pathways in early rheumatoid arthritis. Can Fam Physician. 2006;52:1444–1445. [PMC free article] [PubMed] [Google Scholar]
- Glazier RH, Dalby DM, Badley EM, Hawker GA, Bell MJ, Buchbinder R, Lineker SC. Management of the early and late presentations of rheumatoid arthritis: a survey of Ontario primary care physicians. CMAJ. 1996;155:679–687. [PMC free article] [PubMed] [Google Scholar]
- Deal CL, Hooker R, Harrington T, Birnbaum N, Hogan P, Bouchery E, Klein-Gitelman M, Barr W. The United States rheumatology workforce: supply and demand, 2005-2025. Arthritis Rheum. 2007;56:722–729. doi: 10.1002/art.22437. [DOI] [PubMed] [Google Scholar]
- Wyatt JC. When to use Web-based surveys. J Am Med Inform Assoc. 2000;7:426–429. doi: 10.1136/jamia.2000.0070426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan K. E-mail surveys response rates: a review. Journal of Computer-Mediated Communication. 2006;6:0. http://www.ascusc.org/jcmc/vol4/issue3/ [Google Scholar]
- Ritter P, Lorig K, Laurent D, Matthews K. Internet versus mailed questionnaires: a randomized comparison. J Med Internet Res. 2004;6:e29. doi: 10.2196/jmir.6.3.e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katon WJ, Von Korff M, Lin EH, Simon G, Ludman E, Russo J, Ciechanowski P, Walker E, Bush T. The Pathways Study: a randomized trial of collaborative care in patients with diabetes and depression. Arch Gen Psychiatry. 2004;61:1042–1049. doi: 10.1001/archpsyc.61.10.1042. [DOI] [PubMed] [Google Scholar]
- Greenfield S. Defining and understanding 'co-management' care. Diabetes Care. 2002;25:1657–1658. doi: 10.2337/diacare.25.9.1657. [DOI] [PubMed] [Google Scholar]