Abstract
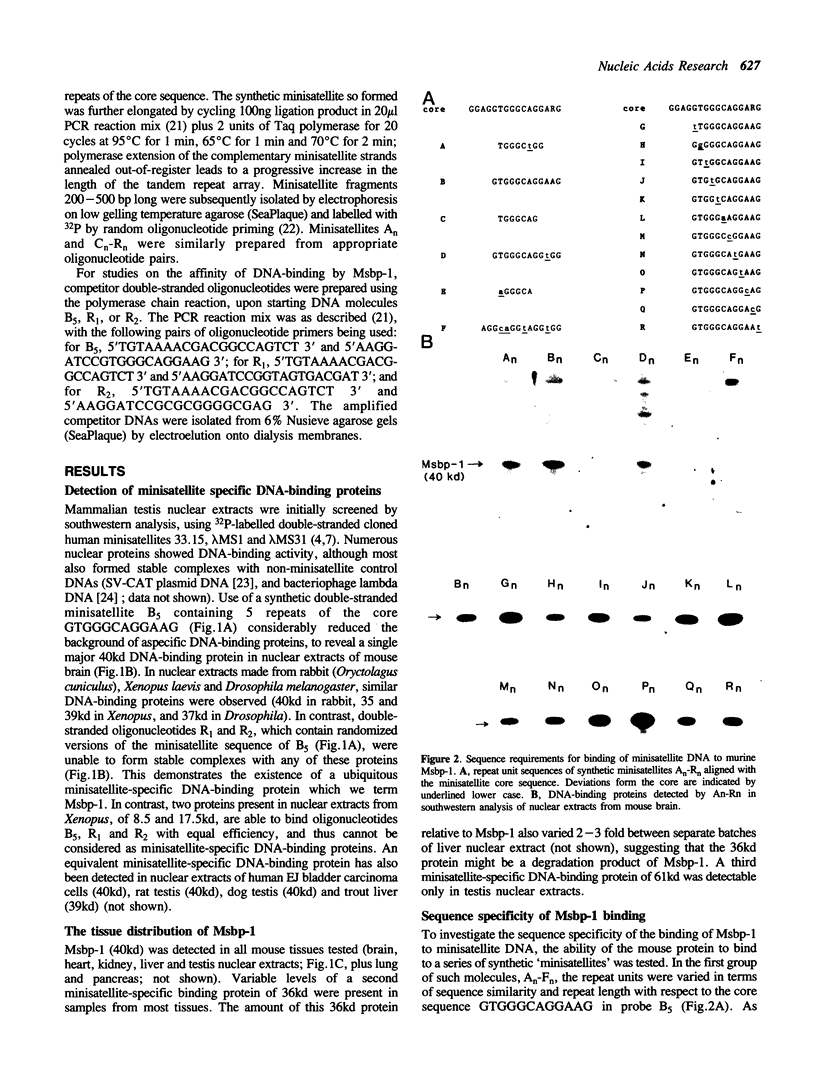
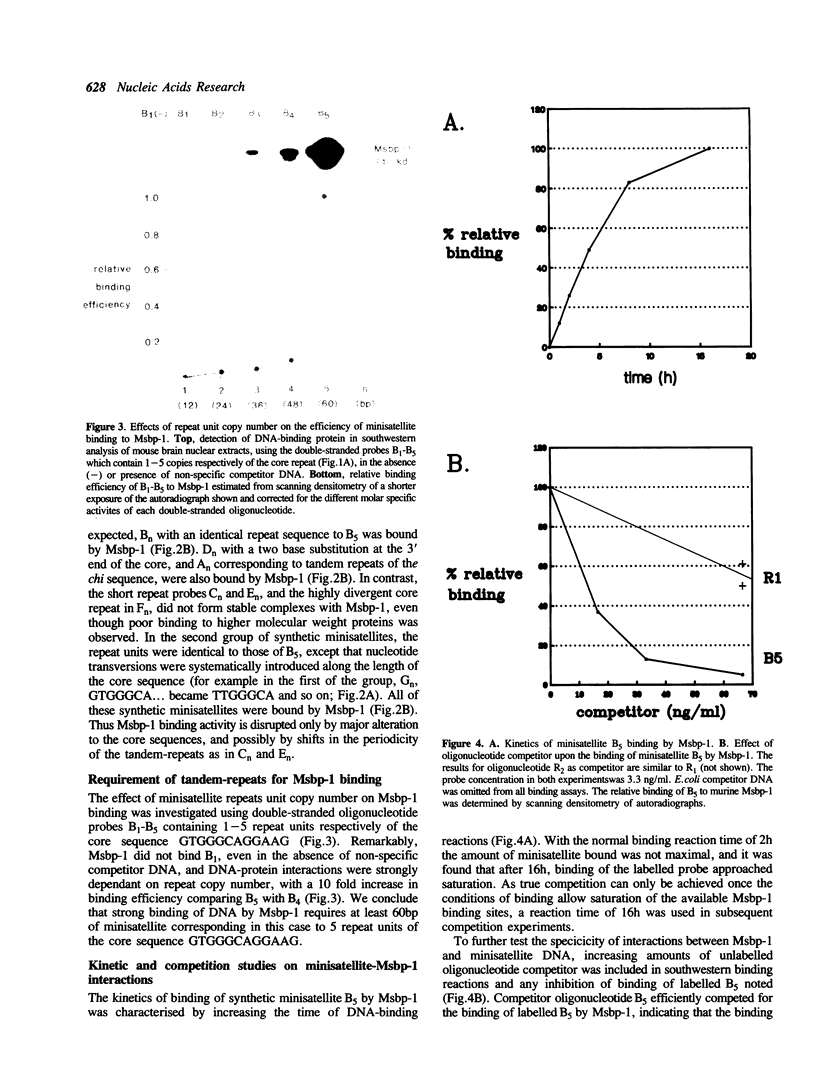
We describe the detection of a ubiquitous DNA-binding protein which appears to interact specifically with tandem-repeated minisatellites. The murine 40 kd protein, which we term Msbp-1, was found to be present in all mouse tissues tested. This protein was bound specifically and with high affinity by double-stranded DNA containing a repeat sequence related to the minisatellite 'core' sequence, and binding required the presence of multiple repeat units. Corresponding minisatellite-specific DNA-binding proteins could also be detected in species ranging from Drosophila to man. This analysis represents the first direct evidence that minisatellites can function as a specific recognition signal for an endogenous DNA-binding protein.
