Abstract
Studies in aquatic systems have shown that habitat complexity may provide refuge or reduce the number of encounters prey have with actively searching predators. For ambush predators, habitat complexity may enhance or have no effect on predation rates because it conceals predators, reduces prey detection by predators, or visually impairs both predators and prey. We investigated the effects of habitat complexity and predation by the ambush predators Toxorhynchites rutilus and Corethrella appendiculata on their mosquito prey Aedes albopictus and Ochlerotatus triseriatus in container analogs of treeholes. As in other ambush predator-prey systems, habitat complexity did not alter the effects of T. rutilus or C. appendiculata whose presence decreased prey survivorship, shortened development time, and increased adult size compared to treatments where predators were absent. Faster growth and larger size were due to predator-mediated release from competition among surviving prey. Male and female prey survivorship were similar in the absence of predators, however when predators were present, survivorship of both prey species was skewed in favor of males. We conclude that habitat complexity is relatively unimportant in shaping predator-prey interactions in this treehole community, where predation risk differs between prey sexes.
Keywords: Container mosquitoes, Population growth measurements, Predator–prey interactions
Introduction
Predation plays a major role in shaping aquatic communities directly by reduction of prey abundance and altering diversity or, indirectly, by modifying the direct interactions among species (e.g., trophic linkage, behavioral, and chemical) (reviewed by Kerfoot and Sih 1987). Interactions between predators and prey may be altered by the physical environment. Predator–prey studies demonstrate that when a predator is present prey may preferentially occupy structured habitats (e.g., vegetation) over more “open” habitats (e.g., Savino and Stein 1982; Greenberg et al. 1995). Structurally complex habitats may provide refuge for prey and reduce predation by lowering the number of encounters with predators. However, general statements about the effects of habitat complexity on predator–prey relations are confounded by differences among systems in predator efficiency (e.g., Ray-Culp et al. 1999), prey behavior (Greenberg et al. 1995; Flynn and Ritz 1999), and probably most importantly, predator foraging strategy (e.g., Coen et al. 1981; Heck and Crowder 1991; Greenberg et al. 1995).
The foraging strategy of a predator (e.g., ambush versus active) may greatly influence its ability to capture prey under varying degrees of habitat complexity. Obstacles may visually impair actively searching predators, interrupt pursuit of prey by decreasing maneuverability, or otherwise reduce overall predator efficiency (Savino and Stein 1982; Manatunge et al. 2000; Spitzer et al. 2000). Most research on actively searching predators and their prey in structured habitats has focused on planktivorous and piscivorous fish, which has shown enhanced, prey survival in habitats with greater complexity (Coen et al. 1981; Savino and Stein 1982; Coull and Wells 1983; Manatunge et al. 2000; Spitzer et al. 2000). Although less is known for ambush predators, habitat complexity may conceal predators (Heck and Orth 1980; Coen et al. 1981) and sometimes enhance predation efficiency in structurally complex habitats (Heck and Crowder 1991; James and Heck 1994; Flynn and Ritz 1999). Thus, it is important to understand the consequences of varying habitat complexity for both predator and prey in a number of systems before generalizations can be made.
Natural and artificial containers (e.g., treeholes, discarded tires, and vases) harbor small, discrete aquatic communities, which have been well studied, although the effects of habitat complexity on predator-prey interactions have received relatively little attention (O‘Flynn and Craig 1982; Juliano 1989). In these communities, temporal and spatial changes in accumulation of leaf litter account for variations in physical structure. Habitat complexity, in the form of leaf litter, may have both direct effects on resource availability and indirect effects on predation risk by impairment or enhancement of predator foraging (Grenouillet et al. 2002, references therein). Water levels may alter habitat complexity if leaf litter present in the container occupies a large proportion of the water column and interrupts the air–water interface when water levels are low. In Florida, the two dominant predators in treeholes and discarded tires are a mosquito Toxorhynchites rutilus and a corethrellid midge Corethrella appendiculata. Larvae of T.rutilus are obligate predators, consuming a wide range of invertebrates including mosquitoes (Campos and Lounibos 2000), and use an ambush strategy for subsurface prey (Steffan and Evenhuis 1981). C. appendiculata is a smaller ambush predator whose 3rd and 4th instars reduce mosquito abundance by consuming small larvae (Lounibos 1983).
The most common mosquito prey encountered by these two predators in Florida includes the Asian tiger mosquito, Aedes albopictus and the eastern treehole mosquito, Ochlerotatus triseriatus. A. albopictus, native to Asia, is the most abundant and widespread of the container mosquito species that have invaded the continental United States in the last few decades. Laboratory studies have shown that A. albopictus larvae outcompete O. triseriatus when food resources are limiting (Novak et al. 1993; Teng and Apperson 2000), but in nature they appear to coexist, attributable in part to habitat segregation among macrohabitats (Lounibos et al. 2001) and in part to predator preference for A. albopictus (Griswold and Lounibos 2005a).
Behavioral, morphological, and physiological differences between male and female insect prey may alter predation rates on the sexes. For prey that are sexually dimorphic and protandrous, such as many mosquitoes (e.g., Brust 1967), predation may differentially affect the sexes. Protandry, here defined as the arrival of males before females into a seasonal breeding population, is common among insects and is predicted to occur most often where females are monogamous (e.g., butterflies and mosquitoes) whereby sexual selection theory predicts males maximize mating opportunities (Wiklund and Fagerström 1977; Nylin et al. 1993; Kleckner et al. 1995; ZijIstra et al. 2002). Natural selection may act differently on male and female mosquitoes, since female fitness is related to fecundity whereas male fitness depends on the number of matings (Steinwascher 1982; Kleckner et al. 1995). Therefore, sex-specific reaction norms, induced by biological interactions (e.g., larval competition and predation) would be expected for aedine mosquitoes. Intraspecific competition studies with A. aegypti and A. albopictus have shown sex-specific differences among population growth measurements such as survivorship, development time, and size (Bedhomme et al. 2003; Alto et al. 2005).
The current study tests the hypothesis that the presence of predators and habitat structure alter population growth of mosquito prey and affect their sexes differentially. We predict that the presence of predators will negatively impact mosquito prey population growth (e.g., survivorship to adulthood, development time to adulthood, and adult mass) and that responses may be more detrimental for females since their development time is greater, thus exposing females longer to predation. Furthermore, the presence of habitat structure may mediate these effects. Specifically, we test the hypothesis that habitat complexity alters predator impact by determining whether there is an interaction between habitat complexity and predation. Finally, we investigate whether there are differential sex-specific effects of size-selective predation and habitat structure on two co-occurring prey species.
Materials and methods
Laboratory experiments were used to evaluate the effects of T. rutilus and C. appendiculata on A. albopictus and O. triseriatus population growth in habitats of different complexity. Our approach was to conduct a series of experiments starting with a simple system (e.g., single predator-prey and single-level habitat complexity) and working towards a more complex system (e.g., single predator-2 prey and variable habitat complexity). Prey species used in the experiments consisted of F1– F2 progeny of field-collected larvae and pupae from discarded tires and other artificial containers in peninsular Florida. Predators, T. rutilus and C. appendiculata, were obtained from laboratory colonies that originated from Florida. Field-collected larvae of both predators were added to colonies of these species at irregular intervals. Experiments were initiated by adding newly hatched first instar (<24 h old) prey to water-filled containers varying in habitat complexity. At the same time, either T. rutilus (<24 h old, first instar) or C. appendiculata (fourth instars of known age and feeding history) were added to half of the containers whereas the remaining half received no predators (i.e., controls). All experiments were performed at 25°C±1 and a photoperiod of 14:10 (L/D). Densities of predator and prey species used in the experiments were within the range encountered in natural treehole communities in Florida (Lounibos 1983; Lounibos et al. 2001).
Experiment 1: T. rutilus predation and single-level habitat complexity effects on A. albopictus prey population growth measurements
Experimental units consisted of plastic cylindrical containers (19.5×20.5 cm, height × diameter) filled with 500 ml filtered oak infusion water (O’Meara et al. 1989), 4000 ml tap water, and 0.2 g of 1:1 yeast/albumin. Large containers were chosen to reflect similar volumes found in large artificial containers, such as discarded tires, where T. rutilus are found. Five days later, 250 A. albopictus larvae were added to each container. Ten containers received 1 T. rutilus larva and the remaining ten containers received none. To provide habitat structure, artificial leaves were made from 76 µm-thick black plastic, from which 20 pieces, each 4×16 cm, were placed in ten of the containers and the remaining ten containers received none (i.e., control) (2×2×5 = 20 total). Twenty artificial leaves were evenly spaced along the entire perimeter of the container by attaching one end of each to the upper edge and leaving the other end unattached to allow some movement in the water column. On the third day of the experiment, supplemental resources were added to each container (500 ml oak infusion and 0.2 g of 1:1 yeast/albumin).
