Abstract
Inborn errors of the genes encoding two of the four human JAKs (JAK3 and TYK2) and three of the six human STATs (STAT1, STAT3, and STAT5B) have been described. We review the disorders arising from mutations in these five genes, highlighting the way in which the molecular and cellular pathogenesis of these conditions has been clarified by the discovery of inborn errors of cytokines, hormones, and their receptors, including those interacting with JAKs and STATs. The phenotypic similarities between mice and humans lacking individual JAK-STAT components suggest that the functions of JAKs and STATs are largely conserved in mammals. However, a wide array of phenotypic differences has emerged between mice and humans carrying bi-allelic null alleles of JAK3, TYK2, STAT1, or STAT5B. Moreover, the high level of allelic heterogeneity at the human JAK3, STAT1, and STAT3 loci has revealed highly diverse immunological and clinical phenotypes, which had not been anticipated.
Introduction
None of the known Janus kinases (JAKs), or Signal Transducer and Activator of Transcription (STAT) molecules, or associated upstream receptors and downstream targets was discovered through investigations of human patients 1. Nevertheless, the discovery of germline mutations in two of the four human JAKs (JAK3 and TYK2) and three of the six human STATs (STAT1, STAT3, STAT5B) has provided considerable biological insight. The phenotypic similarities between mice and humans lacking individual JAK-STAT components suggest that the functions of JAKs and STATs are largely conserved in mammals. However, a wide array of phenotypic differences has emerged between mice and humans with defects in a single JAK-STAT component. Differences in immunological phenotypes may reflect intrinsic mechanistic differences between the two species, as illustrated, for example, by the insertion of a minisatellite into the mouse STAT2 gene, preventing the recruitment of STAT4 in response to IFN-α/β 2. Differences in infectious phenotypes may also reflect differences between experimental infections in mice and natural infections in humans 3. We will review here the inborn errors affecting five human JAKs and STATs, which mostly manifest as immunological and infectious phenotypes, although extrahematological clinical features have also been documented for at least two of these disorders (STAT5B and STAT3). We will specifically focus on the biological lessons learned from these experiments of Nature.
Human severe combined immune deficiency reveals a critical role for JAK3-mediated signaling in lymphoid development and function
In 1950, Glanzman and Riniker described two infants with severe infections, diarrhea and failure to thrive, both of whom died with disseminated candidiasis. Postmortem examination revealed a marked depletion of lymphoid tissue 4. These cases probably represent the first description of severe combined immune deficiency (SCID) in humans, a genetically heterogenous group of conditions characterized by a lack of autologous T cells and extreme susceptibility to infections caused by a broad range of pathogens. SCID is inevitably fatal within the first few years of life, unless immune reconstitution is achieved therapeutically 5. The initial descriptions of SCID were consistent with autosomal recessive (AR) inheritance, but it soon became clear that most of the patients with SCID identified in the United States were male 6, and that SCID was often inherited as an X-linked recessive (XR) trait 7. Patients with XR SCID (X-SCID) typically lack circulating T and NK lymphocytes, but have normal numbers of B cells (T− B+NK− SCID phenotype).
In 1992, Takeshita et al. cloned the IL2RG gene, encoding the γ chain of the interleukin-2 receptor (IL-2R) 8. One year later, Warren Leonard’s group mapped the IL2RG gene to Xq13, where the X-SCID locus had been previously mapped, and showed it to be mutated in patients with X-SCID 9 (Table 1; Figure 1). The IL-2Rγ chain was initially defined as a component of the heterotrimeric high-affinity IL-2R, which also includes IL-2Rα and IL-2Rβ 8. However, over the course of a decade, it was progressively demonstrated that the IL-2Rγ chain is common to the receptors for IL-4, IL-7, IL-9, IL-15 and IL-21 10–15. This led to its being renamed the common γ chain, or γc. None of these cytokine receptors has intrinsic kinase activity, and their ability to mediate signal transduction upon ligand binding is dependent on the activation of JAK1 and JAK3 16. In particular, JAK1 associates with the cytokine-specific receptor subunit, whereas JAK3 binds to the γc 17. This observation led to the discovery that JAK3 mutations account for an AR variant of T− B+ NK− SCID in female and male patients without IL2RG mutations 18,19. The molecular mechanisms accounting for the impaired development of T and NK lymphocytes in patients with defects of γc and JAK3 signaling were unraveled by studies in humans and mice showing that IL-7 serves an essential role for T lymphocyte differentiation 20,21, whereas IL-15 is involved in NK cell development22,23.
TABLE 1.
Inborn errors of five human JAKs and STATs and their related defects
| Gene | Inheritance | Allele | Cytokines | Hormones |
|---|---|---|---|---|
| JAK3 | AR | LOF, HPO | IL-2, IL-4, IL-7, IL-9, IL-15, IL-21 | |
| IL2RG | XR | LOF, HPO | IL-2, IL-4, IL-7, IL-9, IL-15, IL-21 | |
| IL7R | AR | LOF | IL-7 | |
| STAT5B | AR | LOF | IL-2, IL-7, IL-15 | GH |
| IL2R | AR | LOF | IL-2 | |
| GHR | AR | ? | GH | |
| AD | ? | GH | ||
| STAT1 | AR | LOF, HPO | IFN-a/b, IFN-g, IFN-l, IL-27 | |
| AD | LOF | IFN-g | ||
| AD | HPR | IFN-a/b, IFN-g, IFN-l, IL-27 | ||
| IFNGR1 | AR | LOF, HPO | IFN-g | |
| AD | LOF | IFN-g | ||
| IFNGR2 | AR | LOF, HPO | IFN-g | |
| IRF8 | AD | HPO | ||
| IL12B | AR | LOF | IL-12, IL-23 | |
| IL12RB1 | AR | LOF | IL-12, IL-23 | |
| NEMO | XR | HPO | Mulitple | |
| CYBB | XR | HPO | ||
| TYK2 | AR | LOF | IFN-a/b, IFN-l, IL-6, IL-10, IL-12, IL-23… | |
| IL12B | AR | LOF | IL-12, IL-23 | |
| IL12RB1 | AR | LOF | IL-12, IL-23 | |
| IL-10 | AR | ? | IL-10 | |
| IL10RB1 | AR | LOF | IL-10 | |
| IL-10RB2 | AR | LOF | IL-10, IL-22, IFN-l | |
| STAT3 | AD | LOF | IL-6, IL-10, IL-11, IL-21, IL-23, etc. | |
| IL11RA | AR | LOF | IL-11 | |
| IL17F | AD | HPO | IL-17A/F, IL-17F/F# | |
| IL17RA | AR | LOF | IL-17A/A, IL-17A/F, IL-17F/F# | |
| STAT1 | AD | HPR | IFN-a/b, IFN-g, IFN-l, IL-27§ | |
| IL12B | AR | LOF | IL-12, IL-23 | |
| IL12RB1 | AR | LOF | IL-12, IL-23 | |
| IL-10 | AR | ? | IL-10 | |
| IL10RB1 | AR | ? | IL-10 | |
| IL-10RB2 | AR | ? | IL-10, IL-22, IFN-l |
AR: Autosomal recessive
AD: Autosomal dominant
XR: X-linked recessive
LOF: Loss-of-function
HPO: Hypomorphic
HPR: Hypermorphic
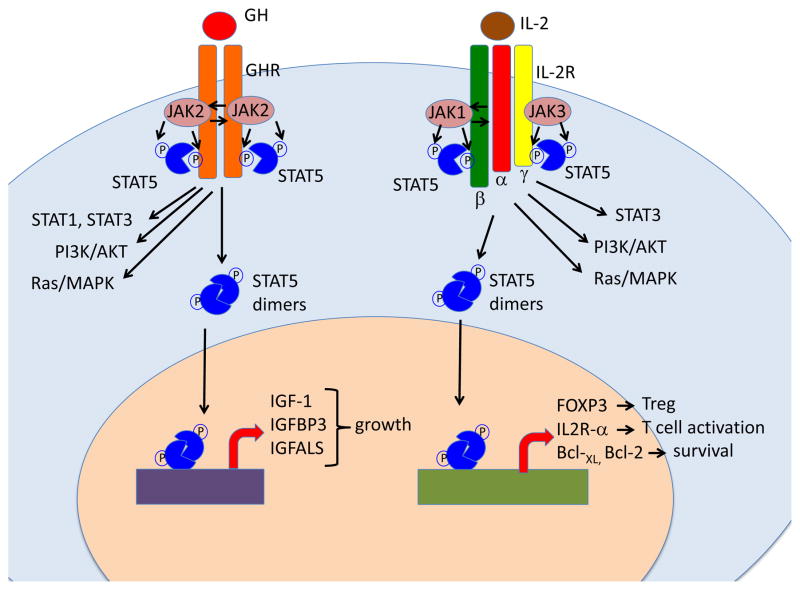
Figure 1. Schematic representation of the role of JAK/STAT5 signaling in response to growth hormone and to interleukin-2.
Left: Binding of growth hormone (GH) to GH receptor (GHR) homodimer triggers activation of the JAK2 kinase that phosphorylates the GHR, creating docking sites for STAT proteins. In the Figure, recruitment and JAK2-mediated phosphorylation of STAT5 is shown, but STAT1 and STAT3 are also activated. In addition, GH also triggers activation of the PI3K/AKT and Ras/MAPK pathways. Phosphorylated STAT5 proteins dimerize and translocate to the nucleus where they drive activation of target genes. Although both STAT5A and STAT5B are activated in response to GH, the genes that encode for Insulin Growth Factor-1 (IGF-1), IGF binding protein 3 (IGFBP3) and for the acid labile subunit of IGF-like binding protein (IGFALS) are under the direct control of STAT5B, and their expression is markedly repressed in STAT5B-mutated patients. This results in severe growth failure.
Right: binding of interleukin-2 (IL-2) to its high-affinity receptor comprising IL-2Rα, -β and -γ chains promotes activation of JAK1 and JAK3 proteins, and recruitment of STAT3 and STAT5 to the phosphorylated IL-2R chains. PI3K/AKT and the Ras/MAPK pathways are also activated. STAT5A and STAT5B are phosphorylated and form homo- and hetero-dimers that translocate to the nucleus. Genes that are directly controlled by STAT5B and whose expression is significantly reduced in STAT5B-mutated patients include: FOXP3 (that promotes development and function of Treg cells), IL2RA (that favors T cell activation), and the genes that encodes for anti-apoptotic factors Bcl-2 and Bcl-XL. Failure to activate these genes in response to IL-2 explains the association of immunodeficiency and immune dysregulation in patients with STAT5B deficiency.
