Synopsis
The IGF axis is a tightly controlled endocrine system that regulates cell growth and development, known to have an important function in cancer biology. IGF1 and IGF2 can promote cancer growth in a GH-independent manner both through paracrine and autocrine secretion and can also confer resistance to chemotherapy and radiation. Many alterations of this system have been found in neoplasias, including increased expression of ligands and receptors, loss of heterozigosity of the IGF2 locus and increased IGF1R gene copy number. The IGF1 network is an attractive candidate for targeted therapy, including receptor blockade with monoclonal antibodies and small molecule inhibitors of receptor downstream signaling. This article reviews the role of the IGF axis in the initiation and progression of cancer, and describes the recent advances in IGF inhibition as a therapeutic tool.
Keywords: IGF1R, IGF1, IGF2, insulin, kinase, cancer
Background
Insulin growth factors (IGF) were initially described as humoral mediators of growth hormone (GH) action[1]. This signaling network is involved in regulation of human development, energy balance and cell growth[2,3]. In addition to its well-characterized role in glucose, protein and lipid metabolism[4], the complex nature of this hormonal axis continues to attract the attention of scientists and physicians given the growing body of evidence that supports its critical role in cancer biology[2,5]. Unlike other trophic factors involved in neoplastic development, insulin growth factors exert endocrine, paracrine and autocrine effects. The components of this cellular network are summarized in Table 1.
Table 1.
Components of the IGF axis and relevant features.
| Insulin Growth Factor 1 (IGF1) | Binds IGF1R and hybrid receptors |
| Insulin Growth Factor 2 (IGF2) | Binds IGF1R, IGF2R, IR-A and hybrid receptors |
| Insulin | Binds IR-A, IR-B and IGF1R |
| Insulin Growth Factor Receptor 1 (IGF1R) | Binds IGF1 and IGF2 with high affinity, and insulin with low affinity |
| Insulin Growth Factor 2/Mannose-6-phosphate Receptor (IGF2/M6P-R or IGF2R) | Binds IGF2 Lacks intracellular signaling |
| Insulin Receptor A (IR-A) | Lacks exon 11 (splice variant) Binds insulin and IGF2 with high affinity |
| Insulin Receptor B (IR-B) | Contains exon 11 (splice variant) Binds insulin with high affinity |
| IGF1R/IR-A | Hybrid Receptor |
| IGF1R/IR-B | Hybrid Receptor |
| IGF binding protein 1–6 (IGFBP1-6) | Bind IGF1 and IGF2, forming tertiary complexes with IGFALS and prolonging growth factors half-life. |
| IGF binding protein 7–8 (IGFBP7-8) | Bind IGF1 with low affinity |
| Acid-labile subunit (IGFALS) | 85 KD protein, member of leucin-rich repeats superfamily. Prolongs half-life of IGFBP3/5 and IGF1 binary complexes. |
In humans, both the insulin and the IGF receptors are composed of two globular extracellular α-subunits that are each linked to a β-subunit and to each other by disulfide bonds. The tyrosine kinase activity resides in the β-subunit [6]. There are also “hybrid” receptors composed of half an insulin receptor and half an IGF receptor (IRαβ linked to IGF1Rαβ) (Fig. 1) [7,8]. Binding of IGF2 to the IGF2/Mannose-6-phosphate receptor triggers internalization and degradation of the ligand, without eliciting downstream signaling. Thus, the IGF2/M6P-R is considered a “tumor suppressor”[9,10], since it functions as a scavenger that reduces IGF2 availability to bind IGF1R.[11,12,13].
Figure 1. Components of the IGF axis.
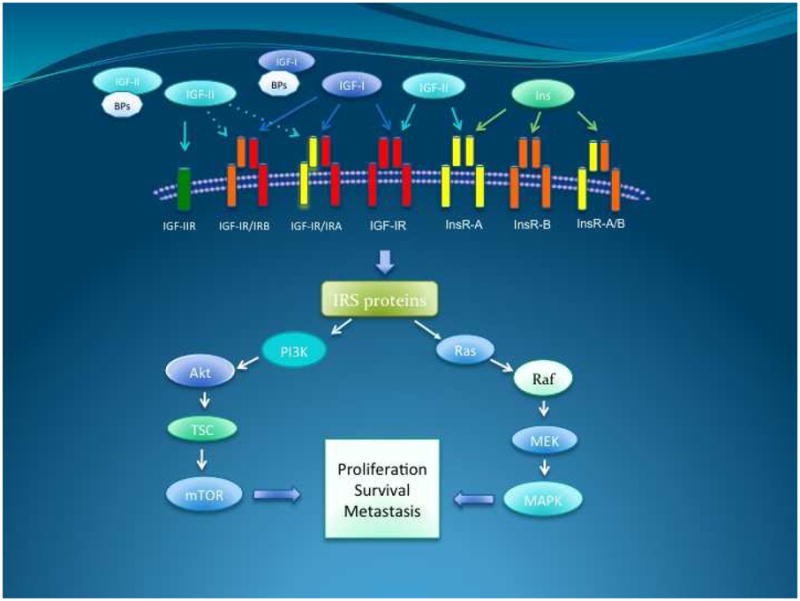
The IGF axis is composed by two IGF ligands, IGF-I and IGF-II. They bind one of the different IGF binding proteins, (BPs). The majority of circulating IGF is bound to IGFBP-3 and a molecule called acid labile subunit. The different combinations of receptors in the axis bind to IGF-I, IGF-II and insulin with different affinities, as represented by the large or small size of the ligand.
Binding of IGF1 to the alpha subunit of IGF1R triggers a conformational change that causes activation of the catalytic domain in the intracellular beta subunit causing tyrosine autophosphorylation and transphosphorylation (Y1131, Y1135, and Y1136) that enhances its tyrosine kinase activity [14]. These events lead to recruitment of adaptor proteins such as IRS, CRK and SHC. Downstream signaling is mostly channeled through the MAPK/Ras-Raf-Erk pathway, the phosphatidylinositol-3-kinase/AKT/mTOR (PI3K/AKT) pathway and the Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway. Ultimately, activation of IGF1R results in increased cell proliferation and decreased apoptosis[5,15,16,17].
The central regulation of the IGF system resides in the hypothalamus. Various stimuli lead to growth-hormone releasing hormone (GHRH) or somatostatin secretion[18,19]. These peptides regulate the secretion of GH by the anterior pituitary gland. Binding of GH to its receptor in the liver results in stimulation of IGF1 synthesis and secretion. In addition, insulin can indirectly increase IGF1 production in part by up regulating GH receptors [3,20,21]. Cancer cells, however can secrete significant quantities of IGF1 in a paracrine or autocrine manner, independently of GH regulation[22]. Thus, the IGF1 axis signals in a hormone-dependent manner to regulate physiological growth, and in a hormone-independent fashion in the context of neoplasia.
Individuals with supra-physiologic levels of GH (acromegaly) appear to have an increased risk for cancer development, possibly via an IGF1-mediated mechanism[3,23]. Conversely, patients with Laron syndrome (IGF1 deficiency and insensitivity to GH) seem have a lower cancer risk than their relatives[24,25]. The influence of circulating IGF1 has been documented in mouse models of tumor development [26]. However, there is a clear distinction between murine and human IGF axis. While mice present high IGF1 and almost undetectable IGF2 levels in the postnatal life[19,27], postnatal human IGF1 remains at low concentrations. In addition, human IGF2 is not only highly secreted but also functionally relevant beyond birth.
Although IGF2 is also secreted in the liver, this process is not regulated by GH. Notably, the IGF2 gene is imprinted. In most tissues, only the paternal allele is expressed. When imprinting of the maternal allele is lost, IGF2 expression is increased. In the case of Beckwith-Widemann syndrome, loss of imprinting results in marked IGF2 overexpression resulting in fetal and neonatal overgrowth. Many tumor types harbor bi-allelic expression of IGF2 via this mechanism, including Wilms tumor, esophageal carcinoma, Ewing’s sarcoma, rhabdomyosarcoma, colorectal carcinoma, osteosarcoma, prostate adenocarcinoma, hepatocellular carcinoma and adrenocortical carcinoma [11,19,28,29,30,31,32,33,34,35,36].
