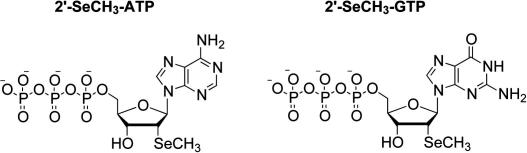
Graphical abstract
Keywords: Selenium, Nucleosides, Nucleoside modifications, RNA, Crystallography
Abstract
Modified nucleoside triphosphates (NTPs) represent powerful building blocks to generate nucleic acids with novel properties by enzymatic synthesis. We have recently demonstrated the access to 2′-SeCH3-uridine and 2′-SeCH3-cytidine derivatized RNAs for applications in RNA crystallography, using the corresponding nucleoside triphosphates and distinct mutants of T7 RNA polymerase. In the present note, we introduce the chemical synthesis of the novel 2′-methylseleno-2′-deoxyadenosine and -guanosine 5′-triphosphates (2′-SeCH3-ATP and 2′-SeCH3-GTP) that represent further candidates for the enzymatic RNA synthesis with engineered RNA polymerases.
1. Introduction
In recent years, selenium-modified RNA1,2 has been frequently used as a powerful derivative for multiple anomalous dispersion (MAD) phasing3,4 of X-ray crystallographic data. In particular, the ribose 2′-methylseleno (2′-SeCH3) modification has been thoroughly explored and was responsible for several important structure determinations of small RNAs (Fig. 1).5–7 Although 2′-SeCH3-RNA is readily available by chemical solid-phase synthesis, the concomitant limitation with respect to the size of the RNA represents a drawback.8 To overcome this limitation, we have recently demonstrated the efficient enzymatic synthesis of 2′-SeCH3-uridine and 2′-SeCH3-cytidine modified RNA using the corresponding pyrimidine nucleoside triphosphates and mutants of T7 RNA polymerase.9
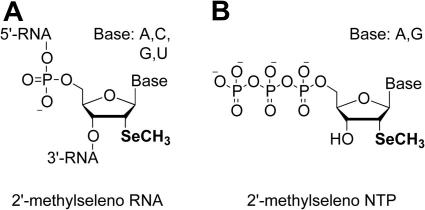
Figure 1.
Constitution of 2′-methylseleno derivatized RNA (A) and 2′-methylseleno ribonucleoside triphosphates of adenosine and guanosine (B).
In this short note, we introduce the synthesis of the novel 2′-methylseleno-2′-deoxyribonucleoside triphosphates of adenosine and guanosine (2′-SeCH3-ATP and 2′-SeCH3-GTP) that represent further potential candidates for enzymatic RNA synthesis with engineered RNA polymerases yet to be evolved or with known polymerase mutants under optimized conditions.10 Moreover, 2′-SeCH3-ATP and 2′-SeCH3-GTP are considered interesting analogues to study ATP- and GTP-dependent regulation processes and signal transduction pathways.
2. Results and discussion
Chemical synthesis is the method of choice for preparing large quantities of nucleoside triphosphates compared to the enzymatic preparation with nucleoside and nucleotide kinases,11 especially if modified nucleosides are targeted, as herein. The first chemical synthesis of NTPs was accomplished in 1949,12 and ever since, diverse chemical approaches have been developed to effectively synthesize nucleoside triphosphates.13–32 Due to the multiple functionalities of nucleosides, protection and deprotection of sugar hydroxyl and nucleobase amino groups has to be carefully considered in order to minimize the formation of by-products and regioisomers. Although challenging, one-pot procedures using minimally protected or completely unprotected nucleosides as substrates receive high attention and are a matter of continuous optimization.14–20,31,32 For the 5′-triphosphate synthesis of the 2′-SeCH3 modified purine nucleosides we have decided for such a strategy and focused on the minimally protected N6-acetyl-2′-methylseleno-2′-deoxyadenosine 1 and N2-acetyl-2′-methylseleno-2′-deoxyguanosine 3 (Scheme 1) as the appropriate precursors.