A. albopictus pupae were removed daily from experimental containers and placed in 20 ml vials with tap water until emergence. Vials were checked daily for newly emerged adults whose sex was recorded. Thus, in this experiment, effects of T. rutilus and habitat structure on A. albopictus were restricted to larval stages and the first 24 h of the pupal stage. The experiment continued until all A. albopictus had pupated or died.
Experiment 2: T. rutilus predation and variable habitat complexity effects on A. albopictus prey population growth measurements
The objectives of experiment 2 were to determine the effects of predation in environments with varying degrees of habitat complexity. Experimental units consisted of plastic cylindrical containers (15.5×14.5 cm, height × diameter). Oak (Quercus virginiana) leaves used as a prey food resource were dried at 65°C for 48 h and ground into a powder in a blender (Vitamix). This eliminated habitat complexity caused by whole leaves, while still providing prey with natural resources. Each container received 3.5 g ground oak leaves, 800 ml sieved (180 µm) water from tires found outdoors, and artificial habitat complexity in the form of 0, 4, 10, 14, or 20 artificial, cloth maple leaves (sold commercially for decorating), each treatment replicated 12 times. For each habitat complexity treatment, we used equal numbers of small (37.13 cm2) and large (57.82 cm2) artificial leaves, whose leaf areas were determined by digital scanning with Scion Image Beta 4.02 (O’Neal et al. 2002). Prior to their addition, the artificial leaves were thoroughly rinsed three times in warm water and soaked for 24 h. The contents of the containers were allowed to incubate for 3 d before the addition to each container of 100 first instar A. albopictus larvae. Thirty containers received 1 T. rutilus larva and the remaining 30 containers received none (5×2×6 = 60 total).
Adult A. albopictus were allowed to emerge in the containers. Containers were covered with nylon mesh (~210 µm) to prevent escape of emerging adults. We recorded emergences and removed adults from the containers daily using an aspirator. The experiment continued until all A. albopictus had developed to adulthood or died as immatures.
Experiment 3: C. appendiculata predation and variable habitat complexity effects on A. albopictus and O. triseriatus population growth measurements
C. appendiculata larvae were collected from laboratory colonies and reared on cultured nematodes until they molted to fourth instars. Teneral fourth instars were fed nematodes ad libitum for 48 h and then starved for 24 h before the experiment. Three days before the start of the experiment, each container (11.0×6.5 cm, height × diameter) received 400 ml sieved (180 µm mesh) tire water, 2.0 g of ground leaves, and the addition of one of four levels of artificial habitat complexity in the form of artificial maple leaves (as in experiment 2) cut in half (18.56 cm2). Treatment levels for habitat complexity consisted of 0, 2, 6, or 10 half-leaves. Treatments consisted of 0 or 1 fourth instar C. appendiculata and four levels of habitat complexity, each replicated five times (4×2×5 = 40 total). Each treatment received 50 first instar A. albopictus and 50 first instar O. triseriatus. Prey larvae were added to each container and allowed to acclimate for 10 min before adding predators. Containers were covered with nylon mesh (~210 µm) to trap emerging adults.
Data analysis
Individual Multivariate Analyses of Variance (MANOVA) were used to determine the treatment effects of predator, habitat complexity, and their interaction on prey population growth measurements: survivorship to adulthood, development time to adulthood, and adult mass. We used data transformations when the raw data did not meet the assumptions of univariate normality and homogeneous variances. Randomization two-way Analyses of Variance (ANOVA) were used (program RT Version 1.02, Manly 1991a, b) when no common transformation improved departures from normality (e.g., λ′, experiments 2 and 3). Standardized canonical coefficients (SCCs) were used to determine the relative contribution of each of the population growth measurements to significant multivariate effects and their relationship to each other (e.g., positive or negative) (SAS Institute 1989; Scheiner 1993). For each experiment, individual ANOVAs for each prey species were used to determine effects of predator, habitat complexity, and interaction terms on an estimated finite rate of population increase (λ′) calculated for each replicate container (Juliano 1998):
| (1) |
where No is the original number of females in a cohort (assumed to be 50%), Ax is the number of females emerging to adulthood on day x, wx is the mean adult female size on day x, and f(wx) describes the relationship between female size and the number of eggs produced. Sizes of adult A. albopictus and O. triseriatus were determined by measuring dry masses (dried at 60°C for >48 h) using a microbalance. D is the number of days from adult female emergence to oviposition. For A. albopictus and O. triseriatus, D is assumed to be 14 and 12 d, respectively (Livdahl and Willey 1991; Nannini and Juliano 1998). We used the following fecundity-size relationships [f(wx)]:
- A. albopictus (Lounibos et al. 2002):
(2) - O. triseriatus (Nannini and Juliano 1998):
(3)
Results
Experiment 1: T. rutilus predation and single-level habitat complexity effects on A. albopictus prey population growth measurements
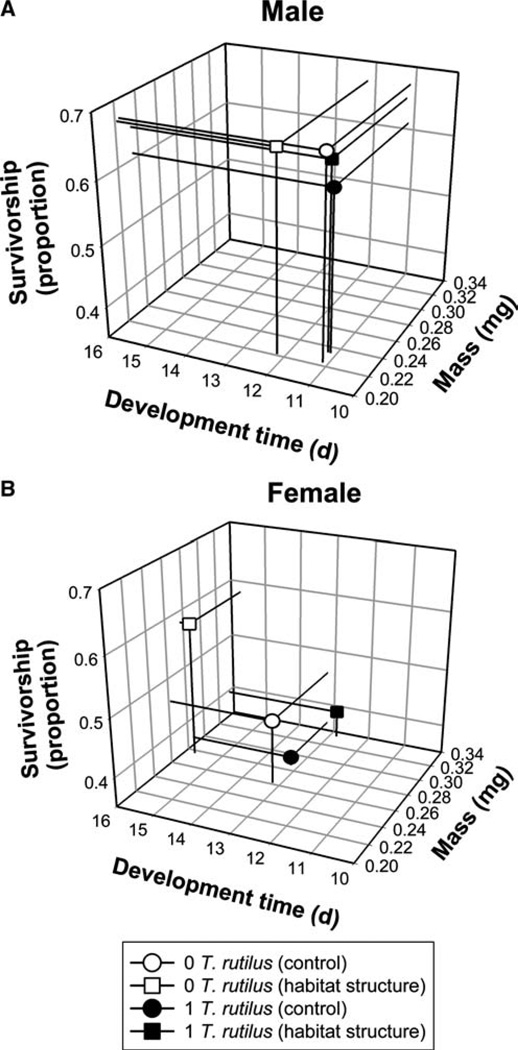
The MANOVA for the analysis of female A. albopictus showed significant treatment effects of the predator, habitat complexity, and interaction (Table 1). For all significant effects, SCCs showed survivorship and development time contributed the most to significant effects and adult mass made only a minor contribution (Table 1). Also, for all significant effects, both survivorship and development time were positively related to each other but negatively related to mass, except for the habitat structure effect, as shown by the signs of the SCCs. For the significant predator effect, A. albopictus females had lower survivorship, shorter development time, and increased mass in the presence of T. rutilus than in its absence (Fig. 1b). A. albopictus females had higher survivorship, longer development time, and increased mass in containers with added artificial leaves compared with containers without artificial leaves (Fig. 1b). A significant interaction resulted from significantly higher survivorship and longer development time in treatments without T. rutilus and with artificial leaves compared with all other treatment combinations (For all contrasts Pillai’s trace >0.61; d.f. = 3, 16; and P < 0.003) (Fig. 1b). No other contrasts of multivariate means were significantly different from each other (All Pillai’s trace < 0.32; d.f. = 3, 16; and P > 0.13). MANOVA for the analysis of male A. albopictus survivorship, development time, and mass showed no treatment effects (Table 1, Fig. 1a). Despite the significant effects on individual growth measurements of females, ANOVA for the analysis of λ′ showed no significant treatment effects (All F1,16 ≤ 0.28 and P > 0.37).