The bioavailability of IGF1 and IGF2 is highly influenced by their binding to insulin growth factor binding proteins (IGFBP) and the acid-labile subunit (IGFALS). Several factors can increase IGFBP synthesis, including estrogens[37], retinoids[38], and vitamin D[39]. In addition, antiproliferative pathways such as TGFβ[38] and p53[40] also increase IGBP concentration. However, there is also some evidence that shows that certain IGFBPs could enhance delivery of these growth factors to the tumor microenvironment [2,41]. Hyperinsulinism indirectly increases hepatic IGF1 secretion and increases its availability by decreasing IGFBP levels[3,42,43].
Oncogenic transcription factors can also regulate IGFBP expression. Notably, EWS-FLI1, an aberrant fusion protein responsible for malignant transformation in Ewing’s Sarcoma Family of Tumors, binds the IGFBP-3 promoter and decreases its expression[44]. Another example is WT1, a tumor suppressor gene that plays a significant role in the development of Wilms Tumor, a pediatric kidney neoplasm. WT1 represses IGF1R expression, thus regulating the activity of the IGF pathway in Wilms tumor [45].
Insulin Growth Factor Axis in Cancer
Epidemiological evidence suggests that obesity and type II diabetes, both clinical conditions where hyperinsulinism is present, are associated with increased cancer risk[3,46,47]. At least for some tumor subtypes, the same holds true if elevated IGF1 is detected[48,49,50,51]. Correlation between IGF1R expression and worse outcome has been suggested in different tumor contexts, including colorectal carcinoma, breast cancer and melanoma[52,53,54]
IGF1R is highly expressed and biologically active in many neoplastic processes. However, gene amplifications were found relatively recently[55,56]. Increased IGF1R copy number was found in small cell lung cancer[57]. In addition, a high level gain of IGF1R at 15q26 was recently described in pediatric high-grade gliomas[58], a disease where IGF2 signaling has a growth-promoting role[59]. Recent studies also suggest elevated IR-A expression in cancer cells[60,61].
IGF1R is often necessary for oncogene-mediated malignant transformation [62,63,64]. In Ewing’s Sarcoma, IGF1R activity is necessary for transformation of fibroblasts with the EWS/FLI1 fusion transcript, as described in 1997. More recently, silencing of the fusion protein revealed that several microRNAs that negatively regulate expression of proteins in the IGF axis are silenced by EWS/FLI1[65].
A remarkable aspect of IGF1R biology is its association with resistance to cytotoxic chemotherapy, radiation therapy and targeted agents[66]. Resistance to cytotoxic agents is documented across tumor types. One of the mechanisms by which this occurs is decreased intracellular concentration of the agent via activation of ATP-dependent efflux pumps such as P-glycoprotein, encoded by the multidrug resistance (MDR) gene. In gastric carcinoma patients, IGF1R was found to correlate with overexpression of MDR-associated protein 1(MRP-1) and with poor clinical prognosis[67].
Many preclinical studies have shown sensitization of cancer cell lines to chemotherapy agents by silencing or blocking IGF1R. Although there is some suggestion about AKT activation playing a role in this process, many of the mechanisms involved are still under investigation. In breast cancer cells, IGF1R blockade improved the response to taxol and doxorubicin[68]. Similar results were obtained with NSCLC cells treated with etoposide and carboplatin[69], with atypical teratoid rhabdoid tumor (ATRT) cells treated with doxorubicin and cisplatin[70], and with gastric cancer cells treated with 5-FU[71]. Other examples include osteosarcoma[72,73], colorectal carcinoma[74], mesothelioma[75], and esophageal carcinoma[76]. Interestingly, a recent study showed increased sensitivity of glioblastoma cell lines to BCNU and cisplatin when the tumor suppressor gene WT1 was silenced[77]. As described before, WT1 negatively regulates IGF1R expression. In hepatocellular carcinoma, IGF-1 reduces the cell sensitivity to doxorubicin by changing the redox potential via up regulation of gluthatione transferase expression[78]. Silencing of the IGF1R gene enhances sensitivity to DNA-damaging agents such as mitoxantrone, etoposide, and nitrogen mustard in prostate cancer cells[79]. An attractive hypothesis to explain this observation considers IGF signaling as a modulator of XRCC5, a DNA double-strand break repair protein involved in non-homologous end joining recombination[80].
Given its link to DNA repair pathway, it is not surprising that IGF1R inhibition can enhance the effect of radiation therapy. IGF1R knockdown results in decreased ATM expression[81], while defective ATM kinase activity is associated with reduced expression of IGF1R. Both conditions lead to increased radiosensitivity[82,83]. These findings are consistent with clinical data in breast cancer, where a higher rate of tumor recurrence after surgery and radiation was seen in patients whose primary tumors showed high IGF1R expression[54].
There are examples of cooperation between hormonal therapy and IGF1 axis inhibition. Anti-estrogens, such as tamoxifen, are widely used for management of estrogen receptor positive breast cancer. Unfortunately, 40 % patients do not respond to this treatment. Several factors play a role in tamoxifen resistance. IGF1R is known to cross talk with the estrogen receptor pathway and could be responsible for the resistant phenotype. Interestingly, tamoxifen-resistant breast cancer cell lines have been shown to have an increased expression of IGF2, which is able to trigger growth and survival signaling via IGF1R[66,84,85]. Moreover, IGF1R inhibitors have been able to inhibit growth of tamoxifen-resistant breast cancer cell lines in vitro [86,87].
IGF1R inhibition can be combined with other molecularly targeted agents. Some cancer types co-express IGF1R and epidermal growth factor receptor (EGFR)[88]. EGFR family members mediate proliferation, differentiation and survival in malignant cells. Forty to eighty percent of non-small cell lung cancers (NSCLC) have EGFR overexpression[89], and 30% of breast cancers overexpress HER-2 [90,91]. EGFR inhibitors such as erlotinib and gefitinib have been successfully developed, but unfortunately resistance to therapy often follows initial response. EGFR/IGFR heterodimers that activate the IGF1R signaling pathway have been found after treating NSCLC cell lines with gefitinib [92,93]. In addition, IGF1R silencing markedly increased apoptosis of gefitinib-treated cell lines[93]. IGF1R has also been found to be a factor in breast cancer resistance to trastuzumab[94], and there is evidence to suggest that HER-2 phosphorylation is influenced by IGF1R signaling[95].
Targeting Insulin Growth Factor Receptor 1
GH antagonists
Pegvisomant is a genetically engineered GH receptor antagonist used in the treatment of acromegaly[96]. Although there is preclinical evidence of some antitumor activity, its clinical use as an antineoplastic agent has been limited[97,98]. Somatostatin, the physiologic antagonist of GH, has also been proposed as an anti cancer agent in the past[99]
Ligand antagonists
IGFPB3 naturally binds the ligands of the IGF axis and decreases their bioavailability in the circulation. Recombinant IGFBP3 has been proposed as a way to decrease IGF1R signaling, and it showed activity in preclinical models[100,101].
MEDI-573 is a human neutralizing IGF1/IGF2 monoclonal antibody that inhibits binding of the growth factors to IGF1R and IR-A. Interestingly, it appears to inhibit IGF1R signaling with virtually no effect in insulin activation of IR-A. Preclinical data shows inhibition of tumor growth in vivo using xenografts of high-expressing IGF1R/IR-A cells [102].
Receptor antagonists
Several neutralizing antibodies against the IGF1R receptor have been extensively studied, and they continue to be evaluated in many clinical trials. A list of the different currently available agents is shown in Table 2. There was a significant concern about hyperglycemia, since blockade of IGF1R causes a compensatory increase in the levels of GH, which can induce insulin resistance and stimulation of gluconeogenesis[2]. Fortunately however, hyperglycemia has not been found to be a significant problem in clinical trials using IGF1R blocking antibodies. Available antibodies are either of IgG1 or IgG2 isotype. Isotype differences in terms of side effects given different capacity to bind Fc gamma receptors has not been clearly established yet[103].
