Steroid hormone receptors are a class of the nuclear receptor family whose mechanism of transcriptional regulation has been under intensive study for over 30 years. Yet beginning even 30 years ago there were suggestions that at least some effects of steroids, particularly proliferative and electrophysiological effects, might be mediated at the plasma membrane (refs. 1–3 for review). The evidence was based largely on immunofluorescence and the rapidity of the effects, but the entire field of nongenomic signaling by nuclear receptors was not widely appreciated until the recent demonstration that both the estrogen receptor and the progesterone receptor (PR) can activate the cytoplasmic tyrosine kinase c-Src and the mitogen-activated protein kinase (MAPK) pathway (4, 5). Very recently this activation of c-Src has been shown to be associated with binding of a proline-rich sequence in PR to the c-Src Src homology 3 domain, leading to reduced autoinhibitory repression of c-Src kinase activity (V. Boonyaratanakornkit, M. Porter-Scott, V. Ribon, L. Sherman, S. M. Anderson, T. W. Miller, and D. P. Edwards, personal communication). The activated c-Src/PR complex then mediates activation of the MAPK pathway. The proline-rich sequence of PR also binds to the Src homology 3 domain of Cbl-associated proteins and other signaling molecules (Boonyaratanakornkit et al., personal communication). c-Src is acylated at its N terminus with myristic acid, a modification thought to direct its localization to the plasma membrane (7). Thus, PR activation of c-Src is a presumed plasma membrane effect of PR. However, active c-Src also has been localized on the nuclear periphery (8), making it unclear where PR activation of c-Src occurs.
Although these nongenomic actions of progesterone are clearly mediated by the nuclear receptor, in other systems it has been assumed that nongenomic actions of steroids are mediated by a distinct receptor located at the cell membrane.
Although these nongenomic actions of progesterone are clearly mediated by the nuclear receptor, in other systems it has been assumed that nongenomic actions of steroids are mediated by a distinct receptor located at the cell membrane.
One example is the modulation of γ-aminobutyric acid (GABA)-ergic transmission by certain steroids, including progestins, which may be important in general anesthesia (9). These effects are thought to reflect steroid binding to GABA receptors. Another example is evident in progesterone stimulation of Ca2+ release and the acrosome reaction in mammalian sperm, which lack nuclear receptors (10, 11). Sperm have GABA receptors but whether these mediate the effect of progesterone on the acrosome reaction is not clear (11). For many steroids that affect GABA-ergic transmission, the effect is not enantioselective and can be produced roughly equally by steroid enantiomers (12). The same lack of enantiomer selectivity is true for progesterone stimulation of the acrosome reaction in human sperm (S. Meizel, J.L.M., and D. Covey, unpublished data). This is different from classical nuclear receptor actions, which generally exhibit strict enantiomer selectivity.
