Abstract
Seamless tubes form intracellularly without cell-cell or autocellular junctions. Such tubes have been described across phyla, but remain mysterious despite their simple architecture. In Drosophila, seamless tubes are found within tracheal terminal cells, which have dozens of branched protrusions extending hundreds of microns. We find that mutations in multiple components of the dynein motor complex block seamless tube growth, raising the possibility that the lumenal membrane forms through minus-end directed transport of apical membrane components along microtubules. Growth of seamless tube is polarized along the proximodistal axis by Rab35 and its apical membrane-localized GAP, Whacked. Strikingly, loss of whacked (or constitutive activation of Rab35) leads to tube overgrowth at terminal cell branch tips, while over-expression of whacked (or dominant negative Rab35) causes formation of ectopic tubes surrounding the terminal cell nucleus. Thus, vesicle trafficking plays key roles in making and shaping seamless tubes.
Three tube types – multicellular, autocellular and seamless – are found in the Drosophila trachea1, 2. Most tracheal cells contribute to multicellular tubes or make themselves into unicellular tubes by wrapping around a lumenal space and forming autocellular adherens junctions, but two specialized tracheal cell types, fusion cells and terminal cells, make “seamless” tubes. 1, 3, 4 How seamless tubes are made and how they are shaped is largely unknown. One hypothesis holds that seamless tubes are built by “cell hollowing,”5, 6 in which vesicles traffic to the center of the cell and fuse to form an internal tube of apical membrane, while an alternative model proposes that apical membrane is extended internally from the site of intercellular adhesion.7 In both models, transport of apical membrane would likely play a key role. Because terminal cells make seamless tubes continuously during larval life, they serve as an especially sensitive model system in which to dissect the genetic program.
Tracheal cells are initially organized into epithelial sacs with their apical surface facing the sac lumen. During tubulogenesis, γ-tubulin becomes localized to the lumenal membrane of each tracheal cell, generating microtubule networks oriented with minus-ends towards the apical membrane.8 Terminal and fusion cells are first selected as tip cells1 that undergo a partial epithelial to mesenchymal transition and initiate branching morphogenesis: they lose all but one or two cell-cell contacts and become migratory.9, 10 Branchless-FGF signaling induces a subpopulation of tip cells to differentiate as terminal cells.9 During larval life, terminal cells ramify on tissues spread across several hundred microns, with branching patterns that reflect local hypoxia.11 A single seamless tube forms within each branched extension of the terminal cell.
How trafficking contributes to seamless tube morphogenesis is unknown. Despite clues that vesicle transport plays a role in the genesis of seamless tubes, the tube morphogenesis genes remain elusive. 7, 11–13 Here we characterize the cytoskeletal polarity of larval terminal cells, show that a minus-end directed microtubule motor complex is required for seamless tube growth, and characterize mutations in whacked that uncouple seamless tube growth from the normal spatial cues. Sequence analysis suggests that whacked encodes a RabGAP, and we show that Rab35 is the essential target of Whacked, and that together, Whacked and Rab35 can polarize the growth of seamless tubes.
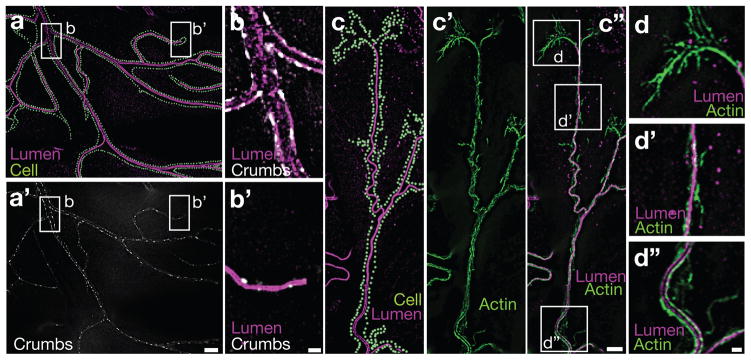
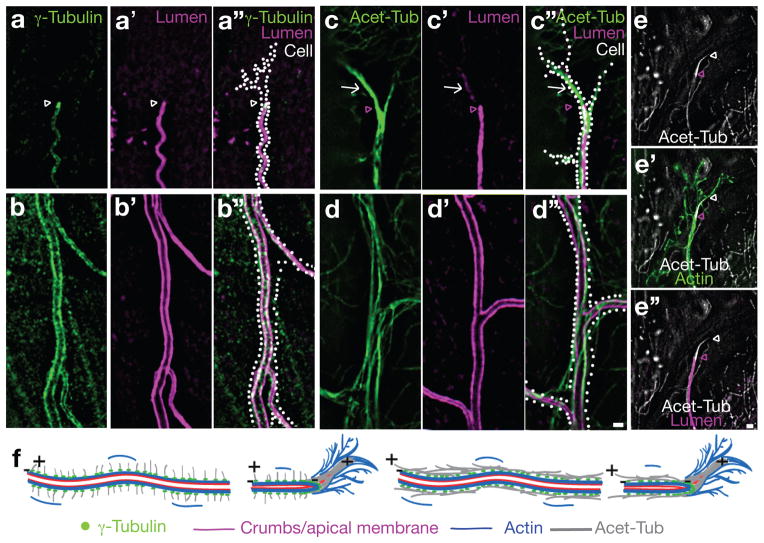
We examined apical-basal polarity and cytoskeletal organization in mature larval terminal cells. The lumenal membrane (Figure S1a, b and Methods) was decorated by puncta of Crumbs, a definitive apical membrane marker 14, 15 (Figure 1a, a′, b, b′). We found actin filaments enriched in three distinct subcellular domains: surrounding seamless tubes, decorating filopodia, and outlining short stretches of basolateral membrane (Figure 1c-c″, d-d″). The microtubule cytoskeleton also appeared polarized, with γ-tubulin lining the seamless tubes and enriched at tube tips (Figure 2a-a″, b-b″). These data are consistent with tracheal studies in the embryo.7 EB1::GFP analyses of growing (plus-ends) microtubules demonstrated that some are oriented towards the soma and others towards branch tips (Movie S1). Stable acetylated microtubules ran parallel to the tubes (Figure 2d-d″) and extended beyond the lumen at branch tips (Figure 2c-c″, arrowhead) where they may template tube growth. Consistent with such a role, we observed microtubule tract-associated fragments of apical membrane distal to the blind ends of the seamless tubes (Figure 2c-c″, arrow). Filopodia extended past the stable microtubules (Figure 2e-e″, white arrowhead) as expected. These data suggest that mature terminal cells maintain the polarity and organization described for embryonic terminal cells.7 Based on γ-tubulin localization, we infer that a subset of microtubules is nucleated at the apical membrane, and that apically-targeted transport along such microtubules would require minus-end motor proteins. Indeed, homozygous mutant Lissencephaly-1 (Lis-1, a Dynein motor-associated protein) embryos have been reported to have seamless tube defects.7 Because γ-tubulin lines the entire apical membrane, growth through minus-end directed transport might be expected to occur all along the length of seamless tubes, and indeed, a pulse of CD8::GFP (transmembrane protein tagged with GFP) synthesis uniformly labeled the apical membrane as it first became detectable (Figure S1c, c′ and supplemental methods).
FIGURE 1. The lumenal membrane of tracheal terminal cells has apical identity.
(a) A portion of a wild type terminal cell is shown, with the cell shape outlined by dots (Cell, light green) – cell outline layer was made by tracing actin::RFP staining with the gain increased sufficiently to visualize basolateral actin. The terminal cell is stained with α-Wkdpep sera, revealing the lumenal membrane (Lumen, magenta) of seamless tubes. (a′) Staining (Crumbs, white) against a functional Crumbs::GFP fusion protein knocked into the endogenous crumbs locus demonstrates that the lumenal membrane of terminal cell seamless tubes is apical. White boxes in (a, a′) labeled b and b′ marquee the terminal cell soma and a branch tip, respectively, and are shown enlarged in (b, b′). Merged images of Crumbs (white) and lumenal membrane (Lumen, magenta) co-staining are shown. Subcellular localization of actin relative to the seamless tube is examined in (c – c″). In (c), a portion of a terminal cell (Cell, light green dots) containing a branched seamless tube (Lumen, magenta) is shown. In (c′), the subcellular distribution of actin (Actin, green) is shown. In (c″), a merged image is shown. The white boxes labeled (d, d′, and d″) highlight distinct domains of actin localization that are shown enlarged in (d-d″): (d) filopodial actin, (d′) basolateral actin, and (d″) apical actin. Scale bars for a, a′ in a′; for c, c′,c″ in c″ = 10 μm and in b′ and d″ for b, b′ and for d, d′, d″, respectively = 2 μm.
FIGURE 2. The terminal cell microtubule cytoskeleton is polarized.
Terminal cell outlines (determined as in Figure 1) are indicated (Cell, white dots). (a, b) The subcellular distribution of γ-tubulin is shown in a typical wild type terminal cell. γ-tubulin (γ-Tub, green) lines the seamless tube and is enriched at the blind-end of the tube found at the tip of the terminal cell (a). Costaining of γ-tubulin and apical membrane (magenta, a′, b′) is shown (a″, b″). (c, d) The subcellular distribution of acetylated microtubules (Acet-Tub, green) is shown. Acetylated microtubule bundles run parallel the apical membrane (magenta, c′, d′). Costaining is shown (c″, d″). We note that apical membrane fragments (white arrow) discontinuous with the seamless tube are found to line acetylated microtubule tracts extending beyond the seamless tube blind end (magenta arrowhead). (e) The relationship between acetylated microtubules (white), apical membrane (Lumen, magenta) and actin rich filopodia (Actin, green) at branch tips is shown. Acetylated microtubules extend beyond (white arrowhead) the blind-end (magenta arrowhead) of the seamless tube. Actin-based filopodial projections extend beyond both the seamless tube and the stable microtubule tract. These data (and those from Figure 1) are summarized in the schematic diagrams shown in (f). In the panel on the left, microtubules are shown oriented perpendicular to the axis of the tube while in the panel on the right, microtubules are shown oriented parallel to the long axis of the tube. Either or both microtubule arrangements may be present. Scale bars for a-d are in d″ and for e, e′ and e″ are in e″ and = 2 μm.