Table 2.
Monoclonal antibodies against IGF1R.
| Agent | Ongoing Trials* | Toxicities | ||
|---|---|---|---|---|
|
AMG 479 Amgen, Thousand Oaks, CA |
ganitumab | Fully human monoclonal IgG1 | EWS, DSRCT, Ovarian Carcinoma, CRC, NSCLC Pancreatic carcinoma (Phase III) |
Thrombocytopenia Hyperglycemia Neutralizing antibodies |
|
RG1507 Roche, Basel, Switzerland |
Fully human monoclonal IgG1 | Development discontinued | Hyperglycemia Lymphopenia CVA |
|
|
IMC-A12 ImClone, New York, NY |
cixutumumab | Fully human monoclonal IgG1 | ACC, thymic carcinoma, SCLC, soft tissue sarcomas, osteosarcoma, EWS, HCC, breast cancer, head and neck carcinoma, prostate cancer, hepatocellular carcinoma, islet cell cancer, pancreatic cancer | Hyperglycemia Anemia Infusion reaction |
|
MK-0646 Merck, Whitehouse Station, NJ |
dalotuzumab | Humanized mouse monoclonal IgG1 | NSCLC, SCLC, CRC, Pancreatic carcinoma, breast cancer, neuroendocrine tumors | Thrombocytopenia GI bleeding Pneumonitis Increased transaminases |
|
CP-751871 Pfizer, New york, NY |
figitumumab | Fully human monoclonal IgG2 | CRC, NSCLC, SCLC, breast cancer Phase III (lung cancer) terminated due to lack of benefit |
Hyperglycemia Anemia Cholestasis Hyperuricemia |
|
SCH717454 Schering-Plough, Kenilworth, NJ |
robatumumab | Fully human monoclonal IgG1 | CRC, EWS, osteosarcoma | |
|
AVE1642 Sanofi-Aventis, Paris, France |
Humanized mouse monoclonal IgG1 | Breast cancer, multiple myeloma, hepatocellular carcinoma | Hyperglycemia Hypersensitivity |
Abbreviations: EWS, Ewing’s Sarcoma; DSRCT, desmoplastic small round cell tumor ; CRC, colorectal carcinoma; NSCLC, non-small cell lung cancer; SCLC, small cell lung cancer; ACC, adrenocortical carcinoma; HCC, hepatocellular carcinoma; CVA, cerebral vascular accident.
Clinicaltrials.gov
The IGF1 axis has clear biological implications in Ewing’s Sarcoma, and it is not surprising that promising responses have been documented this group of patients. Durable responses have been achieved in patients with this disease treated with RG1507[19]. In a phase I trial of RG1507 in patients with advanced solid tumors the drug was well tolerated. Two patients with Ewing’s Sarcoma had confirmed partial responses and thirteen patients (two of them with Ewing’s sarcoma) achieved stable disease[104]. In a recent multi-center phase II study of RG1507 in 115 patients with refractory Ewing’s Sarcoma family of tumors, the overall response rate was ten percent (one complete response and ten partial responses), with a median duration of twenty-nine months. In addition, eight patients had unconfirmed partial responses [in press]. Although the response was overall modest, it is quite possible that it reflects the need to find an accurate predictive biomarker to determine who are the patients who are likely to respond to IGF1R blockade. A phase I trial with a different IGF1R blocking antibody, AMG 479, documented a confirmed complete response and a partial response in two patients with Ewing’s Sarcoma[105]. Similar results were achieved in a phase I trial of figitumumab in patients with sarcomas[106].
Another tumor where blocking IGF1R might be a reasonable strategy is gastrointestinal stromal tumor (GIST)[107,108,109,110]. Both wild-type and KIT-mutant GIST present high expression of IGF1R and preclinical activity against these tumors has been promising. Clinical trials to further evaluate this hypothesis are in progress.
A randomized phase III trial of figitumumab in combination with paclitaxel and carboplatin versus paclitaxel and carboplatin in patients with non-small cell lung cancer (NSCLC) was permanently suspended at 681 patients since the futility analysis favored the paclitaxel-carboplatin arm[111]; despite promising previous phase II trials[112]. The study showed that figitumumab does not improve overall survival and increases the risk for severe toxicities; even for patients with non-adenocarcinoma histology. However, patients with circulating levels of IGF-1 of greater than 1 ng/mL showed improved overall survival; which stresses again the need of appropriate predictive pharmacodynamic biomarkers in targeted-therapy trials.
As mentioned earlier, IGF1R plays a role in resistance to chemotherapy and radiation therapy. Taking advantage of IGF1R inhibition while treating patients with cytotoxic agents and/or radiation therapy is being actively explored. In breast cancer models, there is evidence to support combination of IGF1R inhibitors and EGFR inhibitors given the cross-talk that these two relevant pathways have[94,113,114]. Moreover, adding downstream signaling inhibition to the receptor blockade to potentiate antitumor effect is a strategy that is being used in several trials in solid tumors. mTOR inhibitors such as rapamycin are an interesting choice, since the PI3K/AKT/mTOR pathway is regulated by receptor tyrosine kinases such as IGF1R. In addition, mTOR inhibition induces AKT activation via an IGF dependent mechanism, supporting the rationale for this combination[115]. Initial results are encouraging, and several trials are under development[116].
Small molecule inhibitors
Tyrosine kinase inhibitors targeting IGF1R have been recently developed. A list of these small molecule inhibitors is depicted in table 3. Most of them target IR in addition to IGF1R. Even though this could bring opportunity for occurrence of metabolic side effects, it is also a chance to improve therapeutic efficacy given the cross talk between IGF1R and IR[2,3,15]. With one notable exception, these drugs are in early phases of development. Linsitinib efficacy in adrenocortical carcinoma, a disease with high dependence on IGF signaling, is being evaluated in Phase III. These drugs can become an attractive therapeutic tool given a potentially tolerable toxicity profile, possibility of oral administration and the theoretical advantage of “multi-target” inhibition. However, much has to be learned about these agents and the tumor context where their efficacy can be maximized.
Table 3.
Small molec ule inhibitors targeting IGF1R.
| Agent | Specificity | Ongoing trials* | Toxicities | |
|---|---|---|---|---|
|
XL228 Exelixis, South San Francisco, CA |
IGF1R SFK, BCR-ABL FGFR1–3, AURK A, AURK B |
Phase I (advanced malignancies/CML) | Hyperglycemia Anorexia Fatigue |
|
|
OSI906 (Linsitinib) Astellas, Deerfield, IL |
Reversible ATP- competitive inhibitor | IGF1R/IR | NSCLC, ovarian cancer, breast cancer, head and neck carcinoma, HCC, SCLC (Phase II) ACC (Phase III) |
Increased GH and IGF1R concentrations Hyperinsulinemia Hyperglycemia QTc prolongation Nausea |
|
BIIB022 Biogen, Cambridge, MA |
Phase I Phase Ib (HCC, NSCLC) |
Headache Fatigue Nausea QTc prolongation Hypertension GI bleed Glucose level fluctuation |
||
|
AXL 1717 (picropodophylin) Axelar, Stockholm, Sweden |
Non-ATP competitive autophosphorylation inhibitor at Y1136 | High IGF1R selectivity (no IR inhibition) | Phase I | |
|
BMS-754807 Bristol-Myers, New York, NY |
Reversible, ATP- competitive inhibitor | IGF1R/IR MET, RON, TrkA, TrkB, AURK A, AURK B |
Phase I/II (solid tumors) Breast cancer (phase II) |
Hypoglycemia Hyperglycemia Fatigue |
|
INSM18 (Nordihydroguaiaretic acid) Insmed, Monmouth Junction, NJ |
IGF1R/HER-2 5-α reductase 15-lipoxygenase PDGFR | Phase II: prostate cancer |
Abbreviations: NSCLC, non-small cell lung cancer; SCLC, small cell lung cancer; ACC, adrenocortical carcinoma; HCC, hepatocellular carcinoma; CML, chronic myelogenous leukemia.