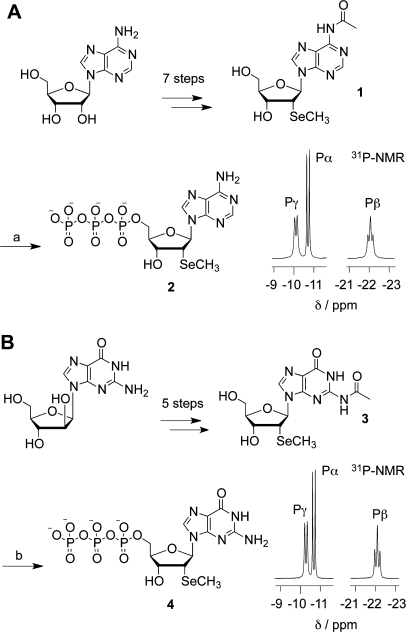
Scheme 1.
Synthesis and 31P NMR spectra of the modified nucleoside triphosphates 2 and 4. Reaction conditions: (a) (i): 1.2 equiv POCl3, 1.2 equiv 1,8-bis(dimethylamino)naphthaline in PO(OMe)3, 0 °C, 2 h; (ii): 5 equiv (HNBu3)2H2P2O7, NBu3 in DMF, 0 °C, 10 min; (iii): 0.2 M TEAB, 30 min; (iv): H2O/NH3, room temperature, 2.5 h; (b) (i): 1.2 equiv POCl3, 1.2 equiv 1,8-bis(dimethylamino)naphthaline in PO(OMe)3, −15 °C, 30 min; (ii): 5 equiv (HNBu3)2H2P2O7, NBu3 in DMF, −15 °C, 45 min; (iii): 0.2 M TEAB, 30 min; (iv): H2O/MeOH/NEt3, room temperature, 14 h.
Precursor compound 1 was prepared in seven steps according to our previously published route for the synthesis of the corresponding phosphoramidite building block for RNA solid-phase synthesis.33 Our route began with the simultaneous protection of the 3′- and 5′-hydroxyl groups of adenosine using 1,3-dichloro-1,1,3,3-tetraisopropyldisiloxane (TIPDSiCl2), followed by protection of the 2′-hydroxyl group as trimethylsilyl ether and reaction with acetyl chloride to furnish the N6-acetyl adenosine derivative. Then, the trimethylsilyl group was cleaved by p-toluenesulfonic acid. Triflation of the ribose 2′-OH gave the corresponding activated intermediate which was converted into the arabino nucleoside in diastereoselective manner by treatment with potassium trifluoroacetate and 18-crown-6-ether. After triflation of the arabinose 2′-OH, the activated derivative was reacted with sodium methyl selenide, producing the desired 2′-methylseleno key diastereomer in high yields. Deprotection of the TIPDS moiety proceeded straightforward using tetrabutylammonium fluoride (TBAF) and acetic acid and provided compound 1, the actual precursor for introducing the triphosphate group (Scheme 1A). Preparation of the 5′-triphosphate was performed according to Yoshikawa′s monophosphorylation using POCl3 with trimethylphosphate in the presence of 1,8-bis(dimethylamino)naphthalene (proton sponge).14 The resulting 2′-SeCH3 adenosine 5′-chlorophosphate intermediate was readily reacted with tris(tetra-n-butylammonium)hydrogenpyrophosphate along the lines of Ludwig and Ötvös.15,16 After ion-exchange chromatography, deprotection of the acetyl group and subsequent reversed-phase purification, the overall yield for the conversion of 1 into 5′-triphosphate 2′-SeCH3 adenosine 2 amounted to 36%.