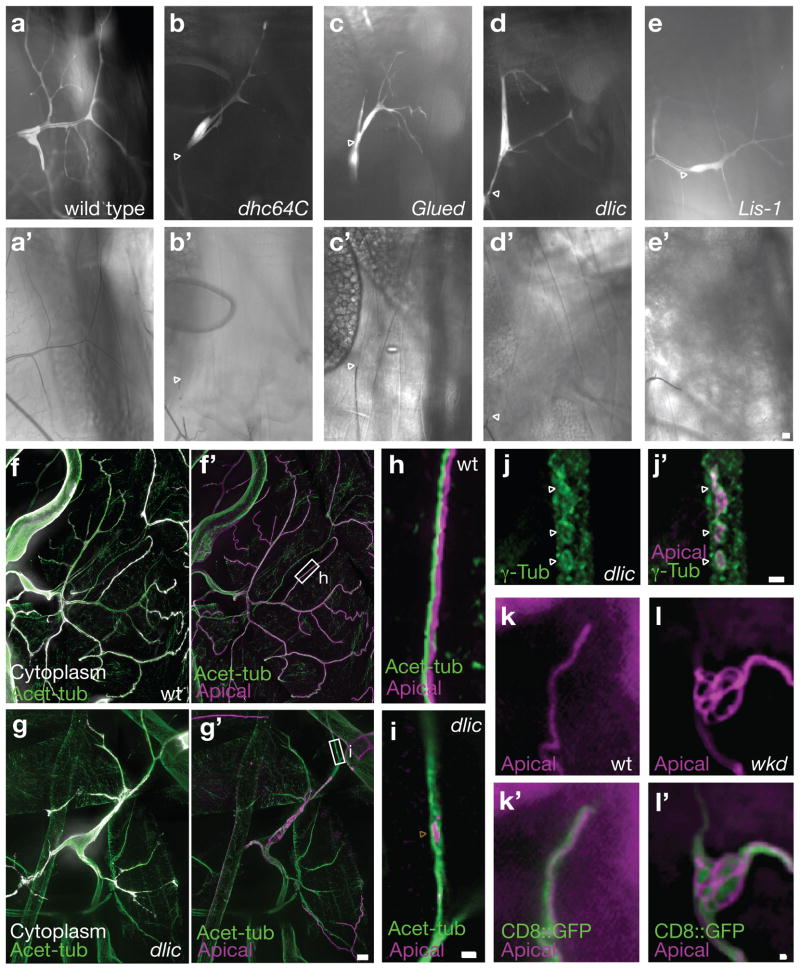
The cytoplasmic Dynein motor complex drives minus-end directed transport of intracellular vesicles in many cell types;16 to test for its requirement in seamless tube formation, we examined terminal cells mutant for any of four dynein motor complex genes: Dynein heavy chain 64C (Dhc64C), Dynein light intermediate chain (dlic), Dynactin p150 (Glued), and Lis-1. Mutant terminal cells showed a cell autonomous requirement for these genes. Mutant terminal cells had thin cytoplasmic branches that lacked air-filling (Figure 3a–e′), and antibody staining revealed that seamless tubes did not extend into these branches although acetylated microtubules often did (Figure 3f–i and data not shown). We also note that formation of filopodia at branch tips is disrupted in dynein motor complex mutants, which may account for the decreased number of branches in mutant terminal cells. Ectopic seamless tubes that were not air-filled were detected near the nucleus, as described below. Interestingly, discontinuous apical membrane fragments (similar to those in Lis-1 embryos)7 were found in terminal branches lacking seamless tubes, and were associated with microtubule tracts (Figure 3h,i). While γ–tubulin was enriched on truncated tubes and on these presumptive seamless tube intermediates, diffuse γ–tubulin staining was detected throughout the mutant cells (Figure 3j, j′; compare to Figure 2a, b), suggesting that assembly of apical membrane is required to establish or maintain γ–tubulin localization. Likewise, Crumbs appeared reduced and aberrantly localized (Figure S1d–f). Reduced acetylated microtubule staining in these cells may reflect loss of apical γ–tubulin (compare 3h, i). Importantly, these data show that stable microtubules extend through cellular projections that lack seamless tubes. Thus, without minus-end directed transport, stable microtubules are insufficient to promote seamless tube formation, but stable cellular projections are formed and maintained in the absence of seamless tubes.
FIGURE 3. Seamless tube growth is blocked or is overly exuberant in dynein motor complex and whacked mutants, respectively.
(a–d) Positively marked (GFP, white) mutant terminal cells were identified in mosaic animals. Cells mutant for dynein motor complex components made branched cellular extensions, but had little or no air-filled seamless tubes (arrowheads indicate the position beyond which gas-filling of tubes is not detected, a′–e′). To determine if acetylated microtubules (green) were present within terminal cells lacking air-filled tubes, wild type control (f, f′) and dlic (g, g′) mosaic animals were filleted, fixed and stained. Homozygous terminal cell clones (f, g) were marked with GFP (white). Apical membrane (Apical – α-Wkdpep – magenta) staining reveals seamless tube remnants near the terminal cell nucleus (g′) of mutant terminal cells, but not near branch tips (compare f′,g′). In (h,i), high magnification images of seamless tubes from wild type and dlic mutant terminal cells (f′, g′; marqueed area), respectively. In wild type (h), continuous seamless tube was present along the acetylated microtubule bundle. In dlic mutant cells, apical membrane was absent except for small bits of discontinuous tube that could be detected adjacent to acetylated microtubules (i, arrowhead). We found that such discontinuous bits of tube were able to organize γ-tubulin around them, but that γ-tubulin localization was mostly lost in dynein complex mutant terminal cells, with staining present diffusely throughout the cell (j, j′ compare to Figure 2a,b). As compared to wild type animals (k, k′), seamless tubes in whacked terminal cells showed excessive growth at branch tips (i, i′), resulting in a “U-turn” phenotype, in which seamless tube extended through the cytoplasm in a series of 180 degree turns. Scale bars for a-e′ in e′; for f-g′ in g′ = 10 μm; for h and i in i, for j,j′ in j′ and for k-l′ in l′ = 2 μm.
In contrast to these defects in seamless tubes generation, mutations in whacked17 confer overly exuberant tube growth (Figure 3k,l and Figure S2a, d, i). Examination of whacked terminal cell tips revealed a “U-turn” phenotype in which seamless tubes executed a series of 180 degree turns – below we entertain the possibility that branch retraction, similar to that observed in talin mutants,18 could contribute to the U-turn defect.
Homozygous whacked animals survived until pharate adult stages, and other than the seamless tube defects, had normal tracheal tubes at the third larval instar. Mosaic analysis revealed a terminal cell autonomous requirement for whacked. Mutant clones in multicellular tubes, and in unicellular tubes that lumenize by making autocellular adherens junctions, were of normal morphology (Figure S2f–h). Strikingly, fusion cells, which also form seamless tubes, were unaffected by loss of whacked.
To determine the molecular nature of whacked we took a positional cloning approach (Figure S3 and methods). Mapping techniques defined a candidate gene interval of ~ 75 kb. We focused on CG5344 as it encodes a protein containing a TBC (Tre2/Bub2/Cdc16) domain characteristic of Rab GTPase activating proteins (GAPs),19–21 and hence was likely to participate in vesicular trafficking, a process that could lie at the heart of seamless tube formation. We identified single nucleotide changes that result in mis-sense (PC24) and nonsense (220) mutations in CG5344 coding sequence. Pan-tracheal knockdown of whacked by RNAi caused terminal cell-specific U-turn defects (other defects characteristic of the EMS alleles of whacked -- see supplemental methods – were detected at a low frequency, data not shown). A genomic rescue construct for CG5344 rescued whacked mutants, confirming gene identity (Figure S2j, m). Based on these results, we conclude that whacked is CG5344 and that it likely regulates vesicular trafficking during seamless tube morphogenesis.
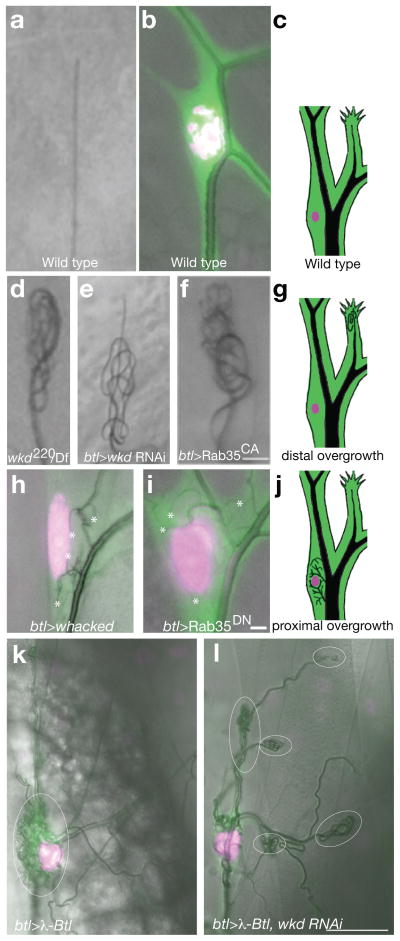
To determine the Rab target(s) of Whacked regulation, we tested whether tracheal expression of constitutively active “GTP-locked” Rab isoforms (henceforth Rab-CA) might phenocopy whacked. Rab-CA for 31 of the 33 Drosophila Rabs22 were tested individually in the tracheal system. Rab35-CA alone conferred terminal cell-specific U-turns defects (Figure 4a–g).