Clinicaltrials.gov
Metformin
Although metformin is not a new targeted agent, its mechanism of action is closely related to the IGF axis. Initially used as an antidiabetic drug that reduces both glucose and insulin plasma concentrations, the role of this agent in cancer therapy is an exciting area of current research that is beyond the limits of this review and has been described elsewhere[117]. Patients treated with metformin appear to have a lower incidence of cancer that untreated controls in epidemiological studies[118,119,120]. This biguanide affects tumor growth by reducing insulin secretion and by inhibiting AMPK-LKB1. Modulation of the IGF axis with combination of drugs including metformin is promising, and currently under intense investigation.
Challenges and Future Directions
Almost thirty years after the IGF1 precursor[121] was sequenced, we are witnessing major advances in the understanding IGF1 biology and the development of new and more sophisticated anti-cancer drugs exploiting this pathway. One of the greatest challenges for the future is the identification of predictive biomarkers to assist in patient selection. IGF1 levels, IGFBP3 levels and novel metabolic imaging techniques are just a few examples of the tools being used at the moment [122,123].
As these agents are being administered to a higher number of patients, it is likely that unforeseen toxicities will become apparent. For example, there is the potential risk of CNS toxicity with prolonged exposure to IGF1R blocking agents, since IGF1 in known to provide a neuroprotective effect in the brain [124].
Determining the most effective ways to combine IGF1R targeted therapy with either cytotoxic chemotherapy or other targeted agents remains a challenge. Finally, as with other newly developed targeted therapies, mechanisms of acquired resistance to IGF1R blockade will be seen, and understanding these mechanisms will be a key for future development of these agents.
Footnotes
Disclosures: “The authors have nothing to disclose”.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Daughaday WH, Hall K, Raben MS, Salmon WD, Jr, van den Brande JL, et al. Somatomedin: proposed designation for sulphation factor. Nature. 1972;235:107. doi: 10.1038/235107a0. [DOI] [PubMed] [Google Scholar]
- 2.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915–928. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 3.Gallagher EJ, LeRoith D. Minireview: IGF, Insulin, and Cancer. Endocrinology. 2011;152:2546–2551. doi: 10.1210/en.2011-0231. [DOI] [PubMed] [Google Scholar]
- 4.Riedemann J, Macaulay VM. IGF1R signalling and its inhibition. Endocr Relat Cancer. 2006;13(Suppl 1):S33–43. doi: 10.1677/erc.1.01280. [DOI] [PubMed] [Google Scholar]
- 5.Pollak M. Insulin-like growth factor-related signaling and cancer development. Recent Results Cancer Res. 2007;174:49–53. doi: 10.1007/978-3-540-37696-5_4. [DOI] [PubMed] [Google Scholar]
- 6.De Meyts P. Insulin and its receptor: structure, function and evolution. Bioessays. 2004;26:1351–1362. doi: 10.1002/bies.20151. [DOI] [PubMed] [Google Scholar]
- 7.Benyoucef S, Surinya KH, Hadaschik D, Siddle K. Characterization of insulin/IGF hybrid receptors: contributions of the insulin receptor L2 and Fn1 domains and the alternatively spliced exon 11 sequence to ligand binding and receptor activation. Biochem J. 2007;403:603–613. doi: 10.1042/BJ20061709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belfiore A. The role of insulin receptor isoforms and hybrid insulin/IGF-I receptors in human cancer. Curr Pharm Des. 2007;13:671–686. doi: 10.2174/138161207780249173. [DOI] [PubMed] [Google Scholar]
- 9.Laube F. Mannose-6-phosphate/insulin-like growth factor-II receptor in human melanoma cells: effect of ligands and antibodies on the receptor expression. Anticancer Res. 2009;29:1383–1388. [PubMed] [Google Scholar]
- 10.Scott CD, Firth SM. The role of the M6P/IGF-II receptor in cancer: tumor suppression or garbage disposal? Horm Metab Res. 2004;36:261–271. doi: 10.1055/s-2004-814477. [DOI] [PubMed] [Google Scholar]
- 11.Jeyaratnaganthan N, Hojlund K, Kroustrup JP, Larsen JF, Bjerre M, et al. Circulating levels of insulin-like growth factor-II/mannose-6-phosphate receptor in obesity and type 2 diabetes. Growth Horm IGF Res. 2010;20:185–191. doi: 10.1016/j.ghir.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Samani AA, Yakar S, LeRoith D, Brodt P. The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev. 2007;28:20–47. doi: 10.1210/er.2006-0001. [DOI] [PubMed] [Google Scholar]
- 13.Denley A, Bonython ER, Booker GW, Cosgrove LJ, Forbes BE, et al. Structural determinants for high-affinity binding of insulin-like growth factor II to insulin receptor (IR)-A, the exon 11 minus isoform of the IR. Mol Endocrinol. 2004;18:2502–2512. doi: 10.1210/me.2004-0183. [DOI] [PubMed] [Google Scholar]
- 14.Favelyukis S, Till JH, Hubbard SR, Miller WT. Structure and autoregulation of the insulin-like growth factor 1 receptor kinase. Nat Struct Biol. 2001;8:1058–1063. doi: 10.1038/nsb721. [DOI] [PubMed] [Google Scholar]
- 15.Buck E, Mulvihill M. Small molecule inhibitors of the IGF-1R/IR axis for the treatment of cancer. Expert Opin Investig Drugs. 2011;20:605–621. doi: 10.1517/13543784.2011.558501. [DOI] [PubMed] [Google Scholar]
- 16.Shelton JG, Steelman LS, White ER, McCubrey JA. Synergy between PI3K/Akt and Raf/MEK/ERK pathways in IGF-1R mediated cell cycle progression and prevention of apoptosis in hematopoietic cells. Cell Cycle. 2004;3:372–379. [PubMed] [Google Scholar]
- 17.Levine AJ, Feng Z, Mak TW, You H, Jin S. Coordination and communication between the p53 and IGF-1-AKT-TOR signal transduction pathways. Genes Dev. 2006;20:267–275. doi: 10.1101/gad.1363206. [DOI] [PubMed] [Google Scholar]
- 18.Pollak MN. Insulin-like growth factors and neoplasia. Novartis Found Symp. 2004;262:84–98. discussion 98–107, 265–108. [PubMed] [Google Scholar]
- 19.Kim SY, Wan X, Helman LJ. Targeting IGF-1R in the treatment of sarcomas: past, present and future. Bull Cancer. 2009;96:E52–60. doi: 10.1684/bdc.2009.0915. [DOI] [PubMed] [Google Scholar]
- 20.Amiel SA, Sherwin RS, Hintz RL, Gertner JM, Press CM, et al. Effect of diabetes and its control on insulin-like growth factors in the young subject with type I diabetes. Diabetes. 1984;33:1175–1179. doi: 10.2337/diab.33.12.1175. [DOI] [PubMed] [Google Scholar]
- 21.Leung KC, Doyle N, Ballesteros M, Waters MJ, Ho KK. Insulin regulation of human hepatic growth hormone receptors: divergent effects on biosynthesis and surface translocation. J Clin Endocrinol Metab. 2000;85:4712–4720. doi: 10.1210/jcem.85.12.7017. [DOI] [PubMed] [Google Scholar]
- 22.Busund LT, Ow KT, Russell P, Crowe PJ, Yang JL. Expression of insulin-like growth factor mitogenic signals in adult soft-tissue sarcomas: significant correlation with malignant potential. Virchows Arch. 2004;444:142–148. doi: 10.1007/s00428-003-0931-y. [DOI] [PubMed] [Google Scholar]