Table 1.
MANOVAs for effects of T. rutilus predation and habitat complexity treatments on female and male A. albopictus for population growth measurements (development time to adulthood, survivorship to adulthood, and adult mass)
| Analysis | Source | d.f. | Pillai’s trace | P | Standardized canonical coefficients | ||
|---|---|---|---|---|---|---|---|
| Development | Survivorship | Mass | |||||
| Experiment 1 | |||||||
| Female | Predator | 3 | 0.68 | 0.0008 | 0.89 | 1.24 | −0.33 |
| A. albopictus | Habitat complexity | 3 | 0.48 | 0.0241 | 1.22 | 0.91 | 0.42 |
| Pred. × Habitat complexity | 3 | 0.45 | 0.0323 | 1.37 | 0.78 | −0.05 | |
| Error d.f. | 16 | ||||||
| Male | Predator | 3 | 0.31 | 0.1376 | |||
| A. albopictus | Habitat complexity | 3 | 0.38 | 0.0691 | |||
| Pred. × Habitat complexity | 3 | 0.25 | 0.2274 | ||||
| Error d.f. | 16 | ||||||
| Experiment 2 | |||||||
| Female | Predator | 3 | 0.94 | <0.0001 | 0.22 | 3.51 | 0.53 |
| A. albopictus | Habitat complexity | 12 | 0.44 | 0.0502 | 2.52 | −2.46 | −0.17 |
| Pred. × Habitat complexity | 12 | 0.18 | 0.7472 | ||||
| Error d.f. | 43 | ||||||
| Male | Predator | 3 | 0.89 | <0.0001 | 0.47 | 2.60 | −0.26 |
| A. albopictus | Habitat complexity | 12 | 0.60 | 0.0007 | 1.24 | −1.36 | 0.04 |
| Pred. × Habitat complexity | 12 | 0.27 | 0.2769 | ||||
| Error d.f. | 49 | ||||||
Fig. 1.
Tri-variate LS means (from MANOVA) for effects of T. rutilus predators and habitat complexity on a male and b female A. albopictus survivorship to adulthood, development time to adulthood, and adult mass
Experiment 2: T. rutilus predation and variable habitat complexity effects on A. albopictus prey population growth measurements
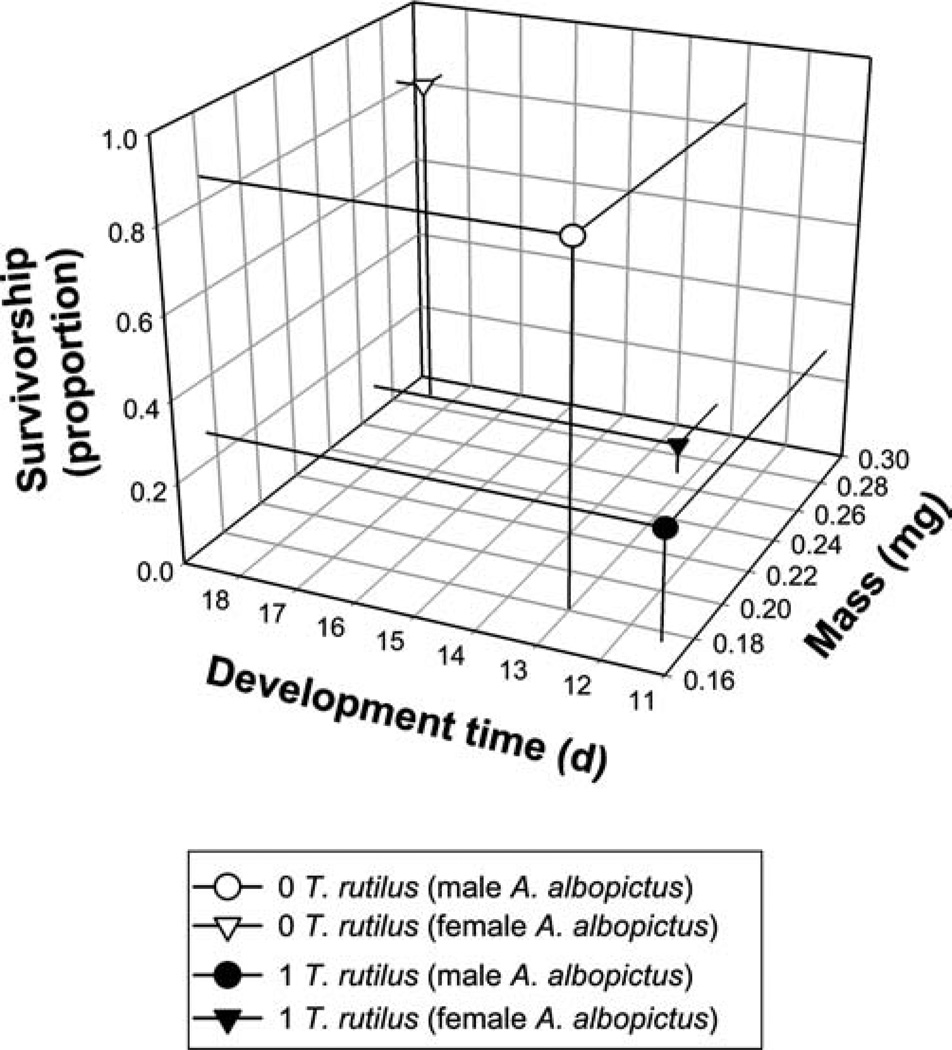
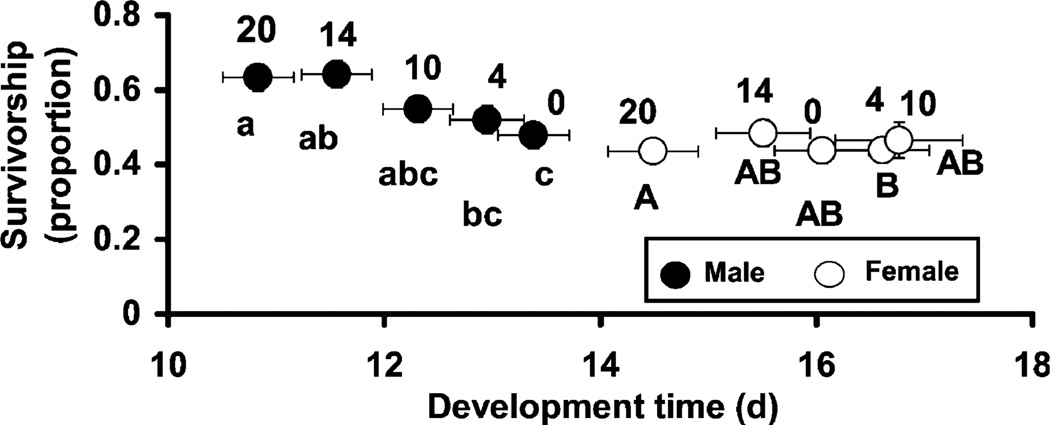
The MANOVAs for the analysis of both female and male A. albopictus showed significant effects of the predator and of habitat complexity but no significant interactions (Table 1). For the predator effect, SCCs showed survivorship accounted for most of the multivariate effect with development time and adult mass contributing far less (Table 1). For predator effects on females, all variables were positively related to one another, whereas for males, development time and survivorship were positively related to each other but negatively related to mass (Table 1). For the habitat complexity effect, SCCs showed that survivorship and development time contributed approximately equally to the overall effect, with adult mass contributing far less. For the significant predator effect, both female and male A. albopictus had lower survivorship, shorter development time, and similar mass in the presence of T. rutilus than in its absence (Fig. 2). For male A. albopictus, increased habitat complexity was associated with higher survivorship and shorter development time (Fig. 3).
Fig. 2.
Tri-variate LS means (from MANOVA) for effects of T. rutilus predators on male and female A. albopictus survivorship to adulthood, development time to adulthood, and adult mass
Fig. 3.