FIGURE 4. Whacked and Rab35 polarize seamless tube growth.
In (a, d-f), brightfield micrographs revealing gas-filled terminal cell seamless tubes are shown. In (b, h,i, k and l), fluorescent micrographs labeling the terminal cell cytoplasm (green) and nucleus (magenta) are superimposed on a brightfield image. In (a) and (b) wild type terminal cell branch tip (a) and soma (b) are shown. In (c), a schematic illustrates the position and distribution of seamless tubes in wild type terminal cells. In wkd (d), wkd RNAi (e), or Rab35CA (f) terminal cell tips, growth of seamless tubes appears to outpace growth of cellular extensions, resulting in a tangle of gas-filled tubes coiled in the tip cytoplasm. In (g), the distal seamless tube overgrowth seen in (d – f) is illustrated in a schematic diagram. In terminal cells over-expressing wkd (h), or Rab35DN (i), ectopic gas-filled seamless tubes (*) are observed surrounding the terminal cell nucleus. This proximal overgrowth phenotype is illustrated in the schematic in (j). In (k), a terminal cell expressing an activated FGFR (λ-breathless) produces ectopic gas-filled seamless tubes (outlined with dashed white oval) surrounding the terminal cell nucleus (magenta), much like (although more extreme than) overexpression of wkd or Rab35DN. In (l), a sibling larva expressing a whacked RNAi transgene in addition to λ-Btl shows abundant ectopic tubes (white ovals) at branch tip positions rather than around the nucleus (magenta). Scale bars in f (for a, d–f), i (for b, h, and i) = 5 microns, and l (for l, k) = 50 microns.
To evaluate Whacked over-expression, UAS-whacked (methods) was expressed in wild type animals in a pan-tracheal pattern. Excess Whacked caused formation of ectopic seamless tubes surrounding the terminal cell nucleus (proximal seamless tube overgrowth) (Figure 4h). At higher levels of expression, small spheres of apical membrane were found adjacent to the nucleus and less abundantly at more distal sites (Figure S4a–l). Consistent with Whacked regulation of vesicle trafficking by modulation of Rab35, expression of a dominant negative Rab35 (henceforth, Rab35-DN) caused formation of ectopic proximal tubules (Figure 4i, j).
We sought to determine whether Rab35 was the essential target of Whacked GAP activity. Whacked primary structure is equally conserved in three human RabGAPs: TBC1D10C (FLJ00332 /Carabin/EPI64C – 32% identity), TBC1D10A (EPI64A – 30% identity), and TBC1D10B (FLJ13130/ EPI64B – 27% identity). All three act as Rab35GAPs,23 although each has been proposed to have additional targets24–26. To further test if Whacked acts as a Rab35GAP, we examined whether Rab35DN could suppress whacked mutants; tracheal-specific expression of Rab35DN strongly suppressed the “U-turn” defects of whacked null animals and surprisingly, also rescued the lethality of whacked (Figure S2k, m). Since mutant Rab35 isoforms phenocopy whacked gain and loss of function, Rab35DN bypasses the requirement for whacked, and human Whacked orthologs are Rab35GAPs, we conclude that the critical function of Whacked is as a GAP for Drosophila Rab35.
In other systems Rab35 is implicated in polarized membrane addition to plasma membrane compartments – e.g immune synapse, cytokinetic furrow, etc. – or, in actin regulation.23, 27–36 Because a role for actin in fusion cell seamless tube formation has been proposed,37 we examined whether Whacked and Rab35 act by modulation of the terminal cell actin cytoskeleton. Because the actin bundling protein Fascin (Drosophila singed) was recently identified biochemically as a Rab35 effector,36 we tested for a role of singed in terminal cell tubes, but found no evidence for one (methods, Figure S4m–r).
Furthermore, over-expression of Whacked (Figure S4t), or of Rab35DN (data not shown), did not significantly alter the terminal cell actin cytoskeleton, leading us to conclude that actin regulation is not a primary function of Wkd/Rab35 during seamless tube morphogenesis.
We found the alternative model – that Rab35 acted in polarized membrane addition – attractive, since extra Rab35-GTP activity promoted seamless tube growth at branch tips whereas depletion of Rab35-GTP promoted tube growth at the cell soma. To test this model, we took advantage of our knowledge that expression of an activated Breathless-FGFR (λ-Btl)38 in terminal cells induces robust growth of ectopic seamless tubes surrounding the nucleus (Figure 4k and Figure S5a, e); we asked if growth of the ectopic tubes could be redirected from the soma to the branch tips by eliminating whacked. The activated FGFR phenotype was not altered in whacked heterozygotes (Figure S5b, e), but in whacked mutant animals (or whacked RNAi animals) the site of ectopic seamless tube growth was strikingly different. In some cells, extra tubes were found throughout the cell (Figure S5d, e) – in the soma and at branch tips – while in others extra tubes were only present at the branch tip (Figure 4l, Figure S5c, e). Thus, the position of seamless tube growth is dependent upon Whacked activity, although Whacked itself is not essential for tube formation. These data argue against branch retraction (as occurs in talin mutants)18 as the mechanism for generating a U-turn phenotype, since branch retraction would not re-direct ectopic tube growth.
To better understand how Whacked and Rab35 determined the site of seamless tube growth, we investigated their subcellular distribution. Pan-tracheal expression of mKate2 tagged Whacked (Wkd::mKate) rescued whacked null animals (Figure S2l, m). The steady-state subcellular localization of Wkd::mKate was restricted to the lumenal membrane with higher accumulation at the growing tips of seamless tubes (direct fluorescence of Wkd::mKate in a fixed larva, Figure 5a). At lower levels, we noted cytoplasmic puncta of Wkd::mKate that could reflect vesicular localization, and labeling of filopodia (immunofluorescence using an antibody against mKate; Figure 5b,c). We found that YFP-Rab35 was distributed in a diffuse pattern throughout the terminal cell cytoplasm with some apical enrichment, and notable localization to filopodia (Figure 5b′, d). We find substantial co-localization of Wkd::mKate with YFP::Rab35 at the apical membrane, in cytoplasmic puncta, and in filopodia (Figure 5b″). Among endosomal Rabs, Rab35 appeared uniquely abundant within filopodia, and showed the greatest overlap with Whacked at the apical membrane (Figure S4u–x). We noted substantial overlap between Wkd/Rab35 and acetylated microtubules, including at positions distal to the blind-end of seamless tubes (Figure 5b″, c, d, e). The enrichment of Whacked along seamless tubes suggests that Rab35 functions in an apical membrane trafficking event, leading us to speculate that recycling endosomes at filopodia might be targeted to the growing seamless tube by minus-end motor transport.
FIGURE 5. Subcellular distribution of Wkd and Rab35, and the role of minus-end transport in seamless tube growth.
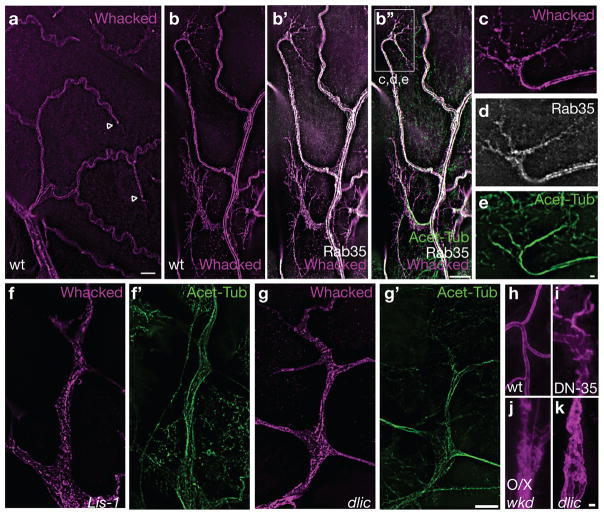
In wild type terminal cells (a–e) Whacked::mKate2 fusion protein (Whacked, magenta), is enriched apically, especially at branch tips (arrowheads). In comparison (b′,b″,d), YFP::Rab35 (white) is more broadly distributed, but also apically enriched. Whacked and Rab35 show extensive co-localization (b′), and also overlay with acetylated microtubule tracts (Acet-Tub, green). At branch tips (marqueed area in b″), Wkd (c) and Rab35 (d) are adjacent to acetylated microtubules (e), but also decorate actin-based filopodial processes (see Figure S3). In dynein motor complex mutants (Lis-1, f,f′; dlic g,g′), Whacked is dispersed throughout the cytoplasm and often detected on basolateral membrane. Although not excluded from areas with acetylated microtubule bundles, Whacked is not enriched along them (f″, g″). In (h–k), α-Wkdpep is used to stain the apical membrane of seamless tubes; in the soma surrounding the terminal cell nucleus of wild type cells (h), single seamless tubes are found growing into each cytoplasmic extension, whereas terminal cells expressing a dominant negative Rab35 isoform (i), overexpressing (O/X) wild type Whacked (j), or mutant for dynein motor complex components such as dlic (k) have ectopic seamless tubes. Scale bars in a for a, in g′ for b–g′ = 10 μm ; in e for c–e = 1 μm; and in k for h–k = 2 μm.