- 23.Loeper S, Ezzat S. Acromegaly: re-thinking the cancer risk. Rev Endocr Metab Disord. 2008;9:41–58. doi: 10.1007/s11154-007-9063-z. [DOI] [PubMed] [Google Scholar]
- 24.Steuerman R, Shevah O, Laron Z. Congenital IGF1 deficiency tends to confer protection against post-natal development of malignancies. Eur J Endocrinol. 2011;164:485–489. doi: 10.1530/EJE-10-0859. [DOI] [PubMed] [Google Scholar]
- 25.Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, Wei M, Madia F, et al. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci Transl Med. 2011;3:70ra13. doi: 10.1126/scitranslmed.3001845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu Y, Cui K, Miyoshi K, Hennighausen L, Green JE, et al. Reduced circulating insulin-like growth factor I levels delay the onset of chemically and genetically induced mammary tumors. Cancer Res. 2003;63:4384–4388. [PubMed] [Google Scholar]
- 27.LeRoith D, Roberts CT., Jr The insulin-like growth factor system and cancer. Cancer Lett. 2003;195:127–137. doi: 10.1016/s0304-3835(03)00159-9. [DOI] [PubMed] [Google Scholar]
- 28.Cui H, Cruz-Correa M, Giardiello FM, Hutcheon DF, Kafonek DR, et al. Loss of IGF2 imprinting: a potential marker of colorectal cancer risk. Science. 2003;299:1753–1755. doi: 10.1126/science.1080902. [DOI] [PubMed] [Google Scholar]
- 29.Zhang L, Zhou W, Velculescu VE, Kern SE, Hruban RH, et al. Gene expression profiles in normal and cancer cells. Science. 1997;276:1268–1272. doi: 10.1126/science.276.5316.1268. [DOI] [PubMed] [Google Scholar]
- 30.Giordano TJ, Kuick R, Else T, Gauger PG, Vinco M, et al. Molecular classification and prognostication of adrenocortical tumors by transcriptome profiling. Clin Cancer Res. 2009;15:668–676. doi: 10.1158/1078-0432.CCR-08-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ravenel JD, Broman KW, Perlman EJ, Niemitz EL, Jayawardena TM, et al. Loss of imprinting of insulin-like growth factor-II (IGF2) gene in distinguishing specific biologic subtypes of Wilms tumor. J Natl Cancer Inst. 2001;93:1698–1703. doi: 10.1093/jnci/93.22.1698. [DOI] [PubMed] [Google Scholar]
- 32.Xu W, Fan H, He X, Zhang J, Xie W. LOI of IGF2 is associated with esophageal cancer and linked to methylation status of IGF2 DMR. J Exp Clin Cancer Res. 2006;25:543–547. [PubMed] [Google Scholar]
- 33.Zhan S, Shapiro DN, Helman LJ. Loss of imprinting of IGF2 in Ewing’s sarcoma. Oncogene. 1995;11:2503–2507. [PubMed] [Google Scholar]
- 34.Li Y, Meng G, Huang L, Guo QN. Hypomethylation of the P3 promoter is associated with up-regulation of IGF2 expression in human osteosarcoma. Hum Pathol. 2009;40:1441–1447. doi: 10.1016/j.humpath.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 35.Fu VX, Dobosy JR, Desotelle JA, Almassi N, Ewald JA, et al. Aging and cancer-related loss of insulin-like growth factor 2 imprinting in the mouse and human prostate. Cancer Res. 2008;68:6797–6802. doi: 10.1158/0008-5472.CAN-08-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu J, Qin Y, Li B, He WZ, Sun ZL. Hypomethylated and hypermethylated profiles of H19DMR are associated with the aberrant imprinting of IGF2 and H19 in human hepatocellular carcinoma. Genomics. 2008;91:443–450. doi: 10.1016/j.ygeno.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 37.Huynh H, Yang X, Pollak M. Estradiol and antiestrogens regulate a growth inhibitory insulin-like growth factor binding protein 3 autocrine loop in human breast cancer cells. J Biol Chem. 1996;271:1016–1021. doi: 10.1074/jbc.271.2.1016. [DOI] [PubMed] [Google Scholar]
- 38.Gucev ZS, Oh Y, Kelley KM, Rosenfeld RG. Insulin-like growth factor binding protein 3 mediates retinoic acid- and transforming growth factor beta2-induced growth inhibition in human breast cancer cells. Cancer Res. 1996;56:1545–1550. [PubMed] [Google Scholar]
- 39.Rozen F, Yang XF, Huynh H, Pollak M. Antiproliferative action of vitamin D-related compounds and insulin-like growth factor-binding protein 5 accumulation. J Natl Cancer Inst. 1997;89:652–656. doi: 10.1093/jnci/89.9.652. [DOI] [PubMed] [Google Scholar]
- 40.Buckbinder L, Talbott R, Velasco-Miguel S, Takenaka I, Faha B, et al. Induction of the growth inhibitor IGF-binding protein 3 by p53. Nature. 1995;377:646–649. doi: 10.1038/377646a0. [DOI] [PubMed] [Google Scholar]
- 41.Duan C, Ren H, Gao S. Insulin-like growth factors (IGFs), IGF receptors, and IGF-binding proteins: roles in skeletal muscle growth and differentiation. Gen Comp Endocrinol. 2010;167:344–351. doi: 10.1016/j.ygcen.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 42.Frystyk J, Vestbo E, Skjaerbaek C, Mogensen CE, Orskov H. Free insulin-like growth factors in human obesity. Metabolism. 1995;44:37–44. doi: 10.1016/0026-0495(95)90219-8. [DOI] [PubMed] [Google Scholar]
- 43.Grinspoon S, Clemmons D, Swearingen B, Klibanski A. Serum insulin-like growth factor-binding protein-3 levels in the diagnosis of acromegaly. J Clin Endocrinol Metab. 1995;80:927–932. doi: 10.1210/jcem.80.3.7533774. [DOI] [PubMed] [Google Scholar]
- 44.Prieur A, Tirode F, Cohen P, Delattre O. EWS/FLI-1 silencing and gene profiling of Ewing cells reveal downstream oncogenic pathways and a crucial role for repression of insulin-like growth factor binding protein 3. Mol Cell Biol. 2004;24:7275–7283. doi: 10.1128/MCB.24.16.7275-7283.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Werner H, Shen-Orr Z, Rauscher FJ, 3rd, Morris JF, Roberts CT, Jr, et al. Inhibition of cellular proliferation by the Wilms’ tumor suppressor WT1 is associated with suppression of insulin-like growth factor I receptor gene expression. Mol Cell Biol. 1995;15:3516–3522. doi: 10.1128/mcb.15.7.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coughlin SS, Calle EE, Teras LR, Petrelli J, Thun MJ. Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. Am J Epidemiol. 2004;159:1160–1167. doi: 10.1093/aje/kwh161. [DOI] [PubMed] [Google Scholar]
- 47.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 48.Ahlgren M, Melbye M, Wohlfahrt J, Sorensen TI. Growth patterns and the risk of breast cancer in women. N Engl J Med. 2004;351:1619–1626. doi: 10.1056/NEJMoa040576. [DOI] [PubMed] [Google Scholar]
- 49.Chan JM, Stampfer MJ, Giovannucci E, Gann PH, Ma J, et al. Plasma insulin-like growth factor-I and prostate cancer risk: a prospective study. Science. 1998;279:563–566. doi: 10.1126/science.279.5350.563. [DOI] [PubMed] [Google Scholar]
- 50.Diorio C, Pollak M, Byrne C, Masse B, Hebert-Croteau N, et al. Insulin-like growth factor-I, IGF-binding protein-3, and mammographic breast density. Cancer Epidemiol Biomarkers Prev. 2005;14:1065–1073. doi: 10.1158/1055-9965.EPI-04-0706. [DOI] [PubMed] [Google Scholar]