Bi-variate LS means (± SE) for habitat complexity effect on male and female A. albopictus survivorship to adulthood and development time to adulthood. Numbers above LS means show habitat complexity treatment (e.g., number of whole cloth maple leaves added). Lower and upper case letters indicate significant differences for males and females, respectively [experiment wise α = 0.05, sequential Bonferroni method (Rice 1989)]. LS means for mass were omitted since they contributed little to the overall habitat structure effect
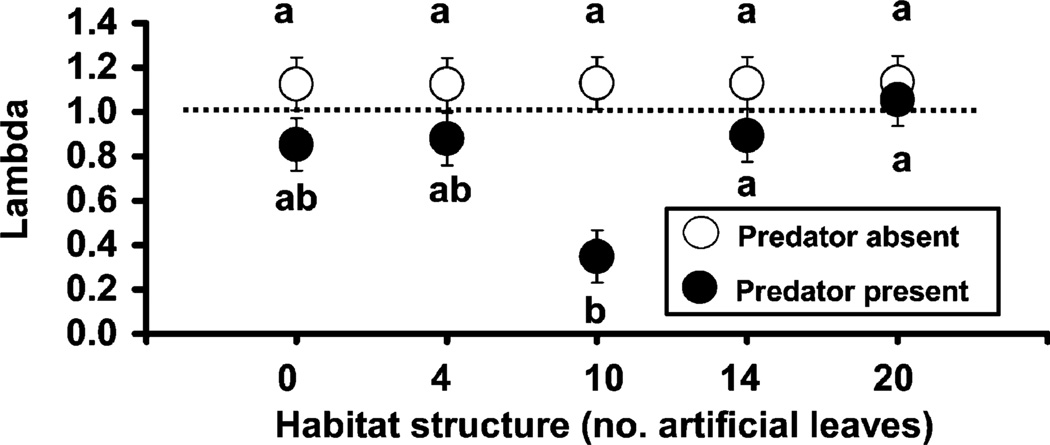
Randomization ANOVA showed significant treatment effects on λ′ of the predator (F1,50 = 18.53, P = 0.001), habitat complexity (F4,50 = 2.56, P = 0.0038), and their interaction (F4,50 = 2.55, P = 0.044). For the predator effect, A. albopictus λ′ was significantly lower in the presence of T. rutilus compared with T. rutilus absent (LS mean ± SE: 0.81 ± 0.05 and 1.13 ± 0.05, respectively). Significant habitat complexity and interaction effects were mainly due to very low λ′ values found in treatments with 10 artificial leaves and T. rutilus present (Fig. 4). For the habitat structure effect, only 10 vs. 20 artificial leaves treatments showed significant differences among λ′ values [LS mean ± SE: 0.74 ± 0.08 and 1.1 ± 0.08, respectively, using a Tukey–Kramer adjustment for multiple comparisons (SAS Institute 1989)]. No other trends among λ′ values appear to be due to habitat complexity (Fig. 4). Among containers with T. rutilus present, a single replicate in each of 0, 4, and 14 habitat treatments had no survivors to adulthood resulting in λ′ = 0 for those replicates. However, four replicates for the 10-leaf habitat treatment had no survivors, so the mean λ′ value was lower than those of other treatments. Reanalysis of the data, leaving out all 10-leaf habitat complexity treatments, showed that predator treatment effect was highly significant whereas the habitat and interaction treatments were not significant (Predator F1,40 = 7.50, P = 0.0092; Habitat F3,40 = 0.40, P = 0.7596; Interaction F3,40 = 0.34, P = 0.7972), thus supporting our claim that a single treatment was driving the habitat and interaction effects, and that predator effect was stronger than the other two factors.
Fig. 4.
LS means (± SE) for significant treatment effects of T. rutilus predators in five habitat structures (0, 4, 10, 14, and 20 artificial leaves added) on A. albopictus λ′ (lambda). Letters indicate significant differences among λ′ values for the significant treatment interaction [Tukey–Kramer adjustment for multiple comparisons (SAS Institute 1989)]
Experiment 3: C. appendiculata predation and variable habitat complexity effects on A. albopictus and O. triseriatus population growth measurements
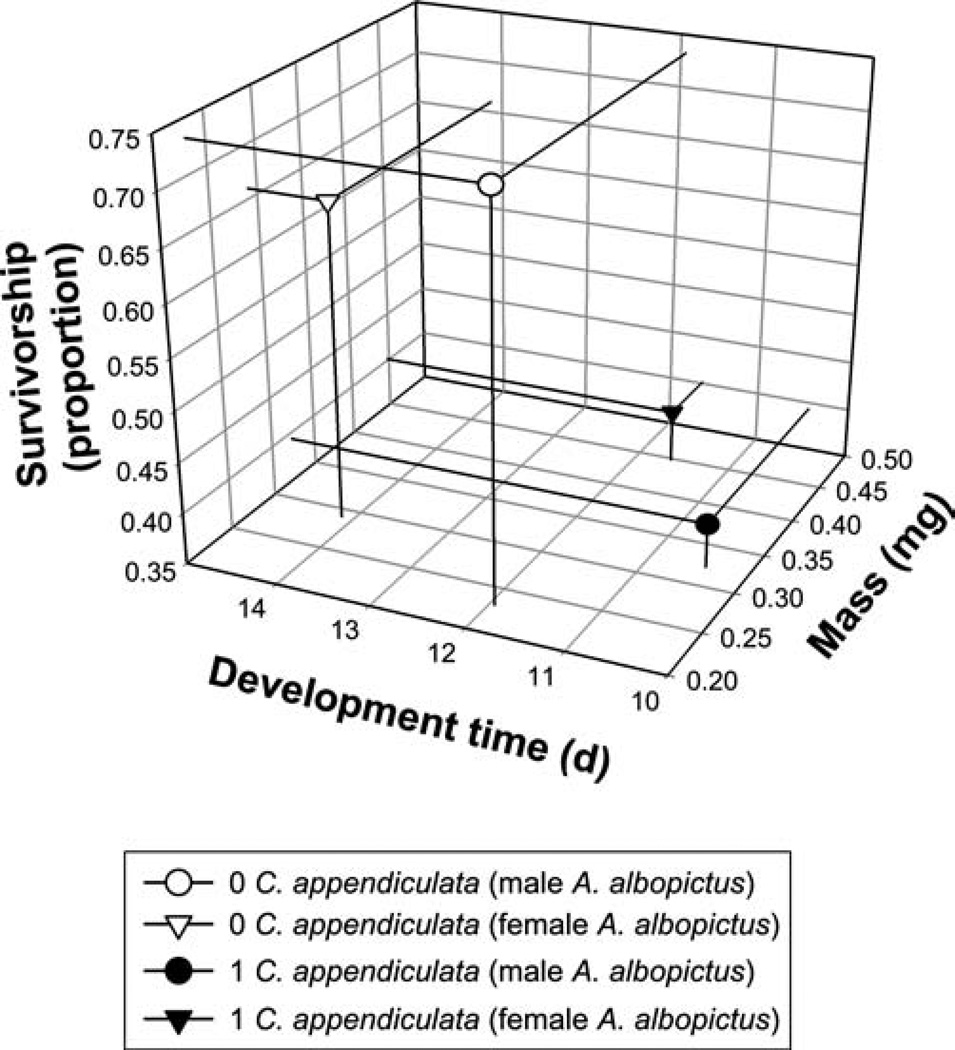
The MANOVA for the analysis of female and male A. albopictus growth showed significant effects of the predator, but not of habitat complexity or their interaction with predation (Table 2). For the significant predator effect, SCCs showed mass contributed the most to significant effects with development time and survivorship contributing similarly, but less than mass (Table 2). For females, development time and survivorship were positively related to each other, whereas, mass was negatively related to the other variables (Table 2). For males, development time and mass were positively related to each other, whereas, survivorship was negatively related (Table 2). In the presence of C. appendiculata, male and female A. albopictus had lower survivorship, shorter development time and greater mass (Fig. 5).
Table 2.