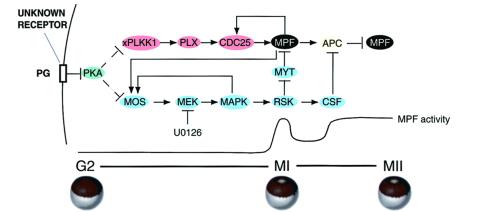
Perhaps the best-characterized example of nongenomic progesterone action is at the membrane of the amphibian oocyte. During growth oocytes are arrested at the G2/prophase border of meiosis I and resume meiosis after progesterone stimulation. Resumption of meiosis is caused by alterations in several signal transduction pathways, including deactivation of cAMP-dependent protein kinase (protein kinase A, PKA) and activation of the MAPK and polo-like kinase pathways (ref. 13, for review). These pathways eventually converge to promote dephosphorylation and activation of cyclin B/Cdc2 (mitosis-promoting factor, MPF), the enzyme that catalyzes entry into M phase of meiosis I (Fig. 1). The polo-like kinase pathway leads to activation of the phosphatase Cdc25C, which dephosphorylates and activates Cdc2/cyclin B (MPF), whereas the MAPK pathway leads to activation of p90RSK, which phosphorylates and inactivates Myt1, the kinase that phosphorylates and inactivates MPF (Fig. 1). The activation of cyclin B/Cdc2 can be monitored indirectly by dissolution of the germinal vesicle (nucleus; germinal vesicle breakdown, GVBD), which is evident by appearance of a white spot on the pigmented animal pole of the oocyte. The concept that this action of progesterone is nongenomic and mediated by a membrane interaction dates from studies beginning 30 or more years ago that showed injection of progesterone is unable to induce GVBD, whereas steroid bound to polymers is able to cause GVBD when applied externally but not when injected or when not touching the oocyte (14, 15). Occasional reports that injected progesterone is effective (16) invariably are found to reflect leakage of injected steroid from the oocyte to the extent that directly adjacent uninjected oocytes also respond (Y. Masui, personal communication). The nongenomic nature of progesterone action is evident from induction of GVBD in the presence of actinomycin D and from cyclin B/Cdc2 (MPF) activation in physically enucleated oocytes treated with progesterone (14).
Figure 1.
Signal transduction pathways regulated by progesterone in meiosis. The model depicts progesterone acting through an unknown membrane receptor to inhibit adenylyl cyclase, reduce the level of cAMP, and decrease the activity of PKA. Through multiple unknown steps decreased PKA leads to translational activation of Mos mRNA and subsequent generation of an active MAPK pathway. A target of MAPK, p90RSK, operates in meiosis I (MI) to promote MPF activation by inhibition of Myt1, and in meiosis II (MII) p90RSK inhibits cyclin B degradation by the anaphase-promoting complex (APC) as an element required for cytostatic factor (CSF)-mediated metaphase arrest. Decreased PKA also leads to activation of the polo-like kinase cascade by multiple unknown steps. This kinase cascade pathway results in activation of Cdc25C, the phosphatase that dephosphorylates and activates MPF (cyclin B/Cdc2) at the G2/M transition in meiosis I. Feedback loops exist in which activated MPF can directly activate Cdc25, and Mos mRNA translation and/or stability can be increased by active MPF and active MAPK via multiple unknown steps. PG, progesterone; xPlkk1, Xenopus polo-like kinase kinase; Plx, Xenopus polo-like kinase; MEK, MAP kinase kinase.
The earliest known biochemical events in oocytes treated with progesterone are a rapid decrease in cAMP due to inhibition of adenylyl cyclase activity (ref. 17, for review) and complex changes in phospholipid metabolism (18). The decrease in cAMP and subsequent decline in PKA activity is both necessary and sufficient to cause GVBD several hours later (19, 20). The adenylyl cyclase inhibition is GTP-dependent, like that seen for numerous receptor-mediated cyclase effects, and is still evident in membranes from oocytes treated with cholera toxin or pertussis toxin. The progesterone-dependent cyclase inhibition observed in purified plasma membrane preparations has an IC50 similar to the EC50 of progesterone for GVBD (17). Recently the effect of progesterone on GVBD was found to be enantioselective with over a 100-fold difference in potency of progesterone enantiomers for the induction of GVBD (J.M. and D. F. Covey, unpublished data). This specificity suggests that progesterone action on the oocyte involves a different receptor binding site than that associated with progestin effects on GABA receptors or mammalian sperm. The concept that the oocyte membrane receptor is likely to be distinct from the conventional nuclear receptor rests on several features of the response. First, the GVBD response does not require transcription. Second, the EC50 for GVBD, typically 200 nM for GVBD within 6 h, is much higher than that required for transcriptional activation by the nuclear receptor (1 nM), suggesting a different hormone binding domain is involved than the one present in nuclear receptors. Third, the structure/function relations of steroids active for GVBD do not fit those required for nuclear receptor function (21). Indeed, GVBD can be stimulated with almost equal potency by progesterone, testosterone, certain glucocorticoids (but not dexamethasone), and certain mineralocorticoids (but not aldosterone). Finally, the nuclear PR antagonist RU486 does not block GVBD induction by progesterone or R5020 and, in fact, acts as a weak agonist (22). The search for a membrane receptor in oocytes and other systems has been ongoing for many years, and several candidates have been proposed (ref. 23, for review). Some have been cloned, but little evidence supports their function as a membrane receptor. The search is hampered by the lipophilic nature of steroids rendering binding in membrane preparations highly nonspecific.