- 51.Gualberto A, Pollak M. Emerging role of insulin-like growth factor receptor inhibitors in oncology: early clinical trial results and future directions. Oncogene. 2009;28:3009–3021. doi: 10.1038/onc.2009.172. [DOI] [PubMed] [Google Scholar]
- 52.All-Ericsson C, Girnita L, Seregard S, Bartolazzi A, Jager MJ, et al. Insulin-like growth factor-1 receptor in uveal melanoma: a predictor for metastatic disease and a potential therapeutic target. Invest Ophthalmol Vis Sci. 2002;43:1–8. [PubMed] [Google Scholar]
- 53.Hakam A, Yeatman TJ, Lu L, Mora L, Marcet G, et al. Expression of insulin-like growth factor-1 receptor in human colorectal cancer. Hum Pathol. 1999;30:1128–1133. doi: 10.1016/s0046-8177(99)90027-8. [DOI] [PubMed] [Google Scholar]
- 54.Turner BC, Haffty BG, Narayanan L, Yuan J, Havre PA, et al. Insulin-like growth factor-I receptor overexpression mediates cellular radioresistance and local breast cancer recurrence after lumpectomy and radiation. Cancer Res. 1997;57:3079–3083. [PubMed] [Google Scholar]
- 55.Hellawell GO, Turner GD, Davies DR, Poulsom R, Brewster SF, et al. Expression of the type 1 insulin-like growth factor receptor is up-regulated in primary prostate cancer and commonly persists in metastatic disease. Cancer Res. 2002;62:2942–2950. [PubMed] [Google Scholar]
- 56.Law JH, Habibi G, Hu K, Masoudi H, Wang MY, et al. Phosphorylated insulin-like growth factor-i/insulin receptor is present in all breast cancer subtypes and is related to poor survival. Cancer Res. 2008;68:10238–10246. doi: 10.1158/0008-5472.CAN-08-2755. [DOI] [PubMed] [Google Scholar]
- 57.Badzio A, Wynes MW, Dziadziuszko R, Merrick DT, Pardo M, et al. Increased insulin-like growth factor 1 receptor protein expression and gene copy number in small cell lung cancer. J Thorac Oncol. 2010;5:1905–1911. doi: 10.1097/JTO.0b013e3181f38f57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bax DA, Mackay A, Little SE, Carvalho D, Viana-Pereira M, et al. A distinct spectrum of copy number aberrations in pediatric high-grade gliomas. Clin Cancer Res. 2010;16:3368–3377. doi: 10.1158/1078-0432.CCR-10-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Soroceanu L, Kharbanda S, Chen R, Soriano RH, Aldape K, et al. Identification of IGF2 signaling through phosphoinositide-3-kinase regulatory subunit 3 as a growth-promoting axis in glioblastoma. Proc Natl Acad Sci U S A. 2007;104:3466–3471. doi: 10.1073/pnas.0611271104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Belfiore A, Pandini G, Vella V, Squatrito S, Vigneri R. Insulin/IGF-I hybrid receptors play a major role in IGF-I signaling in thyroid cancer. Biochimie. 1999;81:403–407. doi: 10.1016/s0300-9084(99)80088-1. [DOI] [PubMed] [Google Scholar]
- 61.Frasca F, Pandini G, Sciacca L, Pezzino V, Squatrito S, et al. The role of insulin receptors and IGF-I receptors in cancer and other diseases. Arch Physiol Biochem. 2008;114:23–37. doi: 10.1080/13813450801969715. [DOI] [PubMed] [Google Scholar]
- 62.Sell C, Rubini M, Rubin R, Liu JP, Efstratiadis A, et al. Simian virus 40 large tumor antigen is unable to transform mouse embryonic fibroblasts lacking type 1 insulin-like growth factor receptor. Proc Natl Acad Sci U S A. 1993;90:11217–11221. doi: 10.1073/pnas.90.23.11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martin MJ, Melnyk N, Pollard M, Bowden M, Leong H, et al. The insulin-like growth factor I receptor is required for Akt activation and suppression of anoikis in cells transformed by the ETV6-NTRK3 chimeric tyrosine kinase. Mol Cell Biol. 2006;26:1754–1769. doi: 10.1128/MCB.26.5.1754-1769.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Toretsky JA, Kalebic T, Blakesley V, LeRoith D, Helman LJ. The insulin-like growth factor-I receptor is required for EWS/FLI-1 transformation of fibroblasts. J Biol Chem. 1997;272:30822–30827. doi: 10.1074/jbc.272.49.30822. [DOI] [PubMed] [Google Scholar]
- 65.McKinsey EL, Parrish JK, Irwin AE, Niemeyer BF, Kern HB, et al. A novel oncogenic mechanism in Ewing sarcoma involving IGF pathway targeting by EWS/Fli1-regulated microRNAs. Oncogene. 2011 doi: 10.1038/onc.2011.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Casa AJ, Dearth RK, Litzenburger BC, Lee AV, Cui X. The type I insulin-like growth factor receptor pathway: a key player in cancer therapeutic resistance. Front Biosci. 2008;13:3273–3287. doi: 10.2741/2925. [DOI] [PubMed] [Google Scholar]
- 67.Ge J, Chen Z, Wu S, Chen J, Li X, et al. Expression levels of insulin-like growth factor-1 and multidrug resistance-associated protein-1 indicate poor prognosis in patients with gastric cancer. Digestion. 2009;80:148–158. doi: 10.1159/000226089. [DOI] [PubMed] [Google Scholar]
- 68.Beech DJ, Parekh N, Pang Y. Insulin-like growth factor-I receptor antagonism results in increased cytotoxicity of breast cancer cells to doxorubicin and taxol. Oncol Rep. 2001;8:325–329. doi: 10.3892/or.8.2.325. [DOI] [PubMed] [Google Scholar]
- 69.Warshamana-Greene GS, Litz J, Buchdunger E, Garcia-Echeverria C, Hofmann F, et al. The insulin-like growth factor-I receptor kinase inhibitor, NVP-ADW742, sensitizes small cell lung cancer cell lines to the effects of chemotherapy. Clin Cancer Res. 2005;11:1563–1571. doi: 10.1158/1078-0432.CCR-04-1544. [DOI] [PubMed] [Google Scholar]
- 70.D’Cunja J, Shalaby T, Rivera P, von Buren A, Patti R, et al. Antisense treatment of IGF-IR induces apoptosis and enhances chemosensitivity in central nervous system atypical teratoid/rhabdoid tumours cells. Eur J Cancer. 2007;43:1581–1589. doi: 10.1016/j.ejca.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 71.Min Y, Adachi Y, Yamamoto H, Imsumran A, Arimura Y, et al. Insulin-like growth factor I receptor blockade enhances chemotherapy and radiation responses and inhibits tumour growth in human gastric cancer xenografts. Gut. 2005;54:591–600. doi: 10.1136/gut.2004.048926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Luk F, Yu Y, Walsh WR, Yang JL. IGF1R-Targeted Therapy and Its Enhancement of Doxorubicin Chemosensitivity in Human Osteosarcoma Cell Lines. Cancer Invest. 2011;29:521–532. doi: 10.3109/07357907.2011.606252. [DOI] [PubMed] [Google Scholar]
- 73.Wang YH, Xiong J, Wang SF, Yu Y, Wang B, et al. Lentivirus-mediated shRNA targeting insulin-like growth factor-1 receptor (IGF-1R) enhances chemosensitivity of osteosarcoma cells in vitro and in vivo. Mol Cell Biochem. 2010;341:225–233. doi: 10.1007/s11010-010-0453-2. [DOI] [PubMed] [Google Scholar]
- 74.Dallas NA, Xia L, Fan F, Gray MJ, Gaur P, et al. Chemoresistant colorectal cancer cells, the cancer stem cell phenotype, and increased sensitivity to insulin-like growth factor-I receptor inhibition. Cancer Res. 2009;69:1951–1957. doi: 10.1158/0008-5472.CAN-08-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kai K, D’Costa S, Sills RC, Kim Y. Inhibition of the insulin-like growth factor 1 receptor pathway enhances the antitumor effect of cisplatin in human malignant mesothelioma cell lines. Cancer Lett. 2009;278:49–55. doi: 10.1016/j.canlet.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 76.Liu YC, Leu CM, Wong FH, Fong WS, Chen SC, et al. Autocrine stimulation by insulin-like growth factor I is involved in the growth, tumorigenicity and chemoresistance of human esophageal carcinoma cells. J Biomed Sci. 2002;9:665–674. doi: 10.1159/000067282. [DOI] [PubMed] [Google Scholar]
