Abstract
We have analyzed the role of the three members of the Pex11 protein family in peroxisome formation in the filamentous fungus Penicillium chrysogenum. Two of these, Pex11 and Pex11C, are components of the peroxisomal membrane, while Pex11B is present at the endoplasmic reticulum. We show that Pex11 is a major factor involved in peroxisome proliferation. We also demonstrate that P. chrysogenum cells deleted for known peroxisome fission factors (all Pex11 family proteins and Vps1) still contain peroxisomes. Interestingly, we find that, unlike in mammals, Pex16 is not essential for peroxisome biogenesis in P. chrysogenum, as partially functional peroxisomes are present in a pex16 deletion strain. We also show that Pex16 is not involved in de novo biogenesis of peroxisomes, as peroxisomes were still present in quadruple Δpex11 Δpex11B Δpex11C Δpex16 mutant cells. By contrast, pex3 deletion in P. chrysogenum led to cells devoid of peroxisomes, suggesting that Pex3 may function independently of Pex16. Finally, we demonstrate that the presence of intact peroxisomes is important for the efficiency of ß-lactam antibiotics production by P. chrysogenum. Remarkably, distinct from earlier results with low penicillin producing laboratory strains, upregulation of peroxisome numbers in a high producing P. chrysogenum strain had no significant effect on penicillin production.
Introduction
Peroxisomes represent a class of important organelles that are characterized by an unprecedented functional plasticity that varies with the organism in which they occur and the environmental conditions. In fungi, peroxisomes play a crucial role in the metabolism of various unusual components used for growth (N- and C-sources), the detoxification of reactive oxygen species as well as the formation of specific secondary metabolites, e.g. ß-lactam antibiotics [1], [2], [3], [4]. In these organisms proliferation of peroxisomes is generally induced when the cells are placed at conditions that require the function of peroxisomal enzymes for growth, such as fatty acids [3].
In general, two modes of peroxisome development have been documented namely de novo synthesis from the endoplasmic reticulum (ER) and multiplication by fission [5], [6]. In the yeast Hansenula polymorpha Pex11 family members are essential for both pathways of peroxisome development [7]. Accordingly, in this yeast peroxisome biogenesis is fully inhibited when both de novo formation from the ER (by deletion of Hp-PEX25) and fission (by deletion of Hp-PEX11) are simultaneously blocked [7].
The molecular details of de novo pathways are still largely unknown. Besides Pex25 in the yeast species Hansenula polymorpha [7] and Saccharomyces cerevisiae [8] the de novo route likely requires Pex3, Pex19, Rho1 and in higher eukaryotes also Pex16 [7], [9], [10].
In contrast, the sequence of events in peroxisome fission is better understood. Of the Pex11 family members, Pex11 is a key protein in peroxisome fission, the main mode of organelle multiplication in budding yeast [11], [12]. Peroxisome abundance in eukaryotes can be readily prescribed by manipulation of the Pex11 levels: absence of the Pex11 protein generally leads to a strong reduction of peroxisome numbers, whereas its overproduction promotes their proliferation [8], [13], [14], [15]. We have recently demonstrated that Pex11 functions in the formation of the initial membrane curvature required for the tubulation of the peroxisomal membrane as the initial step of fission [16]. The Pex11-induced membrane elongation is followed by membrane restriction and scission steps. Components involved in organelle constriction are not yet identified, fission requires the function of dynamin-related proteins (DRPs) like Dnm1 and Vps1 [11], [12], [17], [18], [19], [20], [21], [22]. The function of a third Pex11 family member in H. polymorpha, Pex11C, is still largely unknown [7].
In the filamentous fungus Penicillium chrysogenum, peroxisomes are important for efficient production of ß-lactam antibiotics [23], [24], [25], [26]. P. chrysogenum contains three Pex11 family members termed Pex11, Pex11B and Pex11C [27]. Interestingly, overproduction of Pex11 in low penicillin (PEN) producing strains of P. chrysogenum led to an increased number of peroxisomes in conjunction with enhanced antibiotic production [25]. The function of other Pex11 family members in filamentous fungi is still unknown. Interestingly, P. chrysogenum also contains a Pex16 ortholog [27]. In mammals, Pex16 has been shown to be essential for peroxisome formation and its depletion was manifested by a complete absence of peroxisomal structures [28], [29]. Although the molecular function of Pex16 remains largely unknown, it was proposed that Pex16 may act at a very early stage of peroxisome biogenesis by recruiting the peroxisomal membrane protein Pex3 to the ER [30], [31], [32], [33], [34].
