Abstract
Objectives
1) To report rates of neuropsychiatric symptoms (NPS) in cognitive impairment, no dementia (CIND). 2) To compare the 30-day prevalence of NPS in CIND with that in dementia and cognitively normal individuals. 3) To compare the prevalence of NPS in amnestic MCI (aMCI) with other predementia syndromes.
Design
Comparison of prevalence proportions among several defined groups.
Setting
Population-based study.
Participants
A subsample of the permanent residents of Cache County, Utah, aged 65 years or older in January 1995 (N = 5092) and who had completed clinical assessments and had an informant-completed Neuropsychiatric Inventory.
Measurements
Chi-square statistics, tests for trend, and logistic regression models were used to analyze the three objectives listed earlier.
Results
The most prevalent NPS in those with CIND were depression (16.9%), irritability (9.8%), nighttime behaviors (7.6%), apathy (6.9%), and anxiety (5.4%). Trend analyses confirmed that the CIND group had NPS prevalence rates that fell between the normal and dementia groups for most NPS. Logistic regression models showed no significant difference between aMCI and other CIND participants in the prevalence of any NPS (lowest p: 0.316).
Conclusions
These data confirm the relatively high prevalence of NPS in CIND reported by other studies, especially for affective symptoms. No differences in NPS prevalence were found between aMCI and other types of CIND.
Keywords: agitation, anxiety, CIND, cache county, dementia, depression, MCI, NPI, NPS
Dementia is increasingly recognized as a serious public health concern.1 Fueled by an aging population and an increased public awareness of the signs and symptoms of dementia, there has been a notable increase in the prevalence of dementia.2 In conjunction with this increase has been a growing focus on individuals with notable cognitive impairment at a severity level insufficient for a diagnosis of dementia.3 The terms “cognitive impairment, no dementia” (CIND) and “mild cognitive impairment” (MCI) have been used to describe the collection of symptoms displayed by these individuals, many of whom are in the prodromal stages to dementia. According to the American Association of Geriatric Psychiatry (AAGP) position statement,4 CIND is a clinical syndrome consisting of measurable or evident decline in memory or other cognitive abilities with little effect on day-to-day functioning that does not meet criteria for dementia listed by Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IVTR). AAGP defines MCI as a clinically recognizable subgroup of CIND that is in all likelihood a prodrome of Alzheimer disease.
Although rates of conversion vary,5 transition from MCI to dementia averages 12%–15% per year in community samples. Risk factors for transition from CIND and MCI to dementia have been a subject of growing interest, as it is believed that their identification can focus interventions at earlier stages of dementia thereby preventing the full syndrome. Risk factors for transition from MCI to dementia include cardiovascular risk factors,6 stroke,7 and the ApoE ε4 allele.8
While dementia is defined by the occurrence of cognitive and functional decline, it has become apparent that neuropsychiatric symptoms (NPS) are nearly universal in dementia through the course of illness.9 As a result, and because NPS are associated with a worse prognosis in dementia,4 the occurrence of NPS in MCI/CIND is also of interest. Study of a community sample first suggested a prevalence of NPS in MCI on the order of 50%, intermediate between that seen in persons with dementia and the cognitively normal.10 This estimate was supported by work from a large clinical sample where depression was common in MCI.11 Replication of the high prevalence of NPS in community samples of MCI has since been published from European12 and North American13 population samples, and from a large Argentinean clinical sample.14 Only Geda et al.13 previously examined the prevalence of NPS in different forms of CIND in the United States and found it to be comparable in amnestic and nonamnestic MCI.
In addition to a high prevalence of NPS in MCI/CIND, several studies have shown a link between CIND/MCI and later conversion to dementia.8,15–17 Modrego and Ferrandez11 reported that a diagnosis of major depression doubles the risk of transition from MCI to dementia, while a report from the Kungsholmen project suggested higher transition risk with increasing levels of anxiety.12 These risk relationships were further clarified by Taragano et al.,14 who reported that a form of NPS referred to as “mild behavioral impairment” in patients with MCI or cognitive complaints greatly increases the risk of transition from MCI to Alzheimer dementia (AD), while NPS without cognitive complaints greatly increase the risk of transition to frontotemporal dementia.
We have previously reported findings from the Cache County Study (CCS) of Memory Health and Aging, a unique population-based study of risk factors for the onset and progression of dementia, suggesting that subtypes of CIND, especially with features consistent with amnestic MCI, are associated with a risk of transition to dementia of 55% over 3 years.8 The presence of one or more copies of the ApoE ε4 allele further increased this risk. We now turn to examine the prevalence of NPS in CIND and its subtypes.
Using the Cache County sample, here we 1) report rates of NPS in CIND; 2) compare the 30-day prevalence of NPS in persons with CIND to that in persons with dementia and to cognitively normal elderly controls; 3) compare the prevalence of NPS in the most common subtype of CIND, amnestic MCI (aMCI), to that in persons with other forms of CIND. In regard to the subgroup analyses, we hypothesize that the non-aMCI group, being less well-defined, will have a higher prevalence of NPS. A future paper will estimate the association between NPS and transition rates from CIND to dementia.
