Abstract
This work describes studies of the photodegradation mechanism of 1-Nitropyrene (1-NO2Py) in a chemical model system consisting of an organic solvent and known constituents of an aerosol particle. Photoproducts such as 1-hydroxypyrene (1-OHPy), 1-hydroxy-x-nitropyrenes (1-OH-x-NO2Py), 1-nitrosopyrene and 1,6- and 1,8-pyrenediones were identified by HPLC and HPLC-MS techniques and their quantum yields show a significant dependence on the type of solvent. The photodegradation quantum yield of 1-NO2Py, ϕ(−1−NO2Py), was larger in toluene, benzene and polar protic solvents (10−3) in comparison with nonpolar and polar aprotic solvents, where the yield is in the order of 10−4. In solvents with an abstractable hydrogen atom the products formed in higher yields were 1-OHPy and 1-OH-x-NO2Py. These represent 60 to 80% of the photodestruction yield and result from abstraction and recombination reactions of the pyrenoxy radical, an intermediate postulated to be formed as a result of a nitro-nitrite rearrangement in nitroaromatics. The small O2 effect in the photodegradation yield and the quenching experiments with azulene demonstrate the small contribution of the 3(π,π*) state in the 1-NO2Py photoreaction. The nitrosopyrene product was not observed under these conditions, demonstrating the participation of the 3(π,π*) state in its formation. In the presence various phenols aerosol constituents the photodegradation yield increased by ten fold in all solvents. This effect is partly ascribed by the reaction of 3(π,π*) state with the phenol. The effect of water resulted in the reduction of the 1-NO2Py photodegradation yield and of its photoproducts. The phototodegradation of 1-NO2Py was also studied in a viscous solvent, hexadecane, and it was determined that this medium does not inhibit its photodecay.
Keywords: nitropyrenes, photodegradation and photoproducts quantum yields and mechanism, quenching effects of phenols, water and polycyclic aromatic hydrocarbons
Introduction
Nitropolycyclic aromatic hydrocarbons (nitro-PAHs) are ubiquitous environmental pollutants released into the atmosphere during incomplete combustion of fossil fuels and biomass. It has been demonstrated that, in addition to being formed by direct combustion processes, nitro-PAHs could be formed from the atmospheric reactions of the parent PAH with hydroxyl radicals and nitrogen oxides under conditions that can be found in polluted environments, with subsequent partitioning to the particulate phase.1–3
Nitro-PAHs can be a significant fraction of the direct acting mutagenic and carcinogenic compounds present in extracts of ambient air particles,4 and their persistence in the gas and particle phases may result in human exposure through inhalation.5 They are potent mutagens and carcinogens6–8 and even if present at much lower concentrations (up to pg/m3) than their parent compounds in urban areas, they show toxicological importance.8–11 In fact, more than 90% of the mutagenicity for the total exhaust emitted from a diesel engine was found in the particulate phase, and can be associated to nitro-PAHs.12–13
The chemical transformations of nitro-PAH in the environment have been studied to a limited extent. One environmental fate of some nitro-PAHs is their photodecomposition.14 Studies of nitro-PAHs photodegradation in solution or coated on substrates have been done in the past several years15–20 and it has been shown that some nitro-PAHs can be readily decomposed when exposed to light both in solution and on surfaces. 1-Nitropyrene (1-NO2Py) is the predominant nitrated polycyclic aromatic hydrocarbon emitted in diesel engine exhaust.21–22 Its photodegradation mechanism is still a subject of interest and debate. Previous studies indicate that 1-NO2Py is transformed in the presence of sunlight into compounds that are significantly more or less mutagenic. 1–2 Photoproducts such as hydroxypyrene,3,20 hydroxynitropyrenes,3,14,19,23–24 and pyrenediones16 have been reported, but studies on their formation mechanisms are limited. The formation of five 1-hydroxy-x-nitropyrenes was demonstrated recently both in methanol solutions and in ambient airborne particles.23 The diurnal variability of these hydroxynitropyrenes observed in this study indicated that they originate from vehicle emissions and from secondary formation in the atmosphere, probably via the photoreaction of 1-NO2Py.
Van den Braken19 proposed a photodestruction mechanism for nitropyrene in methanol (Scheme 1, adapted from reference 19) that includes the excitation of the nitropyrene into an excited state (singlet or triplet) from which it can photodissociate to yield NO2 and a pyrene radical (Py) or undergo the nitro-nitrite rearrangement proposed by Chapman.25 Through the first channel, the radical formed can abstract a hydrogen atom from the solvent to form pyrene (Py + HS → PyH + S). Via the second pathway, an excited state, either a receiver 3(n,π*) state or the S1 state26 forms a cyclic intermediate which eventually rearranges into 1-pyrenylnitrite (PyONO). This unstable nitrite decomposes by O-N bond scission to produce a pyrenoxy radical (PyO·), (PyONO → PyO· + NO·). In the photolysis of 9-nitroanthracene, Plaza-Medina et al.27 showed that the anthryloxy radical absorption signal appears within a few picoseconds of the excitation pulse, requiring a fast nitro-nitrite rearrangement process from the S1 state instead of the 3(n,π*). The pyrenoxy radical can abstract a hydrogen atom from the solvent to form hydroxypyrene (PyO· + HS → PyOH + S). Through another route, the intermediate products of the dissociation of the nitrite, PyO· + NO·, can react via the addition of the NO radical to a C-x position on the pyrene ring to form a nitroso substituted compound. This intermediate subsequently oxidizes forming 1-OH-x-NO2 pyrene products.23 Still another reaction is a hydrogen abstraction from the solvent by an excited triplet state.
Scheme 1.
Photodegradation mechanism of 1-NO2Py
The quantum yields of photoreduction of a few nitro-PAHs have been reported. For example, measuring the disappearance of nitrobenzene in 2-propanol, Testa and Hurley28–29 calculated a yield of 0.01. For the photoreduction of nitronaphthalenes in 2-propanol, Hashimoto et al.30 reported a yield of 0.04, while Obi and co workers31 informed a 0.037 quantum yield for the photo reduction of 2-nitronaphthalene. Analogously to aromatic carbonyl compounds, the photoreduction of nitro-PAHs has been proposed to take place through their triplet states. In the case of nitrobenzene its lowest triplet state has been assigned to an n,π* state28–29, and for nitronaphthalene to a π,π*.32 For carbonyl compounds the 3(n,π*) state is more photoreactive than the 3(π,π*) state, thus the higher photoreduction yield in naphthalene was interpreted in terms of a longer triplet life time of the 3(π,π*) state which will compensate for the slower reaction rate of this state.31 In ethanol, 9-nitroanthracene photoreduces with a 0.07 quantum yield.33 They interpreted the small yield in terms of the recombination of the 9-anthryloxy radical with NO to regenerate the 9-nitroanthracene. Although photoreduction is one of the major reported photodegradation routes, there are others, such as photochemical oxidation of the aromatic ring or photodimerization that could significantly contribute to the photodestruction of the nitroarenes.
According to the proposed mechanisms and products reported, the quantum yield of the possible products could be influenced by the feasibility of H abstraction reaction by radical intermediates and the triplet state, on the rigidity of the solvent cage, which will determine the possibility of the geminate radicals (PyO + NO) of escaping from the cage and on the possible orientation of the NO2 group relative to the aromatic ring. Herein, we report studies on the effect of polar protic, polar aprotic and non-polar solvents and of different viscosities on the quantum yield of photodestruction of nitropyrenes, and the yield of formation of the principal products in order to quantify the different photodecomposition channels. We also examined the effect of some additives such as oxygen, phenols, azulene and water, representative components of the aerosol, on the photochemical properties of 1-NO2Py and on the formation of its photoproducts.
Experimental
1-Nitropyrene 99%, 1-hydroxypyrene 98%, phenol 99%, 1-hydroxynaphthalene (1-naphthol) 99%, 2-hydroxynaphthalene (2-naphthol) 99%, 1,4-dihydroxybenzene (hydroquinone) ≥99%, 2,6-dimethoxyphenol (syringol) 99%, 4-hydroxy-3-methoxybenzaldehyde (vanillin), 4-hydroxy-3-methoxybenzoic acid (vanillic acid) 97%, 3-methoxy-4-hydroxyphenyl acetic acid (homovanillic acid), and anthracene ≥99% were from Sigma-Aldrich Chemical Co. 1-Nitropyrene was recrystallized three times with methanol. Purified 1-nitropyrene was examined using the HPLC fluorescence chromatogram (exciting at 350 nm) and observing the decrease in the emission at 400 nm, due to the major impurity, 1-hydroxypyrene.
The solvents hexane ≥99%, benzene ≥99.9% (HPLC grade), carbon tetrachloride (CCl4) ≥99.9% (HPLC grade), 3-methylpentane 99+%, 2-propanol 99.5%, toluene ≥99.5% and ethanol 99.5% were from Sigma Aldrich Chemicals. Methanol (99.9%) and acetonitrile (99.9%) were obtained from Fisher Scientific. Diethyl ether 99% (HPLC grade), ethyl acetate 99.8% (HPLC grade) and isopentane 99+%, were from Alfa Aesar. Hexane was purified using a chromatographic column with activated silica gel. This silica was heated in an oven at 400°C for 10 hours.