The identification of patients with γc and JAK3 defects preceded the generation of mice lacking the corresponding genes 24–27. These mice had a T− B− NK− phenotype, indicating a requirement for γc-JAK3 signaling in B-cell development in mice, but not in humans. The generation of circulating B lymphocytes in humans is not disturbed by defects in γc and JAK3 signaling, but the B cells produced harbor intrinsic abnormalities, with impaired class-switch recombination and defective antibody production 28,29. The molecular basis of B cell autonomous functional abnormalities in patients with defects of γc and JAK3 signaling has been recently unraveled. T follicular helper (TFH) cells secrete IL-21, a potent inducer of proliferation, Ig isotype switching, plasma cell generation and Ab secretion by human B cells 30,31. The binding of IL-21 to a heterodimeric receptor composed of IL-21R and γc induces activation of the JAK-STAT pathway. In particular, IL-21-induced STAT3 activation results in the upregulation of PRDM1 (encoding BLIMP-1) and XBP1, which are required for plasma cell generation 32,33. In vitro stimulation of X-SCID and JAK3-deficient B cells with CD40 ligand (CD40L) and IL-21 does not induce proliferation, plasmablast differentiation or Ab secretion, strongly suggesting that IL-21 is the primary γc-dependent cytokine required for the maturation of human Ab responses 34. Studies of infants with SCID have thus provided insight into the essential role played by γc and JAK3 signaling in the development of T and NK cells, and in the function of B cells.
Whereas null mutations in IL2RG and JAK3 are responsible for SCID, hypomorphic mutations in the same genes may cause other immunodeficiencies, ranging from life-threatening Omenn’s syndrome to milder combined immunodeficiencies 35–38. Mutations in the intracytoplasmic tail of γc allowing residual JAK3 binding have been associated with a delay in the appearance and gradual decline of circulating T cells 39. The development of autologous T cells has also been reported in patients with JAK3 mutations partially permissive for JAK3 expression and for JAK3 and STAT5 phosphorylation 35. The autologous T cells that develop in such patients display an activated phenotype and a restricted TCR repertoire indicative of very low levels of thymopoiesis and homeostatic proliferation 40,41. However, the R222C mutation in the intracytoplasmic tail of γc is associated with normal numbers of circulating T lymphocytes, a normal polyclonal T-cell repertoire and normal thymus morphology 42,43. Some γc mutations may thus be permissive for IL-7-mediated signaling and, hence, for normal thymic T-cell development. However, T cells carrying the R222C γc mutation bind IL-2 much less strongly than wild-type T cells, thus accounting for the patients’ immunodeficiency and infectious diseases 42. Other patients have been identified in whom hypomorphic mutations in the IL2RG, IL7R and JAK3 genes are even associated with prominent clinical features of immune dysregulation, including lymphoproliferation and autoimmunity 44. In one such patient with residual levels of JAK3 protein, the stimulation of circulating T lymphocytes with IL-2 in vitro led to normal proliferation, but no induction of Fas ligand (FasL) expression 44. Observations in patients with mutations of the IL2RA and STAT5B genes neatly confirm the critical role played by IL-2 in immune homeostasis.
Inborn errors of STAT5B, at the crossroads of immunodeficiency and immune dysregulation
In 1997, Sharfe et al. described an infant with severe bacterial, viral and fungal infections 45. Counts of autologous T lymphocytes were moderately low, T cells displayed a weak proliferative response to mitogens in vitro, and the patient displayed no rejection of an allogeneic skin graft. However, unlike children with SCID, the patient not only had circulating T cells but also developed peripheral lymphocytic proliferation and autoimmune primary biliary cirrhosis 46. The disease was caused by a homozygous mutation of the IL2RA gene, which prevented expression of IL-2Rα (CD25) 45 (Table 1; Figure 1). Ten years later, Caudy et al. described another child with biallelic IL2RA gene mutations, and a history of severe viral infections, autoimmune enteropathy, lymphoproliferation, insulin-dependent diabetes, autoimmune neutropenia and eczema 47. The clinical phenotype of these two patients included features of both combined immunodeficiency and severe autoimmunity, reminiscent of immune dysregulation-polyendocrinopathy-enteropathy-X-linked (IPEX) syndrome, an XR disorder caused by mutations of the FOXP3 gene, which is critically required for the development and function of CD4+ CD25hi T regulatory (Treg) cells 48,49. The association of immunodeficiency and autoimmunity in patients with CD25 deficiency reflects the biological role of IL-2-mediated signaling. In fact, interaction of IL-2 with its high-affinity receptor, composed of IL-2Rα, IL-2Rβ and γc is important both for the activation of effector CD4+ and CD8+ T cells and for the generation of Foxp3+ induced Treg (iTreg) lymphocytes from naive peripheral T lymphocytes 50. Moreover, CD25 deficiency impairs survival and the fitness of mature nTregs in mice 51,52.
In 2003, Kofoed et al. described a patient with short stature and growth hormone (GH) insensitivity syndrome (GHIS), facial dysmorphism, severe infections and lymphoid interstitial pneumonitis, with a homozygous missense mutation in the STAT5B gene, which encodes a key component of the IL-2R signaling pathway 53. Several other patients with biallelic STAT5B mutations have since been reported 54, in whom GHIS was associated with susceptibility to various infections, autoimmune manifestations and eczema. Human STAT5A and STAT5B are very similar (>90% identity) in terms of their cDNA and protein product sequences, suggesting that they may have been generated by gene duplication. However, the STAT5A and STAT5B proteins differ in the last six amino acids of the DNA-binding domain and in 20 amino acids of the transactivation domain. These differences have important biological and clinical implications, as demonstrated by the identification of STAT5B-deficient patients. The interaction of GH with its receptor (GHR) triggers JAK2 activation and STAT5B phosphorylation, leading to the production of insulin growth factor 1 (IGF-1), a key factor for body growth (Figure 1). Patients with GHR deficiency display the same extrahematological signs as STAT5B-deficient patients, with postnatal growth retardation and GH insensitivity 55,56. By contrast, mice lacking STAT5B present a loss of sexually dimorphic body growth, such that Stat5b−/− males are similar in size to wild-type females, whereas wild-type males are much larger 57.
STAT5B-deficient patients also display various autoimmune and allergic signs, including autoimmune thyroiditis, idiopathic thrombocytopenic purpura, lymphocytic interstitial pneumonitis and severe eczema 53,54,58–60. Individual targeting of the Stat5a and Stat5b genes in mice is not associated with significant immune abnormalities 57,61. By contrast, Stat5a, Stat5b double-deficient (Stat5a−/−Stat5b−/−) mice have very small numbers of Treg cells in both the thymus and the periphery 62–64, leading to signs of autoimmunity and lymphocytic infiltration in multiple target organs 65. These data are consistent with the role of Stat5 in the IL-2-induced upregulation of Foxp3 66. The very small number of CD4+ CD25hi Foxp3+ cells and the impairment of their function in STAT5B-deficient patients 67 may therefore result from impaired IL-2 signaling, accounting for the signs of immune dysregulation associated with the disease, which are clinically related to those seen in CD25-deficient patients. These patients also display severe infections. So, what is the mechanism underlying this immunodeficiency? Higher rates of T-lymphocyte apoptosis are observed only in mice lacking both STAT5A and STAT5B 68. Nevertheless, an increase in T-lymphocyte apoptosis may contribute to the T-cell lymphopenia observed in STAT5B-deficient patients. Defects of effector T cells may therefore account for the patients’ broad and profound susceptibility to infections. These observations indicate that STAT5A and STAT5B play largely redundant roles in the development and function of the immune and endocrine systems in mice, whereas STAT5B has unique, non-redundant functions in growth and immunity in humans.
Inborn errors of STAT1 immunity: loss-of-function and gain-of-function alleles reveal a double-edged sword
Mutations in STAT1 were first identified in studies of patients with Mendelian susceptibility to mycobacterial diseases (MSMD), who are prone to clinical disease caused by weakly virulent mycobacterial species, such as Bacille Calmette-Guérin (BCG) vaccines and environmental mycobacteria (Figure 2) (Table 1) 69. They are also prone to tuberculosis and salmonellosis, and, more rarely, to other infections caused by intramacrophagic bacteria, fungi and parasites. The first genetic studies, carried out between 1996 and 2000, implicated the IFNGR1 and IFNGR2 genes, encoding the two chains of the IFN-γ receptor (Table 1; Figure 2). Allelic heterogeneity at the IFNGR1 locus defined four forms of IFN-γR1 deficiency, including two forms of complete and two forms of partial deficiency 70. There is also allelic heterogeneity at the IFNGR2 locus, with at least three forms of disease 71. The narrow range of infectious diseases in these patients was surprising, given the broader susceptibility observed in the mouse model 72, although it gradually became apparent that pathogens other than Mycobacterium and Salmonella posed a threat to these patients 70,73.
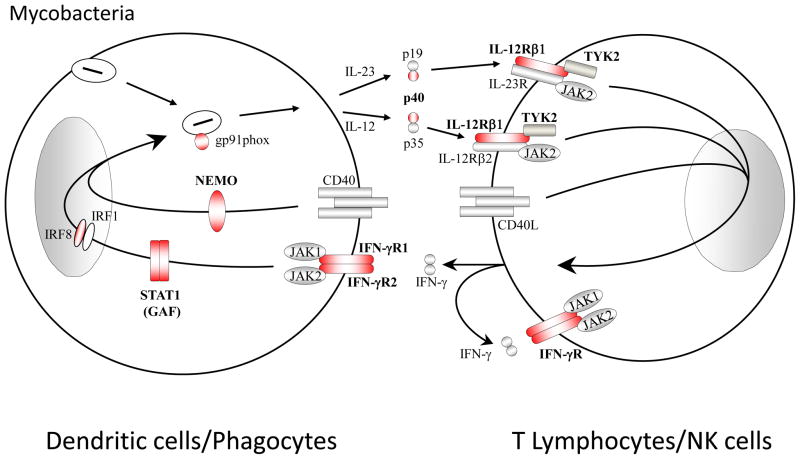
Figure 2. Inborn errors in the IL-12/23-IFN-γ pathway underlie Mendelian susceptibility to mycobacterial diseases (MSMD).
Schematic diagram of cytokine production and cooperation between phagocytes/dendritic myeloid cells and NK/T lymphocytes. The IL-12/IFN-γ circuit, the CD40/CD40L pathway and the oxidative burst (mediated in part by CYBB-encoded gp91, a component of the NADPH phagocyte oxydase) are crucial for protective immunity against mycobacterial infection in humans. Mutations in IFNGR1 or IFNGR2, encoding the ligand-binding and associated chains of the IFN-gR, impair cellular responses to IFN-g. Likewise, heterozygous dominant-negative mutations in STAT1 impair IFN-g but not IFN-a/b responses. Mutations in IL-12p40 or IL-12Rb1 impair IL-12-dependent induction of IFN-g. Mutations in CYBB that selectively impair the respiratory burst in monocyte-derived macrophages are associated with MSMD. Heterozygous dominant-negative mutations in IRF8 impair the development of IL-12-producing CD1cCD11c DCs. Proteins for which mutations in the corresponding genes have been identified and associated with MSMD, are shown in red. The allelic heterogeneity is described in Table 1.