MANOVAs for effects of C. appendiculata predation and habitat complexity on female and male A. albopictus and O. triseriatus population growth measurements (development time to adulthood, survivorship to adulthood, and adult mass)
| Analysis | Source | d.f. | Pillai’s trace | P | Standardized canonical coefficients | ||
|---|---|---|---|---|---|---|---|
| Development | Survivorship | Mass | |||||
| Experiment 3 | |||||||
| Female | Predator | 3 | 0.76 | <0.0001 | 0.40 | 0.63 | −1.32 |
| A. albopictus | Habitat complexity | 9 | 0.34 | 0.2227 | |||
| Pred. × Habitat | 9 | 0.22 | 0.5771 | ||||
| Error d.f. | 32 | ||||||
| Male | Predator | 3 | 0.80 | <0.0001 | 0.57 | −0.75 | 1.37 |
| A. albopictus | Habitat complexity | 9 | 0.24 | 0.5150 | |||
| Pred. × Habitat | 9 | 0.32 | 0.2754 | ||||
| Error d.f. | 32 | ||||||
| Female | Predator | 3 | 0.34 | 0.1327 | |||
| O. triseriatus | Habitat complexity | 9 | 0.32 | 0.8009 | |||
| Pred. × Habitat | 3 | 0.02 | 0.9745 | ||||
| Error d.f. | 15 | ||||||
| Male | Predator | 3 | 0.80 | <0.0001 | 0.08 | 1.10 | −1.38 |
| O. triseriatus | Habitat complexity | 9 | 0.25 | 0.6841 | |||
| Pred. × Habitat | 9 | 0.44 | 0.2131 | ||||
| Error d.f. | 24 | ||||||
Fig. 5.
Tri-variate LS means (from MANOVA) for the effects of C. appendiculata predators on male and female A. albopictus survivorship to adulthood, development time to adulthood, and adult mass
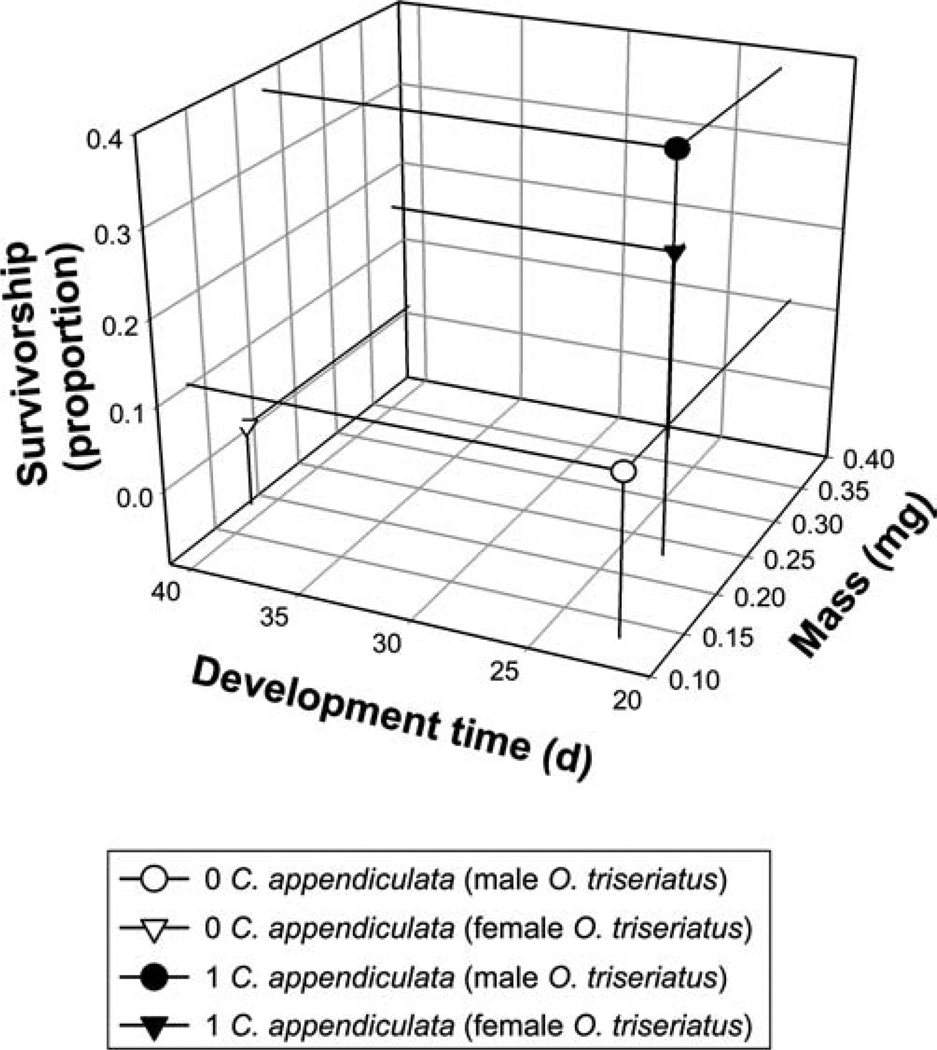
MANOVA for the analysis of male O. triseriatus showed significant effects of the predator, but not habitat complexity or their interaction (Table 2). For the significant predator effect, SCCs showed mass and survivorship contributed the most to significant effects with development time having only a minor contribution (Table 2). Development time and survivorship were positively related to each other, while mass was negatively related to the other variables. In the presence of C. appendiculata, O. triseriatus males had higher survivorship, greater mass, and similar development times (Fig. 6). MANOVA for the analysis of female O. triseriatus showed no significant treatment effects (Table 2). However, this analysis should be interpreted with caution because a large number of containers with C. appendiculata absent resulted in no O. triseriatus survivors. Thus we were unable to calculate development time and mass for many replicates. This drastically reduced sample size and statistical power to detect treatment differences since MANOVA does not adjust for missing data (Scheiner 1993). As a compromise, we analyzed response variables individually by univariate ANOVAs. Results were similar to the MANOVA for males showing significant effects of a predator (For all variables, P < 0.0023 except for development time where P = 0.0525), but not of habitat complexity or interactions of these variables (All P > 0.17). In the presence of C. appendiculata, O. triseriatus females had higher survivorship, greater mass, and shorter development times (Fig. 6).
Fig. 6.
Tri-variate LS means (from MANOVA) for the effects of C. appendiculata predators on male and female O. triseriatus survivorship to adulthood, development time to adulthood, and adult mass
Randomization ANOVA showed no significant effects of predator (F1,32 = 0.97, P = 0.407), habitat (F3,32 = 1.08, P = 0.397), or their interaction (F3,32 = 0.87, P = 0.539) on λ′ values for A. albopictus. There were significant effects of predator (F1,32 = 47.88, P = 0.001), but not habitat complexity (F3,32 = 1.89, P = 0.160) or the predator × habitat interaction (F3,32 = 1.51, P = 0.222) on λ′ values for O. triseriatus. Presence of C. appendiculata resulted in greater λ′ values for O. triseriatus than when the predator was absent (LS mean ± SE; 0.95 ± 0.07 and 0.24 ± 0.07, respectively).
Discussion
Habitat and predator interaction
Our results show that predator presence and habitat complexity altered prey population growth, and the outcomes were dependent on experimental design and predator–prey species combinations. However, habitat complexity did not reduce overall predation rates as seen in previous studies with actively searching predators (Savino and Stein 1982; Manatunge et al. 2000; Spitzer et al. 2000). The phenomenon of reduced predation rates in structurally complex habitats seems to apply most to actively foraging predators (Coen et al. 1981; Heck and Crowder 1991). Others have suggested that habitat complexity may provide ambush predators with camouflage and reduce the ability of prey to detect the predator (Heck and Orth 1980; Coen et al. 1981; James and Heck 1994). Habitat complexity may not be as important for predators that do not chase their prey through structurally complex habitats. Ambush predators may detect prey motion, and thus habitat complexity may have little effect on encounter rate for this foraging strategy. James and Heck (1994) hypothesized that in cases where habitat complexity has no effect on predation, physical structure provides a visual barrier where the predator cannot see the prey and the prey cannot see the predator. C. appendiculata and T. rutilus are most likely to detect prey by tactile and chemical cues (Lounibos et al. 1987; Kesavaraju and Juliano 2004) since compound eyes do not fully develop until after the larval stage (Steffan and Evenhuis 1981). Previous studies investigating ambush predator–prey relationships among habitats varying in structural complexity have been few and largely limited to fish (e.g., James and Heck 1994; Greenberg et al. 1995; Flynn and Ritz 1999). The current study extends the general results of ambush predator-prey fish systems in structured habitats to the two dominant dipteran predators in Florida containers and their associated mosquito prey.