The guanosine derivative 3 was prepared in seven steps according to our previously published route for the synthesis of 2′-SeCH3 guanosine phosphoramidite for RNA solid-phase synthesis.6 Simultaneous protection of the 3′- and 5′-hydroxyl groups of commercially available 9-[d-arabinofuranosyl]guanine using 1,3-dichloro-1,1,3,3-tetra-iso-propyldisiloxane (TIPDSiCl2) was followed by treatment with acetic anhydride to yield a mixture of N2,2′-O-diacetylated and N2,N2,2′-O-triacetylated nucleosides. After protection of the guanine at O6 with a (4-nitrophenyl)ethyl moiety under Mitsunobu conditions, mild basic hydrolysis liberated the arabinose 2′-hydroxyl group while the guanine N2 remained monoacetylated. Then, triflation of the 2′-OH primed diastereoselective introduction of the methylseleno group using sodium methylselenide. Subsequent deprotection of the TIPDS moiety and simultaneous release of the O6-(4-nitrophenyl)ethyl group proceeded straightforward using tetrabutylammonium fluoride (TBAF) and produced the desired precursor 3 in high yield (Scheme 1B). Preparation of the 5’-triphosphate was performed according to Yoshikawa′s procedure for monophosphorylation using POCl3 with trimethylphosphate in the presence of proton sponge.14 Subsequently, the 2′-SeCH3 guanosine 5′-chlorophosphate intermediate was reacted with tris(tetra-n-butylammonium)hydrogenpyrophosphate at low temperature along the lines of Gillerman29 and Howorka.34 Ion-exchange chromatography, deprotection of the acetyl group and reversed-phase purification, resulted in an overall yield 40% for the transformation of 3 into highly pure 5′-triphosphate 2′-SeCH3 guanosine 4. We mention that acetyl deprotection of the 2′-SeCH3 guanosine 5′-triphosphate in aqueous ammonia (as applied for deprotection of the 2′-SeCH3 adenosine 5′-triphosphate) was incomplete, according to analysis by reversed-phase-chromatography and NMR spectroscopy. However, cleavage of the acetyl group proceeded quantitatively when conditions were changed to triethylamine in methanol and water.
In summary, we have generated an efficient protocol for the access to 5′-triphosphates of 2′-SeCH3 adenosine and 2′-SeCH3 guanosine. These modified NTPs represent a solid foundation for our ongoing efforts to engineer the corresponding RNA polymerases by directed evolution based methods and computational protein design.
3. Experimental
3.1. General remarks
Chemical reagents and solvents were purchased from commercial suppliers (Sigma–Aldrich) and used without further purification. Organic solvents for reactions were dried overnight over freshly activated molecular sieves (4 Å). The reactions were carried out under argon atmosphere. Ion exchange chromatography was performed on a GE Healthcare Äktaprime system using a DEAE Sephadex A-25 (GE Healthcare) self-packed column (GE Healthcare, HR 16/10). The LC separation was monitored by ultraviolet (UV) detection at 280 nm. Triethylammonium bicarbonate (TEAB) gradients of 0.05–1.0 M were applied (flow rate: 2 mL/min). Prior to separation the column was equilibrated with 1 M TEAB buffer at room temperature for 1 h and subsequently rinsed with 0.05 M TEAB buffer. Reverse phase chromatography was performed on a GE Healthcare Äktaprime system using a commercial Merck Lobar 310-25 LiChroprep RP-18 (40–63 μm) column. The LC separation was monitored by ultraviolet (UV) detection at 280 nm. Solvent systems: A: triethylammonium acetate (TEAA) (50 mM, pH 8), B: CH3CN. A linear gradient of 0–40% B was applied, flow rate 5 mL/min. 1H, 13C, and 31P NMR spectra were recorded on Bruker 300 MHz and Bruker 600 MHz instruments. The chemical shifts (δ) are reported relative to tetramethylsilane (TMS) and referenced to the residual proton signal of the deuterated solvent D2O: 4.59 ppm for 1H NMR spectra; 31P chemical shifts are relative to external 85% phosphoric acid and were assigned based on comparison to the literature.13,27,32 1H and 13C assignments are based on COSY and HSQC experiments. MS experiments were performed on a 7 Tesla wide bore Fourier transform—ion cyclotron resonance (FT-ICR) mass spectrometer (Bruker Daltronics) with an electrospray ion source. Samples were analyzed in the negative-ion mode. The yields of the triphosphate products were determined by UV spectroscopy (IMPLEN Nanophotometer) using the following extinction coefficients: ε (ATP, 260 nm) 15300; ε (GTP, 260 nm) 11700.