METHODS
Sampling, Screening, and Procedure
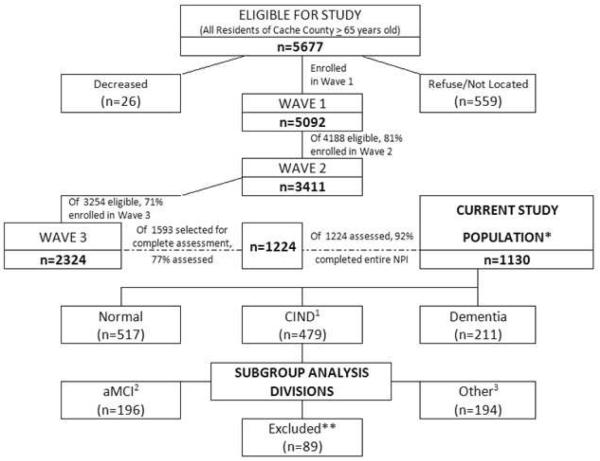
The present study utilized data from the third of four triennial waves of dementia ascertainment in Cache County, Utah (Fig. 1). The methods of the CCS have been detailed extensively elsewhere.18,19 Briefly, all permanent residents of the county who were 65 years or older in January 1995 (N = 5677) were asked to become part of this study. We enrolled 5,092 of these (90%) in Wave 1, all of whom were screened for dementia in a multistaged assessment protocol. The first stage of screening involved either a revised version of the Modified Mini-Mental State Exam (3MS)20,21 or for those unable to complete testing, the Informant Questionnaire of Cognitive Status in the Elderly.22 Those scoring below the predetermined education and sensory-adjusted score were selected for an informant-based interview, which assessed clinical symptoms consistent with dementia.23
Figure 1.
Cache Country and Current Study Design.
Participants whose informant interviews were suggestive of dementia or its prodrome, and members of the designated subsample, were selected to undergo a clinical assessment (CA) with a trained research nurse and a neuropsychological technician. The designated subsample was created at Wave 1 to match each identified case of AD at a 2:1 ratio, matched on age, gender, and ApoE ε4 genotype group, except for participants aged 65–74, whom were matched at a 4:1 ratio.18 Due to attrition, additional individuals were selected in Wave 3 to “replenish” the designated subsample, this time matching each case of dementia or prodromal AD at a 2:1 ratio, with 100% sampling of all persons aged 85 or older. The CA consisted of neuropsychological assessment,24 brief physical examination, and informant interview of the development and course of any clinical symptoms. A geropsychiatrist and neuropsychologist, with members of the examination team, reviewed results of the CA and assigned preliminary diagnoses of dementia or CIND. The diagnostic panel members were sensitive to the overlapping nature of dementia/CIND and depression, differentiating the two based on the different syndromes found in each.
Dating of age at dementia onset was based on when the subject unambiguously met DSM-III-TR criteria for dementia and dementia severity determined by using the Clinical Dementia Rating25 (CDR) as a guide. Participants who met DSM-III-TR criteria for dementia26 or prodromal AD were selected to undergo an MRI scan and laboratory studies to rule out nondegenerative pathology that could explain the present cognitive symptoms. Furthermore, those carrying a dementia diagnosis were invited to complete a geropsychiatry examination.
All data from the CA, neuroimaging and laboratory results, and geropsychiatry examination were subsequently reviewed by an expert panel consisting of study geropsychiatrists, neurologists, neuropsychologists, and a cognitive neuroscientist for final assignment of dementia diagnoses, mild cognitive syndromes, or normal cognitive status. Diagnoses of AD and vascular and other forms of dementia followed standard research criteria. For example, a diagnosis of AD was made according to NINCDS-ADRDA criteria.27 Clinical diagnoses of CIND and aMCI were made according to published criteria. A diagnosis of prodromal AD was assigned when the pattern of clinical symptoms or neuropsychological testing was suggestive of this state. Another requirement was the absence of other medical/psychiatric conditions that would preclude an eventual AD diagnosis.8
For present purposes, the group of participants diagnosed as having CIND was further subdivided into those with aMCI and those with other CIND diagnoses (CIND-Other). For a diagnosis of aMCI, participants met the published criteria for mild ambiguous/prodromal AD,8 MCI by Petersen,28 and/or MCI by CDR criteria.15 By definition, participants who did not qualify for a diagnosis of CIND or dementia were assigned a CDR rating of 0. These subtypes were grouped together due to their use as the CIND types most predictive of conversion to Alzheimer Disease. For a diagnosis of CIND-Other, participants did not meet criteria for a diagnosis of aMCI and did meet criteria for the CSHA definition of CIND29 and/or suffered from CIND related to vascular causes. It is important to note that CIND attributed to other medical conditions, such as surgery or nondegenerative pathology, were not included in the CIND-Other subgroup, whereas they were included in the original CIND group. We did not have adequate sample sizes to evaluate each CIND subtype separately.