Habitat complexity treatment
Although more complex habitats did not lessen effects of a predator on prey population growth, we did observe significant main effects of habitat complexity for male or female A. albopictus in the first two experiments (Table 1). In the first experiment, habitat complexity increased female survivorship and lengthened development time (Fig. 1b). In the second experiment, additional habitat complexity consistently resulted in shorter development time for both sexes and increased survivorship among males (Fig. 3). Differences in the habitat complexity effects on development time between experiments 1 and 2 are, in part, due to substantial differences in water volume and food resources between the two experiments. The yeast/albumin used in experiment 1 was likely a superior food resource compared to oak leaves of experiment 2. In general, among habitat complexity treatments, both sexes developed more rapidly in larger containers and with yeast/albumin resources used in experiment 1 (Figs. 1a, b, 3). The second experiment may be more representative of natural habitats since we used multiple levels of structural complexity along with natural food resources (i.e., oak leaves). Addition of leaves increased the surface area available for browsing by the mosquito larvae. Microorgansims may have accumulated on these artificial surfaces, increasing food supply and availability for the larvae. By increasing habitat complexity, encounters among individual prey are reduced, possibly lessening interference competition (Case and Gilpin 1974; Broadie and Bradshaw 1991; Suutari et al. 2004). Reducing competition would account for shorter development times and greater survivorship as seen in the second experiment (Fig. 3).
Predator treatment
Predators commonly have strong effects on prey population growth (Sih et al. 1985) such as survivorship (Lounibos et al. 2001), development time, and mass (e.g. Grill and Juliano 1996). In most experiments, the presence of a predator resulted in reduced survivorship, shorter development time, greater size at emergence, and in some cases reduced λ′ for the prey. However, in the first experiment, predatory T. rutilus had no observable affect on any population growth measure of A. albopictus males (Table 1). This lack of an effect on males was likely due to a combination of the large container volume and sufficient food resources allowing for rapid development of males, thus limiting their exposure to predation by T. rutilus (mean ± SE time to emergence in the presence and absence of T. rutilus was 11.17 ± 0.15 and 11.67 ± 0.15 d, respectively). Size-structure among prey may alter susceptibility to predation (Werner and Gilliam 1984), and rapidly developing male mosquitoes that achieve larger sizes may be less vulnerable to T. rutilus predation. In the presence of predators, shorter development time occurs in part when rapidly developing larvae survive to emergence and bias the mean development time, however it is also likely due to a release from competition (Morin 1981; Wilbur et al. 1983). For mosquitoes, competition lengthens development time and reduces size and survivorship (Teng and Apperson 2000; Lounibos et al. 2002). In agreement with other mosquito and anuran studies, we show that both intraspecific and interspecific competition among prey may be alleviated when a predator crops prey from the environment (Morin 1981; Wilbur et al. 1983; Chambers 1985; Grill and Juliano 1996).
Predator-dependent outcomes
Predator identity was never a treatment variable within a single experiment. Therefore, drawing conclusions about predator identity by comparisons among the experiments must be done with caution since multiple confounding effects may influence the outcome (e.g., experimental design). However, inspection of results of the three experiments shows that population growth measurements most susceptible to effects of the predator treatments may depend on the particular predator present, as well as the sex of the prey. In the first two experiments, using A. albopictus prey and a T. rutilus predator, SCCs showed that survivorship dominated the predator effect for both sexes, except for males in experiment 1 (Table 1). In experiment 3 with the predator C. appendiculata, adult mass contributed the most to the significant predator effect for males of both prey species and females of A. albopictus (Table 2), suggesting similarities between A. albopictus and O. triseriatus in population growth measurements affected. However, we cannot rule out whether differences in competition due to differences in experimental setup contributed, in part, to differences in prey performance between studies (e.g., intraspecific versus interspecific). Differences in the relative sizes of predators and prey with time may alter predator–prey interactions due to size related energy requirements of predators, so that larger predators may have greater rates of prey consumption (Kurzava and Morin 1998; Griswold and Lounibos 2005b). In the current study, the observed predator-dependent differences in prey population growth measurements most affected in experiments 1 and 2 (e.g., survivorship) vs. 3 (e.g., mass) may be due to the relative sizes of the two predator species. Large T. rutilus cull final instar and pupal mosquito prey (Bradshaw and Holzapfel 1983). Conversely, final-stage C. appendiculata are size-limited, and mosquito prey are relatively invulnerable to predation by this species in their third and fourth instars (Lounibos 1985), so that prey body size acts as an absolute refuge, as in a variety of predator–prey systems (e.g., Persson et al. 1996; Ray-Culp et al. 1999; Wellborn 2002). Therefore, mass may be a larger contributor to the overall predator effect since C. appendiculata eats only early instars of A. albopictus and O. triseriatus and, so, the relative contribution of survivorship to the overall predator effect was reduced.
Sex-dependent outcomes
Differences in treatment effects on females and males in the three experiments may be, in part, attributable to differences in sex-specific development and size because adult male mosquitoes are often smaller than females and the first to emerge to adulthood (e.g., Brust 1967). In experiment 1, size-structured predation (Werner and Gilliam 1984) may have allowed for rapidly developing male A. albopictus to achieve larger sizes that may be less vulnerable to T. rutilus predation. In contrast, the longer development time of females (Briegel and Timmermann 2001) may have made female A. albopictus more vulnerable to predation in experiment 1. Conversely, there were significant treatment effects on male, but not female, O. triseriatus in experiment 3. Although this comparison is between two prey species (A. albopictus versus O. triseriatus), it suggests differences in prey population growth measurements depend upon the sex of the prey. Differences in initial starting conditions among experiments 1–3 could, in part, influence the outcome.
In the first two experiments, male and female A. albopictus survivorship were similar in the absence of T. rutilus, however, when T. rutilus was present, survivorship was disproportionably greater for males (Figs. 1a, b, 2). These results were not observed for A. albopictus with C. appendiculata (Fig. 5), but were observed for O. triseriatus in the presence of C. appendiculata (Fig. 6). Field and laboratory experiments have shown skewed sex ratios favoring male copepod prey attributed to sex-dependent differences in size, activity, and ability to escape attack from a variety of predators (Maly 1970; Hairston et al. 1983; Svensson 1997). For A. albopictus and O. triseriatus in the current study, the results suggest a sex-dependent difference in population growth measurements owing, in part, to the size differences of male and female prey. Differences between A. albopictus and O. triseriatus in male and female survivorship in the presence of C. appendiculata may be due to different contributions from the population growth parameters (Table 2). Although mass contributes the most to the predatory C. appendiculata effect for both male A. albopictus and O. triseriatus, survivorship contributed relatively more to male O. triseriatus as compared to male A. albopictus (SCCs, Table 2).
Predatory C. appendiculata reverses the outcome of prey performance
A. albopictus larvae outcompeted O. triseriatus when nutrients were limiting in laboratory microcosms (Novak et al. 1993; Teng and Apperson 2000). Our third experiment supports this conclusion in that A. albopictus had shorter development time and greater size and survivorship than O. triseriatus in the absence of C. appendiculata (Figs. 5, 6). However, based on survivorship and λ′ measurements, O. triseriatus differentially benefited from the presence of C. appendiculata compared to A. albopictus (Fig. 6), as shown by others (Griswold and Lounibos 2005c). Studies have shown that direct effects of predation (e.g., predator-mediated release from competition) (e.g., Morin 1981), as well as trait-mediated indirect effects of predation (e.g., morphology and behavior) (Werner and Anholt 1996; Relyea 2000) may reverse the outcome of competition. In the current study, predator-mediated release from interspecific competition as well as behavioral differences among the prey in the presence of a predator may largely be responsible (Morin 1981; Werner and Anholt 1996; Griswold and Lounibos, 2005c).
Interactions among competing prey species may be altered when they differ in their behavioral plasticity in response to predator cues (e.g., Werner and Anholt 1996; Peacor and Werner 1997). Predation studies found O. triseriatus, but not A. albopictus capable of behavioral plasticity in response to cues from T. rutilus present in the water (Juliano and Reminger 1992; Juliano and Gravel 2002; Kesavaraju and Juliano 2004). O. triseriatus responds to predator cues by changing to more frequent low-risk behaviors (Kesavaraju and Juliano 2004). Also, A. albopictus was preferred to O. triseriatus in prey preference comparisons with either T. rutilus and C. appendiculata (Griswold and Lounibos 2005a) suggesting that the two species differ in their ability to avoid predation. Future studies should incorporate behavior observations of both predator and prey to determine the mechanisms behind these results.