The disorder is intrinsic to the hematopoietic lineage, as mycobacterial disease can be cured by HSCT, in both mice and humans 74,75. It is however unclear whether IFN-γ is required to activate T cells or phagocytes, or both, during the course of mycobacterial infection, and whether its activation of T cells results indirectly in activation of phagocytes. The intramacrophagic nature of most of the pathogens seen in these patients suggests that human IFN-γ functions more as a macrophage-activating factor than as an antiviral interferon. It has also been shown that human IL-12, which is secreted by phagocytes, is an essential IFN-γ-inducing factor, as patients with IL-12p40 (Prando C. et al., unpublished) or IL-12Rβ1 deficiency 76 display MSMD with poor production of IFN-γ by both NK cells and T cells. The nature of the phagocytic cells producing IL-12 in this process remains unclear, although MSMD patients with AD IRF8 deficiency lack a potent IL-12-producing leukocyte subset, the CD1c+ CD11c+ dendritic cells 77. The genes controlling the microbe-induced production of IL-12 by phagocytes in this process have remained elusive. However, the T cell-dependent, CD40-dependent induction of IL-12 has been shown to be important in this process, as this pathway is disrupted in patients with an XR form of MSMD due to specific mutations in the NEMO gene whereas most other NF-κB pathways are intact 78,79.
In macrophages, IFN-γ controls the constitutive and inducible expression of a wide range of genes. The IFN-γ-inducible target genes in leukocytes, including those operating specifically in macrophages in the control of mycobacteria, remain to be determined. However, clues to the identity of these targets were provided serendipitously by the discovery of MSMD-causing mutations in CYBB, which encodes the gp91 subunit of the phagocyte NADPH oxidase. CYBB alleles selectively deleterious in monocyte-derived macrophages, but not in monocytes and granulocytes, were found in two kindreds with another XR form of MSMD 80. This observation suggests that CYBB and, perhaps, other genes controlling the respiratory burst may be key targets of IFN-γ in host defense against tuberculous mycobacteria. IFN-γ target genes must, in any case, be STAT1-dependent, because various heterozygous mutations of STAT1 were shown, from 2001 onwards, to be associated with the impairment, but not abolition of IFN-γ responses and MSMD 81,82. This finding was surprising, because STAT1 is also required for responses to IFN-α/β. Human STAT1 was the first member of the STAT family to be identified as a key molecule required for cellular responses to IFN-α/β 83, and its role in this and IFN-γ pathways has been clearly described in both human and mouse cells 84. The STAT1 alleles found in these MSMD patients are intrinsically null for both signaling pathways: the activation of both STAT1 homodimers (GAF) and STAT1-STAT2-IRF9 heterotrimers (ISGF3). However, it was dominant for GAF activation (by negative dominance) but recessive for ISGF3 activation (without negative dominance and even without haplo-insufficiency) in heterozygous cells. In other words, heterozygosity for the STAT1 mutations resulted in a normal response of the patients’ cells to IFN-α/β (for ISGF3), but not to IFN-γ (for GAF), accounting for the patients displaying MSMD but no viral phenotype.
Viral diseases have not been documented in patients with AD MSMD carrying STAT1 mutations, and have been reported in only a few patients carrying IFNGR1 or IFNGR2 mutations 70. The observation that STAT1 alleles may underlie MSMD without susceptibility to viral diseases was confirmed indirectly in 2003 by the identification of the first patients with an AR form of complete STAT1 deficiency 85. These patients display overt susceptibility to both mycobacterial and viral infections. Their cells do not respond to either IFN-γ or IFN-α/β. Unlike MSMD patients with STAT1 mutations, whose outcome is favorable, these patients died in the absence of HSCT. The underlying defect was subsequently shown to be broader, impairing responses to IFN-λ and IL-27 86. Other patients with a similarly broad and profound susceptibility to viral infections have since been described 87,88. Hypomorphic alleles underlie partial forms of AR STAT1 deficiency with a milder bacterial and viral phenotype 86,89–91. Patients with either form of AR STAT1 deficiency are broadly susceptible to viruses, including herpes simplex virus-1 (HSV-1) infections, which may cause HSV-1 encephalitis (HSE) (Figure 3) (Table 1) 85,92. The existence of a STAT1-independent IFN-α/β-responsive pathway, together with the action of antiviral molecules other than IFNs, might account for the control of at least some viral infections in patients lacking STAT1 87. Conversely, the contribution of IFN-α/β and IFN-λ to the viral phenotypes seen in patients bearing STAT1 mutations is unknown, as no patient lacking either the IFN-α/β receptor or the IFN-λ receptor has been described 93. However, IL-10Rβ-deficient patients would be expected to be unresponsive to IFN-λ, but not IFN-α/β, and such patients have never yet been reported to display susceptibility to any particular viral disease 94. The cells requiring STAT1 to control viruses have not been identified. The IFN-α/β and –λ target genes controlling viruses including HSV-1 have also remained elusive. The identification of more patients with inborn errors of IFN-α/β and –λ immunity, including AR forms of STAT1 deficiency, should help to define the molecular and cellular basis of human viral infections.
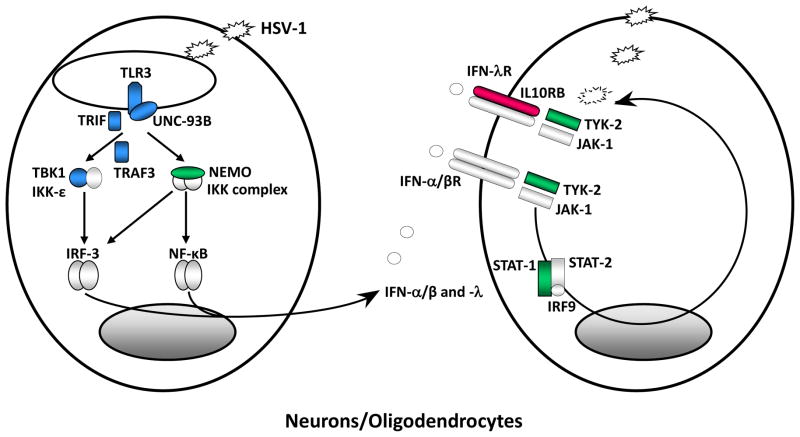
Figure 3. Inborn errors of TLR3-dependent, IFN-a/b and -l immunity underlie childhood herpes simplex virus 1 encephalitis (HSE).
Schematic representation of the production of and response to IFN-α/-β, and IFN-λ in anti-HSV-1 immunity in the central nervous system (CNS), based on the genetic dissection of children with HSE. Like most viruses HSV-1 produce dsRNA intermediates during its replication. TLR3 is an endosomal transmembrane receptor for dsRNA. The recognition of dsRNA by TLR3 induces activation of the IRF-3 and NF-kB pathways via TRIF, leading to IFN-α/-β and/or IFN-λ production. TLR3, UNC-93B, TRIF, TRAF3, TBK1 and NEMO deficiencies are all associated with impaired IFN-α/-β and/or IFN-λ production and predisposition to HSE in the course of primary infection by HSV-1. The binding of IFN-α/β and IFN-λ to their receptors induce the phosphorylation of JAK1 and TYK-2, activating the signal transduction proteins STAT-1, STAT-2 and IRF9. This complex is translocated as a heterotrimer to the nucleus, where it acts as a transcriptional activator, binding to specific DNA response elements in the promoter region of IFN-inducible genes. STAT-1 and TYK2 deficiencies are associated with impaired IFN-α/β responses and, for STAT1, impaired IFN-λ responses and predisposition to HSE. Proteins for which genetic mutations have been identified and associated with susceptibility to isolated HSE are shown in blue. Proteins for which genetic mutations have been identified and associated with susceptibility to mycobacterial, bacterial and viral diseases, including HSE, are shown in green. Proteins for which genetic mutations have been identified but not associated with susceptibility to infectious diseases are shown in red. This figure will be revised as new results are obtained with the genetic and immunological dissection of children with HSE and other viral diseases.
Surprisingly, a whole-exome sequencing study aiming to identify genetic etiologies of chronic mucocutaneous candidiasis (CMC) identified patients with AD CMC carrying heterozygous missense mutations affecting the STAT1 coiled-coil domain (CCD) (Figure 4) (Table 1) 95. A genome-wide linkage analysis led to the independent identification of similar mutations in other patients with CMC 96. These patients seem to be affected by a broader range of fungal diseases, as the same STAT1 mutations were recently found in patients with CMC and disseminated disease caused by Coccidioides immitis and Histoplasma capsulatum (Holland SM, personal communication). An explanation for this paradox was provided by the demonstration that the CMC-causing STAT1 alleles are actually gain-of-function 95. This observation accounts for the development, in some patients with CMC, of autoimmune signs, which may result from enhanced IFN-α/β immunity 97. Nuclear dephosphorylation, rather than hyperphosphorylation in the cytoplasm, is thought to be the principal mechanism underlying the gain of function of these alleles 95. These alleles have been shown to be both gain-of-function and dominant for all cytokines tested: IFN-α/β, IFN-γ, IFN-λ and IL-27. The CMC phenotype can also be accounted for by the very poor development of IL-17-producing T cells 95. Indeed, patients with AD IL-17F or AR IL-17RA deficiency are prone to CMC 98. The mechanism by which STAT1 gain-of-function alleles impair the development of IL-17 T cells remains to be deciphered. One possibility, based on work in the mouse model, is that IFNs and IL-27 strongly inhibit the development of IL-17-producing T cells 99. Alternatively, the gain-of-function STAT1 molecules may divert signals that are normally dependent on STAT3, downstream from IL-6, IL-21 and IL-23, all of which are potent inducers of IL-17 T cells 100. The search for new genetic etiologies of CMC should provide insight into the mechanisms actually involved. In any case, STAT1 is an example of a human gene for which loss-of-function mutations have been shown to cause certain infectious diseases, whereas gain-of-function mutations cause other infectious diseases.
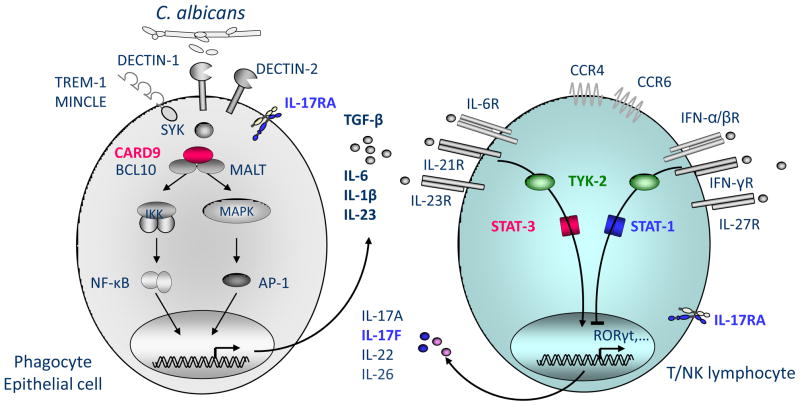
Figure 4. Inborn errors of IL-17 immunity underlie chronic mucocutaenous candidiasis (CMC).