Full text
PDF
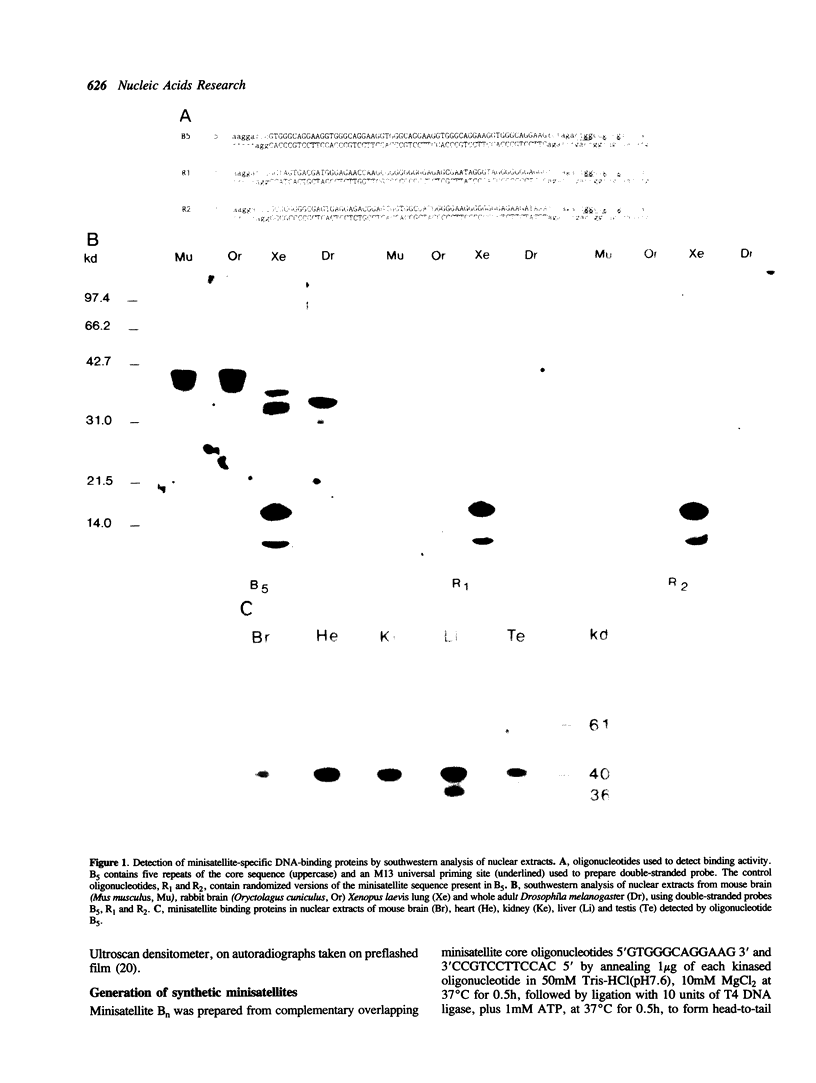

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armour J. A., Patel I., Thein S. L., Fey M. F., Jeffreys A. J. Analysis of somatic mutations at human minisatellite loci in tumors and cell lines. Genomics. 1989 Apr;4(3):328–334. doi: 10.1016/0888-7543(89)90338-8. [DOI] [PubMed] [Google Scholar]
- Chandley A. C., Mitchell A. R. Hypervariable minisatellite regions are sites for crossing-over at meiosis in man. Cytogenet Cell Genet. 1988;48(3):152–155. doi: 10.1159/000132613. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth M. L., Gait M. J., Goelet P., Hong G. F., Singh M., Titmas R. C. Rapid synthesis of oligodeoxyribonucleotides VI. Efficient, mechanised synthesis of heptadecadeoxyribonucleotides by an improved solid phase phosphotriester route. Nucleic Acids Res. 1981 Apr 10;9(7):1691–1706. doi: 10.1093/nar/9.7.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys A. J., Brookfield J. F., Semeonoff R. Positive identification of an immigration test-case using human DNA fingerprints. 1985 Oct 31-Nov 6Nature. 317(6040):818–819. doi: 10.1038/317818a0. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J. Highly variable minisatellites and DNA fingerprints. Biochem Soc Trans. 1987 Jun;15(3):309–317. doi: 10.1042/bst0150309. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Royle N. J., Wilson V., Wong Z. Spontaneous mutation rates to new length alleles at tandem-repetitive hypervariable loci in human DNA. Nature. 1988 Mar 17;332(6161):278–281. doi: 10.1038/332278a0. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Neumann R., Keyte J. Amplification of human minisatellites by the polymerase chain reaction: towards DNA fingerprinting of single cells. Nucleic Acids Res. 1988 Dec 9;16(23):10953–10971. doi: 10.1093/nar/16.23.10953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Thein S. L. Hypervariable 'minisatellite' regions in human DNA. Nature. 1985 Mar 7;314(6006):67–73. doi: 10.1038/314067a0. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Thein S. L. Individual-specific 'fingerprints' of human DNA. Nature. 1985 Jul 4;316(6023):76–79. doi: 10.1038/316076a0. [DOI] [PubMed] [Google Scholar]
- Kelly R., Bulfield G., Collick A., Gibbs M., Jeffreys A. J. Characterization of a highly unstable mouse minisatellite locus: evidence for somatic mutation during early development. Genomics. 1989 Nov;5(4):844–856. doi: 10.1016/0888-7543(89)90126-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Enhanced autoradiographic detection of 32P and 125I using intensifying screens and hypersensitized film. FEBS Lett. 1977 Oct 15;82(2):314–316. doi: 10.1016/0014-5793(77)80609-1. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Leppert M., O'Connell P., Wolff R., Holm T., Culver M., Martin C., Fujimoto E., Hoff M., Kumlin E. Variable number of tandem repeat (VNTR) markers for human gene mapping. Science. 1987 Mar 27;235(4796):1616–1622. doi: 10.1126/science.3029872. [DOI] [PubMed] [Google Scholar]