Predictions about the impact of predators on prey require a clear understanding of mechanisms driving predator–prey interactions. As in other studies, we attempted to understand the mechanisms by comparisons of multiple measures of prey population growth. We showed that the effects of habitat complexity were less important to shaping predator–prey interactions in this community, where predation risk differs between male and female prey. Our multivariate and SCCs analyses suggested predator-specific impacts on prey performance that are consistent with anticipated size-structured predation between two predators of different sizes.
Acknowledgements
We thank H. Lynn for providing us with T. rutilus larvae; R. Escher and N. Nishimura for help in the initial set-up and daily maintenance of the experiments; J. Butler for additional laboratory space; S. Juliano, and S. Yanoviak for useful discussions and ideas leading to habitat complexity manipulation; A. Ellis, G. O’Meara, J. Rey, and S. Yanoviak for helpful comments on the manuscript. All experiments were performed in compliance with the current laws of the USA. This research was supported by a grant from the National Institutes of Health (R01-AI-44793). This is Florida Agricultural Experiment Station Journal Series R-10851.
Contributor Information
Barry W. Alto, Florida Medical Entomology Laboratory, University of Florida, 200 9th St. SE, Vero Beach, FL, 32962 USA, bwalto@ufl.edu, Tel.: + 1-772-7787200, Fax: + 1-772-7787205
Marcus W. Griswold, Entomology and Nematology Department, University of Florida, P.O. Box 110620, Gainesville, FL, 32611 USA
L. Philip Lounibos, Florida Medical Entomology Laboratory, University of Florida, 200 9th St. SE, Vero Beach, FL, 32962 USA.
References
- Alto BW, Yanoviak SP, Lounibos LP, Drake BG. Effects of elevated atmospheric C02 on water chemistry and mosquito growth under competitive conditions in container habitats. Florida Entomol. 2005 doi: 10.1653/0015-4040(2005)88[372:EOEACO]2.0.CO;2. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedhomme S, Agnew P, Sidobre C, Michalakis Y. Sex-specific reaction norms to intraspecific larval competition in the mosquito Aedes aegypti. J Evol Biol. 2003;16:721–730. doi: 10.1046/j.1420-9101.2003.00576.x. [DOI] [PubMed] [Google Scholar]
- Bradshaw WE, Holzapfel CM. Predator-mediated, non-equilibrium coexistence of tree-hole mosquitoes in southeastern North America. Oecologia. 1983;57:239–256. doi: 10.1007/BF00379586. [DOI] [PubMed] [Google Scholar]
- Briegel H, Timmermann SE. Aedes albopictus (Diptera: Culicidae): physiological aspects of development and reproduction. J Med Entomol. 2001;38:566–571. doi: 10.1603/0022-2585-38.4.566. [DOI] [PubMed] [Google Scholar]
- Broadie KS, Bradshaw WE. Mechanisms of interference competition in the western treehole mosquito, Aedes sierrensis. Ecol Entomol. 1991;16:145–154. [Google Scholar]
- Brust RA. Weight and developmental time of different stadia of mosquitoes reared at various constant temperatures. Can Entomol. 1967;99:986–993. [Google Scholar]
- Campos RE, Lounibos LP. Natural prey and digestion times of Toxorhynchities rutilus (Diptera: Culicidae) in southern Florida. Ann Entomol Soc Am. 2000;93:1280–1287. [Google Scholar]
- Case TJ, Gilpin ME. Interference competition and niche theory. Proc Natl Acad Sci USA. 1974;71:3073–3077. doi: 10.1073/pnas.71.8.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RC. Competition and predation among larvae of three species of treehole breeding mosquitoes. In: Lounibos LP, Rey JR, Frank JH, editors. Ecology of mosquitoes: proceedings of a workshop. Vero Beach, FL: Florida Medical Entomology Laboratory; 1985. pp. 25–53. [Google Scholar]
- Coen LD, Heck KL, Abele LG. Experiments on competition and predation among shrimps of seagrass meadows. Ecology. 1981;62:1484–1493. [Google Scholar]
- Coull BC, Wells JBJ. Refuges from fish predation: experiments with phytal meiofauna from the New Zealand rocky intertidal. Ecology. 1983;64:1599–1609. [Google Scholar]
- Flynn AJ, Ritz DA. Effect of habitat complexity and predatory style on the capture success of fish feeding on aggregated prey. J Mar Biol Ass UK. 1999;79:487–494. [Google Scholar]
- Greenberg LA, Paszkowski CA, Tonn WM. Effects of prey species composition and habitat structure on foraging by two functionally distinct piscivores. Oikos. 1995;74:522–532. [Google Scholar]
- Grenouillet G, Pont D, Seip KL. Abundance and species richness as a function of food resources and vegetation structure: juvenile fish assemblages in rivers. Ecography. 2002;25:641–650. [Google Scholar]
- Grill CP, Juliano SA. Predicting species interactions based on behaviour: predation and competition in container-dwelling mosquitoes. J Anim Ecol. 1996;65:63–76. [Google Scholar]
- Griswold MW, Lounibos LP. Does differential predation permit invasive and native mosquito larvae to coexist in Florida? Ecol Entomol. 2005a;30:122–127. doi: 10.1111/j.0307-6946.2005.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griswold MW, Lounibos LP. Predator identity and additive effects in a treehole community. Ecology. 2005b doi: 10.1890/0012-9658(2006)87[987:piaaei]2.0.co;2. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griswold MW, Lounibos LP. Competitive outcomes of aquatic container diptera depend on predation and resource levels. Ann Entomol Soc Am. 2005c doi: 10.1603/0013-8746(2005)098[0673:COOACD]2.0.CO;2. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hairston NG, Walton WE, Jr, Li KT. The causes and consequences of sex-specific mortality in a freshwater copepod. Limnol Oceanogr. 1983;28:935–947. [Google Scholar]
- Heck KL, Crowder LB. Habitat structure and predator–prey interactions in vegetated aquatic systems. In: Bell SS, McCoy ED, Mushinsky HR, editors. Habitat structure: the physical arrangement of objects in space. NY: Chapman and Hall; 1991. pp. 281–299. [Google Scholar]
- Heck KL, Orth RJ. Seagrass habitats: the roles of habitat complexity, competition and predation in structuring associated fish and motile macroinvertebrate assemblages. In: Kennedy VS, editor. Estuarine perspectives. NY: Academic Press; 1980. pp. 449–464. [Google Scholar]
- James PL, Heck KL. The effects of habitat complexity and light intensity on ambush predators within a simulated seagrass habitat. J Exp Mar Biol Ecol. 1994;176:187–200. [Google Scholar]
- Juliano SA. Geographic variation in vulnerability to predation and starvation in larval treehole mosquitoes. Oikos. 1989;56:99–108. [Google Scholar]
- Juliano SA. Species introduction and replacement among mosquitoes: interspecific resource competition or apparent competition? Ecology. 1998;79:255–268. [Google Scholar]
- Juliano SA, Gravel ME. Predation and the evolution of prey behavior: an experiment with tree hole mosquitoes. Behav Ecol. 2002;13:301–311. [Google Scholar]
- Juliano SA, Reminger L. The relationship between vulnerability to predation and behavior of larval treehole mosquitoes: geographic and ontogenetic differences. Oikos. 1992;63:465–476. [Google Scholar]
- Kerfoot WC, Sih A. Predation: direct and indirect impacts on aquatic communities. Hanover, NH: University Press of New England; 1987. [Google Scholar]
- Kesavaraju B, Juliano SA. Differential behavioral responses to water-borne cues to predation in two container-dwelling mosquitoes. Ann Entomol Soc Am. 2004;97:194–201. doi: 10.1603/0013-8746(2004)097[0194:dbrtwc]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner CA, Hawley WA, Bradshaw WE, Holzapfel CM, Fisher IJ. Protandry in Aedes sierrensis: the significance of temporal variation in female fecundity. Ecology. 1995;76:1242–1250. [Google Scholar]