1-Nitrosopyrene was synthesized by the oxidation of 1-aminopyrene.34 In a three bottom flask with a magnetic stirring bar, 1-aminopyrene (0.206g) were dissolved in 50 mL of dry chloroform saturated with nitrogen and cooled with ice (~4°C). The resulting solution was dark brown. Then a solution of m-chloroperoxybenzoic acid dissolved in 25 mL of chloroform was added. The addition took 25 minutes while maintaining a constant temperature. The progress of the reaction was monitored by thin layer chromatography using silica gel plates. The mobile phase was 10% ethyl acetate/90% hexane. The reaction was quenched after 1.5 hours to inhibit the formation of 1-nitropyrene. The reaction crude was extracted with 0.50 M NaOH (3 × 20 mL) and Na2SO4 was used to break the emulsion. The chloroform fraction was extracted twice with water, dried with anhydrous magnesium sulfate, and evaporated in vacuum. The residue was purified by chromatography on silica gel. 1H-NMR (CDCl3, 400 MHz): 10.34 (d, 1H, J = 9.20 Hz), 8.66 (d, 1H, J = 9.60 Hz), 8.52 (d, 1H, J = 7.60 Hz), 8.45 (d, 1H, J = 7.60 Hz), 8.42 (d, 1H, J = 8.80 Hz), 8.24 (t, 1H, J = 8.0 Hz), 8.16 (d, 1H, J = 8.8 Hz), 8.07 (d, 1H, J = 8.40 Hz), 7.05 (d, 1H, J = 8.80 Hz). 13C-NMR (CDCl3, 100 MHz): 137.5, 133.7, 132.3, 131.9, 131.0, 130.8, 128.0, 127.9, 127.3, 127.1, 125.5, 124.5, 124.2, 122.5, 104.3.
1-Hydroxy-2-nitropyrene was synthesized by dissolving 1-hydroxypyrene in CH3COOH (5 M). The temperature of the solution was kept between 15 and 40 °C. Nitric acid (15.8 M) was added slowly (0.033 mL) and the reaction was followed by thin layer chromatography (TLC). The reaction mixture was stirred for 3 hours at room temperature, and then poured into an ice bath to induce the precipitation of the product. After the ice melted, the mixture was filtered and the solid was dried under the vacuum. The solid was purified by column chromatography using silica gel as the stationary phase and hexane/ethyl acetate giving 20 mg. 1H-NMR (CDCl3, 400 MHz) δ: 7.96 (s, 2H), 8.17 (m, 4H), 8.66 (d, 1H, J = 8.8), 8.84 (s, 1H), 11.67 (s, 1H); 13C-NMR (CDCl3, 100 MHz) δ: 119.1, 121.9, 124.3, 125.6, 125.7, 127.3, 124.4, 128.2, 128.6, 132.5, 133.1, 149.8, 180.44.35
1-Hydroxy-6-nitropyrene and 1-hydroxy-8-nitropyrene were synthesized by refluxing pyrene (2.07g) and lead tetraacetate (1.16g) in toluene:acetic acid (9:1) for 6 hours at 80°C to synthetize 1-acetoxyprene. Using a silica gel column the mixture was eluted using 10% benzene:hexane and then recrystallized using a mixture of 10% ethyl:acetate. The percent yield for this reaction was 53%. Nitration of 1-acetoxypyrene (6.6684g) was obtained by using a mixture of nitric and acetic acid at 30°C. The mixture was added to sodium acetate and 50% methanol:THF at room temperature and let it stand for 20 minutes. The reaction produces a mixture of 1-hydroxy-3-nitropyrene, 1-hydroxy-6-nitropyrene and 1-hydroxy-8-nitropyrene.
1,6- and 1,8-Pyrenediones were synthesized using the procedure and methodology of Cho and Harvey.36 A solution of pyrene (2.02g) in acetic anhydrate (100 mL) at 0°C was added to a solution of sodium dichromate dehydrate (8 g) in glacial acetic acid (100 mL). The resulting solution was stirred at room temperature for 1 day, and then poured into water. The precipitate was collected, washed with water, dissolved in chloroform, and passed through a column of basic alumina (30 g). Evaporation resulted in 1,6- and 1,8-pyrenediones (1.20 g). Elution with 2% ethyl acetate-benzene (6.5:1) removed initially a minor contaminant, followed by 1,6-pyrenedione (orange band). Further elution with 10% ethyl acetate-chloroform (1:1) removed a red band containing the 1,8-pyrenedione. UV-Vis spectra were recorded and compared with the spectra published by Fatiadi.37
The characterization of the principal photoproducts was achieved using HPLC/MS (Q-TOF). Atmospheric Pressure Chemical Ionization (APCI) in the negative mode was used as the mode of ionization. Sulfadimethoxine (m/z 309.0658) was used as the mass corrector. Direct injection of the sample was used. In this modality, fractions of the chromatographic peaks of the different photoproducts were collected (~40 times) during HPLC sample runs and preconcentrated to 3mL. To collect the sample fractions from HPLC runs, 30 mL of 1-NO2Py/methanol/N2 solution (5 × 10−5 M) in a Pyrex container were photolyzed for 45 minutes and preconcentrated to 3 mL. The absorption spectra of this sample were taken at different irradiation times until no decrease was observed in the band at 405 nm (43% of photodegradation). All the principal photoproducts were formed during this photolysis and observed in the HPLC chromatogram. Using the APCI in the negative mode the molecular ion for 1-nitropyrene and its principal photoproducts were obtained: 1-hydroxypyrene, 1-hydroxy-2-nitropyrene, 1-hydroxy-3-nitropyrene, 1-hydroxy-6-nitropyrene, 1-hydroxy-8-nitropyrene, 1-nitrosopyrene and 1,6- and 1,8-pyrenediones. The fragmentation patterns of the fractions of the chromatographic peaks of the products were compared with the product standards and the same fragmentation patterns were observed. Also, in the case of the 1-hydroxy-x-nitropyrenes, the fragmentation patterns of the standard and of the sample were compared with the MS/MS data reported by Kameda, et al.23 where the fragment ions were m/z 262 ([M − H]− ), m/z 232 ([M − H − NO]− ) and m/z 216 ([M − H − NO2]− ).
A stock solution of 1-NO2Py in different solvents was prepared by standard procedures. The concentration of the 1-NO2Py solutions was 5.0 × 10−5 M, while the cosolute concentration was approximately 3.0 × 10−4 M. The concentration of 1-NO2Py was lower than the concentration of cosolutes, trying to simulate the environmental conditions. The cosolutes chosen for this investigation included substituted phenols such as 1-naphthol, 2-naphthol, hydroquinone, syringol, homovanillic acid, vanillic acid, and vanillin. The irradiation of the samples was achieved using a Xe-Hg 1000W Oriel arc lamp, ozone free, with a power supply. A 10 cm long cylindrical cell containing water was used as a filter to remove the infrared radiation. The water circulates through the cell to control the temperature in order to minimize thermal degradation. A 405 ± 5 nm interference filter from Edmund Optics (Stock number G48-640) was used in order to overlap with the absorption band corresponding to nitro compounds and to avoid photolysis of the cosolutes or products. For the broad band excitation experiments a 300 – 420 nm filter was used from Gray Glass (Part number POL2-5970/5MM/black). The cells were placed on an optical bench at a distance of 40 cm from the lamp housing. The effect of nitrogen or oxygen on the photolysis was studied by purging solutions with the respective gas for 15 min prior to the irradiation. The samples under nitrogen or oxygen atmospheres were prepared by closing the cell with a septum and using a septum penetration needle to permit the gas flow and a sterile needle to allow the exit of the gas. To determine the incident light intensities for the quantum yield determinations, the potassium ferrioxalate chemical actinometer was used.38 A IL700 radiometer from International Light was used to check for variations of the lamp intensity during the irradiations.
Absorption measurements were carried out in a Varian CARY 1E double beam spectrophotometer. Each analysis was preceded by a baseline measurement to correct for lamp intensity fluctuations. The chromatographic analyses of unirradiated and irradiated 1-NO2Py samples, and the photoproducts identification were done by injecting 50 µL of the irradiated solution into a Shimadzu HPLC (high performance liquid chromatography) equipped with a photodiode array and fluorescence detectors. The column used for the analyses was a Luna 5µ C18(2) reverse phase column from Phenomenex. The chromatographic plots were obtained using a gradient mode starting with methanol/water (70:30, v/v), at a flow rate of 1 mL/min changing the ratio linearly to 100:0 in 15 min and returning to 70:30 after additional 16 min, for a total of 31 min.