- 77.Chen MY, Clark AJ, Chan DC, Ware JL, Holt SE, et al. Wilms’ tumor 1 silencing decreases the viability and chemoresistance of glioblastoma cells in vitro: a potential role for IGF-1R de-repression. J Neurooncol. 2011;103:87–102. doi: 10.1007/s11060-010-0374-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee JY, Han CY, Yang JW, Smith C, Kim SK, et al. Induction of glutathione transferase in insulin-like growth factor type I receptor-overexpressed hepatoma cells. Mol Pharmacol. 2007;72:1082–1093. doi: 10.1124/mol.107.038174. [DOI] [PubMed] [Google Scholar]
- 79.Rochester MA, Riedemann J, Hellawell GO, Brewster SF, Macaulay VM. Silencing of the IGF1R gene enhances sensitivity to DNA-damaging agents in both PTEN wild-type and mutant human prostate cancer. Cancer Gene Ther. 2005;12:90–100. doi: 10.1038/sj.cgt.7700775. [DOI] [PubMed] [Google Scholar]
- 80.Cosaceanu D, Budiu RA, Carapancea M, Castro J, Lewensohn R, et al. Ionizing radiation activates IGF-1R triggering a cytoprotective signaling by interfering with Ku-DNA binding and by modulating Ku86 expression via a p38 kinase-dependent mechanism. Oncogene. 2007;26:2423–2434. doi: 10.1038/sj.onc.1210037. [DOI] [PubMed] [Google Scholar]
- 81.Macaulay VM, Salisbury AJ, Bohula EA, Playford MP, Smorodinsky NI, et al. Downregulation of the type 1 insulin-like growth factor receptor in mouse melanoma cells is associated with enhanced radiosensitivity and impaired activation of Atm kinase. Oncogene. 2001;20:4029–4040. doi: 10.1038/sj.onc.1204565. [DOI] [PubMed] [Google Scholar]
- 82.Peretz S, Jensen R, Baserga R, Glazer PM. ATM-dependent expression of the insulin-like growth factor-I receptor in a pathway regulating radiation response. Proc Natl Acad Sci U S A. 2001;98:1676–1681. doi: 10.1073/pnas.041416598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shahrabani-Gargir L, Pandita TK, Werner H. Ataxia-telangiectasia mutated gene controls insulin-like growth factor I receptor gene expression in a deoxyribonucleic acid damage response pathway via mechanisms involving zinc-finger transcription factors Sp1 and WT1. Endocrinology. 2004;145:5679–5687. doi: 10.1210/en.2004-0613. [DOI] [PubMed] [Google Scholar]
- 84.Wakeling AE, Nicholson RI, Gee JM. Prospects for combining hormonal and nonhormonal growth factor inhibition. Clin Cancer Res. 2001;7:4350s–4355s. discussion 4411s-4412s. [PubMed] [Google Scholar]
- 85.Knowlden JM, Hutcheson IR, Barrow D, Gee JM, Nicholson RI. Insulin-like growth factor-I receptor signaling in tamoxifen-resistant breast cancer: a supporting role to the epidermal growth factor receptor. Endocrinology. 2005;146:4609–4618. doi: 10.1210/en.2005-0247. [DOI] [PubMed] [Google Scholar]
- 86.Parisot JP, Hu XF, DeLuise M, Zalcberg JR. Altered expression of the IGF-1 receptor in a tamoxifen-resistant human breast cancer cell line. Br J Cancer. 1999;79:693–700. doi: 10.1038/sj.bjc.6690112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nicholson RI, Gee JM. Oestrogen and growth factor cross-talk and endocrine insensitivity and acquired resistance in breast cancer. Br J Cancer. 2000;82:501–513. doi: 10.1054/bjoc.1999.0954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cunningham MP, Essapen S, Thomas H, Green M, Lovell DP, et al. Coexpression of the IGF-IR, EGFR and HER-2 is common in colorectal cancer patients. Int J Oncol. 2006;28:329–335. [PubMed] [Google Scholar]
- 89.Mendelsohn J. The epidermal growth factor receptor as a target for cancer therapy. Endocr Relat Cancer. 2001;8:3–9. doi: 10.1677/erc.0.0080003. [DOI] [PubMed] [Google Scholar]
- 90.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 91.Press MF, Bernstein L, Thomas PA, Meisner LF, Zhou JY, et al. HER-2/neu gene amplification characterized by fluorescence in situ hybridization: poor prognosis in node-negative breast carcinomas. J Clin Oncol. 1997;15:2894–2904. doi: 10.1200/JCO.1997.15.8.2894. [DOI] [PubMed] [Google Scholar]
- 92.Morgillo F, Woo JK, Kim ES, Hong WK, Lee HY. Heterodimerization of insulin-like growth factor receptor/epidermal growth factor receptor and induction of survivin expression counteract the antitumor action of erlotinib. Cancer Res. 2006;66:10100–10111. doi: 10.1158/0008-5472.CAN-06-1684. [DOI] [PubMed] [Google Scholar]
- 93.Morgillo F, Kim WY, Kim ES, Ciardiello F, Hong WK, et al. Implication of the insulin-like growth factor-IR pathway in the resistance of non-small cell lung cancer cells to treatment with gefitinib. Clin Cancer Res. 2007;13:2795–2803. doi: 10.1158/1078-0432.CCR-06-2077. [DOI] [PubMed] [Google Scholar]
- 94.Lu Y, Zi X, Zhao Y, Mascarenhas D, Pollak M. Insulin-like growth factor-I receptor signaling and resistance to trastuzumab (Herceptin) J Natl Cancer Inst. 2001;93:1852–1857. doi: 10.1093/jnci/93.24.1852. [DOI] [PubMed] [Google Scholar]
- 95.Balana ME, Labriola L, Salatino M, Movsichoff F, Peters G, et al. Activation of ErbB-2 via a hierarchical interaction between ErbB-2 and type I insulin-like growth factor receptor in mammary tumor cells. Oncogene. 2001;20:34–47. doi: 10.1038/sj.onc.1204050. [DOI] [PubMed] [Google Scholar]
- 96.Thankamony GN, Dunger DB, Acerini CL. Pegvisomant: current and potential novel therapeutic applications. Expert Opin Biol Ther. 2009;9:1553–1563. doi: 10.1517/14712590903449222. [DOI] [PubMed] [Google Scholar]
- 97.Dagnaes-Hansen F, Duan H, Rasmussen LM, Friend KE, Flyvbjerg A. Growth hormone receptor antagonist administration inhibits growth of human colorectal carcinoma in nude mice. Anticancer Res. 2004;24:3735–3742. [PubMed] [Google Scholar]
- 98.McCutcheon IE, Flyvbjerg A, Hill H, Li J, Bennett WF, et al. Antitumor activity of the growth hormone receptor antagonist pegvisomant against human meningiomas in nude mice. J Neurosurg. 2001;94:487–492. doi: 10.3171/jns.2001.94.3.0487. [DOI] [PubMed] [Google Scholar]
- 99.Hejna M, Schmidinger M, Raderer M. The clinical role of somatostatin analogues as antineoplastic agents: much ado about nothing? Ann Oncol. 2002;13:653–668. doi: 10.1093/annonc/mdf142. [DOI] [PubMed] [Google Scholar]
- 100.Jerome L, Alami N, Belanger S, Page V, Yu Q, et al. Recombinant human insulin-like growth factor binding protein 3 inhibits growth of human epidermal growth factor receptor-2-overexpressing breast tumors and potentiates herceptin activity in vivo. Cancer Res. 2006;66:7245–7252. doi: 10.1158/0008-5472.CAN-05-3555. [DOI] [PubMed] [Google Scholar]