- Kurzava LM, Morin PJ. Tests of functional equivalence: complementary roles of salamanders and fish in community organization. Ecology. 1998;79:477–489. [Google Scholar]
- Livdahl TP, Willey MS. Prospects for an invasion: competition between Aedes albopictus and native Aedes triseriatus. Science. 1991;253:189–191. doi: 10.1126/science.1853204. [DOI] [PubMed] [Google Scholar]
- Lounibos LP. The mosquito community of treeholes in subtropical Florida. In: Frank JH, Lounibos LP, editors. Phytotelmata: terrestrial plants as hosts for aquatic insect communities. NJ: Plexus Publishing, Inc.; 1983. pp. 223–246. [Google Scholar]
- Lounibos LP. Interactions influencing production of treehole mosquitoes in south Florida. In: Lounibos LP, Rey JR, Frank JH, editors. Ecology of mosquitoes: proceedings of a workshop. FL: Florida Medical Entomology Laboratory; 1985. pp. 65–77. [Google Scholar]
- Lounibos LP, Frank JH, Machado-Allison CE, Ocanto P, Navarro JC. Survival, development and predatory effects of mosquito larvae in Venezuelan phytotelmata. J Trop Ecol. 1987;3:221–242. [Google Scholar]
- Lounibos LP, O’Meara GF, Escher RL, Nishimura N, Cutwa M, Nelson T, Campos RE, Juliano SA. Testing predictions of displacement of native Aedes by the invasive Asian Tiger mosquito Aedes albopictus in Florida, USA. Biol Invas. 2001;3:151–166. [Google Scholar]
- Lounibos LP, Suárez S, Menéndez Z, Nishimura N, Escher RL, O’Connell SM, Rey JR. Does temperature affect the outcome of larval competition between Aedes aegypti and Aedes albopictus? J Vector Ecol. 2002;27:86–95. [PubMed] [Google Scholar]
- Maly EJ. The influence of predation on the adult sex ratios of two copepod species. Limnol Oceangr. 1970;15:566–573. [Google Scholar]
- Manatunge J, Asaeda T, Priyadarshana T. The influence of structural complexity on fish-zooplankton interactions: a study using artificial submerged macrophytes. Env Biol Fish. 2000;58:425–438. [Google Scholar]
- Manly BFJ. Randomization and Monte Carlo methods in biology. London: Chapman& Hall; 1991a. [Google Scholar]
- Manly BFJ. RT: a program for randomization testing, version 1:02. WY: West Inc.; 1991b. [Google Scholar]
- Morin PJ. Predatory salamanders reverse the outcome of competition among three species of anuran tadpoles. Science. 1981;212:1284–1286. doi: 10.1126/science.212.4500.1284. [DOI] [PubMed] [Google Scholar]
- Nannini MA, Juliano SA. Effects of the facultative predator Anopheles barberi on population performance of its prey Aedes triseriatus (Diptera: Culicidae) Ann Entomol Soc Am. 1998;91:33–42. [Google Scholar]
- Novak MG, Higley LG, Christiansen CA, Rowley WA. Evaluating larval competition between Aedes albopictus and A. triseriatus (Diptera) through replacement series experiments. Environ Entomol. 1993;22:311–318. [Google Scholar]
- Nylin S, Wiklund C, Wickman P-O, Garcia-Barros E. Absence of trade-offs between sexual size dimorphism and early male emergence in a butterfly. Ecology. 1993;74:1414–1427. [Google Scholar]
- O’Flynn MI, Craig GB., Jr Effect of Toxorhynchites brevipalpis on Aedes aegypti (Diptera: Culicidae) in continuousbreeding laboratory populations. J Med Entomol. 1982;19:380–387. doi: 10.1093/jmedent/19.4.380. [DOI] [PubMed] [Google Scholar]
- O’Meara GF, Vose FE, Carlson DB. Environmental factors influencing oviposition by Culex (Culex) (Diptera: Culicidae) in two types of traps. J Med Entomol. 1989;26:528–534. doi: 10.1093/jmedent/26.6.528. [DOI] [PubMed] [Google Scholar]
- O’Neal ME, Landis DA, Isaacs R. An inexpensive, accurate method for measuring leaf area and defoliation through digital image analysis. J Econ Entomol. 2002;95:1190–1194. doi: 10.1603/0022-0493-95.6.1190. [DOI] [PubMed] [Google Scholar]
- Peacor SD, Werner EE. Trait-mediated indirect interactions in a simple aquatic food web. Ecology. 1997;78:1146–1156. [Google Scholar]
- Persson L, Andersson J, Wahlstrom E, Eklov P. Size-specific interactions in lake systems: predator gape limitation and prey growth rate and mortality. Ecology. 1996;77:900–911. [Google Scholar]
- Ray-Culp M, Davis M, Stoner AW. Predation by xanthid crabs on early post-settlement gastropods: the role of prey size, prey density, and habitat complexity. J Exp Mar Biol Ecol. 1999;240:303–321. [Google Scholar]
- Relyea RA. Trait-mediated indirect effects in larval anurans: reversing competition with the threat of predation. Ecology. 2000;81:2278–2289. [Google Scholar]
- Rice WR. Analyzing tables of statistical tests. Evolution. 1989;43:223–225. doi: 10.1111/j.1558-5646.1989.tb04220.x. [DOI] [PubMed] [Google Scholar]
- SAS Institute. SAS/STAT user’s guide, version 6. Cary, NC: SAS Institute; 1989. [Google Scholar]
- Savino JF, Stein RA. Predator-prey interaction between largemouth bass and bluegills as influenced by simulated, submersed vegetation. Trans Am Fish Soc. 1982;111:255–266. [Google Scholar]
- Scheiner SM. Multiple response variables and multispecies interactions. In: Scheiner SM, Gurevitch J, editors. Design and analysis of ecological experiments. NY: Chapman& Hall; 1993. pp. 94–112. [Google Scholar]
- Sih A, Crowley P, McPeek M, Petranka J, Strohmeier K. Predation, competition, and prey communities: a review of field experiments. Ann Rev Ecol Syst. 1985;16:269–311. [Google Scholar]
- Spitzer PM, Mattila J, Heck KL. The effects of vegetation density on the relative growth rates of juvenile pinfish, Lagodon rhomboides (Linneaus), in Big Lagoon, Florida. J Exp Mar Biol Ecol. 2000;244:67–86. [Google Scholar]
- Steffan WA, Evenhuis NL. Biology of Toxorhynchites. Ann Rev Entomol. 1981;26:159–181. [Google Scholar]
- Steinwascher K. Relationship between pupal mass and adult survivorship and fecundity for Aedes aegypti. Environ Entomol. 1982;11:150–153. [Google Scholar]
- Suutari E, Rantala MJ, Salmela J, Suhonen J. Intraguild predation and interference competition on the endangered dragonfly Aeschna viridis. Oecologia. 2004;140:135–139. doi: 10.1007/s00442-004-1559-6. [DOI] [PubMed] [Google Scholar]
- Svensson J-E. Chaoborus predation and sex-specific mortality in a copepod. Limnol Oceanogr. 1997;42:572–577. [Google Scholar]
- Teng HJ, Apperson CS. Development and survival of immature Aedes albopictus and Aedes triseriatus (Diptera: Culicidae) in the laboratory: effects of density, food, and competition in response to temperature. J Med Entomol. 2000;37:40–52. doi: 10.1603/0022-2585-37.1.40. [DOI] [PubMed] [Google Scholar]
- Wellborn GA. Trade-off between competitive ability and antipredator adaptation in a freshwater amphipod species complex. Ecology. 2002;83:129–136. [Google Scholar]
- Werner EE, Anholt BR. Predator-induced behavioral indirect effects: consequences to competitive interactions in anuran larvae. Ecology. 1996;77:157–169. [Google Scholar]
- Werner EE, Gilliam JF. The ontogenetic niche and species interactions in size-structured populations. Ann Rev Ecol Syst. 1984;15:393–425. [Google Scholar]
- Wiklund C, Fagerström T. Why do males emerge before females? A hypothesis to explain the incidence of protandry in butterflies. Oecologia. 1977;31:153–158. doi: 10.1007/BF00346917. [DOI] [PubMed] [Google Scholar]
- Wilbur HM, Morin PJ, Harris RH. Salamander predation and the structure of experimental communities: anuran responses. Ecology. 1983;64:1423–1429. [Google Scholar]
- ZijIstra WG, Kesbeke F, Zwaan BJ, Brakefield PM. Protandry in the butterfly Bicyclus anynana. Evol Ecol Res. 2002;4:1229–1240. [Google Scholar]