Two recent papers in PNAS, Tian et al. (24) and Bayaa et al. (25), challenge the dogma that a novel PR mediates oocyte maturation, and they both present new evidence suggesting the oocyte GVBD response might be mediated by the nuclear PR. Tian et al. (24) have cloned an apparent Xenopus homolog of nuclear PR (xPR) and show that when overexpressed in oocytes xPR modestly accelerates progesterone-induced GVBD and the rate of MAPK activation, due to a more rapid accumulation of Mos, a MAPK kinase kinase. The dose of progesterone required for maximal effect also is reduced. Moreover, injection of antisense oligonucleotides against xPR reduces the percentage of oocytes undergoing GVBD in response to high-dose progesterone. The GVBD response can be partially rescued by subsequent injection of the heat-stable inhibitor of PKA, which acts downstream of the receptor, or importantly, by overexpression of either xPR or human nuclear PR. Rescue correlates with expression of Mos and MAPK activation. The authors conclude xPR is required for oocyte GVBD.
Bayaa et al. (25) cloned a partial cDNA encoding the same gene, including the DNA binding domain and the hormone binding domain. The gene product activates transcription of PR-reporter genes after transfection into COS cells. Those authors have produced an antibody that shows the xPR protein is present in resting oocytes and is extranuclear despite the presence of a conserved nuclear localization signal. However, when overexpressed the receptor is found in both nuclear and cytoplasmic fractions but not in the membrane. They also show that overexpression of xPR modestly accelerates the rate of progesterone-induced GVBD and MAPK activation, and it increases sensitivity to progesterone. The accelerated rate of MAPK activation also can be seen in physically enucleated oocytes. The authors conclude that xPR mediates progesterone-induced oocyte maturation by a nongenomic mechanism.
The results in these two papers are internally consistent. One can infer that the effects on MAPK activation do not require domains in the first 145 amino acids of xPR because these are missing in the partial clone isolated by Bayaa et al. (25). The sequence of xPR has interesting similarities to human and chicken PR, including the proline-rich sequence important for binding the Src homology 3 domain of c-Src and other signaling molecules (Boonyaratanakornkit et al., personal communication). Like chicken PR, xPR lacks the critical cysteine reported to be required for PR binding of RU486; yet Bayaa et al. (25) find that RU486 blocks reporter gene transcription in Cos cells expressing xPR, whereas RU486 induces GVBD by itself, as reported (22). This would be most consistent with RU486 acting on two different PR types in oocytes, the nuclear receptor and a membrane receptor. A caveat in both papers is that the effects reported depend on massive overexpression of xPR, which causes mislocalization of the receptor to both nucleus and cytoplasm; yet the effects on the hormone response are modest–only a 1-h acceleration of GVBD and MAPK activation in a 10-h assay. Tian et al. (24) used antisense oligos to attempt to show endogenous xPR mediates normal GVBD. A more complete analysis will be available when antibodies are used to evaluate loss of endogenous xPr by antisense treatment. Follow-up studies are particularly important because the rescue experiments performed by Tian et al. use PR overexpression requiring oocyte culturing for up to 6 days in a medium with no nutrients, resulting in oocytes that are of uncertain physiological status and often morphologically atypical.