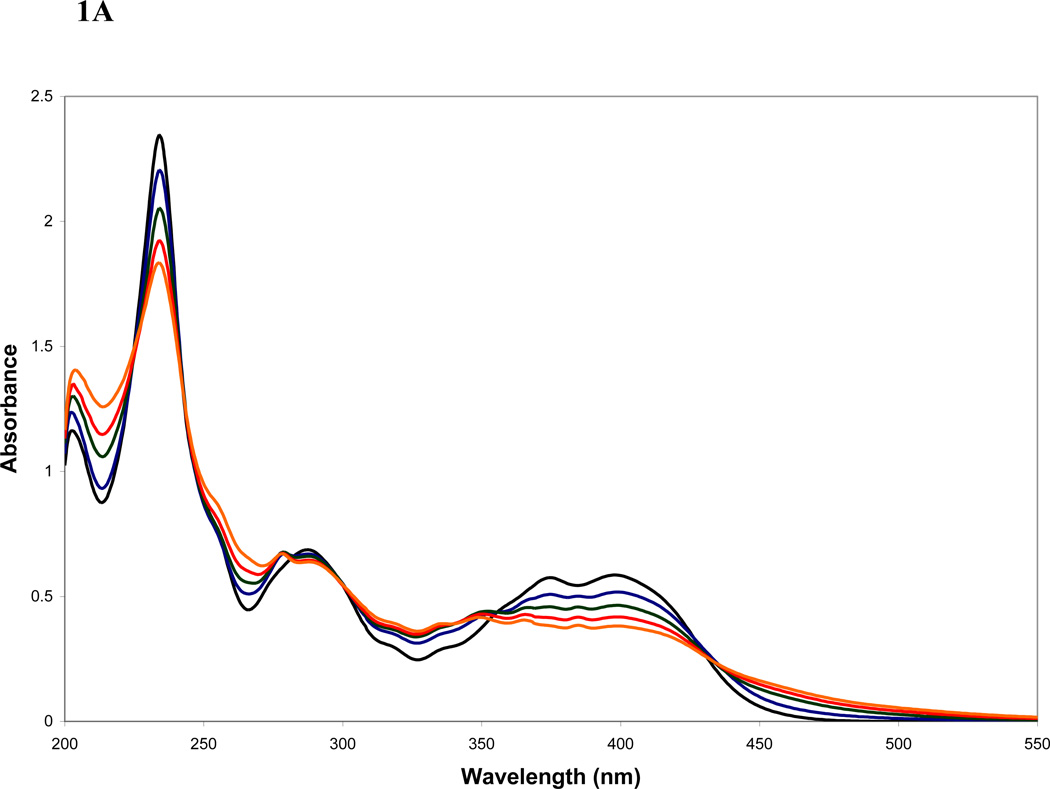
The quantum yields (Φ) for 1-NO2Py photodegradation or for the photoproducts formation were determined using the area of the chromatographic peaks of the corresponding compounds to calculate the number of molecules destroyed or formed during the photolysis process. For the determination of the formation quantum yields, the molar absorption coefficient of each compound was calculated from a calibration curve at 254 nm using HPLC data. In the case of the hydroxynitropyrenes products, the molar absorption coefficient of the standard 1-hydroxy-2-nitropyrene was used. Because the relative intensity of the absorption bands of these isomers (Supplement Information) in the 254 nm wavelength region are similar, it was reasonable to assume equal absorption coefficients. The number of photons absorbed by the sample was determined using the incident intensity measured with the actinometer. Also, the photodegradation quantum yields of 1-NO2Py were determined using data obtained from the absorbance at 405 nm of the 1-NO2Py solution spectrum at different irradiation times using the Cary spectrophotometer. The ϕ(−1−NO2Py)’s were determined at times up to 50% depletion. These quantum yields were 3 to 5 times lower than those calculated with the HPLC data due to the absorbance of the photoproducts at the same wavelength of 1−NO2Py (Figure 1A, B).
Figure 1.
A. Absorption spectra of 1-NO2Py in methanol exposed to O2 at different irradiation times (broad band excitation): 0 s (black), 600 s (blue), 1200 s (green), 1800 s (red) and 2400 s (orange).
B. Absorption spectra of 1-NO2Py in hexane exposed to O2 at different irradiation times (broad band excitation): 0 s (black), 600 s (blue), 1200 s (green), 1800 s (red) and 2400 s (orange).
Results and Discussion
Effect of solvents
The absorption changes observed during the photolysis of a methanol or hexane solution of 1-NO2Py under aerobic conditions using a broad band filter are presented in Figures 1A,B. During the irradiation the following features are evident: a decrease in the intensity of the bands associated with the π,π* transitions from the NO2 group and pyrene’s ring system (450 – 350, 300 – 280, 240 – 220 nm), increases in the wavelength region above 450, around 320 and 250, and the presence of several isosbestic points resulting from absorbing photoproducts. The different absorption changes with irradiation time in these solvents suggest different product distribution and yields.
The photodegradation quantum yields of 1-NO2Py in all solvents were of the order of 10−4–10−3 (Table 1). The higher yields were obtained with benzene, toluene and alcohols (methanol and 2-propanol). For nitropyrenes, the fluorescence quantum yields in some of the solvents are of the order of 10−3–10−4 suggesting an ultrafast intersystem crossing due to couplings with specific triplets. This coupling has been observed for 1-NO2Py and other nitroaromatics and constitutes a limiting factor in the dissociative photochemistry in nitro-PAHs.39–42 Because yields for the 3(π,π*) state are in the range of 0.5 (methanol) to 0.6 (cyclohexane), and the phosphorescence quantum yields43 are of the order of 10−4, the low photodestruction yields suggest a very low reactivity of this triplet state and of the intermediate species that form from either an excited 3(n,π*) or S1 state. Thus, radiationless activation routes or radical recombination reactions, which regenerate the parent compounds, seem to be dominating the photophysics and photochemistry of these nitropyrenes, and could explain the observed small yields.
Table 1.
Quantum yields* (ϕ) of 1-NO2Py photodegradation and product formation in different solvents.
| solvent | ϕ(−1-NO2Py) × 10−3 |
ϕ(1-OHPy) × 10−3 |
ϕ(1-nitrosopyrene) × 10−3 |
ϕ(1,6-pyrenedione) × 10−3 |
ϕ(1,8-pyrenedione) × 10−3 |
|---|---|---|---|---|---|
| benzenea,α | 1.6 ± 0.3 | 0.07 ± 0.01 | |||
| tolueneb,β | 1.5 ± 0.2 | ||||
| 2-propanolc,χ | 1.4 ± 0.2 | 0.57 ± 0.04 | 0.062 ± 0.001 | ||
| methanold,δ | 1.1 ± 0.1 | 0.2 ± 0.1 | 0.28 ± 0.02 | ||
| EPA1 | 0.9 ± 0.3 | 0.28 ± 0.05 | |||
| CCl4ε | 0.9 ± 0.2 | 0.11 ± 0.05 | 0.20 ± 0.07 | ||
| acetonitrilee,γ | 0.77 ± 0.05 | 0.06 ± 0.01 | 0.026 ± 0.003 | ||
| acetonitrile/H2O (50:50) | 0.16 ± 0.05 | 0.025 ± 0.006 | 0.010 ± 0.002 | 0.08 ± 0.01 | |
| 3 -methylpentanef,g,η | 0.41 ± 0.06 | 0.09 ± 0.02 | |||
| hexanef,ι | 0.16 ± 0.04 | 0.09 ± 0.03 | |||
| hexadecaneκ | 0.50 ± 0.05 | 0.08 ± 0.02 | 0.09 ± 0.02 |
| solvent | ϕ(1-OH-2-NO2Py) × 10−3 |
ϕ(1-OH-6-NO2-Py) × 10−3 |
ϕ(1-OH-8-NO2Py) × 10−3 |
ϕ(1-OH-3-NO2Py) × 10−3 |
|---|---|---|---|---|
| benzene | 1.0 ± 0.3 | |||
| toluene | 0.4 ± 0.2 | |||
| 2-propanol | 0.065 ± 0.005 | 0.058 ± 0.007 | 0.06 ± 0.01 | 0.097 ± 0.008 |
| methanol | 0.033 ± 0.005 | 0.06 ± 0.03 | 0.06 ± 0.04 | 0.07 ± 0.05 |
| EPA | ||||
| CCl4 | 0.3 ± 0.1 | |||
| acetonitrile | 0.4 ± 0.2 | 0.010 ± 0.003 | 0.009 ± 0.003 | 0.035 ± 0.001 |
| acetonitrile/H2O (50:50 | 0.020 ± 0.002 | 0.0063 ± 0.0002 | 0.0086 ± 0.0006 | 0.0049 ± 0.0003 |
| 3-methylpentane | ||||
| hexane | 0.036 ± 0.002 | |||
| hexadecane | 0.026 ± 0.005 |
Quantum yield values represent the average of at least three experiments.
EPA: diethyl ether: isopentane: ethanol, 5:5:2 volume ratio.
Hydrogen bond dissociation energy (kJ/mol):
473 (C6H5-H).
376 (C6H5-CH2-H).
381 ((CH3)2CH-OH).
402 (OHCH2-H).
393 (NC-CH2-H).
394 (secondary carbons), 411 (primary carbons).
381 (tertiary carbons).
Viscosity (× 10−3 Poise):
0.649.
0.585.
2.04.
0.59.
0.969.
0.345.
0.307.
0.313.
33.4.