In a similar vein, we speculate that vesicles might be transported from the soma towards branch tips in a process regulated by Whacked and Rab35. Disruption of such transport might explain why over-expression of whacked leads to ectopic seamless tube growth in the soma. We asked if Wkd::mKate localization was compromised in dynein motor complex mutants. Because these cells have branches that lack apical membrane/seamless tubes, we anticipated disruption in the localization pattern of Wkd, but wondered whether co-localization with acetylated tubulin would be intact, indicative of a microtubule association independent of dynein motor transport. We find that Wkd::mKate2 is broadly distributed throughout the cytoplasm of dynein motor complex mutants, and does not show enrichment on acetylated microtubule tracts (Figure 5f,f′, g, g′), indeed, we detected substantial basal enrichment of Wkd::mKate. If Whacked/Rab35 dependent trafficking of apical vesicles was dynein motor complex dependent, we might expect to see ectopic seamless tubes in the soma of dynein motor complex mutants, similar to those seen with whacked over-expression or expression of DN-Rab35. In fact, we consistently find such ectopic tubes in the dynein motor complex mutants (Figure 5h–k), consistent with dynein-dependent trafficking of Rab35 vesicles. We cannot rule out the possibility that these defects are due to dynein-dependent processes unrelated to Wkd and Rab35; however, we did test whether the ectopic tubes could be redirected distally by expression of CA-Rab35, or elimination Wkd (Figures S1g-g″, h-h″). The motor complex ectopic tube phenotype could not be altered, suggesting that the phenotype does not arise as an indirect consequence of altered Wkd localization or Rab35 activity.
The roles of RabGAP proteins have only started to come into focus in recent years. Historically, it has been difficult to determine which Rab proteins are substrates of specific RabGAPs.39 Tests of in vitro GAP activity produced conflicting results, and in some cases did not appear indicative of in vivo function.40 Indeed, the specificity of Carabin (aka, Whacked ortholog TBC1D10C) has been controversial: it was first shown to act as a RasGAP,24 while later studies indicate a Rab35-specific GAP activity23, 30, 32. Our in vivo genetic data for whacked, together with recent studies characterizing the function of all three human Whacked-like TBC proteins (TBC1D10A-C),23 make a compelling case that this family of proteins acts as GAPs for Rab35. Further, our study establishes a role for classical vesicle trafficking proteins in seamless tube growth. Since seamless tubes but not multicellular or autocellular tracheal tubes are affected by mutations in whacked and Rab35, our study also establishes an in vivo cell type-specific requirement for trafficking genes in tube morphogenesis.
We conclude that Whacked and Rab35 regulate polarized growth of seamless tubes, and speculate that Whacked and Rab35 direct transport of apical membrane vesicles to the distal tip of terminal cell branches (when equilibrium is shifted toward active Rab35-GTP), or to a central location adjacent to the terminal cell nucleus (when equilibrium is shifted towards inactive Rab35-GDP). Analogous to its previously described roles in targeting vesicles to the immune synapse in T cells, the cytokinetic furrow in Drosophila S2 cells, and the neuromuscular junction in motor neurons, Rab35 would promote transport of vesicles from a recycling endosome compartment to the apical membrane. We further speculate that Breathless-FGFR activation at branch tips may couple terminal cell branching with seamless tube growth within that new branch (Figure S5f).
METHODS
Fly strains
EMS alleles of whacked were generated elsewhere17 and are characterized below. Minos allele of whacked (http://flybase.org) is available from the Bloomington stock center (http://flystocks.bio.indiana.edu). Mosaic analyses: FRT82B, wkd220, FRTG13, Lis1G10.14; Dhc64C4–19, FRT2A; Glued1, FRT2A; and dlic1 and dlic2 FRT19A (kindly provided by Dr. Tadashi Uemura). Alleles of singed used sn36a, snX2, and Df(1)c128. UAS-YFP::Rab (wild type, CA, and DN) strains were a gift from Jun Zhang and Matt Scott.22 UAS-whacked RNAi strain was obtained from the VDRC (http://stockcenter.vdrc.at/control/main). UAS-λBtl has been described,38 and a second chromosome insertion was generated (ASG) by P-element mobilization. crumbs::GFP was a gift from Yang Hong.41 UAS-wkd was generated by digesting whacked cDNA RE26521 (DGRC) with XhoI and BamH1 and ligating to pUAST cut with XhoI and partially digested with BamH1. Orientation of the insert was confirmed by testing for the presence of the EcoR1 site in the pUAST polylinker. Whacked::mKate2 fusion – the whacked cDNA was subcloned into pKS-bluescript, an NcoI site was introduced in place of the whacked stop codon. pmKate2 (Evrogen) was digested with NcoI and Not I, and inserted, in frame, downstream the whacked coding sequence. The whacked::mKate2 fusion was cloned into pUAST. whacked genomic rescue construct: a 3909bp PCR product was amplified from a w1118 genomic DNA (Qiagen DNeasy Kit) template using Phusion high fidelity DNA polymerase (New England Biolabs). The PCR product was cloned using a TopoTA cloning kit (Invitrogen). Clones were sequenced and mutant-free DNA was excised from the pCRII-Topo with Kpn I, and placed into pCasper4. UAS and genomic constructs were injected to generate transgenic strains according to standard protocols42, or by Genetic Services, Inc. pWG9.2, a second chromosome insertion of the whacked genomic DNA construct, rescued the tracheal defects and lethality associated with wkd220/Df(3R)Exel6276 and wkdMINOS/Df(3R)Exel6276 flies. Deficiency strains (http://flybase.org) are available from the Bloomington stock center, except for Df(3R)pros235, Df(3R)pros640, and Df(3R) thoRI, which were gifts from the Engels lab. whacked alleles. Although the U-turn phenotype is the predominant defect in whacked mutant animals, in our initial characterization of whacked we found that terminal cell seamless tubes often came to premature dead-ends (similar to the dynein motor mutants), and that the overall number of branches in each terminal cell was reduced (Supplemental Figure S2)17. Truncated tubes terminated in irregular shapes, leading us to name the gene whacked, to reflect the appearance that tubes had been crudely lopped off. However, extensive outcrossing of the whacked220 strain resulted in a simplified phenotype in which a robust U-turn defect persisted but all other tube and branching defects were greatly reduced in frequency – this suggests that the principle phenotype of whacked mutants is exuberant seamless tube growth at branch tips, and also that loss of whacked sensitizes formation of seamless tubes to genetic background. Interestingly, these other phenotypes were also rescued by the wkd genomic rescue construct (see below).
Positional cloning
Meiotic recombination placed whacked in the interval defined by the recessive markers curled (cu) and stripe (sr). Complementation tests against deficiency strains spanning the interval between cu and sr showed that whacked was uncovered by Df(3R)MKX1 which deletes polytene bands 86C1 to 87B1-5. Recombinant chromosomes in which cross-overs occurred between cu and sr were typed with various RFLP SNP markers including 86E5 and 86F10 which defined a smaller (~300 kb) candidate interval for whacked. Strains with small defined chromosomal deletions spanning 86E to 86F10 were tested for complementation, establishing a ~75 kb segment of the chromosome in 86E14-17 as the location of the whacked gene.
Primers
(1) whacked genomic rescue construct
Forward genomic primer 5′–GGTACC CGTAAACTTGAACGTTGCCACC-3′ and
Reverse genomic primer 5′-GGTACC GGTCTATGCACGTGAGCG-3′,
(2,3) RFLP snp mapping – 86E5 (MspI: 2FRT parental chromosome is uncut, but the ru h th st cu sr e ca chromosome is cut).
pros1507F: 5′-CCACCAAACTTCGGAATGCC-3′
pros2235B: 5′-TGGGGGTCGCTTATGCTTAGAC-3′
86F10 (NlaIV: 2FRT parental chromosome is cut, but the ru h th st cu sr e ca chromosome is uncut).
86F10F: 5′-ATTACGATGCCTTCGGTCCAC-3′
86F10B: 5′-AATGTCCTCACTTGTGCCACTG-3′
(4–9) whacked transcription unit sequence analysis:
TBC1F: 5′-TGCCACCTGGTTTTTGCTCTAC-3′
TBC1B: 5′-CGAATGGGGGAAATCTCAATG-3′
TBC2F: 5′-TTTCACTGTCCCATTCCGTTTG-3′
TBC2B: 5′-ATGTAGAGCCACTTCTTCTCCCGC-3′
TBC3F: 5′-CGAAATGGCTTCTATGGCGG-3′
TBC3B: 5′-TTCTTCAGCAGACCCTCCAGGATG-3′
TBC4F: 5′-GTCAGCGTGTGCGATGTGTAAG-3′
TBC4B: 5′-TGAACCAGTTTTGGGGTCACTC-3′
TBC5F: 5′-AAGGTGGCTCTGGTCATTATTGG-3′
TBC5B: 5′-AAACTTTTCAGGCTCGGGGG-3′
TBC6F: 5′-ATGATTACCACCCTGAGGCAGC-3′
TBC6B: 5′-TTTGGACGATGATGGCGACG-3′
Sequence analayses
whacked DNA and control DNA from the parental strain upon which whacked mutations were induced, were amplified by PCR and sequenced. In DNA from whackedPC24 homozygous animals, a single nucleotide change from the parental chromosome was detected that corresponded to a T to A transversion, a missense mutation causing an amino acid substitution in the conserved TBC domain of Whacked. In DNA from whacked220 animals, a C to T transition was detected, resulting in a nonsense mutation at the sixth codon position in the whacked coding sequence. Further support for the identity of whacked came from the subsequent identification of a third mutant allele, this one generated by the insertion of a Minos transposable element into the coding sequence of CG5344.43 Terminal cells mutant for the Minos allele of whacked displayed a decreased number of branches and the U-turn phenotype.
