Abstract
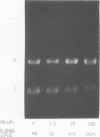
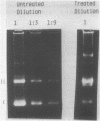
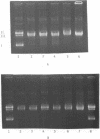
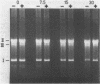
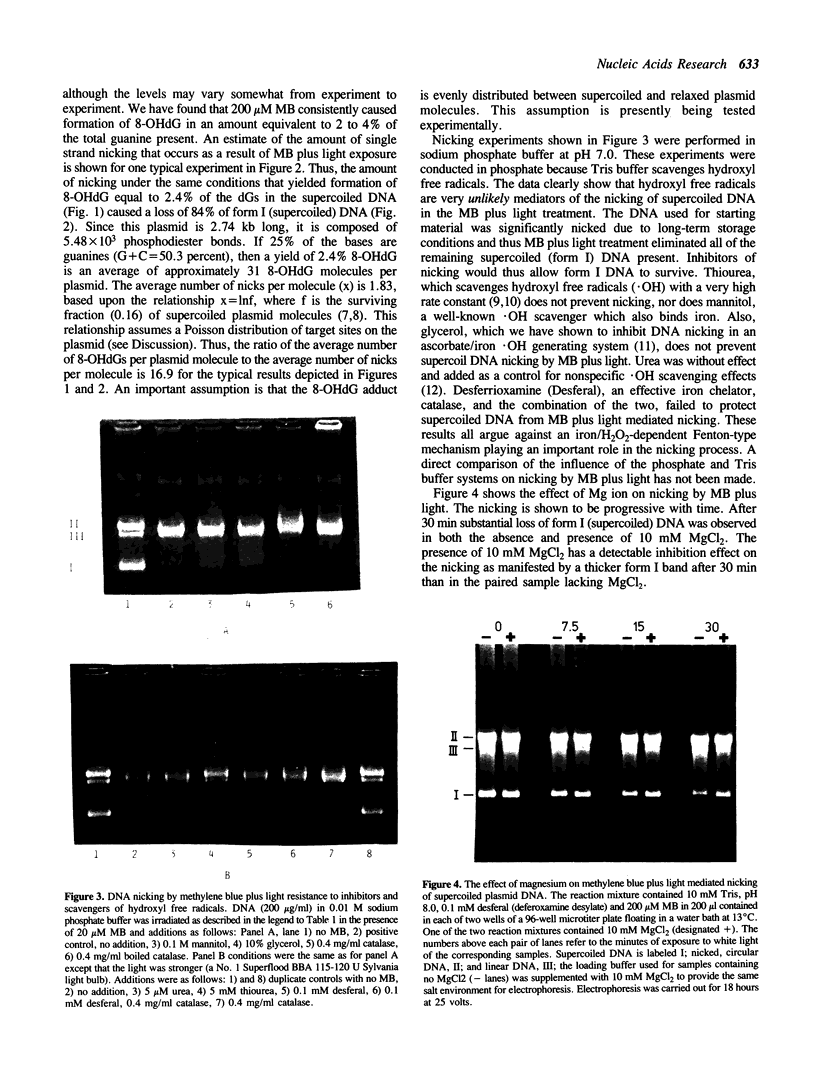
Methylene blue (MB) plus light, in the presence of oxygen, mediates formation of 8-hydroxyguanine in DNA. The yield of 8-hydroxyguanine may be as much as from 2 to 4% of the guanines present. The results presented here show that treatment of supercoiled plasmid DNA with methylene blue plus light causes single-stranded nicks. However, single-stranded nicking occurs approximately 17-fold less frequently than does formation of 8-hydroxyguanine. The nicking rate is reduced in the presence of Mg ion but is not prevented by inhibitors of the iron-catalyzed Fenton reaction or by scavengers of hydroxyl free radicals. Extensive exposure of DNA to light in the presence of MB produces no detectable thiobarbital reactive material thus implicating that single strand nicking does not occur by hydroxyl free radical attack on deoxyribose. Formation of 8-hydroxyguanine is apparently not dependent upon intercalative binding of MB to DNA, since it is formed in polydeoxyguanylic acid.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blok J., Loman H. The effects of gamma-radiation in DNA. Curr Top Radiat Res Q. 1973 Dec;9(2):165–245. [PubMed] [Google Scholar]
- Denq R. Y., Fridovich I. Formation of endonuclease III-sensitive sites as a consequence of oxygen radical attack on DNA. Free Radic Biol Med. 1989;6(2):123–129. doi: 10.1016/0891-5849(89)90109-3. [DOI] [PubMed] [Google Scholar]
- Di Mascio P., Wefers H., Do-Thi H. P., Lafleur M. V., Sies H. Singlet molecular oxygen causes loss of biological activity in plasmid and bacteriophage DNA and induces single-strand breaks. Biochim Biophys Acta. 1989 Mar 1;1007(2):151–157. doi: 10.1016/0167-4781(89)90033-x. [DOI] [PubMed] [Google Scholar]
- Floyd R. A., Watson J. J., Wong P. K., Altmiller D. H., Rickard R. C. Hydroxyl free radical adduct of deoxyguanosine: sensitive detection and mechanisms of formation. Free Radic Res Commun. 1986;1(3):163–172. doi: 10.3109/10715768609083148. [DOI] [PubMed] [Google Scholar]
- Floyd R. A., West M. S., Eneff K. L., Schneider J. E. Methylene blue plus light mediates 8-hydroxyguanine formation in DNA. Arch Biochem Biophys. 1989 Aug 15;273(1):106–111. doi: 10.1016/0003-9861(89)90167-7. [DOI] [PubMed] [Google Scholar]