In toluene and benzene, the quantum yield of 1-hydroxypyrene (1-OHPy) was null and only the 1-hydroxy-2-nitropyrene (1-OH-2-NO2Py) was observed as the principal product, 27% for toluene and 63% for benzene (in the case of benzene a nitrosopyrene product was also seen with a low yield of 4%). The major photodecomposition route of 1-NO2Py in these solvents is through a nitro-nitrite rearrangement where the nitrite intermediate favors the channel of the dissociation and recombination of the NO group to the position 2 of the molecule to form the 1-OH-2-NO2Py product (Schemes 1 and 2). This route is favored because a hydrogen atom abstraction from benzene or toluene by the pyrenoxy radical, PyO, is not favorable due to the high C-H bond dissociation energy in benzene or to the large activation energy of this reaction. The formation of 1-nitrosopyrene is accounted by a hydrogen atom abstraction of the nitro pyrene triplet state (Schemes 1 and 2).
Scheme 2.
Photochemical channels for 1-NO2Py in benzene.
In organic glasses a T1 triplet energy of 184 kJ/mol was calculated from the first maximum of the phosphorescence spectra, while from theoretical calculations the 3(n,π*) triplet energy of 1-NO2Py was estimated in the order of 290 kJ/mol. Because in general the C-H bond energies in these solvents are higher than 300 kJ/mol, this abstraction reaction will be endothermic even at a low activation energy. Two products with absorption spectrum that suggest the loss of the NO2 group (~400 nm) were also seen.
In alcohols such as methanol and 2-propanol, the products formed were 1-OHPy, 1-nitrosopyrene, 1-OH-2-NO2Py, 1-OH-6-NO2Py, 1-OH-8-NO2Py and 1-OH-3-NO2Py (Table 1) (Figures S1-S5, Supplementary Information). The product with the highest yield for both solvents was 1-OHPy, being close to 20 to 40% of the destruction yield. This implies that the hydrogen atom abstraction by the pyrenoxy radical (Schemes 1 and 3), PyO + HS → PyOH + S, is more favorable in these solvents than in the others, and this can be correlated with bond dissociation energies of the C-H bond in these molecules, and the higher relative yield of the PyO radical in alcohols.43 Other major products observed in these alcohols were the 1-OH-x-NO2Py (20%) and 1-nitrosopyrene (26%, methanol). The relative yield of the 1-OHPy was higher than the 1-OH-x-NO2Py’s by a factor of two in 2-propanol and equal in methanol suggesting that the rate constant for the hydrogen abstraction reaction by the pyrenoxy radical can compete favorably with the rearrangement process in these solvents. The higher yield of 1-OHPy in 2-propanol versus methanol can be explained in terms of a weaker C-H bond in this solvent, thus a higher rate of H-abstraction by the PyO radical is expected. The slight increase in yield of 1-OH-x-NO2Py in 2-propanol can be rationalized in terms of a higher viscosity and more rigid solvent cage which induces to a rapid recombination of the PyO and NO in this solvent. Although DFT calculations for the pyrenoxy radical23 show that positions 2, 5, 6, 8 and 9 on the pyrene ring have similar spin densities, an increase in the C-2 position product in 2-propanol compared to methanol was observed which can be explained in terms of the higher viscosity of 2-propanol which favors a faster recombination in the closer position to the C-1. Nonetheless, similar yields for the other hydroxynitropyrene isomers were determined. On the other hand, the appearance of the nitrosopyrene photoproduct in these solvents suggest a hydrogen abstraction reaction by the 3(π,π*) state. Nanosecond laser transient absorption studies demonstrated the presence of an intermediate species assigned to a PyNO2H radical, a precursor of the nitrosopyrene.43 The PyNO2H radical can abstract a hydrogen atom from the solvent to transform into a hydroxylamine which in turn transforms in 1-nitrosopyrene. The transformation of nitrosoPAHs into aminoPAHs has been reported.44–45 Because aminopyrene was not detected in this work, this implies that the nitrosopyrene intermediate is very stable compared to other nitrosoPAHs.
Scheme 3.
Photochemical channels for 1-NO2Py in alcohols (2-propanol).
When 1-NO2Py was irradiated in nonpolar aliphatic solvents such as 3-methylpentane and hexane, the photodestruction yield was reduced by 87% compared to benzene and alcohols (Table 1). In these aliphatic solvents, the 1-OHPy yield was 20% to 60% of the total destruction yield. In hexane, the only products observed were 1-OHPy (57%) and 1-OH-2-NO2Py (22%), suggesting an H-atom abstraction by PyO radical and a restricted or limited rearrangement reaction to the C-2 position in comparison to other carbon positions as observed in polar solvents (Schemes 1 and 4). We have reported that the yield of a long-lived transient absorption with maximum at 420 nm and assigned to a PyO radical is lower in aliphatic solvents than in polar solvents.43 Thus, a lower yield of PyO radicals will result in a smaller yield of PyOH and in a reduction in the photodestruction yield. The lower yield of this radical can be accounted for if in the nonpolar solvent the S1 precursor26 has a shorter lifetime. In degassed EPA (diethyl ether: isopentane: ethanol, 5:5:2 volume ratio) solutions, the only product observed was 1-OHPy and its yield is approximately 30% of the 1-NO2Py destruction yield.
Scheme 4.
Photochemical channels for 1-NO2Py in aliphatic solvents (RH).
For irradiations in CCl4 (Table 1) the photodestruction yield was reduced by 56% in comparison to benzene, and as expected no 1-OHPy or 1-nitrosopyrene products were formed. In this solvent, the principal product was the 1-OH-2-NO2Py (33% of the total) while 1,6- and 1,8-pyrenediones were also formed at 12 and 22% yields, respectively. In CCl4, the nitrite intermediate transforms into the 1-OH-2-NO2 pyrene due to the increase in viscosity which results in a rapid recombination of PyO + NO to form 1-OH-2-NO2Py, or alternatively, the pyrenoxy radical oxidizes to form the diones. Because the competing pathway for the formation of the diones is less efficient than that for formation of the 1-OH-2-NO2Py product, this will result in a decrease of the photodestruction yield. Furthermore, the decrease in the photodestruction quantum yield can be explained in terms of an external heavy atom effect which induces a rapid conversion of the pyrene's excited triplet states into the ground state singlet.
The photoreaction in acetonitrile showed a reduction of 48% in the photodestruction yield when compared to benzene or toluene, and the 1-OHPy yield (8%) was low compared to solvents where H atom abstraction reaction is more favorable, such as alcohols or alkanes (Scheme 5). In this solvent, the relative yield of 1-OH-2-NO2Py formation was at least seven times as large as the 1-OHPy yield, thus demonstrating the low rate of H atom abstraction by the pyrenoxy radical in acetonitrile. Furthermore, the formation of 1-nitrosopyrene was observed, indicating the possibility of H atom abstraction by the 3(π,π*) state. The formation of 1-OH-6-NO2Py, 1-OH-8-NO2Py and 1-OH-3-NO2Py was also observed in this aprotic polar solvent and the sum of their yields was only 7% of the photodegradation yield of 1-NO2Py.
Scheme 5.
Photochemical channels for 1-NO2Py in acetonitrile.
Effect of oxygen
In the presence of O2 the photodestruction yield was reduced in the range of 27% in methanol to 10% in acetonitrile (Table 2). Because the decay lifetime of the 3(n,π*) state of 1-nitropyrene has been estimated to be of the order of a few picoseconds46, it is easily understood that O2 quenching through a diffusion controlled process will not affect the yield if the transformation reactions have this state as precursor or the S1. Thus, oxygen could only affect subsequent processes in which other intermediates participate. For example, the decay rate of 3(π,π*) state is greatly enhanced (k = 1 × 109 M−1s−1) in the presence of O2,43 while for the great majority of the solvents the destruction yield was not affected to the same extent. This implies that participation of this state in the principal destruction routes is small. It is interesting to note that while the yield of 1-OHPy decreased in the presence of oxygen, the relative yield of 1-OH-2-NO2Py increased closely in the same proportion, particularly in methanol and 2-propanol (also, the quantum yields of the 1-OH-x-NO2Py isomers increased). Thus, another possibility is that O2 can scavenge the PyO radicals that have escaped the solvent cage, but can not scavenge those PyO radicals inside the cage that recombine with the NO fragment, thus resulting in a decrease in PyOH. The nitrosopyrene product was observed in solvents under N2 saturated conditions, while in the presence of O2 its yield was reduced to zero, demonstrating the participation of the 3(π,π*) state in the formation of this product.
Table 2.