Immunohistochemistry
Antibodies used in these studies include: mouse α-mRFP (1:1000, Abcam ab65856-100), rabbit α-tRFP (anti-mKate2, 1:2000, Evrogen AB234), chick α-GFP (1:1000, Invitrogen A10262), mouse α-Ac-tubulin monoclonal antibody (1:2000, Sigma T6793), mouse α-γ-tubulin monoclonal antibody (1:1000, GTU-88, Sigma T6557). The Wkdpep rabbit polyclonal antibody was generated against the Wkd peptide, EHTRQKARRAKQKAQQE, but is used as a marker of terminal cell lumenal membrane. Sera is not specific for Wkd as lumenal membrane staining is still detected in wkd null (220/Df) and wkdMINOS larvae (the Minos insertion is within the last wkd exon, 5′ of the nucleotides coding for the Wkd peptide antigen). DsRED, GFP and mKate2 were visualized by direct fluorescence in heat killed or fixed larvae, or by antibody staining of fixed and filleted larvae.
Fascin and Egalitarian studies
We carried out tests to determine whether singed might be the essential Rab35 effector in tracheal terminal cells for seamless tube morphogenesis. First, we asked whether terminal cells lacking Fascin displayed defects consistent with loss of Rab35 activity. We found that singed null terminal cells were entirely wild type in appearance (Figure S5b). Loss of singed also did not suppress the whacked mutant phenotype (Figure S5c,d). Likewise, loss of singed showed no apparent alteration of actin organization in terminal cells (Figure S5f,j). Furthermore, overexpression of Whacked (Figure S5h,i), or Rab35DN (data not shown), did not alter the terminal cell actin cytoskeleton, leading us to conclude that actin regulation is not a primary function of Wkd/Rab35 during seamless tube morphogenesis.
To test for a requirement for egalitarian, which serves as an adaptor for apically localized mRNA transport, we examined third instar larvae homozygous for egl1. Mutant terminal cells were indistinguishable from wild type (data not shown); however, we cannot rule out the possibility that maternal egl mRNA and protein masked a requirement for Egl function in seamless tube formation.
Double mutant analyses
The following crosses were carried out: btl-Gal4, UAS-GFP; wkd220/TM3Sb, TubGal80 flies were crossed with UAS-λbtl/CyO, UAS-DsRED; Df(3R)EXEL/TM6B, UAS-DsRED, or with UAS-Rab35DN/CyO, UAS-DsRED; Df(3R)EXEL/TM6B, UAS-DsRED. The progeny of these crosses that lacked DsRED expression were the experimental genotype, while sibling DsRED expressing larvae that lacked the Tubby phenotype served as wkd loss of function controls. The sn and wkd double mutant analysis was carried out as follows: Df(1)c128/FM7aGFP; Df(3R)EXEL6276/TM6B females were crossed to singedX2; wkdMI/TM6B males. The experimental genotypes were those progeny that lacked GFP and Tubby, while female siblings expressing GFP but lacking Tubby were used as wkd loss of function controls. In order to generate tracheal cells deficient for dlic and wkd, dlic1/FM7; wkd220/+ females were crossed to UAS-GFP RNAi, FRT19A FLP122; btl-Gal4, UAS-GFP; Df(3R)EXEL6276/+ males and progeny were heat shocked at 38.5°C for one hour after a four-hour egg collection. The wkd220/Df progeny were identified by the their tracheal phenotype and were screened for dlic clones (GFP positive). To generate animals in which constitutive active Rab35 was expressed in dlic mutant terminal cells, dlic1/FM7; UAS-Rab35CA females were crossed to TubGal80 FRT19A FLP122; btl-Gal4 UAS-GFP males and the progeny were heat shocked at 38.5°C for one hour after a four-hour egg collection. Positively marked clones were identified and scored for phenotype.
Pulsed CD8::GFP experiment
TubGal80ts flies were crossed with btl-Gal4, UAS-CD8-GFP flies, and the larvae were maintained at 18 °C until third larval instar. Larvae were then subjected to 1 hour heat shock at 38.5 °C to inactivate Gal80 and allow for btl-Gal4 driven expression of CD8-GFP. Larvae were filleted, fixed and stained (as described in methods) at 1 hour, 2 hours and 3 hours after temperature shift.
Transgene Rescue of whacked viability
The following crosses were carried out: (1) Rab35DN: UAS-Rab35DN; Df(3R)EXEL6276/TM6B X breathless-Gal4>DsRED/CyO; wkd220/TM6B. (2) Genomic Rescue: Two crosses were carried out – a) genomic rescue/S; Df(3R)EXEL6276 males were crossed to virgins that were breathless-Gal4>DsRED/CyO; wkd220/TM6B. (3) Whacked::mKate2: btl-Gal4, UAS-wkd::mkate2/CyO; Df(3R)EXEL6276/MKRS X Sp/CyO; wkd220/MKRS. Adults with the rescuing transgenes were scored.
Live imaging of EB1::GFP
Third-instar larvae of the genotype 4Xsrf-Gal4, UAS-EB1GFP were anesthetized with ether for fifteen minutes and imaged for up to 20 minutes on an Olympus Spinning Disk Confocal Microscope. Images were acquired with a 60X, 1.2 NA UPlanApo water immersion objective on a spinning disk confocal consisting of a Yokogawa CSU-X1 confocal scanner attached to an Olympus IX-81 microscope. The camera was an Andor iXon3 EMCCD camera and acquisition was controlled by MetaMorph 7.7. Images were collected at 2 frames/second for one minute. Direction of EB1 movement was determined by manual tracking of GFP fluorescence over a 10–20 second interval.
Supplementary Material
In all panels, staining with the Rabbit α-Wkdpep sera is shown in magenta. In (a) PH-GFP (green) highlights the terminal cell branch outline and filopodia. Scale bar = 10 microns. In (b) lum-GFP 17 accumulates in the liquid-filled tip of a terminal cell tube. Note that the α-Wkdpep staining surrounds the lum-GFP, but is excluded from the lumen of the tube. Scale bar = 2 microns. In (c, c′) a terminal cell from a tubGal80ts; btl-Gal4, UAS-CD8::GFP larvae is shown (CD8::GFP, green). Expression of CD8::GFP was induced by temperature shift in the third larval instar. After 3 hrs, the larvae was filleted, fixed, and stained. Note that CD8::GFP is detectable at low levels throughout the terminal cell, while scattered bright puncta of staining may represent newly added CD8::GFP that has not mixed by lateral diffusion. (d–f) Endogenous Crumbs::GFP (green) expression in three dlic mutant terminal cells. Crumbs localizes to rudimentary tubes in the soma of dlic mutants (d) but does not consistently overlap with discontinuous pieces of tube found more distally (d–f). Crumbs expression around tube fragments was often patchy (e) and could also be observed in regions devoid of our lumenal membrane marker (f). Cell shape is outlined by a white-dotted line (traced from a captured image showing expression of cytoplasmic DsRed). Scale bar = 10 microns. Seamless tube defects in dlic mutants are not the result of Whacked mislocalization. (g-g″) A terminal cell double mutant for dlic and whacked. Double mutant cells are indistinguishable from dlic mutants alone (compare to Figure 2g and 2g′). Moreover, tracheal expression of constitutively active Rab35 is not sufficient to rescue the dlic mutant phenotype (h-h″). cytoplasmic GFP in white, nuclear DsRed or acetylated-tubulin in green. Scale bar = 10 microns.
Meiotic recombination mapping (a) placed whacked into the interval defined by the recessive markers curled (cu) and stripe (sr). Complementation tests against chromosomal deficiency strains further refined the map position of whacked, although anomalous results were obtained with Df(3R)T-32 and T-61. The use of SNP markers (see M&M) verified and further refined the whacked candidate gene interval (b), and a series of small overlapping chromosomal deficiency strains with molecularly defined breakpoints were used to identify a final candidate interval of ~ 78 kb. Sequence analysis revealed that whacked corresponds to the predicted gene CG5344, with each allele of whacked showing a single nucleotide change, as compared to parental DNA, resulting in mis-sense and nonsense changes in the coding sequence. In (c), a schematic of the predicted 363 amino acid Whacked protein, with a central TBC domain, is shown. Mutations in the 220 and PC24 alleles are predicted to truncate the protein prior to the TBC domain, or to alter the TBC domain, respectively. In (d), a ClustalW alignment of Whacked, its three human homologues, and the TBC consensus sequence is shown. Alignment reveals high sequence similarity within the putative RabGAP domain, and conservation of the invariant “dual finger” R and Q residues (indicated in red); position of the M to K mutation in PC24 is indicated (K in green).