- Patel S. B., Cook P. R. The DNA-protein cross: a method for detecting specific DNA-protein complexes in crude mixtures. EMBO J. 1983;2(1):137–142. doi: 10.1002/j.1460-2075.1983.tb01395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptashne M. How eukaryotic transcriptional activators work. Nature. 1988 Oct 20;335(6192):683–689. doi: 10.1038/335683a0. [DOI] [PubMed] [Google Scholar]
- Royle N. J., Clarkson R. E., Wong Z., Jeffreys A. J. Clustering of hypervariable minisatellites in the proterminal regions of human autosomes. Genomics. 1988 Nov;3(4):352–360. doi: 10.1016/0888-7543(88)90127-9. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Hong G. F., Hill D. F., Petersen G. B. Nucleotide sequence of bacteriophage lambda DNA. J Mol Biol. 1982 Dec 25;162(4):729–773. doi: 10.1016/0022-2836(82)90546-0. [DOI] [PubMed] [Google Scholar]
- Singh H., LeBowitz J. H., Baldwin A. S., Jr, Sharp P. A. Molecular cloning of an enhancer binding protein: isolation by screening of an expression library with a recognition site DNA. Cell. 1988 Feb 12;52(3):415–423. doi: 10.1016/s0092-8674(88)80034-5. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Stephan D., Fischer Lindahl K. Gene organization and recombinational hotspots in the murine major histocompatibility complex. Cell. 1986 Mar 28;44(6):895–904. doi: 10.1016/0092-8674(86)90012-7. [DOI] [PubMed] [Google Scholar]
- Vinson C. R., LaMarco K. L., Johnson P. F., Landschulz W. H., McKnight S. L. In situ detection of sequence-specific DNA binding activity specified by a recombinant bacteriophage. Genes Dev. 1988 Jul;2(7):801–806. doi: 10.1101/gad.2.7.801. [DOI] [PubMed] [Google Scholar]
- Wolff R. K., Nakamura Y., White R. Molecular characterization of a spontaneously generated new allele at a VNTR locus: no exchange of flanking DNA sequence. Genomics. 1988 Nov;3(4):347–351. doi: 10.1016/0888-7543(88)90126-7. [DOI] [PubMed] [Google Scholar]
- Wolff R. K., Plaetke R., Jeffreys A. J., White R. Unequal crossingover between homologous chromosomes is not the major mechanism involved in the generation of new alleles at VNTR loci. Genomics. 1989 Aug;5(2):382–384. doi: 10.1016/0888-7543(89)90076-1. [DOI] [PubMed] [Google Scholar]
- Wong Z., Wilson V., Patel I., Povey S., Jeffreys A. J. Characterization of a panel of highly variable minisatellites cloned from human DNA. Ann Hum Genet. 1987 Oct;51(Pt 4):269–288. doi: 10.1111/j.1469-1809.1987.tb01062.x. [DOI] [PubMed] [Google Scholar]