The effect of O2 on the quantum yields.
| solvent | ϕ(−1-NO2Py) × 10−3 |
ϕ(1-OHPy) × 10−3 |
ϕ(1-nitrosopyrene) × 10−3 |
ϕ(1,6-pyrenedione) × 10−3 |
ϕ (1,8-pyrenedione) × 10−3 |
|---|---|---|---|---|---|
| benzene | 1.4 ± 0.1 | ||||
| toluene | 1.3 ± 0.1 | ||||
| 2-propanol | 1.2 ± 0.1 | 0.20 ± 0.04 | |||
| methanol | 0.8 ± 0.1 | 0.17 ± 0.07 | |||
| EPA | 1.1 ± 0.3 | 0.4 ± 0.1 | |||
| CCl4 | 0.8 ± 0.1 | 0.12 ± 0.04 | 0.14 ± 0.06 | ||
| acetonitrile | 0.7 ± 0.1 | 0.03 ± 0.01 | |||
| 3-methylpentane | 0.19 ± 0.06 | 0.07 ± 0.02 | |||
| hexane | 0.13 ± 0.06 | 0.033 ± 0.02 |
| solvent | ϕ(1-OH-2-NO2Py) × 10−3 |
ϕ(1-OH-6-NO2-Py) × 10−3 |
ϕ(1-OH-8-NO2Py) × 10−3 |
ϕ(1-OH-3-NO2Py) × 10−3 |
|---|---|---|---|---|
| benzene | 1.2 ± 0.3 | |||
| toluene | 0.8 ± 0.2 | |||
| 2-propanol | 0.12 ± 0.02 | 0.14 ± 0.03 | 0.14 ± 0.03 | 0.21 ± 0.04 |
| methanol | 0.14 ± 0.06 | 0.09 ± 0.03 | 0.13 ± 0.03 | 0.18 ± 0.02 |
| EPA | 0.015 ± 0.005 | 0.02 ± 0.02 | 0.07 ± 0.02 | |
| CCl4 | 0.2 ± 0.1 | |||
| acetonitrile | 0.4 ± 0.2 | 0.023 ± 0.007 | 0.1 ± 0.1 | 0.03 ± 0.03 |
| 3-methylpentane | 0.05 ± 0.02 | |||
| hexane | 0.07 ± 0.01 |
In benzene and toluene an increase in the yield of 1-OH-2-NO2Py was also observed in the presence of oxygen, while in CCl4 only a minor decrease in the photodestruction yield was observed.
Effect of phenols
It has been reported47 that phenols (ArOH), encountered in the atmospheric aerosol, accelerate the photodestruction of nitropyrene. Indeed, we reported43 that in the presence of several phenols the 3(π,π*) state decays faster and that the quenching rate constant is of the order of 107–109 M−1 s−1. These results have been interpreted in terms of a quenching reaction of the triplet state via a hydrogen abstraction reaction that goes through an electron transfer with a hydrogen-bonded triplet exciplex followed by a proton transfer process. In those experiments the formation of PyNO2H radicals absorbing in the wavelength region of 400 nm was also observed.43 The net photodestruction yield, as well as, the yield of formation of the principal products was determined in the presence of several phenols in four different solvents, hexane, acetonitrile, 2-propanol and CCl4. These results are included in Table 3, and in general there was an increase in the photodestruction yield and in the 1-OHPy and 1-nitrosopyrene product yields in the presence of phenols. New products were observed which could form from radical-radical reactions of the PyNO2H and ArOH radicals.
Table 3.
Effect of phenols on quantum yields
| quencher | ϕ(−1-NO2Py) × 10−3 |
ϕ(1-OHPy) × 10−3 |
ϕ(1-nitrosopyrene) × 10−3 |
ϕ(1-OH-2-NO2Py) × 10−3 |
ϕ(pyrene) × 10−3 |
|---|---|---|---|---|---|
| 2-propanol | |||||
| no phenol | 1.4 ± 0.2 | 0.57 ± 0.04 | 0.062 ± 0.001 | 0.065 ± 0.005 | |
| 1-naphthol | 2.5 ± 0.5 | 1.8 ± 0.5 | 0.13±0.07 | ||
| hydroquinone | 3.2 ± 0.7 | 1.6 ± 0.6 | 1.1 ± 0.5 | ||
| syringol | 3 ± 1 | 1.3 ± 0.3 | 0.4 ± 0.3 | ||
| vanillin | 2.0 ± 0.7 | 1.1 ± 0.6 | 0.1 ± 0.1 | 0.06 ± 0.01 | |
| vanillic acid | 2.5 ± 0.9 | 1.81 ± 0.01 | 0.4 ± 0.2 | ||
| homovanillic acid | 2.6 ± 0.5 | 1.5 ± 0.7 | 0.3 ± 0.1 | ||
| phenol | 1.9 ± 0.4 | 1.0 ± 0.4 | 0.3 ± 0.2 | 0.016 ± 0.002 | |
| acetonitrile | |||||
| no phenol | 0.77 ± 0.05 | 0.06 ± 0.01 | 0.026 ± 0.003 | 0.4 ± 0.2 | |
| 1-naphthol | 1.5 ± 0.3 | 0.8 ± 0.3 | 0.3 ± 0.1 | ||
| hydroquinone | 1.9 ± 0.6 | 0.7 ± 0.4 | 0.8 ± 0.2 | ||
| syringol | 1.5 ± 0.7 | 0.8 ± 0.6 | 0.5 ± 0.2 | 0.020 ± 0.007 | |
| vanillin | 0.55 ± 0.04 | 0.058 ± 0.008 | 0.10 ± 0.01 | 0.09 ± 0.05 | |
| vanillic acid | 0.86 ± 0.03 | 0.039 ± 0.008 | 0.13 ± 0.04 | 0.8 ± 0.2 | |
| homovanillic acid | 0.78 ± 0.06 | 0.19 ± 0.01 | 0.21 ± 0.05 | 0.008 ± 0.002 | |
| phenol | 0.59 ± 0.03 | 0.02 ± 0.01 | 0.09 ± 0.0.3 | 0.23 ± 0.05 | |
| CCl4 | |||||
| no phenol | 0.9 ± 0.2 | 0.3 ± 0.1 | |||
| 1-naphthol | 1.6 ± 0.1 | 0.44 ± 0.03 | |||
| hydroquinone | 1.2 ± 0.2 | 0.17 ± 0.09 | 0.3 ± 0.2 | ||
| syringol | 1.1 ± 0.2 | 0.21 ± 0.04 | 0.18 ± 0.09 | 0.025 ± 0.008 | 0.0815 ± 0.0001 |
| vanillic acid | 1.00 ± 0.09 | 0.026 ± 0.004 | 0.8 ± 0.2 | ||
| homovanillic acid | 1.03 ± 0.01 | 0.04 ± 0.02 | 0.49 ± 0.08 | ||
| phenol | 1.2 ± 0.1 | 0.15 ± 0.05 | 0.24 ± 0.08 | ||
| hexane | |||||
| no phenol | 0.16 ± 0.04 | 0.09 ± 0.03 | 0.036 ± 0.002 | ||
| 1-naphthol | 0.99 ± 0.02 | 0.36 ± 0.05 | 0.4 ± 0.1 | ||
| quencher | ϕ(1,6-pyrenedione) × 10−3 |
ϕ(1,8pyrenedione) × 10−3 |
ϕ(1-OH-6-NO2Py) × 10−3 |
ϕ(1-OH-8-NO2Py) × 10−3 |
ϕ(1-OH-3-NO2Py) × 10−3 |
|---|---|---|---|---|---|
| 2-propanol | |||||
| no phenol | 0.058 ± 0.007 | 0.06 ± 0.01 | 0.097 ± 0.008 | ||
| no phenol | |||||
| 1-naphthol | |||||
| hydroquinone | |||||
| syringol | |||||
| vanillin | 0.08 ± 0.06 | 0.03 ± 0.02 | 0.08 ± 0.06 | ||
| vanillic acid | 0.018 ± 0.002 | 0.014 ± 0.004 | 0.027 ± 0.004 | ||
| homovanillic acid | |||||
| phenol | 0.03 ± 0.01 | 0.012 ± 0.002 | 0.04 ± 0.01 | ||
| acetonitrile | |||||
| no phenol | |||||
| 1-naphthol | |||||
| hydroquinone | |||||
| syringol | |||||
| vanillin | 0.029 ± 0.006 | 0.06 ± 0.05 | |||
| vanillic acid | |||||
| homovanillic acid | |||||
| phenol | |||||
| CCl4 | |||||
| no phenol | 0.11 ± 0.05 | 0.20 ± 0.07 | |||
| 1-naphthol | |||||
| hydroquinone | 0.014 ± 0.003 | 0.08 ± 0.05 | |||
| syringol | 0.019 ± 0.005 | 0.04 ± 0.02 | |||
| vanillic acid | 0.0212 ± 0.0008 | 0.07 ± 0.05 | |||
| Homovanillic acid | 0.020 ± 0.002 | 0.09 ± 0.06 | |||
| phenol | 0.0399 ± 0.0009 | 0.2 ± 0.1 | |||
| hexane | |||||
| no phenol | |||||
| 1-naphthol | |||||
It is interesting to observe that while the yield of the lowest 3(π,π*) state is of the order of 0.4 to 0.6,43 the net photodestruction yields in the presence of phenol were of the order of 10−3, much lower than the triplet yields. This means that either the triplet state presents effective alternate non-radiative pathways before reacting or that once the triplet-ArOH exciplex forms, it deactivates efficiently before the electron-proton transfer reaction is completed (possible back electron process). Nonetheless, interesting solvent effects and differences in reactivity with the phenols were observed.