- 101.Alami N, Page V, Yu Q, Jerome L, Paterson J, et al. Recombinant human insulin-like growth factor-binding protein 3 inhibits tumor growth and targets the Akt pathway in lung and colon cancer models. Growth Horm IGF Res. 2008;18:487–496. doi: 10.1016/j.ghir.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 102.Gao J, Chesebrough JW, Cartlidge SA, Ricketts SA, Incognito L, et al. Dual IGF-I/II-neutralizing antibody MEDI-573 potently inhibits IGF signaling and tumor growth. Cancer Res. 2011;71:1029–1040. doi: 10.1158/0008-5472.CAN-10-2274. [DOI] [PubMed] [Google Scholar]
- 103.Schneider-Merck T, Lammerts van Bueren JJ, Berger S, Rossen K, van Berkel PH, et al. Human IgG2 antibodies against epidermal growth factor receptor effectively trigger antibody-dependent cellular cytotoxicity but, in contrast to IgG1, only by cells of myeloid lineage. J Immunol. 2010;184:512–520. doi: 10.4049/jimmunol.0900847. [DOI] [PubMed] [Google Scholar]
- 104.Kurzrock R, Patnaik A, Aisner J, Warren T, Leong S, et al. A phase I study of weekly R1507, a human monoclonal antibody insulin-like growth factor-I receptor antagonist, in patients with advanced solid tumors. Clin Cancer Res. 2010;16:2458–2465. doi: 10.1158/1078-0432.CCR-09-3220. [DOI] [PubMed] [Google Scholar]
- 105.Tolcher AW, Sarantopoulos J, Patnaik A, Papadopoulos K, Lin CC, et al. Phase I, pharmacokinetic, and pharmacodynamic study of AMG 479, a fully human monoclonal antibody to insulin-like growth factor receptor 1. J Clin Oncol. 2009;27:5800–5807. doi: 10.1200/JCO.2009.23.6745. [DOI] [PubMed] [Google Scholar]
- 106.Olmos D, Postel-Vinay S, Molife LR, Okuno SH, Schuetze SM, et al. Safety, pharmacokinetics, and preliminary activity of the anti-IGF-1R antibody figitumumab (CP-751,871) in patients with sarcoma and Ewing’s sarcoma: a phase 1 expansion cohort study. Lancet Oncol. 2010;11:129–135. doi: 10.1016/S1470-2045(09)70354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pantaleo MA, Astolfi A, Nannini M, Biasco G. The emerging role of insulin-like growth factor 1 receptor (IGF1r) in gastrointestinal stromal tumors (GISTs) J Transl Med. 2010;8:117. doi: 10.1186/1479-5876-8-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Braconi C, Bracci R, Cellerino R. Molecular targets in Gastrointestinal Stromal Tumors (GIST) therapy. Curr Cancer Drug Targets. 2008;8:359–366. doi: 10.2174/156800908785133169. [DOI] [PubMed] [Google Scholar]
- 109.Janeway KA, Zhu MJ, Barretina J, Perez-Atayde A, Demetri GD, et al. Strong expression of IGF1R in pediatric gastrointestinal stromal tumors without IGF1R genomic amplification. Int J Cancer. 2010;127:2718–2722. doi: 10.1002/ijc.25247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tarn C, Rink L, Merkel E, Flieder D, Pathak H, et al. Insulin-like growth factor 1 receptor is a potential therapeutic target for gastrointestinal stromal tumors. Proc Natl Acad Sci U S A. 2008;105:8387–8392. doi: 10.1073/pnas.0803383105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jassem J, Langer CJDDK, Mok T, Benner RJ, Green SJ, Park K, Novello S, Strausz J, Gualberto A. Randomized, open label, phase III trial of figitumumab in combination with paclitaxel and carboplatin versus paclitaxel and carboplatin in patients with non-small cell lung cancer (NSCLC) Journal of Clinical Oncology. 2010;28(suppl):abstr 7500. doi: 10.1200/JCO.2013.54.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Karp DD, Paz-Ares LG, Novello S, Haluska P, Garland L, et al. Phase II study of the anti-insulin-like growth factor type 1 receptor antibody CP-751,871 in combination with paclitaxel and carboplatin in previously untreated, locally advanced, or metastatic non-small-cell lung cancer. J Clin Oncol. 2009;27:2516–2522. doi: 10.1200/JCO.2008.19.9331. [DOI] [PubMed] [Google Scholar]
- 113.Buck E, Eyzaguirre A, Rosenfeld-Franklin M, Thomson S, Mulvihill M, et al. Feedback mechanisms promote cooperativity for small molecule inhibitors of epidermal and insulin-like growth factor receptors. Cancer Res. 2008;68:8322–8332. doi: 10.1158/0008-5472.CAN-07-6720. [DOI] [PubMed] [Google Scholar]
- 114.Guix M, Faber AC, Wang SE, Olivares MG, Song Y, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in cancer cells is mediated by loss of IGF-binding proteins. J Clin Invest. 2008;118:2609–2619. doi: 10.1172/JCI34588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wan X, Harkavy B, Shen N, Grohar P, Helman LJ. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. 2007;26:1932–1940. doi: 10.1038/sj.onc.1209990. [DOI] [PubMed] [Google Scholar]
- 116.Naing A, Kurzrock R, Burger A, Gupta S, Lei X, et al. Phase I trial of cixutumumab combined with temsirolimus in patients with advanced cancer. Clin Cancer Res. 2011;17:6052–6060. doi: 10.1158/1078-0432.CCR-10-2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pollak M. Metformin and other biguanides in oncology: advancing the research agenda. Cancer Prev Res (Phila) 2010;3:1060–1065. doi: 10.1158/1940-6207.CAPR-10-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Monami M, Colombi C, Balzi D, Dicembrini I, Giannini S, et al. Metformin and cancer occurrence in insulin-treated type 2 diabetic patients. Diabetes Care. 2011;34:129–131. doi: 10.2337/dc10-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen TM, Lin CC, Huang PT, Wen CF. Metformin associated with lower mortality in diabetic patients with early stage hepatocellular carcinoma after radiofrequency ablation. J Gastroenterol Hepatol. 2011;26:858–865. doi: 10.1111/j.1440-1746.2011.06664.x. [DOI] [PubMed] [Google Scholar]
- 120.Bo S, Ciccone G, Rosato R, Villois P, Appendino G, et al. Cancer mortality reduction and metformin. A retrospective cohort study in type 2 diabetic patients. Diabetes Obes Metab. 2011 doi: 10.1111/j.1463-1326.2011.01480.x. [DOI] [PubMed] [Google Scholar]
- 121.Jansen M, van Schaik FM, Ricker AT, Bullock B, Woods DE, et al. Sequence of cDNA encoding human insulin-like growth factor I precursor. Nature. 1983;306:609–611. doi: 10.1038/306609a0. [DOI] [PubMed] [Google Scholar]
- 122.Heskamp S, van Laarhoven HW, Molkenboer-Kuenen JD, Franssen GM, Versleijen-Jonkers YM, et al. ImmunoSPECT and immunoPET of IGF-1R expression with the radiolabeled antibody R1507 in a triple-negative breast cancer model. J Nucl Med. 2010;51:1565–1572. doi: 10.2967/jnumed.110.075648. [DOI] [PubMed] [Google Scholar]
- 123.Gualberto A, Pollak M. Clinical development of inhibitors of the insulin-like growth factor receptor in oncology. Curr Drug Targets. 2009;10:923–936. doi: 10.2174/138945009789577945. [DOI] [PubMed] [Google Scholar]
- 124.Freude S, Schilbach K, Schubert M. The role of IGF-1 receptor and insulin receptor signaling for the pathogenesis of Alzheimer’s disease: from model organisms to human disease. Curr Alzheimer Res. 2009;6:213–223. doi: 10.2174/156720509788486527. [DOI] [PubMed] [Google Scholar]