Individuals with a designation of dementia or mild ambiguous prodromal AD were invited to complete an 18-month follow-up CA and expert panel review to confirm diagnoses. Participants without dementia at the end of each wave were eligible for participation in the subsequent wave. In both Waves 3 and 4, the 3MS cut score was raised (to increase the sensitivity for identification of mild cognitive syndromes), the second screening stage of informant interview was eliminated (as this information was gathered at the third stage, or CA), and the designated subsample was replenished as discussed earlier. (Wave 2 3MS cut score: 86/87 for subjects younger than 80 years and 83/84 for subjects older than 80 years; Wave 3 and wave 4 3MS cut score: 90/91 for all ages). All study procedures were approved by the institutional review boards at Utah State University, Duke University, and the Johns Hopkins University.
Because the more detailed diagnostic categories of CIND were not rendered until the third study wave, the final sample for the present investigation included only those study participants who completed the Neuropsychiatric Inventory (NPI) at the CA in Wave 3 (Fig. 1). Of the original 5,092 participants enrolled in wave 1, 368 participants with dementia diagnoses (with an additional 536 participants that died) were excluded from Wave 2. Of the 4,188 eligible, 3,411 (81%) participated in Wave 2. Of the original 5,092 participants, there were 567 excluded with dementia prior to Wave 3 and an additional 1,271 that died prior to the start of Wave 3. Of the 3,254 eligible, 2,324 (71%) participated in Wave 3. There were 1,224 (of 1,593 selected) participants who completed a CA at Wave 3. Of these, 1,130 (92%) completed the entire NPI. For each individual neuropsychiatric symptom, information was included from up to 77 additional participants that did not complete the entire NPI, forming the final sample for the analysis.
Assessment of Neuropsychiatric Symptoms
Neuropsychiatric symptoms were evaluated at the CA using the NPI.30 No other measures of NPS were used, as the NPI is an organized way to measure multiple domains. The research nurse interviewed a knowledgeable informant about the presence of 12 specific domains: delusions, hallucinations, agitation/aggression, depression/dysphoria, anxiety, elation/euphoria, apathy/indifference, disinhibition, irritability/lability, aberrant motor behavior, disturbances of sleep, and disturbances of appetite/eating. The presence of each symptom in the past 30 days was queried with a screening question. If this was endorsed, follow-up was performed with a series of specific questions to clarify the nature of the symptom, the frequency, severity, degree of change from premorbid characteristics, and treatment. As in previous studies,10,13 we defined each outcome variable as either the presence or absence of a given NPS.
Analysis
The present analyses were carried out using STATA (SE) Version 10.1. Test for trend analyses were used to compare the demographics of the three groups (normal versus CIND versus dementia). Of note, CDR ratings were not completed on 79 participants, because they had normal (N = 77) or other non-CIND diagnoses (depression, other medical condition that did not appear to affect cognitive status). The prevalence of neuropsychiatric symptoms by NPI domain were analyzed using 3-way Pearson chi-square (normal versus CIND versus dementia), as well as test for trend analyses and Fisher's exact statistics. All prevalence analyses were based on presence within the past 30 days. Similar analyses were carried out for the aMCI subgroup of CIND (normal versus aMCI versus dementia) and for the CIND-Other subgroup of CIND (normal versus other versus dementia).
Three logistic regression models were designed to compare each of the groupings with one another (e.g., CIND versus Normal). The models use the cognitive groups as the predictors and presence/absence of each NPS as the outcome. Model 1 was an unadjusted logistic regression model used to compare the prevalence of the various domains of the NPI. Model 2 was a logistic regression model adjusted for age, education, and ApoE ε4 status. Model 3 was a logistic regression model adjusted for age, education, ApoE ε4 status, 3MS, and CDR.
RESULTS
A total of 1,224 Cache County residents were enrolled in Wave 3 of the CCS and completed the CA. Of these, 522 were found to be cognitively normal, while 218 suffered from dementia, and 484 were found to have CIND. Within the CIND group, 196 (8.40%*) individuals met criteria for aMCI and 194 (8.35%*) for other types of CIND (*Percentages considering 2,324 participants in Wave 3). As mentioned in the Methods, individuals with CIND attributed to other medical conditions, such as surgery or nondegenerative pathology, were not included in the subgroup analysis, whereas they were included in the initial CIND group.
Table 1 compares demographic data among the groups. As expected, age increased moving from cognitively normal to CIND to dementia. All groups had a slightly larger proportion of women than men, but differences in proportion between the groups were not significant. Mean duration of education was highest in the cognitively normal and lowest in the dementia group. General medical health rating (GMHR)31 values decreased (indicating an increase in medical morbidity), whereas the proportion of participants with at least one ApoE ε4 allele increased, in progressive ratings of severity from cognitively normal to CIND to dementia. Scores on CDR and 3MS also differed as expected across the three groups. CDR is a dementia rating and increased as expected from normal to CIND to dementia. 3MS is a measure of cognitive functioning and, conversely to CDR, decreased as expected. Because of these differences, in later multivariable models, we controlled for age, education, and ApoE ε4 status (Model 2 and Model 3) and for 3MS and CDR (Model 3). Please note that in Table 1, data are presented for all 1,224 participants in Wave 3, not just for the 1,130 completing the entire NPI. No differences were observed between those completing the entire NPI and those not (data not shown).