For example, in hexane the increase in the photodestruction yields in the presence of 1-naphthol was much larger than in polar solvents (488% increase in hexane, versus 195% in acetonitrile or 179% in 2-propanol). In nonpolar solvents the hydrogen atom on the ArOH is free to interact with the NO2 group in the triplet exciplex in comparison to solvents in which this group can either form hydrogen bonds with the phenols, or strongly solvate the NO2 group. Thus, in hexane the increase in photoreactivity can be explained in these terms. The further increase in the yield in the presence of hydroquinone (1,4-dihydroxybenzene) is due to the larger probability of reaction of nitro pyrene with two OH groups instead of only one as in 1-naphthol. No significant steric effects were observed on the photodegradation yield in the presence of o-methoxyphenols (syringol, vanillin, vanillic acid and homovanillic acid) which will make the H-atom of the OH group less accessible for exciplex formation.
In terms of the photoproducts a very significant increase in the yield of 1-OHPy was observed in the presence of the majority of the phenols, for example, in the case of 1-naphthol an increase of 1368% in acetonitrile, 300% in hexane and 319% in 2-propanol was observed. These increases can be interpreted in terms of a fast hydrogen atom abstraction reaction of the PyO radical, formed through the nitro-nitrite rearrangement, with the ArOH: PyO + HOAr → PyOH +ArO. This will imply that the rate of hydrogen abstraction by PyO from the hydrogen donor is faster than the rate for the transformation of the PyO...NO cage pair into 1-OH-2-NO2Py. Indeed, a significant decrease in the relative yield of the 1-OH-2-NO2Py product was observed in the presence of phenols. Otherwise, in CCl4, although there was also an increase in the photodestruction of 1-NO2Py, the product 1-OHPy was not observed as one of the products in the presence of phenols except for syringol solutions. This implies that this product is not formed from a transformation of the PyNO2H radical in this solvent (see below). However, 1-OH-2-NO2Py appeared as the principal photoproduct along with nitrosopyrene in CCl4. The formation of 1-OH-2-NO2Py can be rationalized in terms of higher viscosity of this solvent that favors the recombination of PyO and NO inside the cage. The pyrenediones were also observed and there was a decrease of 15 to 40% in their yields.
Because the reaction of the lowest triplet state with phenols yields a PyNO2H radical which can transform into a nitrosopyrene product, an increase in the yield of this product was expected and confirmed (Table 3). The largest increase in the yield of this product was observed in acetonitrile in comparison to the alcohols. This could be explained if in 2-propanol the ground state 1-NO2Py is weakly H-bonded to 2-propanol, not allowing for the approach of the ArOH.48
The rate constant for the hydrogen abstraction reaction was determined by varying the 1-naphthol concentration and using a Stern Volmer kinetic relationship (Figure 2) and the following reaction scheme:
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
where ArOH is the 1-naphthol. This scheme leads to the following linear equation
from which the slope and intercept can be equated to kd/kr and kr + kq/kr, respectively. ϕ is the photodegradation quantum yield of 1-NO2Py in the presence of 1-naphthol, ϕ0 is the triplet yield, and kr, kq and kd are the rate constants for the hydrogen abstraction reaction, for the quenching by the ArOH and for the deactivation of the excited 1-NO2Py, respectively. Using values of kd = 0.2 × 10−5 s−1 and kq = 0.32 × 109 M−1s−1 obtained from laser photolysis experiments43, a value of 5.7 × 105 M−1s−1 was calculated for kr in the H atom transfer process.
Figure 2.
Reciprocal of the photodegradation quantum yield of 1-NO2Py in acetonitrile with varying concentrations of 1-naphthol.
Quenching of the T(π,π*) of 1-NO2Py
From phosphorescence studies43, we established that the triplet energy for the 3(π,π*) state of 1-NO2Py is 187 kJ/mol. In order to study the participation of this state in the phototransformation process and yield of a specific photoproduct, azulene with a triplet energy of 163 kJ/mol and lacking an absorption band at 405 nm (the wavelength used to photolyze the 1-NO2Py solutions) was used as a triplet quencher. The concentration of azulene in the irradiated solution was five times higher than that of 1-NO2Py. Using laser flash photolysis (355 nm – Nd:YAG laser third harmonic) and observing at a wavelength where the 1-NO2Py triplet absorbs, an increase in its decay was observed in the presence of azulene, thus indicating an effective quenching of the 3(π,π*) state. In nonpolar hexane, in the presence of azulene, no effect was observed in the destruction yield of 1-NO2Py or in the formation yield of the major photoproducts (Table 4). In contrast, in the presence of O2 which can act as a triplet quencher of this state, although no net effect on the disappearance yield was detected a significant decrease in the yield of 1-OHPy from 0.09 to 0.03 (× 10−3) was observed (Table 2). This was interpreted in terms of a rapid scavenging reaction by O2 molecules by PyO radicals. Thus, one can conclude that in nonpolar aliphatic solvents there is not a significant contribution of a 3(π,π*) state to the photodegradation of 1-NO2Py.
Table 4.
Quenching of the T(π,π*) of 1-NO2Py.
| ϕ(−1-NO2Py) × 10−3 |
ϕ(1-OHPy) × 10−3 |
ϕ(1-nitrosopyrene) × 10−3 |
|
|---|---|---|---|
| methanol | |||
| no quencher with quencher |
1.1 ± 0.1 0.814 ± 0.002 |
0.2 ± 0.1 0.54 ± 0.04 |
0.28 ± 0.02 |
| hexane | |||
| no quencher with quencher |
0.16 ± 0.04 0.15 ± 0.02 |
0.09 ± 0.03 0.07 ± 0.01 |
|
| acetonitrile | |||
| no quencher with quencher |
0.77 ± 0.05 0.49 ± 0.05 |
0.06 ± 0.01 0.42 ± 0.01 |
0.026 ± 0.003 |
| ϕ(1-OH-2-NO2Py) × 10−3 |
ϕ(1-OH-6-NO2Py) × 10−3 |
ϕ(1-OH-8-NO2Py) × 10−3 |
ϕ(1-OH-3-NO2Py) × 10−3 |
|
|---|---|---|---|---|
| methanol | ||||
| no quencher with quencher |
0.033 ± 0.005 0.10 ± 0.02 |
0.06 ± 0.03 0.04 ± 0.02 |
0.06 ± 0.04 0.023 ± 0.002 |
0.07 ± 0.05 0.07 ± 0.02 |
| hexane | ||||
| no quencher with quencher |
0.036 ± 0.002 0.034 ± 0.003 |
|||
| acetonitrile | ||||
| no quencher with quencher |
0.4 ± 0.2 0.419 ± 0.002 |
0.010 ± 0.003 | 0.009 ± 0.003 | 0.035 ± 0.001 |
Surprisingly in polar aprotic acetonitrile (36%) and in polar protic methanol (29%) decreases in the photodestruction yields were observed in the presence of azulene. This effect can be rationalized if azulene forms a strong ground state complex with 1-NO2Py, due to π-π interactions, which deactivates the 3(n,π*) or S1 excited states in these polar solvents. Furthermore, a significant increase in the yields of 1-OHPy and 1-OH-2-NO2Py (Table 4) were observed; not seen in the presence of O2 or in nonpolar solvents (Table 2). This increase can be accounted for by the decrease in the yield of nitrosopyrene product (Table 4) or if in the polar solvents the channels that result in the formation of the pyrenoxy radical are favored over a competing rapid intersystem crossing process. It has been suggested24 that the precursor of nitro-nitrite rearrangement is an S1 state instead of the 3(n,π*) state.
Effect of water
The photoreactivity of PAH compounds is influenced by the composition of an atmospheric aerosol.49–51 A linear relationship was observed for water uptake by diesel soot, automobile exhaust, and wood smoke particles. These results indicated that particle water content increases with increasing relative humidity and that the photodegradation rates of some PAHs increase with increasing particle water content.52 Thus, the water content in aerosols and the sunlight can influence the decay of PAHs, however the mechanism of this effect and on the formation of different products has not been studied. Thus, it was our interest to determine the effect of water on the photodegradation of 1-NO2Py and on the yield of its photoproducts.
The effect of water as an additive in a 1-NO2Py/acetonitrile solution was demonstrated in the reduction of the 1-NO2Py photodegradation quantum yield and of the formation quantum yield of the photoproducts (Table 1). The photodegradation quantum yield for 1-NO2Py decreased by 82%, while for the photoproducts a decrease of 60% for 1-OHPy and of about one order of magnitude for 1-OH-2-NO2Py were determined. Two additional products were identified as 1,6- and 1,8-pyrenedione. Nitrosopyrene was not observed, while in the absence of water its formation was evident. It was demonstrated in transient intermediates studies48 that water stabilizes the lowest triplet yield of 1-NO2Py (3(π,π)) by increasing its lifetime while decreasing its initial triplet yield. Thus, a lower yield of this triplet state could lead to a drastic decrease in the yield of the nitrosopyrene (not observed). Water molecules can interact strongly with acetonitrile molecules or with the NO2 group as demonstrated by the bathochromic shift and quenching of the fluorescence of 1-NO2Py.48 Thus, these interactions can alter the composition of the solvent cage in which the PyO + NO radicals are formed, affecting the reactivity of the PyO radical and rearrangement process resulting in a significant decrease in the yields of 1-OHPy and 1-OH-NO2Py products.