Upon C. albicans recognition via various cell surface receptors, adaptor molecules SYK and CARD9 mediate the induction of pro-inflammatory cytokines by myeloid and epithelial cells. Pro-inflammatory cytokines, such as IL-6, IL-21 or IL-23, activate T lymphocytes via STAT3 resulting in their differentiation into IL-17-producing T cells. These cells constitute a major component of the immune defense against C. albicans, as mutations in IL-17F or IL-17RA underlie CMC. Gain of function mutations in STAT1 inhibit this differentiation by mechanisms that have remained elusive. Enhanced stimulation via IFN-a/b, IFN-g, IFN-d and IL-27 might be responsible for this phenotype. The molecule TYK2 is known to act upstream of STAT1 and STAT3. It is unclear whether patients with AR TYK2 deficiency display CMC. TYK2 deficient patients. Proteins represented in red are mutated in patients with CMC only. Proteins represented in blue are mutated in patients with CMC and other infections.
Inborn errors of STAT3 have hematological and extrahematological consequences
The AD form of hyper IgE syndrome (HIES), first described as Job’s syndrome in 1966, is characterized by the triad of eczema and recurrent staphylococcal skin and lung infections 101. In 1972, IgE elevation was added to the syndrome 102. Closer examination also revealed that these patients had somatic features, such as a characteristic facial appearance, for which simple immunologic explanations were unconvincing. The range of infections in these patients is relatively limited, restricted principally to a number of bacteria and fungi, including Staphylococcus aureus and Candida albicans. High IgE levels 102,103 and the impairment of antibody synthesis 33,104 and neutrophil chemotaxis 103,105 in some patients have been documented, but T-cell function was normal 103. The transmission of HIES in multiplex families suggested an AD mode of inheritance 103,106.
The seminal observation of TYK2 deficiency with atopy, staphylococcal disease and high levels of IgE, prompted detailed exploration of the IL-6 signaling pathway in patients with AD HIES 107, leading to the identification of dominant-negative STAT3 mutations as the cause of AD HIES (Figure 5) (Table 1) 108,109. The clinical penetrance of these alleles appeared to be complete, as sporadic cases were caused by de novo mutations. Most of the HIES-causing mutations in STAT3 are missense or in-frame deletions in the SH2 or DNA-binding domains. The mutations are intrinsically loss-of-function but result in inhibition of STAT3 function in a dominant-negative manner 108. Homozygous Stat3 deficiency led to the death of deficient mouse embryos, whereas heterozygotes had no reported phenotype. Therefore, Stat3 was necessary for survival, but there was no overt haploinsufficiency 110. This finding is consistent with the observation that dominant-negative STAT3 mutations decrease STAT3 homodimer activity to about 25% of the normal level 108,111. The embryonic lethality of Stat3−/− mice has made it necessary to explore various hematological and extrahematological tissue-specific deletions. These deletions are important models for STAT3 function in various tissues, but are far from exact mimics of AD HIES, which is partial and affects all tissues simultaneously.
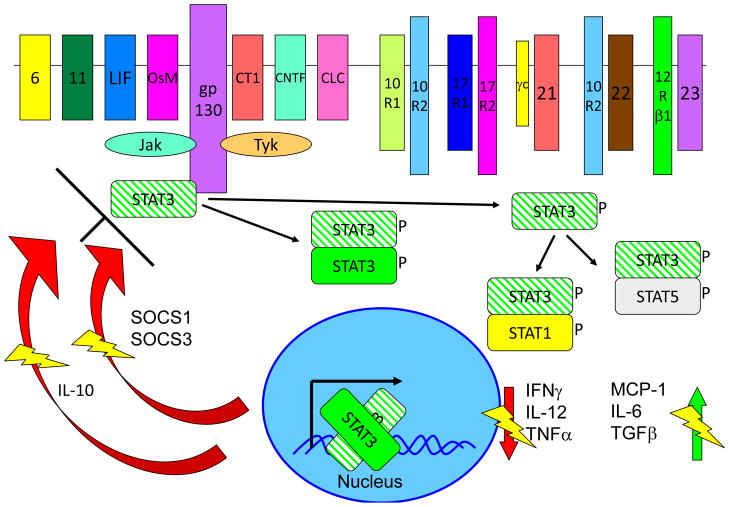
Figure 5. Multiple receptors activate the STAT3 signaling pathway.
The signaling and inhibitions of STAT3 are shown, with areas of special emphasis for the dominant negative mutants shown. Crosshatched STAT3 depicts the AD-mutant form of the molecule, with each heterodimer in which it participates being inhibited from function. The thunderbolts are to indicate where a normal function is being inhibited. STAT3 is both involved in IL-10 signal transduction and in IL-10 expression, both of which are affected in Job’s syndrome.
STAT3 was discovered on the basis of sequence similarities to STAT1, and was initially recognized as a signal transducer for IL-6 and epidermal growth factor (EGF), but not for IFN-γ 112. STAT3 is directly involved in signaling from a multitude of hematological and extrahematological receptors, especially those using the common β chain, gp130 113–116. At least six classes of receptors other than gp130-dependent receptors are known to activate STAT3, which has been implicated in the signal transduction pathways involving γc-dependent cytokines, type I and II interferons, the IL-10 family of cytokines, IL-12 and- 23, receptor tyrosine kinases, and other stimuli 116–119. Following stimulation of the cell, JAKs phosphorylate a key tyrosine residue of STAT3 and the resulting phosphorylated STAT3 forms homo- and heterodimers that are translocated to the nucleus, where they activate a complex array of genes, depending on the stimulus and cell type. There is an extraordinarily high diversity of genes being regulated by STAT3, in a wide range of cell types and tissues.
So, what does the discovery of STAT3 mutations tell us about the pathogenesis of infectious diseases in patients with AD HIES? In a murine T-cell transfer model, STAT3 is required for the development of Th17 cell-mediated colitis 120, due to the STAT3 dependence of cytokines, which induce Th17 development. The development of IL-17 CD4 T cells is thus profoundly impaired in patients with HIES 121–124. The observation of CMC in patients with AD IL-17F or AR IL-17RA deficiency suggests that the development of CMC in AD HIES patients results from impaired IL-17 immunity 95,98. Interestingly, heterozygous loss-of-function STAT3 alleles and heterozygous gain-of-function STAT1 alleles are both associated with impaired development of IL-17 CD4 T cells. Respiratory epithelial cells and keratinocytes, unlike endothelial cells and fibroblasts, have been shown to be tightly dependent on IL-17 for the induction of antimicrobial target genes, probably accounting for CMC 124. The observation that patients lacking IL-17A and IL-17F immunity mostly present with CMC 98 suggests that other mechanisms probably underlie the pathogenesis of pulmonary lesions in HIES. The development of severe staphylococcal diseases in HIES patients, particularly in the lungs, remains unexplained, as infections of this type are not observed in patients with other inborn errors of IL-17 immunity. A high proportion of AD HIES patients were recently shown to have recurrent episodes of varicella zoster infection, which was attributed to the impairment of memory T cells 125. Patients had fewer CD4+ and CD8+ central memory T cells than normal, and these cells displayed low levels of proliferation in vitro. They also displayed specific impairment of the control of varicella zoster and Epstein Barr viruses, but normal control of cytomegalovirus and herpes simplex virus.
What molecular mechanisms underlie some of the hematological and immunological phenotypes not directly related to the infectious diseases seen in HIES patients? The much higher risk of aggressive, predominantly B-cell lymphomas in patients with AD HIES 126 is surprising, since constitutive STAT3 gain-of-function somatic mutations are found in many tumors, including lymphomas 127. The patients’ B cell anomalies have begun to be unraveled. T follicular helper (Tfh) cells are critical for the formation of germinal centers, and the differentiation of both Tfh cells and B cells is controlled by IL-21 34,128,129. Human IL-21 uses STAT3 and IRF-4 to drive B cell differentiation into plasma cells 33,130. AD HIES patients have very low numbers of antigen-specific memory B cells despite intact class switch recombination 33,131,132. However, their germinal centers are relatively normal and the contribution of these B-cell abnormalities to clinical disease remains unclear. B-cell immaturity is strongly linked to the preferential production of IgE in mice, and might explain the IgE elevation in patients 133. Finally, we must consider atopy. Patients with AD HIES have normal numbers of nTreg cells with normal activity, but their ability to respond to IL-10 and the development of iTregs are impaired, potentially accounting for the atopic and inflammatory complications that are so common in this disease 134. Residual IL-10 responses, whether dependent on or independent of STAT3, may account for the inflammatory diseases of HIES patients being less severe than those of patients with deficiencies of IL-10, IL-10RA or IL-10RB 94,135. Atopy is exacerbated by a high skin staphylococcal burden and improved by the control of these bacteria with antibiotics or topical antiseptics.
Many primary immunodeficiencies have extrahematological aspects 136, but the variety and complexity of such manifestations in HIES were initially confusing and the identification of STAT3 as the morbid gene has been startling. One of the most striking features of AD HIES is the characteristic pulmonary cyst formation after pneumonia. The need for lung epithelial cells to migrate, orient and ciliate after injury are severely impaired in mice with lung epithelium-restricted STAT3 and gp130 deficiencies 137. STAT3 also regulates the expression of matrix metalloproteinases (MMPs), which are involved in tissue response to injury, and are aberrant in HIES 138. Arterial remodeling and inflammation are tightly controlled by the TNF-α-induced production of RANTES, which is dependent on both NF-κB and STAT3, ultimately resulting in vascular smooth muscle cell proliferation and atherosclerosis 139. Whether this explains the high rate of coronary artery tortuosity and aneurysm formation seen in HIES patients, and the associated aneurysms in cerebral vessels and lesions in brain white matter 140,141 remains to be determined. A similar process may operate in CMC patients heterozygous for gain-of-function STAT1 mutations, who are also prone to cerebral aneurysms 95. Chandesris et al. demonstrated a direct link between STAT3 and aneurysm formation in mice. Interestingly, HIES patients are not at high risk of atherosclerosis, suggesting that STAT3 deficiency causes arterial aneurysm for reasons distinct from atherosclerosis 142(Chandesris et al., unpublished). Finally, the reasons for the delayed primary dental deciduation and craniosynostosis observed in HIES patients remained a mystery for many years. The recent identification of families with isolated mutations in the gp130-associated IL-11RA who have a similar phenotype 143 suggests that the IL-11 pathway is indeed impaired in STAT3-deficient patients and that this defect may explain some of the craniofacial aspects of Job’s syndrome.