Table 1.
Demographic and Population Descriptors of Participants
| MEAN (SD) / NO. (%) | MEAN (SD) / No. (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Normal (n=522) | CIND1 (n=484) | Dementia (n=218) | Test for Trend | Characteristic | Normal (n=522) | CIND1 (n=484) | Dementia (n=218) | Test for Trend | ||
| Z Statistic | P Value | Z Statistic | P Value | ||||||||
| Age, mean, y | 81.2 (5.0) | 83.3 (5.4) | 85.7(6.3) | 9.69 | <0.001 | MMSE3, mean | 28.1 (1.9) | 26.2 (2.7) | 21.0 (5.4) | −20.89 | <0.001 |
| Gender, no. | −0.5 | 0.618 | 3MS4, mean | 93.1 (5.0) | 87.1 (7.2) | 74.3 (12.8) | −21.85 | <0.001 | |||
| Male | 230 (44.1) | 233 (48.1) | 87 (39.9) | CDR5, No. | 28.68 | <0.001 | |||||
| Female | 292 (55.9) | 251 (51.9) | 131 (60.1) | 0 | 398 (88.6) | 56 (11.7) | 0 (0.0) | ||||
| Education, mean, y | 14.1 (2.8) | 13.3 (3.0) | 13.1 (2.9) | −4.88 | <0.001 | 0.5 | 51 (11.4) | 409 (88.6) | 51 (23.4) | ||
| GMHR2, No. | −13.89 | <0.001 | 1 | 0 | 10 (2.0) | 123 (56.4) | |||||
| Poor/fair | 55 (10.5) | 152 (31.4) | 115 (52.8) | 2 | 0 | 3 (0.6) | 26 (11.9) | ||||
| Good | 300 (57.5) | 273 (56.4) | 94 (43.2) | 3 | 0 | 0 | 14 (6.4) | ||||
| Excellent | 167 (32.0) | 59 (12.2) | 9 (4.0) | 4 | 0 | 0 | 4 (1.8) | ||||
| ApoE ε4 Genotype, No. | 140 (26.9) | 149 (30.98) | 80 (37.0) | 2.27 | 0.006 | ||||||
CIND: cognitive impairment, no dementia;
GMHR: General Medical Health Rating scale;
MMSE: mini-mental state exam;
3MS: modified mini-mental state exam;
CDR: clinical dementia rating;
Table 2 compares the 30-day prevalence of NPS by NPI domain in individuals who were cognitively normal or who were diagnosed with CIND or dementia. The percentages of participants with at least one NPS were as follows: Normal—15.1%, CIND—31%, and Dementia—60.1%. Depression was the most prevalent NPS in CIND followed by irritability/lability and nighttime behaviors. In cognitively normal participants, nighttime behaviors showed the most prevalence. Depression/dysphoria and irritability/lability were the only other NPS domains that had an occurrence of more than 1% in cognitively normal participants. In all cases, the prevalence of NPS increased from normal to CIND to dementia (test for trend analyses, df = 2, p <0.05), except for nighttime behaviors (test for trend, df = 2, p = 0.508) (data not shown).
Table 2.
Neuropsychiatric Symptoms by NPI2 Domain
| No. (%) | χ2 Comparison of Normal vs. CIND1 vs. Dementia | ||||
|---|---|---|---|---|---|
| NPI2 Domain | Normal (n=517) | CIND1 (n=479) | Dementia (n=211) | Statistic (df=2) | P Value |
| Delusions | 0 (0) | 5 (1.1) | 33 (15.6) | 131.467 | <0.001 |
| Hallucinations | 1 (0.2) | 5 (1.1) | 21 (10.1) | 71.101 | <0.001 |
| Agitation/aggression | 4 (0.8) | 17 (3.6) | 36 (17.1) | 90.782 | <0.001 |
| Depression/dysphoria | 25 (4.9) | 81 (16.9) | 63 (29.9) | 83.093 | <0.001 |
| Anxiety | 5 (1.0) | 26 (5.4) | 29 (13.7) | 52.135 | <0.001 |
| Elation/euphoria | 0 (0) | 1 (0.2) | 2 (1.0) | 5.481 | 0.065 |
| Apathy/indifference | 4 (0.8) | 33 (6.9) | 45 (21.3) | 99.829 | <0.001 |
| Disinhibition | 1 (0.2) | 4 (0.8) | 18 (8.5) | 60.592 | <0.001 |
| Irritability/lability | 16 (3.1) | 47 (9.8) | 42 (19.9) | 54.553 | <0.001 |
| Motor Disturbance | 0 (0) | 1 (0.2) | 10 (4.7) | 41.525 | <0.001 |
| Nighttime Behaviors | 37 (7.3) | 36 (7.6) | 18 (8.8) | 0.512 | 0.774 |
| Appetite/eating | 5 (1.0) | 24 (5.0) | 24 (11.4) | 39.368 | <0.001 |
| NPI, ≥1 domain | 79 (15.1) | 150 (31.0) | 131 (60.1) | 150.672 | <0.001 |
CIND: cognitive impairment, no dementia;
NPI: neuropsychiatric inventory.