Effect of viscosity
The photochemistry of the nitro-PAHs adsorbed on combustion particles occur in an organic layer surrounding the graphitic particle core.47,49 This layer has been found to behave as a viscous liquid in combustion particles as well as in the secondary organic aerosol. Thus, it is important to compare the photodegradation of 1-NO2Py in a viscous and in a nonviscous solvent in order to determine the effect of this solvent property. For that purpose, hexadecane, a constituent of diesel exhaust, was used because its viscosity is 100 times higher than for other aliphatic solvents used in this work, such as hexane. Our results indicate that a viscous solvent did not inhibit the photodegradation of 1-NO2Py. (Table 1) As a matter of fact, the quantum yield for 1-NO2Py in hexadecane is similar to that of hexane or 3MP solutions (same order of magnitude) and there is little difference in the yields of the principal photoproducts. The same behavior was observed for 1-NO2Py in diisooctylphthalate (DOP)47 where this viscous solvent did not inhibit the photodegradation 1-NO2Py.
Broad band excitation
Because the great majority of the photoproducts absorb in the wavelength region where the 1-NO2Py absorbs (Figure S1-S5, Supplementary Information) irradiations were performed using a broad band filter (300 – 420 nm) to examine the effect of possible photodegradation of the primary products and of using a higher irradiance (10−2 W/cm2). Under the later conditions the irradiation times were shortened from 3 hours to 30 minutes, thus resulting in larger photodegradation percentages (65% in non polar aliphatic solvents, 70% in acetonitrile, 90% in alcohols and close to 100% in benzene, toluene and CCl4).
Although the same principal products (1-OHPy, 1-OH-2-NO2Py and 1-nitrosopyrene) were observed in aliphatic and polar protic solvents under broad band excitation, significant differences were observed. For example, nitrosopyrene was observed in deaerated hexane and 3-methylpentane solutions, although not seen at the lower irradiance (10−3 W/cm2).
Pyrenediones were detected as well as unidentified products presenting absorption bands in the 210 – 290 nm wavelengths region in alcohols. In acetonitrile as the solvent (N2 atmosphere), the products with the highest yields were the 1,6- and 1,8-pyrenediones (not seen when using the interference filter) along with 1-OHPy. Moreover in the presence of O2, an increase in the yield (3–5 times higher) of the pyrenediones and of 1-OH-2-NO2Py was detected with a simultaneous disappearance of the 1-OHPy. Additional products lacking the characteristic absorption band of the nitro group and presenting loss of the aromatic character (λmax: 210 – 220 nm) were detected as well as in irradiation in the alcohols.
In summary, the must relevant observations for irradiations performed under broad band excitation were that a higher irradiance induces an increase in the nitrosopyrene which results from a hydrogen abstraction reaction of the 3(π,π*) state, and that the relative yield of 1-OHPy decreased while that of pyrenediones increased. Indeed, the irradiation of 1-OHPy in methanol or in hexane under similar irradiation conditions resulted in the formation of 1,6- and 1,8-pyrenediones as the principal products and that their yield increased in the presence of O2. Moreover, the photolysis of 1-OH-2-NO2Py in 2-propanol did not evidence any degradation even after a prolonged irradiation.
Conclusions
The transformations of nitropyrenes continue to be an area of great interest due to the persistence of these contaminants in the atmosphere and their consequences to human health due to their mutagenic and carcinogenic properties. In this work, we focused on the phototransformations of 1-NO2Py in a chemical model system consisting of an organic solvent with different properties such as polarity, hydrogen donor abilities and viscosity, additives representing some of the constituents of an aerosol particle and different irradiation conditions having a major interest on the effects of these variables in the phototransformation mechanism.
The photodegradation yields of 1-NO2Py are small (10−4 – 10−3) in all solvents even when the lowest 3(π,π*) state has a yield of 0.4 – 0.6.43 Because the phosphorescence yield is in the order of 10−4,43 this indicates that the lowest triplet state decays mainly through radiation less deactivation paths. The major participation of this state is under conditions where a suitable hydrogen donor is available such as aliphatic alcohols or phenols from which this state can abstract a hydrogen atom to form 1-nitrosopyrene, not observed under aerobic conditions. The results indicate that major products result from a nitro-nitrite rearrangement.19 No evidence of a direct NO2 dissociation of the first excited singlet or triplet state into a pyrenyl radical was obtained since pyrene, a product that could result from a hydrogen atom abstraction from the solvent by this radical was not detected (Scheme 1). This product was reported by Stark17. Others reported products such as aminopyrene45, methoxypyrene20 and hydroxymethylpyrene20 were not observed in this study. The aminopyrene is a possible photoreduction product formed from the transformation of the PyNO2H radical from the hydrogen atom abstraction of the 3(π,π*) state, while the methoxy and hydroxymethyl pyrene products could suggest the participation of the pyrenoxy radical (Scheme 1). However, none of these products were observed in our study probably because their yields (<10−5) were so low that they could not be detected, or simply the formation of these products was not favored under our reaction conditions.
One of the principal contributions of this work is the evidence on the effect of solvent polarity and rigidity of the solvent cage on the relative yields of the previous reported principal photoproducts (1-OHPy3,20 and 1-OH-x-NO2Py3,14,19,23–24). According to the postulated mechanism (Scheme 1) these are formed as reaction products of intermediates produced from the dissociation of a nitrite intermediate (PyONO → PyO· + NO·). The relative yield of 1-OHPy reflects the reactivity of the PyO radicals, observed in transient studies,43 toward a hydrogen atom abstraction from the solvent, while the yield of 1-OH-x-NO2Py represents a competing reaction of PyO or NO radicals escaping the cage versus rearranging inside the cage. In terms of the participation of the PyO radical in the photodestruction mechanism, although the lowest quantum yield of this intermediate was determined in nonpolar hexane, its contribution to the net photochemical transformation processes of 1-NO2Py is larger in this solvent (79%) than in the others (63 – 68%). If the precursor of the pyrenoxy radical is the S1 state26, its smaller yield in nonpolar solvents is the result of the shorter lifetime of this state in these solvents.26. Furthermore, an interesting observation is that in nonpolar solvents the geminate NO radical adds only to C-2 position of the pyrene ring while in polar solvents addition to other positions was observed. This suggests that polar solvents stabilize the PyO allowing the NO radical to add to other possible positions with similar spin densities23 in the ring before the radicals escape the solvent cage. The 1-OH-2-NO2Py photoproduct was observed in all solvents tested under N2 or O2 atmospheres, while the other 1-OH-x-NO2Py isomers were detected only in polar protic (alcohols) and polar aprotic solvents. Interestingly, the quantum yields of these hydroxynitropyrenes are similar in the alcohols, while in acetonitrile the yield of the 1-OH-2-NO2Py isomer is much higher (10 to 45 times in N2 saturated solutions and 4 to 17 times in O2 saturated solutions). Because 1-OH-2-NO2Py is found in ambient air but not in diesel particles23, while the other isomers have been detected only in diesel particles12, our results suggest that the photochemical formation of the 1-OH-2-NO2Py in air particles will be favorably formed under conditions in which 1-NO2Py is encountered in a polar aprotic environment under low humidity conditions.
The formation of pyrenediones as primary products in CCl4 and in an acetonitrile/water mixture is reported for the first time and also as products of the photodegradation of 1-OHPy in other solvents.
In terms of environmental implications, the nitro-PAHs are found in atmospheric particulate matter and are exposed to different aerosol components that can affect their photochemical degradation. In this study, our results demonstrated that the fate of these pollutants will be highly dependent on the chemical composition of the aerosol. Aerosol constituents such as phenols accelerated the photodegradation of 1-NO2Py while the incorporation of water reduces its photodestruction. The photoproducts distribution is affected by the environmental properties and their identification is of great importance due their degree of toxicity.
Supplementary Material
Acknowledgment
This project was supported by the NIH SCoRE (1SC1ES017352). The authors are grateful to Luis Piñero, University of Puerto Rico – Humacao Campus, for the synthesis of all the products used as standards. We also thank Dra. Olga Álvarez for her helpful assistance with the HPLC-MS instrument and to Melanie Burgos for her technical assistance.