Inborn errors of TYK2 immunity: clinical and immunological phenotypes; progress to date
We arrived at a more complicated page in the story of human inborn errors of the JAK-STAT pathway in 2006, with the description of a Japanese patient with AR TYK2 deficiency (Figures 2, 3, 4, 5) (Table 1) 107. Consistent with a previous classification of HIES on the basis of mode of inheritance into two distinct forms, AD and AR 144, TYK2 deficiency was considered to be an AR genetic etiology of HIES. This was based on the observation that the patient lacking wild-type TYK2 had atopy, susceptibility to cutaneous staphylococcal diseases and high serum concentrations of IgE. However, like MSMD patients, this patient was also susceptible to intramacrophagic bacteria, such as BCG and Salmonella in particular. This infectious phenotype is not classically seen in patients with AD HIES due to dominant-negative STAT3 mutations 106,111,145. The TYK2-deficient patient also developed viral diseases, including recurrent cutaneous herpes simplex virus disease in particular, which is not a characteristic feature of HIES. However, patients with STAT3 mutations have also recently been reported to be prone to viral diseases that reactivate from latency, including VZV and EBV, and this susceptibility has been attributed to an exhaustion of their memory T cells rather than the impairment of IFN responses 125. Thus, clinically, this TYK2-deficient patient may be considered to have HIES. However, the absence of the multiple, non hematological, developmental signs of AD HIES, and the presence of intramacrophagic infections suggest that TYK2 deficiency may be an AR genetic etiology of a phenotype related to, but different from HIES.
Consistent with this view, a Turkish patient was recently found to have TYK2 deficiency with no hematological or extrahematological features of HIES 146. This adult patient had never developed staphylococcal disease, had no history of atopy, and his serum IgE titers never reached the values seen in HIES patients, including the previously described TYK2-deficient patient. However, like this previous patient, he was susceptible to the reactivation of cutaneous viral infections, with recurrent varicella zoster virus lesions. He was also prone to intramacrophagic bacterial infections. Indeed, he initially presented with disseminated BCG disease and later suffered from neurobrucellosis, which resolved but resulted in cognitive impairment. These two TYK2-deficient patients also differed somewhat in terms of fungal susceptibility, as the Japanese patient displayed mild CMC, which was not documented in the Turkish patient. With only two TYK2-deficient patients displaying such overlapping but nonetheless different phenotypes, it is difficult to delineate the clinical hallmark of TYK2 deficiency.
The biological role of human TYK2 in various pathways was investigated in cells from the Japanese patient. TYK2 is a member of the JAK kinase family 147, but it has proved difficult to determine its precise role in various signaling pathways in the mouse model 148. TYK2 seems to be non-redundant in mice, for cellular responses to receptors for at least two classes of cytokines, including IL-12 and IFN-α/β 149,150. Residual responses to mouse cytokines were observed in the complete absence of TYK2. The cells of the Japanese patient did not respond to IFN-α/β, providing a plausible basis for the susceptibility to viral infection observed in the two patients. The cells of this patient also failed to respond to IL-12, possibly accounting for vulnerability to Salmonella, Mycobacterium and Brucella. These responses were rescued by transfection with wild-type TYK2. TYK2-deficient patients can therefore be seen almost as immunological and clinical phenocopies of patients with a partial form of AR STAT1 deficiency, displaying impaired, but not abolished IFN-γ and IFN-α/β immunity and a particular susceptibility to diseases caused by intracellular bacterial and viral pathogens 86,91. The cellular response to other members of the IFN-α/β and IL-12 receptor families has not been investigated. The Japanese TYK2-deficient patient also had impaired responses to IL-10 107,134, but neither he nor the Turkish patient displayed the early-onset, severe colitis observed in patients with IL-10, IL-10R1 and IL-10R2 deficiencies 94,135, suggesting that there are residual, TYK2-independent responses to IL-10. The atopy seen in the Japanese patient may result from an impaired, but not abolished IL-10 response 134. Cellular responses to IFN-λ and other members of the IL-10 family were not tested. Finally, the Japanese patient responded poorly to IL-6; responses to the other members of the IL-6 family of cytokines were not tested. Overall, the atopy, staphylococcal disease and high IgE titers documented in the Japanese patient and their absence in the Turkish patient remain largely unexplained. In any case, the lack of extrahematological manifestations in the two TYK2-deficient patients implies that the STAT3-dependent pathways responsible for such phenotypes, such as the IL-11 pathway, are not TYK2-dependent 143.
Concluding remarks
The characterization of inborn errors of JAK3, TYK2, STAT1, STAT5B and STAT3 in humans has provided answers to key questions about the function of these molecules. Some of these answers preceded or confirmed observations in mouse or human cells in vitro, and observations in mice in vivo. More surprising observations include the association of gain-of-function STAT1 mutations with AD CMC, due to inhibition of the development of IL-17-producing T cells, the profound endocrinological and immunological phenotype of STAT5B-deficient patients, and the association of loss-of-function STAT3 mutations with AD HIES, a complex disorder combining hematological and extrahematological signs. The dissection of clinical and immunological phenotypes associated with germline mutations in related receptors engaging JAKs and STATs has been essential, to decipher the pathogenesis of the disorders of these five genes. For example, we would not understand some dental and skeletal manifestations of AD HIES if patients with AR IL-11RA deficiency had not been identified, and the immune dysregulation of STAT5B-deficient patients if AR IL-2RA deficiency had not been identified. The identification of other inborn errors of immunity, affecting receptors that do not engage JAKs and STATs, has also been illuminating. We would not understand the basis of CMC in patients with gain-of-function STAT1 mutations if we had not identified patients with inborn errors of IL-17F or IL-17RA. In turn, the discovery of germline mutations in human JAK and STAT genes has raised new questions. Many of the immunological and clinical phenotypes seen in these patients remain unexplained at the molecular and cellular levels. For example, we still do not understand the molecular and cellular basis of staphylococcal abscesses in patients with HIES. The advent of whole-exome and whole-genome sequencing will, undoubtedly, facilitate the discovery of new inborn errors of known and unknown genes, shedding light on the pathogenesis of the disorders involving these five genes. It is also probable that germline mutations in the remaining two JAKs (JAK1 and JAK2) and the remaining three STATs (STAT2, STAT4, STAT5A) will soon be discovered. Despite 20 years of outstanding research on these molecules, it remains difficult to predict, with any degree of confidence, the phenotypes of the corresponding patients. With an expanding world population that has already reached seven billion people, increasingly efficient and widespread “phenotyping” by physicians worldwide, a mean mutation rate in the germline of about 10−8, a human genome of about 3.2 billion bp, approximately 2% of which corresponds to 25,000 RNA and protein genes, and a cost of whole-genome sequencing already approaching $1,000, you do not need to be a mathematician to work out that it is now only a matter of years, decades at most, until germline mutations in most of the human genes controlling JAK- and STAT-dependent responses will have been collected and analyzed. Exciting times lie ahead.
Acknowledgments
We thank A. Puel, S-Y. Zhang, S. Boisson-Dupuis, C. Picard and A. Abhyankar for critical reading. We apologize to the many colleagues we could not cite owing to space limitations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.O’Shea JJ, Gadina M, Kanno Y. Cytokine signaling: birth of a pathway. J Immunol. 2011;187:5475–5478. doi: 10.4049/jimmunol.1102913. 187/11/5475 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farrar JD, et al. Selective loss of type I interferon-induced STAT4 activation caused by a minisatellite insertion in mouse Stat2. Nat Immunol. 2000;1:65–69. doi: 10.1038/76932. [DOI] [PubMed] [Google Scholar]
- 3.Casanova JL, Abel L. The human model: a genetic dissection of immunity to infection in natural conditions. Nat Rev Immunol. 2004;4:55–66. doi: 10.1038/nri1264. [DOI] [PubMed] [Google Scholar]
- 4.Glanzmann E, Riniker P. [Essential lymphocytophthisis; new clinical aspect of infant pathology] Annales paediatrici International review of pediatrics. 1950;175:1–32. [PubMed] [Google Scholar]
- 5.Gennery AR, et al. Transplantation of hematopoietic stem cells and long-term survival for primary immunodeficiencies in Europe: entering a new century, do we do better? J Allergy Clin Immunol. 2010;126:602–610. e601–611. doi: 10.1016/j.jaci.2010.06.015. S0091-6749(10)00992-9 [pii] [DOI] [PubMed] [Google Scholar]
- 6.Gitlin D, Craig JM. The Thymus, Other Lymphoid Tissues in Congenital AgammaglobulinemiaI Thymic Alymphoplasia and Lymphocytic Hypoplasia and Their Relation to Infection. Pediatrics. 1963;32:517–530. [PubMed] [Google Scholar]
- 7.Rosen FS, Gotoff SP, Craig JM, Ritchie J, Janeway CA. Further observations on the Swiss type of agammaglobulinemia (alymphocytosis), The effect of syngeneic bone-marrow cells. The New England journal of medicine. 1966;274:18–21. doi: 10.1056/NEJM196601062740104. [DOI] [PubMed] [Google Scholar]
- 8.Takeshita T, et al. Cloning of the gamma chain of the human IL-2 receptor. Science. 1992;257:379–382. doi: 10.1126/science.1631559. [DOI] [PubMed] [Google Scholar]
- 9.Noguchi M, et al. Interleukin-2 receptor gamma chain mutation results in X-linked severe combined immunodeficiency in humans. Cell. 1993;73:147–157. [PubMed] [Google Scholar]