3.2. 2′-Methylseleno-2′-deoxyadenosine 5′-triphosphate (2)
Compound 133 (74 mg, 0.19 mmol) was coevaporated with anhydrous pyridine (2 mL) three times and dried over P2O5 in a desiccator for four hours. Trimethylphosphate (1.5 mL) and 1,8-bis(dimethylamino)naphthalene (61 mg, 0.23 mmol) were added and the solution was cooled to 0 °C. Then, phosphoryl chloride (22 μL, 0.23 mmol) was added dropwise. After 2 h at 0 °C, a mixture of 0.5 M tris(tetrabutylammonium) hydrogen pyrophosphate in DMF (1.9 mL) and tributylamine (0.19 mL) was added and stirred for 10 min at room temperature. Then, 0.2 M TEAB buffer (15 ml, pH 7.5) was added, stirring continued for 30 min, and the mixture was evaporated to dryness. The residue was dissolved in a minimal volume of water and purified using ion-exchange-chromatography. The product fraction was collected and evaporated, then dissolved in H2O (7 mL) and saturated NH4OH solution (20 ml) and stirred for two and a half hours at room temperature. Finally, compound 2 was purified using reversed-phase-chromatography and isolated as triethylammonium salt (by lyophilization from TEAA containing product fractions). Yield: 68.4 μmol as a white foam (36%). 1H NMR (600 MHz, D2O): δ 1.93 (s, 3H, SeCH3); 3.82 (dd, J = 5.5 Hz, J = 9.2 Hz, 1H, H–C(2′)) 4.27 (m, 2H; H1-C(5′), H2-C(5′)); 4.43 (m, 1H, H–C(4′)); 4.74 (m, 1H, H–C(3′)); 6.37 (d, J = 9.3 Hz, 1H, H–C(1′)); 8.27 (s, 1H, H–C(8)); 8.57 (s, 1H, H–C(2)) ppm. 13C NMR (150 MHz, D2O): δ 3.03 (SeCH3); 8.24 (NCH2CH3); 23.23 (CH3COO); 46.66 (NCH2CH3); 47.28 (C(2′)); 65.76 (C(5′)); 73.04 (C(3′)); 85.79 (C(4′)); 88.95 (C(1′)); 140.16 (C(2); 149.12; 153.04 (C(8)); 155.68; 181.20; ppm. 31P NMR (121 MHz, D2O): δ −10.01 (d, J = 19.6 Hz, 1P, Pγ); −10.79 (d, J = 19.9 Hz, 1P, Pα); −22.62 (triplettoid, J = 19.7 Hz, J = 19.9 Hz, 1P, Pβ) ppm. ESI-HRMS (m/z): [M−H]− calcd for C11H17N5O12P3Se, 583.92581; found 583.92581.
3.3. 2′-Methylseleno-2′-deoxyguanosine 5′-triphosphate (4)
Compound 36 (71 mg, 0.18 mmol) was coevaporated with anhydrous pyridine (2 mL) three times and dried over P2O5 in a desiccator for 4 h. Trimethylphosphate (1.5 mL) and 1,8-bis(dimeth-ylamino)naphthalene (57 mg, 0.26 mmol) were added and the solution was cooled to −15 °C. Then, phosphoryl chloride (24 μL, 0.25 mmol) was added dropwise. After 30 min at −15 °C, a mixture of 0.5 M tris(tetrabutylammonium)hydrogen pyrophosphate in DMF (1.6 mL) and tributylamine (0.13 mL) was added and stirred for 45 min at room temperature. Then, 0.2 M TEAB buffer (16 ml, pH 7.5) was added at room temperature, stirring was continued for 30 min, and the mixture was evaporated to dryness. The residue was dissolved in a minimal volume of water and purified using ion-exchange-chromatography. The product fraction was collected and evaporated and the residue was dissolved in H2O/MeOH/NEt3 (3 mL; 7/3/1) and stirred 14 h at room temperature. Compound 4 was finally purified using reversed-phase-chromatography and isolated as triethylammonium salt (by lyophilization from TEAA containing product fractions). Yield: 71 μmol as a white foam (40%). 1H NMR (500 MHz, D2O): δ 1.69 (s, 3H, SeCH3); 4.06 (dd, J = 4.5 Hz, J = 7.9 Hz, 1H, H–C(2′)); 4.15 (m, 2H; H1-C(5′), H2-C(5′)); 4.18 (m, 1H, H–C(4′)); 4.60 (m, 1H, H–C(3′)); 6.08 (d, J = 9.3 Hz, 1H, H–C(1′)); 8.06 (s, 1H, H–C(2)) ppm. 13C NMR (150 MHz, D2O): δ 2.96 (SeCH3); 8.27 (NCH2CH3); 23.11 (CH3COO); 46.70 (NCH2CH3); 45.97 (C(2′)); 65.71 (C(5′)); 73.03 (C(3′)); 85.62 (C(4′)); 89.35 (C(1′)); 116.30; 138.10 (C(2); 151.90; 154.02; 158.95; 181.01 ppm. 31P NMR (121 MHz, D2O): δ −10.21 (d, J = 19.8 Hz, 1P, Pγ); −10.71 (d, J = 20.1 Hz, 1P, Pα); −22.54 (triplettoid, J = 19.9 Hz, J = 19.9 Hz, 1P, Pβ) ppm. ESI-HRMS (m/z): [M−H]− calcd for C10H16N2O14P3Se, 599.92073; found 599.92043.