- Friedmann T., Brown D. M. Base-specific reactions useful for DNA sequencing: methylene blue--sensitized photooxidation of guanine and osmium tetraoxide modification of thymine. Nucleic Acids Res. 1978 Feb;5(2):615–622. doi: 10.1093/nar/5.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge J. M. Ferrous-salt-promoted damage to deoxyribose and benzoate. The increased effectiveness of hydroxyl-radical scavengers in the presence of EDTA. Biochem J. 1987 May 1;243(3):709–714. doi: 10.1042/bj2430709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge J. M., Hou Y. Y. Iron complexes and their reactivity in the bleomycin assay for radical-promoting loosely-bound iron. Free Radic Res Commun. 1986;2(3):143–151. doi: 10.3109/10715768609088066. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M. Thiobarbituric acid-reactivity following iron-dependent free-radical damage to amino acids and carbohydrates. FEBS Lett. 1981 Jun 15;128(2):343–346. doi: 10.1016/0014-5793(81)80113-5. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Toeg D. Iron-dependent free radical damage to DNA and deoxyribose. Separation of TBA-reactive intermediates. Int J Biochem. 1982;14(10):891–893. doi: 10.1016/0020-711x(82)90071-4. [DOI] [PubMed] [Google Scholar]
- Kuchino Y., Mori F., Kasai H., Inoue H., Iwai S., Miura K., Ohtsuka E., Nishimura S. Misreading of DNA templates containing 8-hydroxydeoxyguanosine at the modified base and at adjacent residues. Nature. 1987 May 7;327(6117):77–79. doi: 10.1038/327077a0. [DOI] [PubMed] [Google Scholar]
- Kuhnlein U., Penhoet E. E., Linn S. An altered apurinic DNA endonuclease activity in group A and group D xeroderma pigmentosum fibroblasts. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1169–1173. doi: 10.1073/pnas.73.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwint A. W., Aubry J. M., Arwert F., Kortbeek H., Herzberg S., Joenje H. Inability of chemically generated singlet oxygen to break the DNA backbone. Free Radic Res Commun. 1985;1(1):1–9. doi: 10.3109/10715768509056532. [DOI] [PubMed] [Google Scholar]
- OhUigin C., McConnell D. J., Kelly J. M., van der Putten W. J. Methylene blue photosensitised strand cleavage of DNA: effects of dye binding and oxygen. Nucleic Acids Res. 1987 Sep 25;15(18):7411–7427. doi: 10.1093/nar/15.18.7411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan G. J., Gutteridge J. M. Oxygen radical damage to DNA by rifamycin SV and copper ions. Biochem Pharmacol. 1987 Nov 1;36(21):3629–3633. doi: 10.1016/0006-2952(87)90012-8. [DOI] [PubMed] [Google Scholar]
- Richter C., Park J. W., Ames B. N. Normal oxidative damage to mitochondrial and nuclear DNA is extensive. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6465–6467. doi: 10.1073/pnas.85.17.6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider J. E., Browning M. M., Floyd R. A. Ascorbate/iron mediation of hydroxyl free radical damage to PBR322 plasmid DNA. Free Radic Biol Med. 1988;5(5-6):287–295. doi: 10.1016/0891-5849(88)90099-8. [DOI] [PubMed] [Google Scholar]