- 10.Kondo M, et al. Sharing of the interleukin-2 (IL-2) receptor gamma chain between receptors for IL-2 and IL-4. Science. 1993;262:1874–1877. doi: 10.1126/science.8266076. [DOI] [PubMed] [Google Scholar]
- 11.Russell SM, et al. Interleukin-2 receptor gamma chain: a functional component of the interleukin-4 receptor. Science. 1993;262:1880–1883. doi: 10.1126/science.8266078. [DOI] [PubMed] [Google Scholar]
- 12.Noguchi M, et al. Interleukin-2 receptor gamma chain: a functional component of the interleukin-7 receptor. Science. 1993;262:1877–1880. doi: 10.1126/science.8266077. [DOI] [PubMed] [Google Scholar]
- 13.Kimura Y, et al. Sharing of the IL-2 receptor gamma chain with the functional IL-9 receptor complex. Int Immunol. 1995;7:115–120. doi: 10.1093/intimm/7.1.115. [DOI] [PubMed] [Google Scholar]
- 14.Giri JG, et al. Utilization of the beta and gamma chains of the IL-2 receptor by the novel cytokine IL-15. Embo J. 1994;13:2822–2830. doi: 10.1002/j.1460-2075.1994.tb06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asao H, et al. Cutting edge: the common gamma-chain is an indispensable subunit of the IL-21 receptor complex. J Immunol. 2001;167:1–5. doi: 10.4049/jimmunol.167.1.1. [DOI] [PubMed] [Google Scholar]
- 16.Ghoreschi K, Laurence A, O’Shea JJ. Janus kinases in immune cell signaling. Immunological reviews. 2009;228:273–287. doi: 10.1111/j.1600-065X.2008.00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Russell SM, et al. Interaction of IL-2R beta and gamma c chains with Jak1 and Jak3: implications for XSCID and XCID. Science. 1994;266:1042–1045. doi: 10.1126/science.7973658. [DOI] [PubMed] [Google Scholar]
- 18.Macchi P, et al. Mutations of Jak-3 gene in patients with autosomal severe combined immune deficiency (SCID) Nature. 1995;377:65–68. doi: 10.1038/377065a0. [DOI] [PubMed] [Google Scholar]
- 19.Russell SM, et al. Mutation of Jak3 in a patient with SCID: essential role of Jak3 in lymphoid development. Science. 1995;270:797–800. doi: 10.1126/science.270.5237.797. [DOI] [PubMed] [Google Scholar]
- 20.von Freeden-Jeffry U, et al. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J Exp Med. 1995;181:1519–1526. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puel A, Ziegler SF, Buckley RH, Leonard WJ. Defective IL7R expression in T-B+NK+ severe combined immunodeficiency. Nature Genet. 1998;20:394–397. doi: 10.1038/3877. [DOI] [PubMed] [Google Scholar]
- 22.Kennedy MK, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. The Journal of experimental medicine. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lodolce JP, et al. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 24.Cao X, et al. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity. 1995;2:223–238. doi: 10.1016/1074-7613(95)90047-0. [DOI] [PubMed] [Google Scholar]
- 25.DiSanto JP, Muller W, Guy-Grand D, Fischer A, Rajewsky K. Lymphoid development in mice with a targeted deletion of the interleukin 2 receptor gamma chain. Proc Natl Acad Sci U S A. 1995;92:377–381. doi: 10.1073/pnas.92.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nosaka T, et al. Defective lymphoid development in mice lacking Jak3. Science. 1995;270:800–802. doi: 10.1126/science.270.5237.800. [DOI] [PubMed] [Google Scholar]
- 27.Park SY, et al. Developmental defects of lymphoid cells in Jak3 kinase-deficient mice. Immunity. 1995;3:771–782. doi: 10.1016/1074-7613(95)90066-7. [DOI] [PubMed] [Google Scholar]
- 28.Buckley RH. B-cell function in severe combined immunodeficiency after stem cell or gene therapy: a review. The Journal of allergy and clinical immunology. 2010;125:790–797. doi: 10.1016/j.jaci.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.White H, Thrasher A, Veys P, Kinnon C, Gaspar HB. Intrinsic defects of B cell function in X-linked severe combined immunodeficiency. Eur J Immunol. 2000;30:732–737. doi: 10.1002/(SICI)1521-4141(200003)30:03<732::AID-IMMU732>3.0.CO;2-A. [pii] 10.1002/1521-4141(200003)30:3<732::AID-IMMU732>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 30.Vinuesa CG, Tangye SG, Moser B, Mackay CR. Follicular B helper T cells in antibody responses and autoimmunity. Nat Rev Immunol. 2005;5:853–865. doi: 10.1038/nri1714. nri1714 [pii] [DOI] [PubMed] [Google Scholar]
- 31.Pene J, et al. Cutting edge: IL-21 is a switch factor for the production of IgG1 and IgG3 by human B cells. Journal of immunology. 2004;172:5154–5157. doi: 10.4049/jimmunol.172.9.5154. [DOI] [PubMed] [Google Scholar]
- 32.Diehl SA, et al. STAT3-mediated up-regulation of BLIMP1 Is coordinated with BCL6 down-regulation to control human plasma cell differentiation. Journal of immunology. 2008;180:4805–4815. doi: 10.4049/jimmunol.180.7.4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Avery DT, et al. B cell-intrinsic signaling through IL-21 receptor and STAT3 is required for establishing long-lived antibody responses in humans. The Journal of experimental medicine. 2010;207:155–171. doi: 10.1084/jem.20091706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Recher M, et al. IL-21 is the primary common gamma chain-binding cytokine required for human B-cell differentiation in vivo. Blood. 2011;118:6824–6835. doi: 10.1182/blood-2011-06-362533. blood-2011-06-362533 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Candotti F, et al. Structural and functional basis for JAK3-deficient severe combined immunodeficiency. Blood. 1997;90:3996–4003. [PubMed] [Google Scholar]
- 36.Notarangelo LD, et al. Of genes and phenotypes: the immunological and molecular spectrum of combined immune deficiency. Defects of the gamma(c)-JAK3 signaling pathway as a model. Immunol Rev. 2000;178:39–48. doi: 10.1034/j.1600-065x.2000.17812.x. [DOI] [PubMed] [Google Scholar]
- 37.Chen M, et al. Complex effects of naturally occurring mutations in the JAK3 pseudokinase domain: evidence for interactions between the kinase and pseudokinase domains. Mol Cell Biol. 2000;20:947–956. doi: 10.1128/mcb.20.3.947-956.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Puck JM, et al. Mutation analysis of IL2RG in human X-linked severe combined immunodeficiency. Blood. 1997;89:1968–1977. [PubMed] [Google Scholar]
- 39.Schmalstieg FC, Goldman AS. Immune consequences of mutations in the human common gamma-chain gene. Mol Genet Metab. 2002;76:163–171. doi: 10.1016/s1096-7192(02)00042-2. S1096719202000422 [pii] [DOI] [PubMed] [Google Scholar]
- 40.Mella P, et al. Development of autologous T lymphocytes in two males with X-linked severe combined immune deficiency: molecular and cellular characterization. Clin Immunol. 2000;95:39–50. doi: 10.1006/clim.2000.4842. [DOI] [PubMed] [Google Scholar]
- 41.Brugnoni D, et al. Development of autologous, oligoclonal, poorly functioning T lymphocytes in a patient with autosomal recessive severe combined immunodeficiency caused by defects of the Jak3 tyrosine kinase. Blood. 1998;91:949–955. [PubMed] [Google Scholar]
- 42.Sharfe N, Shahar M, Roifman CM. An interleukin-2 receptor gamma chain mutation with normal thymus morphology. J Clin Invest. 1997;100:3036–3043. doi: 10.1172/JCI119858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poliani PL, et al. Early defects in human T-cell development severely affect distribution and maturation of thymic stromal cells: possible implications for the pathophysiology of Omenn syndrome. Blood. 2009;114:105–108. doi: 10.1182/blood-2009-03-211029. blood-2009-03-211029 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frucht DM, et al. Unexpected and variable phenotypes in a family with JAK3 deficiency. Genes Immun. 2001;2:422–432. doi: 10.1038/sj.gene.6363802. [DOI] [PubMed] [Google Scholar]
- 45.Sharfe N, Dadi HK, Shahar M, Roifman CM. Human immune disorder arising from mutation of the alpha chain of the interleukin-2 receptor. Proc Natl Acad Sci U S A. 1997;94:3168–3171. doi: 10.1073/pnas.94.7.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aoki CA, et al. IL-2 receptor alpha deficiency and features of primary biliary cirrhosis. Journal of autoimmunity. 2006;27:50–53. doi: 10.1016/j.jaut.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 47.Caudy AA, Reddy ST, Chatila T, Atkinson JP, Verbsky JW. CD25 deficiency causes an immune dysregulation, polyendocrinopathy, enteropathy, X-linked-like syndrome, and defective IL-10 expression from CD4 lymphocytes. J Allergy Clin Immunol. 2007;119:482–487. doi: 10.1016/j.jaci.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 48.Wildin RS, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 49.Bennett CL, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 50.Davidson TS, DiPaolo RJ, Andersson J, Shevach EM. Cutting Edge: IL-2 is essential for TGF-beta-mediated induction of Foxp3+ T regulatory cells. Journal of immunology. 2007;178:4022–4026. doi: 10.4049/jimmunol.178.7.4022. [DOI] [PubMed] [Google Scholar]
- 51.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nature immunology. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 52.D’Cruz LM, Klein L. Development and function of agonist-induced CD25+Foxp3+ regulatory T cells in the absence of interleukin 2 signaling. Nature immunology. 2005;6:1152–1159. doi: 10.1038/ni1264. [DOI] [PubMed] [Google Scholar]
- 53.Kofoed EM, et al. Growth hormone insensitivity associated with a STAT5b mutation. N Engl J Med. 2003;349:1139–1147. doi: 10.1056/NEJMoa022926. [DOI] [PubMed] [Google Scholar]
- 54.Nadeau K, Hwa V, Rosenfeld RG. STAT5b deficiency: an unsuspected cause of growth failure, immunodeficiency, and severe pulmonary disease. The Journal of pediatrics. 2011;158:701–708. doi: 10.1016/j.jpeds.2010.12.042. [DOI] [PubMed] [Google Scholar]
- 55.Duquesnoy P, et al. A single amino acid substitution in the exoplasmic domain of the human growth hormone (GH) receptor confers familial GH resistance (Laron syndrome) with positive GH-binding activity by abolishing receptor homodimerization. The EMBO journal. 1994;13:1386–1395. doi: 10.1002/j.1460-2075.1994.tb06392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ayling RM, et al. A dominant-negative mutation of the growth hormone receptor causes familial short stature. Nature genetics. 1997;16:13–14. doi: 10.1038/ng0597-13. [DOI] [PubMed] [Google Scholar]