In (a–e), the terminal cell is marked by CD8-GFP (green) and the seamless tubes running through the cells are visualized by staining against α-Wkdpep (magenta). Merged images (lower middle panels) and schematic drawings illustrating the phenotypes (bottom panels). (a) wild type and (b–e) whacked220/Df mutant terminal cells. In (b), a portion of a whacked mutant terminal cell is shown; note the presence of fewer side branches, and that tube lumens are prematurely truncated (arrowheads); some branches of the terminal cell (*) completely lack lumens. In another whacked terminal cell (c), the seamless tubes within the terminal branches are discontinuous (arrowheads indicate deadends of proximal tubes and arrows indicate start of distal tubes). In (d, same as Figure 3i), a high magnification view of the tip of a whacked terminal cell branch reveals a tangled tube that appears to execute a series of U-turns within the branch tip cytoplasm. In other whacked terminal cells (e), distal dilations in a terminal branches are observed (arrowheads), in which the associated seamless tube looks highly irregular and rough in appearance. In f – h, a mosaic analysis of whacked is shown in which homozygous mutant cells are labeled with GFP and all tracheal nuclei are marked with DsRED2nls (magenta). The fluorescent images are superimposed on brightfield images that allow assessment of gas-filling. Dorsal trunk clones (f), stalk cell clones (g, *), and fusion cell clones (h, **) appear normal but terminal cell clones (g, ^) show the spectrum of defects described above. Bars: a – c, e – 5 microns; d – 1 micron. f – h, 10 microns. (i-l) Third-instar larval terminal cells mutant for whacked220/Df display an overgrowth of seamless tube at the distal tips of terminal branches (i, arrowheads) and a reduction in the number of branches. These defects can be rescued or suppressed by addition of a whacked genomic rescue construct (j), tracheal expression of Rab35DN (k), or tracheal expression of Wkd::mKate2 (l). Scale bar = 10 microns. (m) Table of whacked mutant rescue data. *OG = overgrowth
(a–c) Gross overexpression of UAS-wkd in the tracheal system leads to the production of discontinuous membrane spheres in terminal cells at the expense of gas-filled tubes (cytoplasmic GFP - white (a) green (c), α-Wkdpep - white (b) magenta (c)). These ectopic spheres have apical identity as revealed by co-localization with Crumbs::GFP (green, d–f) and the ability to recruit actin (green, g–i). (j–l) These cells often showed discontinuous pieces of tube associated with microtubule tracts, similar to what we see with dynein motor complex mutants (acetylated microtubules, green). (m–p) Fascin is not the critical effector of Rab35 in tracheal terminal cells. Terminal cells mutant for singed (n) are morphologically indistinguishable from wildtype (m). Terminal cells mutant for whackedMINOS/Df and heterozygous (o) or mutant for singed (p) were indistinguishable from whacked mutants alone. Furthermore, no obvious defects in apical and filopodial localization of actin in terminal cells of sn and whacked mutants exists (q–r, branch tips top panels, more proximal positions bottom panels). Although wkd RNAi and Wkd overexpression (O/X) cause tube defects, actin is localized to the apical membrane of these tubes (s, t - bottom) and filopodia still decorate the tips of branches (s, t - top). However, the filopodia in wkd mutants (data not shown) and wkd RNAi (s) are frequently less branched than wild type (q). (u–x) Analysis of endocytic Rab::Wkd co-localization (immunostaining against mKate2 and YFP) at seamless tubes and in filopodial processes. Rab35 (u) is uniquely enriched in filopodia (top panels) and broadly co-localizes with Wkd (u′) at seamless tubes (bottom panels). Only small amounts of Rab5 (early endosome, v), Rab7 (late endosome, w) and Rab11 (recycling endosome, x) localize with Wkd in filopodia, highlighting a unique relationship between Rab35 and Wkd at the distal tips of branches. Puncta of Wkd do occasionally co-localize (arrowheads) with Rab5, Rab7, and Rab11 along seamless tubes (bottom panels). Scale bar = 10 microns (m–t) and 5 microns (c for a–l, and m, q for u–x).
FIGURE S5. The Rab35/Whacked pathway regulates polarized growth of seamless tubes. (a) As shown in Figure 4k, activation of FGFR signaling in tracheal terminal cells results in the generation of ectopic tubes adjacent to the nucleus of the cell. In a whacked heterozygous background (b), the activated Breathless phenotype is unaltered. In a whacked mutant background (c, d), such ectopic tubes arise at more distal positions, sometimes exclusively at the branch tip, and sometimes more broadly distributed. In a wkd mutant background extra seamless tubes near the nucleus (n) are absent (c) or greatly reduced in number (d). (e) Table of data presented in a–d. (f) Model for Wkd/Rab35 polarization of seamless tube growth. In (f′) we show an idealized terminal cell branch extending from near the terminal cell nucleus (Nuc) out to the growing tip of the terminal cell. The lumenal membrane is shown in black, with elipses (…) representing the space between the soma and branch tip. We hypothesize (see f″, KEY) that activation of Breathless, which triggers outgrowth of cytoplasmic extensions, also regulates Wkd so as to polarize seamless tube growth distally by catalyzing Rab35 GTP hydrolysis, resulting in liberation of vesicles from the transport apparatus. We propose that loss of dynein motor activity (f‴) results in a failure to transport membrane components apically but also Rab35-vesicles distally, and so subsequently, these vesicles are added to the tube at nucleus proximal positions. In (f″″) the absence of Wkd Rab35GAP, or if Rab35 is constitutively active, seamless tube growth will be polarized at the cell tip. If Rab35 dominant negative is expressed, or excess Wkd Rab35GAP is expressed, or induced by constitutively active Breathless-FGFR (f‴″), then transport of vesicles is blocked and addition to seamless tubes will occur proximal to the nucleus. Seamless tube will still be present distal to the soma since other vesicle transport pathways are able to promote tube growth in the absence of Rab35 activity. If Breathless-FGFR is constitutively activated but Whacked is absent from the cell (f″″″), then the ectopic seamless tube formation promoted by FGFR will be directed to the branch tips.
MOVIE S1. EB1:GFP dynamics in tracheal terminal cells. EB1:GFP comets can be seen moving in a bi-directional manner in regions adjacent to the terminal cell nucleus as well as more distal regions of terminal cell branches. Comets can also be seen extending from the apical membrane at a few branch positions more proximal to the cell nucleus. Near the soma, growth was towards branch tips at a 3:2 ratio (n= 50 comets scored), in medial positions growth was equally likely in either direction (~ 1:1, n = 41 comets scored), and near the end of the seamless tubes, there was a 2:1 distal bias (n = 28 comets scored). The first half of the movie extends over a 60 second interval and the second half of the movie extends over a 66 second interval and is being displayed at 10 frames per second.
Acknowledgments
The authors would like to acknowledge: Danielle Willis, a former Stanford undergraduate student who helped with the rough mapping of whacked; Boaz Levi, who helped with the third chromosome screen; and Mark Krasnow, in whose lab the screen and early phases of these studies were carried out. We also thank Dr. Jun Zhang and the laboratories of Dr. Matt Scott and Dr. Hugo Bellen for making CA-Rab and DN-Rab stocks available to us prior to publication, the Engels lab for sharing Deficiency strains, and Mark Metzstein for sharing 4x-SRF-Gal4 flies. We thank Steve DiNardo, Chris Burd, Erfei Bi, and members of the Ghabrial and DiNardo labs for fruitful discussions. We thank Drs. Alondra Schweizer Burguete, Boaz Levi, and Nancy Speck for comments on the manuscript. JS-R was supported by NIH training grant 5-T32-HD007516-12 and subsequently, by an NIH postdoctoral fellowship (NRSA –GM090438-01). ASG gratefully acknowledges support from the University of Pennsylvania and the NIH (1R01GM089782-01A1). This work was supported in part by Basil O’Connor Starter Scholar Research Award Grant No. 5-FY09-43 from the March of Dimes Foundation.
Footnotes
AUTHOR CONTRIBUTIONS
JS-R and ASG conceived of and carried out all experiments described here. ASG wrote the manuscript with input from JS-R. Figures were assembled by JS-R and ASG.