Table 3 displays prevalence data for the CIND subgroups (aMCI versus CIND-Other). Exact p values were used to compare the prevalence of individual NPS between the two groups. The percentage of participants with at least one NPS was: aMCI—26% and CIND-other—26.3%. The lowest p value comparing the two groups (χ2, df = 1, p = 0.261) was for irritability/lability, suggesting that NPS overall and individual NPS were not different between the two groups.
Table 3.
Neuropsychiatric Symptoms by NPI2 Domain
| No. (%) CIND1 | χ2 Comparison of Normal vs. aMCI3 vs. Dementia | χ2 Comparison of Normal vs. Other4 vs. Dementia | χ2 Comparison of Other vs. aMCI | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NPI2 Domain | Normal (n=517) | aMCI3 (n=196) | Other4 (n=194) | Dementia (n=211) | Statistic (df=2) | P Value | Statistic (df=2) | P Value | Statistic (df=1) | P Value |
| Delusions | 0 (0) | 1 (0.5) | 3 (1.6) | 33 (15.6) | 110.007 | <0.001 | 100.902 | <0.001 | 1.032 | 0.31 |
| Hallucinations | 1 (0.2) | 1 (0.5) | 0 (0) | 21 (10.1) | 63.266 | <0.001 | 67.655 | <0.001 | 0.987 | 0.32 |
| Agitation/aggression | 4 (0.8) | 5 (2.6) | 7 (3.7) | 36 (17.1) | 88.565 | <0.001 | 83.075 | <0.001 | 0.377 | 0.539 |
| Depression/dysphoria | 25 (4.9) | 24 (12.3) | 28 (14.6) | 63 (29.9) | 87.605 | <0.001 | 85.597 | <0.001 | 0.431 | 0.512 |
| Anxiety | 5 (1.0) | 9 (4.6) | 8 (4.2) | 29 (13.7) | 55.075 | <0.001 | 56.236 | <0.001 | 0.046 | 0.829 |
| Elation/euphoria | 0 (0) | 1 (0.5) | 0 (0) | 2 (1.0) | 4.425 | 0.109 | 6.735 | 0.0034 | 0.987 | 0.32 |
| Apathy/indifference | 4 (0.8) | 10 (5.1) | 8 (4.2) | 45 (21.3) | 106.275 | <0.001 | 110.458 | <0.001 | 0.202 | 0.653 |
| Disinhibition | 1 (0.2) | 1 (0.5) | 0 (0) | 18 (8.5) | 52.325 | <0.001 | 56.616 | <0.001 | 0.987 | 0.32 |
| Irritability/lability | 16 (3.1) | 14 (7.2) | 20 (10.4) | 42 (19.9) | 59.009 | <0.001 | 55.747 | <0.001 | 1.265 | 0.261 |
| Motor Disturbance | 0 (0) | 1 (0.5) | 0 (0) | 10 (4.7) | 29.462 | <0.001 | 33.876 | <0.001 | 0.987 | 0.32 |
| Nighttime Behaviors | 37 (3.7) | 15 (7.8) | 11 (5.8) | 18 (8.8) | 0.506 | 0.777 | 1.342 | 0.511 | 0.616 | 0.432 |
| Appetite/eating | 5 (1.0) | 6 (3.1) | 8 (4.2) | 24 (11.4) | 44.834 | <0.001 | 42.019 | <0.001 | 0.341 | 0.559 |
| NPI, ≥1 domain | 79 (15.1) | 51 (26.0) | 51 (26.3) | 131 (60.1) | 154.993 | <0.001 | 154.695 | <0.001 | 0.004 | 0.952 |
CIND: cognitive impairment, no dementia;
NPI: neuropsychiatric inventory;
aMCI: amnestic-MCI;
Other: CIND types other then aMCI.
Table 4 shows the results of the most adjusted multivariate model (Model 3, described under methods) comparing the prevalence of NPS in CIND and participants with dementia or those who were cognitively normal. The results of Model 3 will be discussed in detail and can be found in Table 4. Results from Model 1 and Model 2 are not presented, but yielded similar results to Model 3. Table 4 also displays 95% confidence intervals (CIs) for all comparisons. When comparing CIND to normal, odds ratios (ORs) ranged from 10.0 (95% CI: 3.3–29.7, p <0.001, df = 1) for apathy to 1.1 (95% CI: 0.6–1.9, p = 0.746, df = 1) for nighttime behaviors. The NPS that were significantly different in CIND when compared to normal participants were agitation/aggression, depression/dysphoria, anxiety, apathy/indifference, irritability/lability, and appetite/eating. When comparing dementia to CIND, ORs ranged from 9.5 (95% CI: 3.0–30.7, p <0.001, df = 1) for delusions to 1.3 (95% CI: 0.6–2.9, p = 0.453, df = 1) for nighttime behavior. The NPS that were significantly different in dementia when compared to CIND were delusions, hallucinations, apathy/indifference, disinhibition, irritability/lability, and appetite/eating. When comparing aMCI to CIND-Other, ORs ranged from 1.6 (95% CI: 0.6–4.6, p = 0.388, df = 1) for anxiety to 0.7 (see table 4) for a number of NPI domains. None of the comparisons of aMCI to CIND-Other revealed significant differences.