- 57.Udy GB, et al. Requirement of STAT5b for sexual dimorphism of body growth rates and liver gene expression. Proc Natl Acad Sci U S A. 1997;94:7239–7244. doi: 10.1073/pnas.94.14.7239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bernasconi A, et al. Characterization of immunodeficiency in a patient with growth hormone insensitivity secondary to a novel STAT5b gene mutation. Pediatrics. 2006;118:e1584–1592. doi: 10.1542/peds.2005-2882. [DOI] [PubMed] [Google Scholar]
- 59.Hwa V, Nadeau K, Wit JM, Rosenfeld RG. STAT5b deficiency: lessons from STAT5b gene mutations. Best practice & research Clinical endocrinology & metabolism. 2011;25:61–75. doi: 10.1016/j.beem.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 60.Pugliese-Pires PN, et al. A novel STAT5B mutation causing GH insensitivity syndrome associated with hyperprolactinemia and immune dysfunction in two male siblings. Eur J Endocrinol. 2010;163:349–355. doi: 10.1530/EJE-10-0272. EJE-10-0272 [pii] [DOI] [PubMed] [Google Scholar]
- 61.Liu X, et al. Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes Dev. 1997;11:179–186. doi: 10.1101/gad.11.2.179. [DOI] [PubMed] [Google Scholar]
- 62.Antov A, Yang L, Vig M, Baltimore D, Van Parijs L. Essential role for STAT5 signaling in CD25+CD4+ regulatory T cell homeostasis and the maintenance of self-tolerance. Journal of immunology. 2003;171:3435–3441. doi: 10.4049/jimmunol.171.7.3435. [DOI] [PubMed] [Google Scholar]
- 63.Burchill MA, et al. Distinct effects of STAT5 activation on CD4+ and CD8+ T cell homeostasis: development of CD4+CD25+ regulatory T cells versus CD8+ memory T cells. Journal of immunology. 2003;171:5853–5864. doi: 10.4049/jimmunol.171.11.5853. [DOI] [PubMed] [Google Scholar]
- 64.Yao Z, et al. Nonredundant roles for Stat5a/b in directly regulating Foxp3. Blood. 2007;109:4368–4375. doi: 10.1182/blood-2006-11-055756. blood-2006-11-055756 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Snow JW, et al. Loss of tolerance and autoimmunity affecting multiple organs in STAT5A/5B-deficient mice. J Immunol. 2003;171:5042–5050. doi: 10.4049/jimmunol.171.10.5042. [DOI] [PubMed] [Google Scholar]
- 66.Murawski MR, Litherland SA, Clare-Salzler MJ, Davoodi-Semiromi A. Upregulation of Foxp3 expression in mouse and human Treg is IL-2/STAT5 dependent: implications for the NOD STAT5B mutation in diabetes pathogenesis. Annals of the New York Academy of Sciences. 2006;1079:198–204. doi: 10.1196/annals.1375.031. [DOI] [PubMed] [Google Scholar]
- 67.Cohen AC, et al. Cutting edge: Decreased accumulation and regulatory function of CD4+ CD25(high) T cells in human STAT5b deficiency. Journal of immunology. 2006;177:2770–2774. doi: 10.4049/jimmunol.177.5.2770. [DOI] [PubMed] [Google Scholar]
- 68.Behbod F, et al. Specific inhibition of Stat5a/b promotes apoptosis of IL-2-responsive primary and tumor-derived lymphoid cells. Journal of immunology. 2003;171:3919–3927. doi: 10.4049/jimmunol.171.8.3919. [DOI] [PubMed] [Google Scholar]
- 69.Casanova JL, Abel L. Genetic dissection of immunity to mycobacteria: the human model. Annu Rev Immunol. 2002;20:581–620. doi: 10.1146/annurev.immunol.20.081501.125851. [DOI] [PubMed] [Google Scholar]
- 70.Dorman SE, et al. Clinical features of dominant and recessive interferon gamma receptor 1 deficiencies. Lancet. 2004;364:2113–2121. doi: 10.1016/S0140-6736(04)17552-1. [DOI] [PubMed] [Google Scholar]
- 71.Vogt G, et al. Complementation of a pathogenic IFNGR2 misfolding mutation with modifiers of N-glycosylation. J Exp Med. 2008;205:1729–1737. doi: 10.1084/jem.20071987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van den Broek MF, Müller U, Huang S, Zinkernagel RM, Aguet M. Immune defence in mice lacking ype I and/or type II interferon receptors. Immunol Rev. 1995;148:5–18. doi: 10.1111/j.1600-065x.1995.tb00090.x. [DOI] [PubMed] [Google Scholar]
- 73.Vinh DC, Masannat F, Dzioba RB, Galgiani JN, Holland SM. Refractory disseminated coccidioidomycosis and mycobacteriosis in interferon-gamma receptor 1 deficiency. Clin Infect Dis. 2009;49:65. doi: 10.1086/605532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rottman M, et al. IFN-gamma mediates the rejection of haematopoietic stem cells in IFN-gammaR1-deficient hosts. PLoS Med. 2008;5:e26. doi: 10.1371/journal.pmed.0050026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Roesler J, et al. Hematopoietic stem cell transplantation for complete IFN-gamma receptor 1 deficiency: a multi-institutional survey. J Pediatr. 2004;145:806–812. doi: 10.1016/j.jpeds.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 76.de Beaucoudrey L, et al. Revisiting human IL-12Rbeta1 deficiency: a survey of 141 patients from 30 countries. Medicine (Baltimore) 2010;89:381–402. doi: 10.1097/MD.0b013e3181fdd832. 00005792-201011000-00002 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hambleton S, et al. IRF8 Mutations and Human Dendritic-Cell Immunodeficiency. N Engl J Med. 2011 doi: 10.1056/NEJMoa1100066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Filipe-Santos O, et al. X-linked susceptibility to mycobacteria is caused by mutations in NEMO impairing CD40-dependent IL-12 production. J Exp Med. 2006;203:1745–1759. doi: 10.1084/jem.20060085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bustamante J, Picard C, Boisson-Dupuis S, Abel L, Casanova JL. Genetic lessons learned from X-linked Mendelian susceptibility to mycobacterial diseases. Ann N Y Acad Sci. 2011;1246:92–101. doi: 10.1111/j.1749-6632.2011.06273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bustamante J, et al. Germline CYBB mutations that selectively affect macrophages in kindreds with X-linked predisposition to tuberculous mycobacterial disease. Nat Immunol. 2011;12:213–221. doi: 10.1038/ni.1992. ni.1992 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dupuis S, et al. Impairment of mycobacterial but not viral immunity by a germline human STAT1 mutation. Science. 2001;293:300–303. doi: 10.1126/science.1061154. [DOI] [PubMed] [Google Scholar]
- 82.Sampaio EP, et al. A Novel STAT1 Mutation Associated with Disseminated Mycobacterial Disease. J Clin Immunol. 2012 doi: 10.1007/s10875-012-9659-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schindler C, Fu XY, Improta T, Aebersold R, Darnell JE., Jr Proteins of transcription factor ISGF-3: one gene encodes the 91-and 84-kDa ISGF-3 proteins that are activated by interferon alpha. Proc Natl Acad Sci U S A. 1992;89:7836–7839. doi: 10.1073/pnas.89.16.7836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 85.Dupuis S, et al. Impaired response to interferon-alpha/beta and lethal viral disease in human STAT1 deficiency. Nat Genet. 2003;33:388–391. doi: 10.1038/ng1097. [DOI] [PubMed] [Google Scholar]
- 86.Chapgier A, et al. A partial form of recessive STAT1 deficiency in humans. J Clin Invest. 2009;119:1502–1514. doi: 10.1172/JCI37083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chapgier A, et al. Human complete Stat-1 deficiency is associated with defective type I and II IFN responses in vitro but immunity to some low virulence viruses in vivo. J Immunol. 2006;176:5078–5083. doi: 10.4049/jimmunol.176.8.5078. [DOI] [PubMed] [Google Scholar]
- 88.Vairo D, et al. Severe impairment of IFN-gamma and IFN-alpha responses in cells of a patient with a novel STAT1 splicing mutation. Blood. 2011;118:1806–1817. doi: 10.1182/blood-2011-01-330571. blood-2011-01-330571 [pii] [DOI] [PubMed] [Google Scholar]
- 89.Averbuch D, Chapgier A, Boisson-Dupuis S, Casanova JL, Engelhard D. The Clinical Spectrum of Patients with Deficiency of Signal Transducer and Activator of Transcription-1. Pediatr Infect Dis J. 2011;30:352–355. doi: 10.1097/INF.0b013e3181fdff4a. [DOI] [PubMed] [Google Scholar]
- 90.Kristensen IA, Veirum JE, Moller BK, Christiansen M. Novel STAT1 Alleles in a Patient with Impaired Resistance to Mycobacteria. J Clin Immunol. 2010 doi: 10.1007/s10875-010-9480-8. [DOI] [PubMed] [Google Scholar]
- 91.Kong XF, et al. A novel form of human STAT1 deficiency impairing early but not late responses to interferons. Blood. 2010;116:5895–5906. doi: 10.1182/blood-2010-04-280586. blood-2010-04-280586 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sancho-Shimizu V, Perez de Diego R, Jouanguy E, Zhang SY, Casanova JL. Inborn errors of anti-viral interferon immunity in humans. Curr Opin Virol. 2011;1:487–496. doi: 10.1016/j.coviro.2011.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang SY, et al. Inborn errors of interferon (IFN)-mediated immunity in humans: insights into the respective roles of IFN-alpha/beta, IFN-gamma, and IFN-lambda in host defense. Immunol Rev. 2008;226:29–40. doi: 10.1111/j.1600-065X.2008.00698.x. IMR698 [pii] [DOI] [PubMed] [Google Scholar]
- 94.Glocker EO, et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med. 2009;361:2033–2045. doi: 10.1056/NEJMoa0907206. NEJMoa0907206 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu L, et al. Gain-of-function human STAT1 mutations impair IL-17 immunity and underlie chronic mucocutaneous candidiasis. J Exp Med. 2011;208:1635–1648. doi: 10.1084/jem.20110958. jem.20110958 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.van de Veerdonk FL, et al. STAT1 mutations in autosomal dominant chronic mucocutaneous candidiasis. N Engl J Med. 2011;365:54–61. doi: 10.1056/NEJMoa1100102. [DOI] [PubMed] [Google Scholar]
- 97.Crow YJ. Type I interferonopathies: a novel set of inborn errors of immunity. Ann N Y Acad Sci. 2011;1238:91–98. doi: 10.1111/j.1749-6632.2011.06220.x. [DOI] [PubMed] [Google Scholar]
- 98.Puel A, et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332:65–68. doi: 10.1126/science.1200439. science.1200439 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen M, et al. Regulatory effects of IFN-beta on production of osteopontin and IL-17 by CD4+ T Cells in MS. Eur J Immunol. 2009;39:2525–2536. doi: 10.1002/eji.200838879. [DOI] [PubMed] [Google Scholar]
- 100.Zhou L, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. ni1488 [pii] [DOI] [PubMed] [Google Scholar]
- 101.Davis SD, Schaller J, Wedgwood RJ. Job’s Syndrome. Recurrent, “cold”, staphylococcal abscesses. Lancet. 1966;1:1013–1015. doi: 10.1016/s0140-6736(66)90119-x. [DOI] [PubMed] [Google Scholar]
- 102.Buckley RH, Wray BB, Belmaker EZ. Extreme hyperimmunoglobulinemia E and undue susceptibility to infection. Pediatrics. 1972;49:59–70. [PubMed] [Google Scholar]
- 103.Buckley RH, Becker WG. Abnormalities in the regulation of human IgE synthesis. Immunol Rev. 1978;41:288–314. doi: 10.1111/j.1600-065x.1978.tb01469.x. [DOI] [PubMed] [Google Scholar]