References
- 1.Samakovlis C, et al. Development of the Drosophila tracheal system occurs by a series of morphologically distinct but genetically coupled branching events. Development. 1996;122:1395–1407. doi: 10.1242/dev.122.5.1395. [DOI] [PubMed] [Google Scholar]
- 2.Ribeiro C, Neumann M, Affolter M. Genetic control of cell intercalation during tracheal morphogenesis in Drosophila. Curr Biol. 2004;14:2197–2207. doi: 10.1016/j.cub.2004.11.056. [DOI] [PubMed] [Google Scholar]
- 3.Buechner M. Tubes and the single C. elegans excretory cell. Trends Cell Biol. 2002;12:479–484. doi: 10.1016/s0962-8924(02)02364-4. [DOI] [PubMed] [Google Scholar]
- 4.Bar T, Guldner FH, Wolff JR. “Seamless” endothelial cells of blood capillaries. Cell Tissue Res. 1984;235:99–106. doi: 10.1007/BF00213729. [DOI] [PubMed] [Google Scholar]
- 5.Lubarsky B, Krasnow MA. Tube morphogenesis: making and shaping biological tubes. Cell. 2003;112:19–28. doi: 10.1016/s0092-8674(02)01283-7. [DOI] [PubMed] [Google Scholar]
- 6.Uv A, Cantera R, Samakovlis C. Drosophila tracheal morphogenesis: intricate cellular solutions to basic plumbing problems. Trends Cell Biol. 2003;13:301–309. doi: 10.1016/s0962-8924(03)00083-7. [DOI] [PubMed] [Google Scholar]
- 7.Gervais L, Casanova J. In vivo coupling of cell elongation and lumen formation in a single cell. Curr Biol. 2010;20:359–366. doi: 10.1016/j.cub.2009.12.043. [DOI] [PubMed] [Google Scholar]
- 8.Brodu V, Baffet AD, Le Droguen PM, Casanova J, Guichet A. A developmentally regulated two-step process generates a noncentrosomal microtubule network in Drosophila tracheal cells. Dev Cell. 2010;18:790–801. doi: 10.1016/j.devcel.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 9.Sutherland D, Samakovlis C, Krasnow MA. branchless encodes a Drosophila FGF homolog that controls tracheal cell migration and the pattern of branching. Cell. 1996;87:1091–1101. doi: 10.1016/s0092-8674(00)81803-6. [DOI] [PubMed] [Google Scholar]
- 10.Ribeiro C, Ebner A, Affolter M. In vivo imaging reveals different cellular functions for FGF and Dpp signaling in tracheal branching morphogenesis. Dev Cell. 2002;2:677–683. doi: 10.1016/s1534-5807(02)00171-5. [DOI] [PubMed] [Google Scholar]
- 11.Jarecki J, Johnson E, Krasnow MA. Oxygen regulation of airway branching in Drosophila is mediated by branchless FGF. Cell. 1999;99:211–220. doi: 10.1016/s0092-8674(00)81652-9. [DOI] [PubMed] [Google Scholar]
- 12.Berry KL, Bulow HE, Hall DH, Hobert O. A C. elegans CLIC-like protein required for intracellular tube formation and maintenance. Science. 2003;302:2134–2137. doi: 10.1126/science.1087667. [DOI] [PubMed] [Google Scholar]
- 13.Ulmasov B, Bruno J, Gordon N, Hartnett ME, Edwards JC. Chloride intracellular channel protein-4 functions in angiogenesis by supporting acidification of vacuoles along the intracellular tubulogenic pathway. Am J Pathol. 2009;174:1084–1096. doi: 10.2353/ajpath.2009.080625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grawe F, Wodarz A, Lee B, Knust E, Skaer H. The Drosophila genes crumbs and stardust are involved in the biogenesis of adherens junctions. Development. 1996;122:951–959. doi: 10.1242/dev.122.3.951. [DOI] [PubMed] [Google Scholar]
- 15.Wodarz A, Hinz U, Engelbert M, Knust E. Expression of crumbs confers apical character on plasma membrane domains of ectodermal epithelia of Drosophila. Cell. 1995;82:67–76. doi: 10.1016/0092-8674(95)90053-5. [DOI] [PubMed] [Google Scholar]
- 16.Waterman-Storer CM, et al. The interaction between cytoplasmic dynein and dynactin is required for fast axonal transport. Proc Natl Acad Sci U S A. 1997;94:12180–12185. doi: 10.1073/pnas.94.22.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghabrial AS, Levi BP, Krasnow MA. A systematic screen for tube morphogenesis and branching genes in the Drosophila tracheal system. PLoS Genet. 2011;7:e1002087. doi: 10.1371/journal.pgen.1002087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levi BP, Ghabrial AS, Krasnow MA. Drosophila talin and integrin genes are required for maintenance of tracheal terminal branches and luminal organization. Development. 2006;133:2383–2393. doi: 10.1242/dev.02404. [DOI] [PubMed] [Google Scholar]
- 19.Albert S, Gallwitz D. Two new members of a family of Ypt/Rab GTPase activating proteins. Promiscuity of substrate recognition. J Biol Chem. 1999;274:33186–33189. doi: 10.1074/jbc.274.47.33186. [DOI] [PubMed] [Google Scholar]
- 20.Albert S, Will E, Gallwitz D. Identification of the catalytic domains and their functionally critical arginine residues of two yeast GTPase-activating proteins specific for Ypt/Rab transport GTPases. EMBO J. 1999;18:5216–5225. doi: 10.1093/emboj/18.19.5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strom M, Vollmer P, Tan TJ, Gallwitz D. A yeast GTPase-activating protein that interacts specifically with a member of the Ypt/Rab family. Nature. 1993;361:736–739. doi: 10.1038/361736a0. [DOI] [PubMed] [Google Scholar]
- 22.Zhang J, et al. Thirty-one flavors of Drosophila rab proteins. Genetics. 2007;176:1307–1322. doi: 10.1534/genetics.106.066761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsu C, et al. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J Cell Biol. 2010;189:223–232. doi: 10.1083/jcb.200911018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan F, et al. Feedback inhibition of calcineurin and Ras by a dual inhibitory protein Carabin. Nature. 2007;445:433–436. doi: 10.1038/nature05476. [DOI] [PubMed] [Google Scholar]
- 25.Itoh T, Fukuda M. Identification of EPI64 as a GTPase-activating protein specific for Rab27A. J Biol Chem. 2006;281:31823–31831. doi: 10.1074/jbc.M603808200. [DOI] [PubMed] [Google Scholar]
- 26.Ishibashi K, Kanno E, Itoh T, Fukuda M. Identification and characterization of a novel Tre-2/Bub2/Cdc16 (TBC) protein that possesses Rab3A-GAP activity. Genes Cells. 2009;14:41–52. doi: 10.1111/j.1365-2443.2008.01251.x. [DOI] [PubMed] [Google Scholar]
- 27.Chevallier J, et al. Rab35 regulates neurite outgrowth and cell shape. FEBS Lett. 2009 doi: 10.1016/j.febslet.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 28.Chua CE, Lim YS, Tang BL. Rab35--a vesicular traffic-regulating small GTPase with actin modulating roles. FEBS Lett. 2010;584:1–6. doi: 10.1016/j.febslet.2009.11.051. [DOI] [PubMed] [Google Scholar]
- 29.Echard A. Membrane traffic and polarization of lipid domains during cytokinesis. Biochem Soc Trans. 2008;36:395–399. doi: 10.1042/BST0360395. [DOI] [PubMed] [Google Scholar]
- 30.Gao Y, et al. Recycling of the Ca2+-activated K+ channel, KCa2.3, is dependent upon RME-1, Rab35/EPI64C, and an N-terminal domain. J Biol Chem. 2010;285:17938–17953. doi: 10.1074/jbc.M109.086553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kouranti I, Sachse M, Arouche N, Goud B, Echard A. Rab35 regulates an endocytic recycling pathway essential for the terminal steps of cytokinesis. Curr Biol. 2006;16:1719–1725. doi: 10.1016/j.cub.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 32.Patino-Lopez G, et al. Rab35 and its GAP EPI64C in T cells regulate receptor recycling and immunological synapse formation. J Biol Chem. 2008;283:18323–18330. doi: 10.1074/jbc.M800056200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sato M, et al. Regulation of endocytic recycling by C. elegans Rab35 and its regulator RME-4, a coated-pit protein. EMBO J. 2008;27:1183–1196. doi: 10.1038/emboj.2008.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shim J, et al. Rab35 mediates transport of Cdc42 and Rac1 to the plasma membrane during phagocytosis. Mol Cell Biol. 2010;30:1421–1433. doi: 10.1128/MCB.01463-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uytterhoeven V, Kuenen S, Kasprowicz J, Miskiewicz K, Verstreken P. Loss of skywalker reveals synaptic endosomes as sorting stations for synaptic vesicle proteins. Cell. 2011;145:117–132. doi: 10.1016/j.cell.2011.02.039. [DOI] [PubMed] [Google Scholar]
- 36.Zhang J, Fonovic M, Suyama K, Bogyo M, Scott MP. Rab35 controls actin bundling by recruiting fascin as an effector protein. Science. 2009;325:1250–1254. doi: 10.1126/science.1174921. [DOI] [PubMed] [Google Scholar]
- 37.Lee S, Kolodziej PA. The plakin Short Stop and the RhoA GTPase are required for E-cadherin-dependent apical surface remodeling during tracheal tube fusion. Development. 2002;129:1509–1520. doi: 10.1242/dev.129.6.1509. [DOI] [PubMed] [Google Scholar]
- 38.Lee T, Hacohen N, Krasnow M, Montell DJ. Regulated Breathless receptor tyrosine kinase activity required to pattern cell migration and branching in the Drosophila tracheal system. Genes Dev. 1996;10:2912–2921. doi: 10.1101/gad.10.22.2912. [DOI] [PubMed] [Google Scholar]
- 39.Pfeffer S. Filling the Rab GAP. Nat Cell Biol. 2005;7:856–857. doi: 10.1038/ncb0905-856. [DOI] [PubMed] [Google Scholar]
- 40.Fuchs E, et al. Specific Rab GTPase-activating proteins define the Shiga toxin and epidermal growth factor uptake pathways. J Cell Biol. 2007;177:1133–1143. doi: 10.1083/jcb.200612068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang J, Zhou W, Dong W, Watson AM, Hong Y. Directed, efficient, and versatile modifications of the Drosophila genome by genomic engineering. Proc Natl Acad Sci U S A. 2009;106:8284–8289. doi: 10.1073/pnas.0900641106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rubin GM, Spradling AC. Genetic transformation of Drosophila with transposable element vectors. Science. 1982;218:348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- 43.Bellen HJ, et al. The BDGP gene disruption project: single transposon insertions associated with 40% of Drosophila genes. Genetics. 2004;167:761–781. doi: 10.1534/genetics.104.026427. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
In all panels, staining with the Rabbit α-Wkdpep sera is shown in magenta. In (a) PH-GFP (green) highlights the terminal cell branch outline and filopodia. Scale bar = 10 microns. In (b) lum-GFP 17 accumulates in the liquid-filled tip of a terminal cell tube. Note that the α-Wkdpep staining surrounds the lum-GFP, but is excluded from the lumen of the tube. Scale bar = 2 microns. In (c, c′) a terminal cell from a tubGal80ts; btl-Gal4, UAS-CD8::GFP larvae is shown (CD8::GFP, green). Expression of CD8::GFP was induced by temperature shift in the third larval instar. After 3 hrs, the larvae was filleted, fixed, and stained. Note that CD8::GFP is detectable at low levels throughout the terminal cell, while scattered bright puncta of staining may represent newly added CD8::GFP that has not mixed by lateral diffusion. (d–f) Endogenous Crumbs::GFP (green) expression in three dlic mutant terminal cells. Crumbs localizes to rudimentary tubes in the soma of dlic mutants (d) but does not consistently overlap with discontinuous pieces of tube found more distally (d–f). Crumbs expression around tube fragments was often patchy (e) and could also be observed in regions devoid of our lumenal membrane marker (f). Cell shape is outlined by a white-dotted line (traced from a captured image showing expression of cytoplasmic DsRed). Scale bar = 10 microns. Seamless tube defects in dlic mutants are not the result of Whacked mislocalization. (g-g″) A terminal cell double mutant for dlic and whacked. Double mutant cells are indistinguishable from dlic mutants alone (compare to Figure 2g and 2g′). Moreover, tracheal expression of constitutively active Rab35 is not sufficient to rescue the dlic mutant phenotype (h-h″). cytoplasmic GFP in white, nuclear DsRed or acetylated-tubulin in green. Scale bar = 10 microns.