Table 4.
| CIND1 vs. Normal | Dementia vs. CIND1 | Dementia vs. Normal | aMCI3 vs. Other4 | |||||
|---|---|---|---|---|---|---|---|---|
| NPI2 Domain | OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value |
| Delusions | N/A^ | − | 9.5 (3.0–30.7) | <0.001 | N/A^ | − | 0.8 (0.05–10.7) | 0.842 |
| Hallucinations | 3.9 (0.4–38.1) | 0.239 | 4.6 (1.4–14.6) | 0.01 | 38.9 (4.5–334.6) | 0.001 | N/A^ | − |
| Agitation/Aggression | 3.5 (1.1–11.3) | 0.038 | 2.0 (0.9–4.5) | 0.081 | 12.7 (3.6–44.3) | <0.001 | 0.7 (0.2–2.4) | 0.631 |
| Depression/dysphoria | 3.8 (2.3–6.4) | <0.001 | 1.5 (0.9–2.5) | 0.117 | 8.7 (4.4–17.1) | <0.001 | 0.8 (0.5–1.5) | 0.595 |
| Anxiety | 5.8 (2.0–16.3) | 0.001 | 1.9 (0.9–4.1) | 0.103 | 11.1 (3.4–36.5) | <0.001 | 1.6 (0.6–4.6) | 0.388 |
| Elation/euphoria | N/A^ | − | 1.5 (0.1–42.6) | 0.811 | N/A^ | − | N/A^ | − |
| Apathy/indifference | 10.0 (3.3–29.7) | <0.001 | 2.0 (1.1–3.8) | 0.03 | 28.1 (8.6–91.0) | <0.001 | 1.2 (0.5–3.2) | 0.708 |
| Disinhibition | 2.8 (0.3–29.9) | 0.398 | 4.2 (1.1–16.6) | 0.037 | 22.1 (2.3–212.0) | 0.007 | N/A^ | − |
| Irritability/lability | 3.5 (1.9–6.5) | <0.001 | 1.9 (1.0–3.5) | 0.034 | 11.8 (5.1–27.2) | <0.001 | 0.7 (0.3–1.5) | 0.375 |
| Motor Disturbance | N/A^ | − | 5.5 (0.5–60.1) | 0.16 | N/A^ | − | N/A^ | − |
| Nighttime Behaviors | 1.1 (0.6–1.9) | 0.746 | 1.3 (0.6–2.9) | 0.453 | 1.2 (0.5–3.1) | 0.72 | 1.5 (0.7–3.6) | 0.316 |
| Appetite/eating | 5.6 (2.0–15.9) | 0.001 | 2.3 (1.1–4.9) | 0.029 | 12.0 (3.7–38.6) | <0.001 | 0.7 (0.3–2.2) | 0.604 |
CIND: cognitive impairment, no dementia;
NPI: neuropsychiatric inventory;
aMCI: amnestic-MCI;
Other: CIND types other then aMCI;
ORs were adjusted for age, education, ApoE ε4 status, CDR, and 3MS;
A value of “N/A” listed next to an NPI domain indicates that one of the groups in that comparison had a value of “0.”
DISCUSSION
Because of its population base, sample size, and comprehensive participant characterization, the CCS can provide insights into the prevalence of NPS in different forms of CIND. Our data confirm the high prevalence of NPS in CIND reported by other population studies, especially for affective symptoms.10,13 We report an overall past month prevalence of NPS in CIND (31%) that falls intermediate to that of the cognitively normal (15.1%) and those with dementia (60.1%). Comparison of subgroups of CIND, show no differences between those with aMCI and other forms of CIND.
When looking strictly at prevalence, much higher rates of depression were found in both CIND and dementia, with CIND rates falling intermediate. The most distinguishing features between the cognitively normal and CIND were depression, anxiety, apathy/indifference, irritability/lability, and appetite/eating. The most distinguishing feature between those with CIND and those with dementia was the presence of delusions. In fact, delusions were rare in all groups except dementia, where they were present in 15.6% of individuals.
The present study can be compared with two prior population-based studies (the Cardiovascular Health Study [CHS] and the Mayo Clinic Study of Aging [MCSA]) that analyzed the frequency of NPS in MCI.10,13 Like our study, both these studies were population-based and used similar instruments to measure NPS. One main difference between this and the previous two studies is that our study offers a complete comparison within a single population. The CHS study used normal participants from the CCS, and the MCSA did not include comparisons with dementia participants.