Perhaps the greatest question that comes from these studies concerns how or whether xPR is linked to the action of progesterone on the plasma membrane adenylyl cyclase. No xPR was detected in membrane fractions by Western blotting even after overexpression (25). The absence of xPR in the cellular compartment where progesterone signaling is initiated casts doubt on whether it mediates progesterone-induced oocyte maturation. This issue merits further study with more sensitive reagents such as green fluorescent protein–xPR, etc. Another question concerns the binding parameters of xPR. Neither report shows that xPR binds progesterone and with what Kd. The apparent EC50 for transcriptional activation by overexpressed xPR in Cos cells (10 nM) is lower than that usually required for GVBD (200 nM), but direct comparison is complicated because the temperatures and time parameters of the two assays are different. Of particular importance is whether the binding specificity of xPR for other steroids fits the unusual profile of steroids able to induce GVBD. Also, does RU486 bind to xPR and compete for progesterone binding despite the absence of the critical cysteine residue implicated in RU486 binding to human PR?
An alternative explanation for the results of Tian et al. (24) and Bayaa et al. (25) is evident based on the known effects of MAPK activation on progesterone-induced GVBD. Previous work demonstrated that microinjection of pp60v-Src into oocytes activates p90RSK, a target of MAPK, and this leads to acceleration of the rate of GVBD (26). Several other effects of MAPK in oocytes are mediated solely by the activation of p90RSK (27) and, indeed, accelerated GVBD and increased sensitivity to progesterone are also evident in oocytes expressing a constitutively active form of p90RSK (S. D. Gross and J.L.M., unpublished work). Thus, if xPR interacts with Xenopus c-Src and activates its kinase activity, this could account for both early MAPK activation and acceleration of GVBD through p90Rsk independently of a membrane steroid receptor. The accelerated appearance of Mos in oocytes overexpressing xPR noted in both papers could derive from an established feedback loop between active MAPK and activation of mos mRNA translation (6). If cytoplasmic xPR affects oocyte maturation only indirectly via c-Src and MAPK activation, then a prediction is that some MAPK activation would be evident in xPR-overexpressing oocytes treated with progesterone in the presence of cycloheximide. Moreover, inhibition of adenylyl cyclase by progesterone should be unaffected by xPR overexpression, which appears to act distal to the membrane. Further work is clearly necessary to evaluate these possibilities. Even if xPR turns out not to be the receptor that mediates oocyte maturation, these papers establish that cytoplasmic actions of xPR, including possibly c-Src and MAPK activation, may be ideally studied in the oocyte, which lacks the complications of transcriptional effects and nuclear localization of xPR.
Footnotes
References
- 1.Watson C S, Gametchu B. Proc Soc Exp Biol Med. 1999;220:9–19. doi: 10.1046/j.1525-1373.1999.d01-2.x. [DOI] [PubMed] [Google Scholar]
- 2.Wehling M. Annu Rev Physiol. 1997;59:365–393. doi: 10.1146/annurev.physiol.59.1.365. [DOI] [PubMed] [Google Scholar]
- 3.Revelli A, Massoborio M, Tesarik J. Endocr Rev. 1998;19:3–17. doi: 10.1210/edrv.19.1.0322. [DOI] [PubMed] [Google Scholar]