Acknowledgments
Funding by the Austrian Science Fund FWF (I317) and the DFG is gratefully acknowledged. We thank Dr. K. Breuker for FT-ICR mass measurements and Dr. C. Kreutz for NMR support.
Footnotes
Supplementary data (1H and 13C NMR spectra for compounds 2 and 4) associated with this article can be found, in the online version, at doi:10.1016/j.bmc.2012.01.044.
Supplementary data
Spectral data
References and notes
- 1.Lin L., Sheng J., Huang Z. Chem. Soc. Rev. 2011;40:4591. doi: 10.1039/c1cs15020k. [DOI] [PubMed] [Google Scholar]
- 2.Du Q., Carrasco N., Teplova M., Wilds C.J., Egli M., Huang Z. J. Am. Chem. Soc. 2002;124:24. doi: 10.1021/ja0171097. [DOI] [PubMed] [Google Scholar]
- 3.Su L., Chen L., Egli M., Berger J.M., Rich A. Nat. Struct. Biol. 1999;6:285. doi: 10.1038/6722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wild K., Weichenrieder O., Leonard G.A., Cusack S. Structure. 1999;7:1345. doi: 10.1016/s0969-2126(00)80024-6. [DOI] [PubMed] [Google Scholar]
- 5.Serganov A., Keiper S., Malinina L., Tereshko V., Skripkin E., Höbartner C., Polonskaia A., Phan A.T., Wombacher R., Micura R., Dauter Z., Jäschke A., Patel D.J. Nat. Struct. Mol. Biol. 2005;12:218. doi: 10.1038/nsmb906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moroder H., Kreutz C., Lang K., Serganov A., Micura R. J. Am. Chem. Soc. 2006;128:9909. doi: 10.1021/ja0621400. [DOI] [PubMed] [Google Scholar]
- 7.Freisz S., Lang K., Micura R., Dumas P., Ennifar E. Angew. Chem., Int. Ed. 2008;47:4110. doi: 10.1002/anie.200800726. [DOI] [PubMed] [Google Scholar]
- 8.Höbartner C., Rieder R., Kreutz C., Puffer B., Lang K., Polonskaia A., Serganov A., Micura R. J. Am. Chem. Soc. 2005;127:12035. doi: 10.1021/ja051694k. [DOI] [PubMed] [Google Scholar]
- 9.Siegmund V., Santner T., Micura R., Marx A. Chem. Sci. 2011;2:2224. [Google Scholar]
- 10.Burmeister P.E., Lewis S.D., Silva R.F., Preiss J.R., Horwitz L.R., Pendergrast P.S., McCauley T.G., Kurz J.C., Epstein D.M., Wilson C., Keefe A.D. Chem. Biol. 2005;12:25. doi: 10.1016/j.chembiol.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 11.Schultheisz H.L., Szymczyna B.R., Scott L.G., Williamson J.R. J. Am. Chem. Soc. 2010;133:297. doi: 10.1021/ja1059685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baddiley J., Michelson A.M., Todd A.R. J. Chem. Soc. London. 1949;7:582. [Google Scholar]
- 13.Burgess K., Cook D. Chem. Rev. 2000;100:2047. doi: 10.1021/cr990045m. [DOI] [PubMed] [Google Scholar]
- 14.Yoshikawa M., Kato T., Takenishi T. Tetrahedron Lett. 1967;50:5065. doi: 10.1016/s0040-4039(01)89915-9. [DOI] [PubMed] [Google Scholar]