- 104.Sheerin KA, Buckley RH. Antibody responses to protein, polysaccharide, and phi X174 antigens in the hyperimmunoglobulinemia E (hyper-IgE) syndrome. J Allergy Clin Immunol. 1991;87:803–811. doi: 10.1016/0091-6749(91)90126-9. 0091-6749(91)90126-9 [pii] [DOI] [PubMed] [Google Scholar]
- 105.Donabedian H, Gallin JI. The hyperimmunoglobulin E recurrent-infection (Job’s) syndrome. A review of the NIH experience and the literature. Medicine (Baltimore) 1983;62:195–208. doi: 10.1097/00005792-198307000-00001. [DOI] [PubMed] [Google Scholar]
- 106.Grimbacher B, et al. Hyper-IgE syndrome with recurrent infections--an autosomal dominant multisystem disorder. N Engl J Med. 1999;340:692–702. doi: 10.1056/NEJM199903043400904. [DOI] [PubMed] [Google Scholar]
- 107.Minegishi Y, et al. Human tyrosine kinase 2 deficiency reveals its requisite roles in multiple cytokine signals involved in innate and acquired immunity. Immunity. 2006;25:745–755. doi: 10.1016/j.immuni.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 108.Minegishi Y, et al. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature. 2007;448:1058–1062. doi: 10.1038/nature06096. [DOI] [PubMed] [Google Scholar]
- 109.Holland SM, et al. STAT3 Mutations in the Hyper-IgE Syndrome. N Engl J Med. 2007 doi: 10.1056/NEJMoa073687. [DOI] [PubMed] [Google Scholar]
- 110.Takeda K, et al. Targeted disruption of the mouse Stat3 gene leads to early embryonic lethality. Proc Natl Acad Sci U S A. 1997;94:3801–3804. doi: 10.1073/pnas.94.8.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Minegishi Y. Hyper-IgE syndrome. Curr Opin Immunol. 2009;21:487–492. doi: 10.1016/j.coi.2009.07.013. S0952-7915(09)00165-4 [pii] [DOI] [PubMed] [Google Scholar]
- 112.Zhong Z, Wen Z, Darnell JE., Jr Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994;264:95–98. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]
- 113.White UA, Stephens JM. The gp130 receptor cytokine family: regulators of adipocyte development and function. Curr Pharm Des. 2011;17:340–346. doi: 10.2174/138161211795164202. BSP/CPD/E-Pub/000346 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Donnelly RP, et al. Interleukin-26: an IL-10-related cytokine produced by Th17 cells. Cytokine Growth Factor Rev. 2010;21:393–401. doi: 10.1016/j.cytogfr.2010.09.001. S1359-6101(10)00064-X [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Minegishi Y, Saito M. Molecular mechanisms of the immunological abnormalities in hyper-IgE syndrome. Ann N Y Acad Sci. 2011;1246:34–40. doi: 10.1111/j.1749-6632.2011.06280.x. [DOI] [PubMed] [Google Scholar]
- 116.Tangye SG, Cook MC, Fulcher DA. Insights into the Role of STAT3 in Human Lymphocyte Differentiation as Revealed by the Hyper-IgE Syndrome. J Immunol. 2009;182:21–28. doi: 10.4049/jimmunol.182.1.21. [DOI] [PubMed] [Google Scholar]
- 117.O’Shea JJ, Murray PJ. Cytokine signaling modules in inflammatory responses. Immunity. 2008;28:477–487. doi: 10.1016/j.immuni.2008.03.002. S1074-7613(08)00117-9 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.O’Shea JJ, Gadina M, Schreiber RD. Cytokine signaling in 2002: new surprises in the Jak/Stat pathway. Cell. 2002;109(Suppl):S121–131. doi: 10.1016/s0092-8674(02)00701-8. S0092867402007018 [pii] [DOI] [PubMed] [Google Scholar]
- 119.Levy DE, Darnell JE., Jr Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3:651–662. doi: 10.1038/nrm909. nrm909 [pii] [DOI] [PubMed] [Google Scholar]
- 120.Durant L, et al. Diverse targets of the transcription factor STAT3 contribute to T cell pathogenicity and homeostasis. Immunity. 2010;32:605–615. doi: 10.1016/j.immuni.2010.05.003. S1074-7613(10)00169-X [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.de Beaucoudrey L, et al. Mutations in STAT3 and IL12RB1 impair the development of human IL-17-producing T cells. J Exp Med. 2008;205:1543–1550. doi: 10.1084/jem.20080321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ma CS, et al. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J Exp Med. 2008;205:1551–1557. doi: 10.1084/jem.20080218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Milner JD, et al. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452:773–776. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Minegishi Y, et al. Molecular explanation for the contradiction between systemic Th17 defect and localized bacterial infection in hyper-IgE syndrome. J Exp Med. 2009;206:1291–1301. doi: 10.1084/jem.20082767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Siegel AM, et al. A Critical Role for STAT3 Transcription Factor Signaling in the Development and Maintenance of Human T Cell Memory. Immunity. 2011;35:806–818. doi: 10.1016/j.immuni.2011.09.016. S1074-7613(11)00464-X [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Leonard GD, et al. Non-Hodgkin’s lymphoma in Job’s syndrome: a case report and literature review. Leuk Lymphoma. 2004;45:2521–2525. doi: 10.1080/10428190400004463. XQCA9VWWPYG0A4CR [pii] [DOI] [PubMed] [Google Scholar]
- 127.Bowman T, Garcia R, Turkson J, Jove R. STATs in oncogenesis. Oncogene. 2000;19:2474–2488. doi: 10.1038/sj.onc.1203527. [DOI] [PubMed] [Google Scholar]
- 128.Vogelzang A, et al. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity. 2008;29:127–137. doi: 10.1016/j.immuni.2008.06.001. S1074-7613(08)00274-4 [pii] [DOI] [PubMed] [Google Scholar]
- 129.Nurieva RI, et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. S1074-7613(08)00273-2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kwon H, et al. Analysis of interleukin-21-induced Prdm1 gene regulation reveals functional cooperation of STAT3 and IRF4 transcription factors. Immunity. 2009;31:941–952. doi: 10.1016/j.immuni.2009.10.008. S1074-7613(09)00511-1 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Speckmann C, et al. Reduced memory B cells in patients with hyper IgE syndrome. Clin Immunol. 2008;129:448–454. doi: 10.1016/j.clim.2008.08.002. S1521-6616(08)00767-5 [pii] [DOI] [PubMed] [Google Scholar]
- 132.Meyer-Bahlburg A, et al. Heterozygous signal transducer and activator of transcription 3 mutations in hyper-IgE syndrome result in altered B-cell maturation. J Allergy Clin Immunol. 2011 doi: 10.1016/j.jaci.2011.09.017. S0091-6749(11)01488-6 [pii] [DOI] [PubMed] [Google Scholar]
- 133.Wesemann DR, et al. Immature B cells preferentially switch to IgE with increased direct Smu to S{varepsilon} recombination. J Exp Med. 2011;208:2733–2746. doi: 10.1084/jem.20111155. jem.20111155 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Saito M, et al. Defective IL-10 signaling in hyper-IgE syndrome results in impaired generation of tolerogenic dendritic cells and induced regulatory T cells. J Exp Med. 2011;208:235–249. doi: 10.1084/jem.20100799. jem.20100799 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Glocker EO, et al. Infant colitis--it’s in the genes. Lancet. 2010;376:1272. doi: 10.1016/S0140-6736(10)61008-2. S0140–6736(10)61008-2 [pii] [DOI] [PubMed] [Google Scholar]
- 136.Al-Herz W, et al. Primary immunodeficiency diseases: an update on the classification from the International Union of Immunological Societies Expert Committee for Primary Immunodeficiency. Front Immunol. 2011 Nov 08; doi: 10.3389/fimmu.2011.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kida H, et al. GP130-STAT3 regulates epithelial cell migration and is required for repair of the bronchiolar epithelium. Am J Pathol. 2008;172:1542–1554. doi: 10.2353/ajpath.2008.071052. S0002-9440(10)61914-1 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sekhsaria V, et al. Plasma metalloproteinase levels are dysregulated in signal transducer and activator of transcription 3 mutated hyper-IgE syndrome. J Allergy Clin Immunol. 2011;128:1124–1127. doi: 10.1016/j.jaci.2011.07.046. S0091-6749(11)01232-2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kovacic JC, et al. Stat3-dependent acute Rantes production in vascular smooth muscle cells modulates inflammation following arterial injury in mice. J Clin Invest. 2010;120:303–314. doi: 10.1172/JCI40364. 40364 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Freeman AF, et al. Coronary artery abnormalities in Hyper-IgE syndrome. J Clin Immunol. 2011;31:338–345. doi: 10.1007/s10875-011-9515-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chandesris MO, et al. Frequent and Widespread Vascular Abnormalities in Human STAT3 Deficiency. Circ Cardiovasc Genet. 2011 doi: 10.1161/CIRCGENETICS.111.961235. CIRCGENETICS.111.961235 [pii] [DOI] [PubMed] [Google Scholar]
- 142.Cohen P, O’Gara PT. Coronary artery aneurysms: a review of the natural history, pathophysiology, and management. Cardiol Rev. 2008;16:301–304. doi: 10.1097/CRD.0b013e3181852659. 00045415-200811000-00004 [pii] [DOI] [PubMed] [Google Scholar]
- 143.Nieminen P, et al. Inactivation of IL11 signaling causes craniosynostosis, delayed tooth eruption, and supernumerary teeth. Am J Hum Genet. 2011;89:67–81. doi: 10.1016/j.ajhg.2011.05.024. S0002-9297(11)00217-5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Renner ED, et al. Autosomal recessive hyperimmunoglobulin E syndrome: a distinct disease entity. J Pediatr. 2004;144:93–99. doi: 10.1016/S0022-3476(03)00449-9. [DOI] [PubMed] [Google Scholar]
- 145.Freeman AF, Holland SM. Clinical manifestations of hyper IgE syndromes. Dis Markers. 2010;29:123–130. doi: 10.3233/DMA-2010-0734. F2548X82T72242PK [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kilic S, et al. A Tyk2-deficient patient without hyper IgE syndrome. The Journal of Pediatrics. 2012 In press. [Google Scholar]
- 147.Velazquez L, Fellous M, Stark GR, Pellegrini S. A protein tyrosine kinase in the interferon alpha/beta signaling pathway. Cell. 1992;70:313–322. doi: 10.1016/0092-8674(92)90105-l. 0092-8674(92)90105-L [pii] [DOI] [PubMed] [Google Scholar]
- 148.Strobl B, Stoiber D, Sexl V, Mueller M. Tyrosine kinase 2 (TYK2) in cytokine signalling and host immunity. Front Biosci. 2012;17:3214–3232. doi: 10.2741/3908. 3908 [pii] [DOI] [PubMed] [Google Scholar]
- 149.Shimoda K, et al. Tyk2 plays a restricted role in IFN alpha signaling, although it is required for IL-12-mediated T cell function. Immunity. 2000;13:561–571. doi: 10.1016/s1074-7613(00)00055-8. S1074-7613(00)00055-8 [pii] [DOI] [PubMed] [Google Scholar]
- 150.Karaghiosoff M, et al. Partial impairment of cytokine responses in Tyk2-deficient mice. Immunity. 2000;13:549–560. doi: 10.1016/s1074-7613(00)00054-6. S1074-7613(00)00054-6 [pii] [DOI] [PubMed] [Google Scholar]