Abbreviations
- 1-NO2Py
1-nitropyrene
- 1-OHPy
1-hydroxypyrene
- 1-OH-x-NO2Py
1-hydroxy-x-nitropyrenes (x = 2, 3, 6, or 8)
- ArOH
phenols
Footnotes
Supporting Information Available. Absorption spectra of different photoproducts 1-nitrosopyrene, 1-OH-2-NO2Py, 1-OH-6-NO2Py, 1-OH-8-NO2Py and 1-OH-3-NO2Py. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Pitts J. Environ. Health. Perspec. 1983;47:115–140. doi: 10.1289/ehp.8347115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arey J, Zielinska B, Atkinson R, Winer AM, Ramdahl T, Pitts JN. Atmos. Environ. 1986;20:2339–2345. [Google Scholar]
- 3.Nielsen T, Seitz B, Ramdahl T. Atmos. Environ. 1984;18:2159–2165. [Google Scholar]
- 4.Bamford HA, Bezabeh DZ, Schantz MM, Wise SA, Baker JE. Chemosphere. 2003;50:575–587. doi: 10.1016/s0045-6535(02)00667-7. [DOI] [PubMed] [Google Scholar]
- 5.Bamford HJ, Baker JE. Atmos. Environ. 2003;37:2077–2091. [Google Scholar]
- 6.Imaida K. Cancer Res. 1991;51:2902–2907. [PubMed] [Google Scholar]
- 7.Chae YH. Cancer Res. 1999;59:1473–1480. [PubMed] [Google Scholar]
- 8.King LC, Jackson M, Ball LM, Lewtas J. Cancer Lett. 1983;19:241–245. doi: 10.1016/0304-3835(83)90091-5. [DOI] [PubMed] [Google Scholar]
- 9.Zielinska B, Arey J, Atkinson R, Winer AM. Atmos. Environ. 1989;23:223–229. [Google Scholar]
- 10.Hayakawa K, Murahashi T, Butoh M, Miyazaki M. Environ. Sci. Technol. 1995;29:928–932. doi: 10.1021/es00004a012. [DOI] [PubMed] [Google Scholar]
- 11.Reisen F, Arey J. Environ. Sci. Technol. 2005;39:64–73. [PubMed] [Google Scholar]
- 12.Schuetzle D. Environ. Health Persp. 1983;47:65–80. doi: 10.1289/ehp.834765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yaffe D, Cohen Y, Arey J, Grosovsky AJ. Risk Anal. 2001;21:275–294. doi: 10.1111/0272-4332.212111. [DOI] [PubMed] [Google Scholar]
- 14.Fan Z, Kamens RM, Hu J, Zhang J, McDow S. Environ. Sci. Technol. 1996;30:1358–1364. [Google Scholar]
- 15.Yasuhara A, Fuwa K. Chem. Letters. 1983;12:347–348. [Google Scholar]
- 16.Benson JM, Brooks AL, Cheng YS. Atmos. Environ. A. 1985;19:1169–1174. [Google Scholar]
- 17.Stärk G, Stauff J, Miltenburger HG, Stumm-Fischer I. Mutat. Res. 1985;155:27–33. doi: 10.1016/0165-1218(85)90021-7. [DOI] [PubMed] [Google Scholar]
- 18.Holloway MP, Biaglow MC, McCoy EC, Anders M, Rosenkranz HS, Howard PC. Mutat. Res. 1987;187:199–207. doi: 10.1016/0165-1218(87)90037-1. [DOI] [PubMed] [Google Scholar]
- 19.Van den Braken-van Leersum AM, Tintel C, van’t Zelfde M, Cornelisse J, Lugtenburg J. Recl. Trav. Chim. Pays-Bas. 1987;106:120–128. [Google Scholar]
- 20.Muck A, Kubát P, Oliveira A, Vieira LP, Cvaĉka J, Civiš S, Zelinger Z, Barek J, Zima J. J. Hazard. Mater. 2002;95:175–184. doi: 10.1016/s0304-3894(02)00120-6. [DOI] [PubMed] [Google Scholar]
- 21.Bamford HA, Bezabeh DZ, Schantz MM, Wise SA, Baker JE. Chemosphere. 2003;50:575–587. doi: 10.1016/s0045-6535(02)00667-7. [DOI] [PubMed] [Google Scholar]
- 22.Kawanaka Y, Sakamoto K, Wang N, Yun S-J. J. Chromatogr. A. 2007;1163:312–317. doi: 10.1016/j.chroma.2007.06.038. [DOI] [PubMed] [Google Scholar]
- 23.Kameda T, Akiyama A, Toriba A, Tang N, Hayakawa K. Environ. Sci. Technol. 2011;45:3325–3332. doi: 10.1021/es1042172. [DOI] [PubMed] [Google Scholar]
- 24.Yu H. J. Environ. Sci. Heal. C. 2002;20:149–183. doi: 10.1081/GNC-120016203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chapman OL, Heckert DC, Reasoner JW, Thackaberry SP. J. Am. Chem. Soc. 1966;88:5550–5554. [Google Scholar]
- 26.Plaza-Medina EF, Rodríguez-Córdoba W, Peón J. J. Phys. Chem. A. 2011;115:9782–9789. doi: 10.1021/jp204321h. [DOI] [PubMed] [Google Scholar]
- 27.Plaza-Medina EF, Rodríguez-Córdoba W, Morales-Cueto R, Peón J. J. Phys. Chem. A. 2011;115:577–585. doi: 10.1021/jp109041y. [DOI] [PubMed] [Google Scholar]
- 28.Hurley R, Testa AC. J. Am. Chem. Soc. 1966;88:4330–4332. [Google Scholar]
- 29.Hurley R, Testa AC. J. Am. Chem. Soc. 1967;89:6917–6919. [Google Scholar]
- 30.Hashimoto S, Kano K. Kog. Kagaku Zasshi. 1969;72:188. [Google Scholar]
- 31.Obi K, Bottenheim JW, Tanaka I. B. Chem. Soc. Jpn. 1973;46:1060–1063. [Google Scholar]
- 32.Trotter W, Testa AC. J. Am. Chem. Soc. 1968;90:7044–7046. [Google Scholar]
- 33.Hamanoue K, Nakayama T, Kajiwara K, Yamanaka S, Ushida K. J. Chem. Soc. Faraday T. 1992;88:3145–3151. [Google Scholar]
- 34.Howard PC. Carcinogenesis. 1983;4:985–990. doi: 10.1093/carcin/4.8.985. [DOI] [PubMed] [Google Scholar]
- 35.Hashimoto Y, Shudo K. Chem. Pharm. Bull. 1984;32:1992–1994. doi: 10.1248/cpb.32.4300. [DOI] [PubMed] [Google Scholar]
- 36.Cho H, Harvey RG. J. Chem. Soc. Perk. T1. 1976;8:836–839. [PubMed] [Google Scholar]
- 37.Fatiadi AJ. J. Chromatogr. 1965;20:319–324. [Google Scholar]
- 38.Calvert JG, Pitts JN. Photochemistry. New York: John Wiley & Sons, Inc.; 1966. [Google Scholar]
- 39.Morales-Cueto R, Esquivelzeta-Rabell M, Saucedo-Zugazagoitia J, Peón J. J. Phys. Chem. A. 2007;111:552–557. doi: 10.1021/jp065364d. [DOI] [PubMed] [Google Scholar]
- 40.Reichardt C, Aaron Vogt R, Crespo-Hernández CE. J. Chem. Phys. 2009;131:224518. doi: 10.1063/1.3272536. [DOI] [PubMed] [Google Scholar]
- 41.Zugazagoitia JS, Collado-Fregoso E, Plaza-Medina EF, Peón J. J. Phys. Chem. A. 2009;113:805–810. doi: 10.1021/jp8087397. [DOI] [PubMed] [Google Scholar]
- 42.Murudkar S, Mora AK, Singh PK, Nath S. J. Phys. Chem. A. 2011;115:10762–10766. doi: 10.1021/jp205946c. [DOI] [PubMed] [Google Scholar]
- 43.Arce R, Pino EF, Valle C, Ágreda J. J. Phys. Chem. A. 2008;112:10294–10304. doi: 10.1021/jp803051x. [DOI] [PubMed] [Google Scholar]
- 44.Horspool WM, Song P. CRC Handbook of Organic Photochemistry and Photobiology. United States: CRC Press Inc.; 1995. [Google Scholar]
- 45.Gilbert A, Baggott J. Essentials of Molecular Photochemistry. Oxford: Blackwell Scientific Publications; 1991. [Google Scholar]
- 46.Crespo-Hernández C, Burdzinski G, Arce R. J. Phys. Chem. A. 2008;112:6313–6319. doi: 10.1021/jp803847q. [DOI] [PubMed] [Google Scholar]
- 47.Feilberg A, Nielsen T. Environ. Sci. Technol. 2001;35:108–113. doi: 10.1021/es990834l. [DOI] [PubMed] [Google Scholar]
- 48.Arce R, Pino EF, Valle C, Negrón-Encarnación I, Morel M. J. Phys. Chem. A. 2011;115:152–160. doi: 10.1021/jp108652p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McDow SR, Sun Q, Vartialnen M, Hong Y, Yao Y, Fister T, Yao R, Kamens RM. Environ. Sci. Technol. 1994;28:2147–2153. doi: 10.1021/es00061a024. [DOI] [PubMed] [Google Scholar]
- 50.Kamens RM, Guo Z, Fulcher JN, Bell DA. Environ. Sci. Technol. 1988;22:103–108. doi: 10.1021/es00166a012. [DOI] [PubMed] [Google Scholar]
- 51.Feilberg A, Nielsen T. Environ. Sci. Technol. 2000;34:789–797. [Google Scholar]
- 52.McDow SR, Vartiainen M, Sun Q, Hong Y, Yao Y, Kamens RM. Atmos. Environ. 1995;29:791–797. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.