Meiotic recombination mapping (a) placed whacked into the interval defined by the recessive markers curled (cu) and stripe (sr). Complementation tests against chromosomal deficiency strains further refined the map position of whacked, although anomalous results were obtained with Df(3R)T-32 and T-61. The use of SNP markers (see M&M) verified and further refined the whacked candidate gene interval (b), and a series of small overlapping chromosomal deficiency strains with molecularly defined breakpoints were used to identify a final candidate interval of ~ 78 kb. Sequence analysis revealed that whacked corresponds to the predicted gene CG5344, with each allele of whacked showing a single nucleotide change, as compared to parental DNA, resulting in mis-sense and nonsense changes in the coding sequence. In (c), a schematic of the predicted 363 amino acid Whacked protein, with a central TBC domain, is shown. Mutations in the 220 and PC24 alleles are predicted to truncate the protein prior to the TBC domain, or to alter the TBC domain, respectively. In (d), a ClustalW alignment of Whacked, its three human homologues, and the TBC consensus sequence is shown. Alignment reveals high sequence similarity within the putative RabGAP domain, and conservation of the invariant “dual finger” R and Q residues (indicated in red); position of the M to K mutation in PC24 is indicated (K in green).
In (a–e), the terminal cell is marked by CD8-GFP (green) and the seamless tubes running through the cells are visualized by staining against α-Wkdpep (magenta). Merged images (lower middle panels) and schematic drawings illustrating the phenotypes (bottom panels). (a) wild type and (b–e) whacked220/Df mutant terminal cells. In (b), a portion of a whacked mutant terminal cell is shown; note the presence of fewer side branches, and that tube lumens are prematurely truncated (arrowheads); some branches of the terminal cell (*) completely lack lumens. In another whacked terminal cell (c), the seamless tubes within the terminal branches are discontinuous (arrowheads indicate deadends of proximal tubes and arrows indicate start of distal tubes). In (d, same as Figure 3i), a high magnification view of the tip of a whacked terminal cell branch reveals a tangled tube that appears to execute a series of U-turns within the branch tip cytoplasm. In other whacked terminal cells (e), distal dilations in a terminal branches are observed (arrowheads), in which the associated seamless tube looks highly irregular and rough in appearance. In f – h, a mosaic analysis of whacked is shown in which homozygous mutant cells are labeled with GFP and all tracheal nuclei are marked with DsRED2nls (magenta). The fluorescent images are superimposed on brightfield images that allow assessment of gas-filling. Dorsal trunk clones (f), stalk cell clones (g, *), and fusion cell clones (h, **) appear normal but terminal cell clones (g, ^) show the spectrum of defects described above. Bars: a – c, e – 5 microns; d – 1 micron. f – h, 10 microns. (i-l) Third-instar larval terminal cells mutant for whacked220/Df display an overgrowth of seamless tube at the distal tips of terminal branches (i, arrowheads) and a reduction in the number of branches. These defects can be rescued or suppressed by addition of a whacked genomic rescue construct (j), tracheal expression of Rab35DN (k), or tracheal expression of Wkd::mKate2 (l). Scale bar = 10 microns. (m) Table of whacked mutant rescue data. *OG = overgrowth
(a–c) Gross overexpression of UAS-wkd in the tracheal system leads to the production of discontinuous membrane spheres in terminal cells at the expense of gas-filled tubes (cytoplasmic GFP - white (a) green (c), α-Wkdpep - white (b) magenta (c)). These ectopic spheres have apical identity as revealed by co-localization with Crumbs::GFP (green, d–f) and the ability to recruit actin (green, g–i). (j–l) These cells often showed discontinuous pieces of tube associated with microtubule tracts, similar to what we see with dynein motor complex mutants (acetylated microtubules, green). (m–p) Fascin is not the critical effector of Rab35 in tracheal terminal cells. Terminal cells mutant for singed (n) are morphologically indistinguishable from wildtype (m). Terminal cells mutant for whackedMINOS/Df and heterozygous (o) or mutant for singed (p) were indistinguishable from whacked mutants alone. Furthermore, no obvious defects in apical and filopodial localization of actin in terminal cells of sn and whacked mutants exists (q–r, branch tips top panels, more proximal positions bottom panels). Although wkd RNAi and Wkd overexpression (O/X) cause tube defects, actin is localized to the apical membrane of these tubes (s, t - bottom) and filopodia still decorate the tips of branches (s, t - top). However, the filopodia in wkd mutants (data not shown) and wkd RNAi (s) are frequently less branched than wild type (q). (u–x) Analysis of endocytic Rab::Wkd co-localization (immunostaining against mKate2 and YFP) at seamless tubes and in filopodial processes. Rab35 (u) is uniquely enriched in filopodia (top panels) and broadly co-localizes with Wkd (u′) at seamless tubes (bottom panels). Only small amounts of Rab5 (early endosome, v), Rab7 (late endosome, w) and Rab11 (recycling endosome, x) localize with Wkd in filopodia, highlighting a unique relationship between Rab35 and Wkd at the distal tips of branches. Puncta of Wkd do occasionally co-localize (arrowheads) with Rab5, Rab7, and Rab11 along seamless tubes (bottom panels). Scale bar = 10 microns (m–t) and 5 microns (c for a–l, and m, q for u–x).
FIGURE S5. The Rab35/Whacked pathway regulates polarized growth of seamless tubes. (a) As shown in Figure 4k, activation of FGFR signaling in tracheal terminal cells results in the generation of ectopic tubes adjacent to the nucleus of the cell. In a whacked heterozygous background (b), the activated Breathless phenotype is unaltered. In a whacked mutant background (c, d), such ectopic tubes arise at more distal positions, sometimes exclusively at the branch tip, and sometimes more broadly distributed. In a wkd mutant background extra seamless tubes near the nucleus (n) are absent (c) or greatly reduced in number (d). (e) Table of data presented in a–d. (f) Model for Wkd/Rab35 polarization of seamless tube growth. In (f′) we show an idealized terminal cell branch extending from near the terminal cell nucleus (Nuc) out to the growing tip of the terminal cell. The lumenal membrane is shown in black, with elipses (…) representing the space between the soma and branch tip. We hypothesize (see f″, KEY) that activation of Breathless, which triggers outgrowth of cytoplasmic extensions, also regulates Wkd so as to polarize seamless tube growth distally by catalyzing Rab35 GTP hydrolysis, resulting in liberation of vesicles from the transport apparatus. We propose that loss of dynein motor activity (f‴) results in a failure to transport membrane components apically but also Rab35-vesicles distally, and so subsequently, these vesicles are added to the tube at nucleus proximal positions. In (f″″) the absence of Wkd Rab35GAP, or if Rab35 is constitutively active, seamless tube growth will be polarized at the cell tip. If Rab35 dominant negative is expressed, or excess Wkd Rab35GAP is expressed, or induced by constitutively active Breathless-FGFR (f‴″), then transport of vesicles is blocked and addition to seamless tubes will occur proximal to the nucleus. Seamless tube will still be present distal to the soma since other vesicle transport pathways are able to promote tube growth in the absence of Rab35 activity. If Breathless-FGFR is constitutively activated but Whacked is absent from the cell (f″″″), then the ectopic seamless tube formation promoted by FGFR will be directed to the branch tips.
MOVIE S1. EB1:GFP dynamics in tracheal terminal cells. EB1:GFP comets can be seen moving in a bi-directional manner in regions adjacent to the terminal cell nucleus as well as more distal regions of terminal cell branches. Comets can also be seen extending from the apical membrane at a few branch positions more proximal to the cell nucleus. Near the soma, growth was towards branch tips at a 3:2 ratio (n= 50 comets scored), in medial positions growth was equally likely in either direction (~ 1:1, n = 41 comets scored), and near the end of the seamless tubes, there was a 2:1 distal bias (n = 28 comets scored). The first half of the movie extends over a 60 second interval and the second half of the movie extends over a 66 second interval and is being displayed at 10 frames per second.