- 4.Migliaccio A, Di Domenico M, Castoria G, de Falco A, Bontempo P, Nola E, Auricchio F. EMBO J. 1996;15:1292–1300. [PMC free article] [PubMed] [Google Scholar]
- 5.Migliaccio A, Piccoli D, Castoria G, Di Domenico M, Bilancio A, Lombardi M, Gong W, Beato M, Auricchio F. EMBO J. 1998;17:2008–2018. doi: 10.1093/emboj/17.7.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matten W T, Copeland T D, Ahn N G, Vande Woude G F. Dev Biol. 1996;179:485–492. doi: 10.1006/dbio.1996.0277. [DOI] [PubMed] [Google Scholar]
- 7.Cross F R, Garber E A, Pellman D, Hanafusa H. Mol Cell Biol. 1984;4:1834–1842. doi: 10.1128/mcb.4.9.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Resh M D, Erikson R L. J Cell Biol. 1985;100:409–417. doi: 10.1083/jcb.100.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Covey D F, Nathan D, Kalkbrenner M, Nilsson K R, Hu Y, Zorumski C F, Evers A S. J Pharmacol Exp Ther. 2000;293:1009–1016. [PubMed] [Google Scholar]
- 10.Sabeur K, Edwards D P, Meizel S. Biol Reprod. 1996;54:993–1001. doi: 10.1095/biolreprod54.5.993. [DOI] [PubMed] [Google Scholar]
- 11.Meizel S. Biol Reprod. 1997;56:569–574. doi: 10.1095/biolreprod56.3.569. [DOI] [PubMed] [Google Scholar]
- 12.Nilsson K B, Zorumski C F, Covey D F. J Med Chem. 1998;41:2604–2613. doi: 10.1021/jm980148h. [DOI] [PubMed] [Google Scholar]
- 13.Palmer A, Nebreda A R. Prog Cell Cycle Res. 2000;4:131–143. doi: 10.1007/978-1-4615-4253-7_12. [DOI] [PubMed] [Google Scholar]
- 14.Masui Y, Markert C L. J Exp Zool. 1971;177:129–146. doi: 10.1002/jez.1401770202. [DOI] [PubMed] [Google Scholar]
- 15.Smith L D, Ecker R E. Dev Biol. 1971;25:233–247. doi: 10.1016/0012-1606(71)90029-7. [DOI] [PubMed] [Google Scholar]
- 16.Tso J, Thibier O, Mulner O, Ozon R. Proc Natl Acad Sci USA. 1982;79:5552–5556. doi: 10.1073/pnas.79.18.5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sadler S E, Maller J L. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1985;19:179–194. [PubMed] [Google Scholar]
- 18.Morrill G A, Kostellow A B. Steroids. 1999;64:157–167. doi: 10.1016/s0039-128x(98)00093-2. [DOI] [PubMed] [Google Scholar]
- 19.Maller J L, Krebs E G. J Biol Chem. 1977;252:1712–1718. [PubMed] [Google Scholar]
- 20.Huchon D, Ozon R, Fischer E H, DeMaille J G. Mol Cell Endocrinol. 1981;22:211–222. doi: 10.1016/0303-7207(81)90092-7. [DOI] [PubMed] [Google Scholar]
- 21.Morrill G A, Bloch E. J Steroid Biochem. 1977;8:133–139. doi: 10.1016/0022-4731(77)90036-x. [DOI] [PubMed] [Google Scholar]
- 22.Sadler S E, Maller J L. J Steroid Biochem. 1985;22:419–426. doi: 10.1016/0022-4731(85)90448-0. [DOI] [PubMed] [Google Scholar]
- 23.Maller J L. Biol Cell. 1998;90:453–460. [PubMed] [Google Scholar]
- 24.Tian J, Kim S, Hellig E, Ruderman J V. Proc Natl Acad Sci USA. 2000;97:14358–14363. doi: 10.1073/pnas.250492197. . (First Published December 12, 2000; 10.1073/pnas.250492197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bayaa M, Booth R A, Sheng Y, Liu X J. Proc Natl Acad Sci USA. 2000;97:12607–12612. doi: 10.1073/pnas.220302597. . (First Published October 24, 2000; 10.1073/pnas.220302597) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spivack J G, Erikson R L, Maller J L. Mol Cell Biol. 1984;4:1631–1634. doi: 10.1128/mcb.4.8.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gross S D, Schwab M S, Taieb F E, Lewellyn A L, Qian Y-W, Maller J L. Curr Biol. 2000;10:430–438. doi: 10.1016/s0960-9822(00)00425-5. [DOI] [PubMed] [Google Scholar]