- 15.Ludwig J. Acta Biochim. Biophys. Acad. Sci. Hung. 1981;16:131. [PubMed] [Google Scholar]
- 16.Kovacs T., Ötvös L. Tetrahedron Lett. 1988;29:4525. [Google Scholar]
- 17.Ludwig J., Eckstein F. J. Org. Chem. 1989;54:631. [Google Scholar]
- 18.Ludwig J., Eckstein F. J. Org. Chem. 1991;56:1777. [Google Scholar]
- 19.He K., Hasan A., Krzyzanowska B., Shaw B.R. J. Org. Chem. 1998;63:5769. doi: 10.1021/jo972002g. [DOI] [PubMed] [Google Scholar]
- 20.He K., Porter K.W., Hasan A., Briley J.D., Shaw B.R. Nucleic Acids Res. 1999;27:1788. doi: 10.1093/nar/27.8.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lebedev A.V., Koukhareva L.L., Beck T., Vaghefi M.M. Nucleosides, Nucleotides, Nucleic Acids. 2001;20:1403. doi: 10.1081/NCN-100002565. [DOI] [PubMed] [Google Scholar]
- 22.Wu W., Bergstrom D.E., Davisson V.J. J. Org. Chem. 2003;68:3860. doi: 10.1021/jo020745i. [DOI] [PubMed] [Google Scholar]
- 23.Wu W., Freel Meyers C.L., Borch R.F. Org. Lett. 2004;6:2257. doi: 10.1021/ol049267j. [DOI] [PubMed] [Google Scholar]
- 24.Koukhareva I., Lebedev A., Vaghefi M. CRC Press; 2005. p. 39. (Chemistry Biotechnology and Biological Applications). [Google Scholar]
- 25.Horhota A.T., Szostak J.W., McLaughlin L.W. Org. Lett. 2006;8:5345. doi: 10.1021/ol062232u. [DOI] [PubMed] [Google Scholar]
- 26.Sun Q., Edathil J.P., Wu R., Smidansky E.D., Cameron C.E., Peterson B.R. Org. Lett. 2008;10:1703. doi: 10.1021/ol8003029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warnecke S., Meier C. J. Org. Chem. 2009;74:3024. doi: 10.1021/jo802348h. [DOI] [PubMed] [Google Scholar]
- 28.Zlatev I., Lavergne T., Debart F., Vasseur J.J., Manoharan M., Morvan F. Org. Lett. 2010;12:2190. doi: 10.1021/ol1004214. [DOI] [PubMed] [Google Scholar]
- 29.Gillerman I., Fischer B. Nucleosides, Nucleotides, Nucleic Acids. 2010;29:245. doi: 10.1080/15257771003709569. [DOI] [PubMed] [Google Scholar]
- 30.Jansen R.S., Rosing H., Schellensand J.H., Beijnen J.H. Fundam. Clin. Pharmacol. 2011;25:172. doi: 10.1111/j.1472-8206.2010.00823.x. [DOI] [PubMed] [Google Scholar]
- 31.Caton-Williams J., Lin L., Smith M., Huang Z. Chem. Commun. 2011;47:8142. doi: 10.1039/c1cc12201k. [DOI] [PubMed] [Google Scholar]
- 32.Caton-Williams J., Smith M., Carrasco N., Huang Z. Org. Lett. 2011;13:4156. doi: 10.1021/ol201073e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Puffer B., Moroder H., Aigner M., Micura R. Nucleic Acids Res. 2008;36:970. doi: 10.1093/nar/gkm880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borsenberger V., Kukwikila M., Howorka S. Org. Biomol. Chem. 2009;7:3826. doi: 10.1039/b906956a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Spectral data