Both CHS and MCSA report the most prevalent NPS in MCI to be depression (CHS 20%, MCSA 27%), apathy (CHS 15%, MCSA 18.5%), and irritability (CHS 15%, MCSA 19.4%). Our participants with CIND had a similar prevalence of depression/dysphoria, but a lower prevalence of other NPS, including apathy and irritability. This may be explained by selection bias, as our study was population-based. However, as in the previous two studies, affective symptoms seem to predominate. When comparing the ORs in our study with those from the MCSA, similar domains had the highest associations with CIND: depression, apathy, irritability, anxiety, and appetite. Lastly, the MCSA analysis split MCI into aMCI and non-aMCI. They found the prevalence of apathy, agitation, and irritability to be slightly higher in persons with aMCI and depression, anxiety, delusion, and disinhibition to be slightly higher in persons with non-aMCI. In our analysis, no differences were found in NPS prevalence between aMCI participants and those with other types of CIND. Because aMCI is a purer cognitive disorder, it was expected that these participants would have fewer behavior symptoms.
Strengths/Limitations
The strengths of this study include its substantial sample size, extensively tested and well-designed measurements of neuropsychiatric symptoms (the NPI), and the carefully determined diagnoses of CIND/MCI. In addition, this is a study with a very high participation rate. In terms of limitations, the cross-sectional nature of this study is of note, as is the use of the NPI, which utilizes data provided by the caregiver, not the patient himself. Also, the Cache County population is predominantly Caucasian, limiting the generalizability of the results to populations with otherwise similar attributes.
Conclusions/Future
This analysis confirmed other population studies indicating high rates of neuropsychiatric symptoms in different forms of CIND. However, unlike some other studies, there was little difference between subtypes of CIND. The clinical implication of these results is that NPS, as measured by the NPI, are prevalent in all types of CIND and may be a beneficial tool in identification of such patients. Our next step will be to ascertain whether these symptoms can be used to predict a higher rate of incident dementia.
Acknowledgments
Grants: Cache County Memory Study and Dementia Progression Study and Bryan ADR: R01AG11380, R01AG21136, and AG02377.
Authors have no conflicts of interest unless listed: Dr. Rosenberg receives research support from Pfizer, Merck, Elan, Lilly, NIA, and the American Foundation for Aging research. Dr. Lyketsos receives grant support (research or CME) from NIMH, NIA, Associated Jewish Federation of Baltimore, Weinberg Foundation, Forest, GlaxoSmithKline, Eisai, Pfizer, Astra-Zeneca, Lilly, Ortho-McNeil, Bristol-Myers; is a consultant/advisor for Astra-Zeneca, GlaxoSmithKline, Eisai, Novartis, Forest, Supernus, Adlyfe, Takeda, Wyeth, Lundbeck, Merz, Lilly, Genentech; Honorarium or travel support from Pfizer, Forest, GlaxoSmithKline.
Footnotes
Previous Presentation: Information presented at 2009 AAGP Conference in Honolulu, Hawaii.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer's disease in the United States and the public health impact of delaying disease onset. Am J Public Health. 1998;88(9):1337–1342. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rabins PV, Lyketsos CG, Steele CD. Practical Dementia Care. 2nd ed. Oxford University Press; New York: 2006. [Google Scholar]
- 3.Petersen RC, Stevens JC, Ganguli M, et al. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56(9):1133–1142. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- 4.Lyketsos CG, Colenda CC, Beck C, et al. Position statement of the American Association for Geriatric Psychiatry regarding principles of care for patients with dementia resulting from Alzheimer disease. Am J Geriatr Psychiatry. 2006;14(7):561–572. doi: 10.1097/01.JGP.0000221334.65330.55. [DOI] [PubMed] [Google Scholar]
- 5.Tuokko H, Frerichs RJ. Cognitive impairment with no dementia (CIND): longitudinal studies, the findings, and the issues. Clin Neuropsychol. 2000;14(4):504–525. doi: 10.1076/clin.14.4.504.7200. [DOI] [PubMed] [Google Scholar]
- 6.Mielke MM, Rosenberg PB, Tschanz J, et al. Vascular factors predict rate of progression in Alzheimer disease. Neurology. 2007;69(19):1850–1858. doi: 10.1212/01.wnl.0000279520.59792.fe. [DOI] [PubMed] [Google Scholar]
- 7.De Ronchi D, Palmer K, Pioggiosi P, et al. The combined effect of age, education, and stroke on dementia and cognitive impairment no dementia in the elderly. Dement Geriatr Cogn Disord. 2007;24(4):266–273. doi: 10.1159/000107102. [DOI] [PubMed] [Google Scholar]
- 8.Tschanz JT, Welsh-Bohmer KA, Lyketsos CG, et al. Conversion to dementia from mild cognitive disorder: the Cache County Study. Neurology. 2006;67(2):229–234. doi: 10.1212/01.wnl.0000224748.48011.84. [DOI] [PubMed] [Google Scholar]
- 9.Steinberg M, Shao H, Zandi P, et al. Point and 5-year period prevalence of neuropsychiatric symptoms in dementia: the Cache County Study. Int J Geriatr Psychiatry. 2008;23(2):170–177. doi: 10.1002/gps.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lyketsos CG, Lopez O, Jones B, et al. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA. 2002;288(12):1475–1483. doi: 10.1001/jama.288.12.1475. [DOI] [PubMed] [Google Scholar]
- 11.Modrego PJ, Ferrandez J. Depression in patients with mild cognitive impairment increases the risk of developing dementia of Alzheimer type: a prospective cohort study. Arch Neurol. 2004;61(8):1290–1293. doi: 10.1001/archneur.61.8.1290. [DOI] [PubMed] [Google Scholar]
- 12.Palmer K, Berger AK, Monastero R, et al. Predictors of progression from mild cognitive impairment to Alzheimer disease. Neurology. 2007;68(19):1596–1602. doi: 10.1212/01.wnl.0000260968.92345.3f. [DOI] [PubMed] [Google Scholar]
- 13.Geda YE, Roberts RO, Knopman DS, et al. Prevalence of neuropsychiatric symptoms in mild cognitive impairment and normal cognitive aging: population-based study. Arch Gen Psychiatry. 2008;65(10):1193–1198. doi: 10.1001/archpsyc.65.10.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taragano FE, Allegri RF, Krupitzki H, et al. Mild behavioral impairment and risk of dementia: a prospective cohort study of 358 patients. J Clin Psychiatry. 2009;70(4):584–592. doi: 10.4088/jcp.08m04181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morris JC, Storandt M, Miller JP, et al. Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol. 2001;58(3):397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- 16.Tuokko H, Frerichs R, Graham J, et al. Five-year follow-up of cognitive impairment with no dementia. Arch Neurol. 2003;60(4):577–582. doi: 10.1001/archneur.60.4.577. [DOI] [PubMed] [Google Scholar]
- 17.Bennett DA, Wilson RS, Schneider JA, et al. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59(2):198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- 18.Breitner JC, Wyse BW, Anthony JC, et al. APOE-epsilon4 count predicts age when prevalence of AD increases, then declines: the Cache County Study. Neurology. 1999;53(2):321–331. doi: 10.1212/wnl.53.2.321. [DOI] [PubMed] [Google Scholar]
- 19.Lyketsos CG, Steinberg M, Tschanz JT, et al. Mental and behavioral disturbances in dementia: findings from the Cache County Study on Memory in Aging. Am J Psychiatry. 2000;157(5):708–714. doi: 10.1176/appi.ajp.157.5.708. [DOI] [PubMed] [Google Scholar]
- 20.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48(8):314–318. [PubMed] [Google Scholar]
- 21.Tschanz JT, Welsh-Bohmer KA, Plassman BL, et al. An adaptation of the modified mini-mental state examination: analysis of demographic influences and normative data: the cache county study. Neuropsychiatry Neuropsychol Behav Neurol. 2002;15(1):28–38. [PubMed] [Google Scholar]
- 22.Jorm AF, Jacomb PA. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): socio-demographic correlates, reliability, validity and some norms. Psychol Med. 1989;19(4):1015–1022. doi: 10.1017/s0033291700005742. [DOI] [PubMed] [Google Scholar]
- 23.Kawas C, Segal J, Stewart WF, et al. A validation study of the Dementia Questionnaire. Arch Neurol. 1994;51(9):901–906. doi: 10.1001/archneur.1994.00540210073015. [DOI] [PubMed] [Google Scholar]
- 24.Tschanz JT, Welsh-Bohmer KA, Skoog I, et al. Dementia diagnoses from clinical and neuropsychological data compared: the Cache County study. Neurology. 2000;54(6):1290–1296. doi: 10.1212/wnl.54.6.1290. [DOI] [PubMed] [Google Scholar]
- 25.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 26.Diagnostic and statistical manual of mental disorders: DSM-III-R. American Psychiatric Association; Washington, DC: 1987. [Google Scholar]
- 27.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 28.Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 29.Ebly EM, Hogan DB, Parhad IM. Cognitive impairment in the nondemented elderly. Results from the Canadian Study of Health and Aging. Arch Neurol. 1995;52(6):612–619. doi: 10.1001/archneur.1995.00540300086018. [DOI] [PubMed] [Google Scholar]
- 30.Cummings JL. The Neuropsychiatric Inventory: assessing psychopathology in dementia patients. Neurology. 1997;48(5)(suppl 6):S10–S16. doi: 10.1212/wnl.48.5_suppl_6.10s. [DOI] [PubMed] [Google Scholar]
- 31.Lyketsos CG, Galik E, Steele C, et al. The General Medical Health Rating: a bedside global rating of medical comorbidity in patients with dementia. J Am Geriatr Soc. 1999;47(4):487–491. doi: 10.1111/j.1532-5415.1999.tb07245.x. [DOI] [PubMed] [Google Scholar]