In this work we have analyzed the mechanisms of peroxisome development in the filamentous fungus P. chrysogenum. First, we studied the role of the individual Pex11 family members in this process. In contrast to the yeast H. polymorpha, the de novo peroxisome biogenesis route in P. chrysogenum is independent of all Pex11 family members and Pex16. In contrast, deletion of pex3 in P. chrysogenum leads to cells devoid of peroxisomes, suggesting that in this fungus Pex3 may function independently of Pex16. Moreover, we demonstrate that the presence of intact peroxisomes is important for the efficiency of ß-lactam antibiotics production, while affecting peroxisome numbers in a high PEN producing strain does not affect antibiotic production.
Materials and Methods
Strains and growth conditions
Penicillium chrysogenum strains used in this study are listed in Table S1 and were grown at 25°C. Media used to cultivate P. chrysogenum strains are listed in Methods S1.
Escherichia coli DH5α (Stratagene) and DB3.1 (Invitrogen) were used for cloning purposes. Cells were grown at 37°C in LB medium (1% Bacto tryptone (Becton, Dickinson and Company), 0.5% Yeast Extract (Becton, Dickinson and Company) and 0.5% NaCl) supplemented with 50 µg/ml kanamycin, 100 µg/ml ampicillin, 15 µg/ml chloramphenicol or 25 µg/ml zeocin.
For the estimation of antibiotics production by bioassays the β-lactam sensitive strain Micrococcus luteus ATCC 9341 was used [35], cultivated in 2*TY medium (2% bacto-tryptone, 1% yeast extract and 1% NaCl) at 30°C.
Molecular techniques
Plasmids and oligonucleotides used in this study are listed in Tables S2 and S3, respectively. Standard recombinant DNA manipulations were carried out according to Sambrook et al. [36]. Protoplasting of P. chrysogenum and transformation of protoplasts was performed using established procedures [37]. Restriction enzymes (Fermentas, Roche) and other DNA modifying enzymes were used in agreement with the instruction of the suppliers. Preparative polymerase chain reactions (PCR) were performed with Phusion polymerase (Fermentas). Cloned PCR fragments were confirmed by sequencing (Service XS). Initial selection of transformants by colony PCR was performed using Phire polymerase (Fermentas). Southern blotting was performed according to the DIG High Prime Labeling and Detection kit (Roche). In silico analysis of DNA sequences and construction of vector maps was carried out using Clone Manager 5 software (Scientific and Educational Software, Durham). Plasmid constructions are listed in Methods S1.
β-Lactam bioassay
Estimation of the amount of antibiotics produced by P. chrysogenum strains was performed as described previously [35], using culture supernatants after 3 and 6 days of batch cultivation in penicillin production medium (PPM) supplemented with phenylacetic acid. HPLC determination of penicillin G was performed in duplo as described previously [23].
Biochemical techniques
Crude extracts of P. chrysogenum cells were prepared as described previously [38]. Protein concentrations were determined using the RC/DC Protein Assay kit (Bio-Rad) using bovine serum albumin as a standard. SDS-polyacrylamide gel electrophoresis and western blotting were performed in accordance with established protocols using specific polyclonal antibodies against translation elongation factor 1-α (eEF1A), isopenicillin N-acyltransferase (IAT), isopenicillin N synthase (IPNS), Pex11, Pex11B and Pex11C. Polyclonal antisera against the P. chrysogenum Pex11B and Pex11C proteins were prepared using sequence specific peptides and immunization in rabbits (Eurogentec). These antisera recognized the respective proteins in P. chrysogenum extracts only upon their overproduction.
Ultrastructural analysis
Confocal laser scanning microscopy (CLSM) images were obtained using a Zeiss LSM510 equipped with Zeiss Plan-Neofluar 100×/1.3 oil and Zeiss Plan-Apochromatic 63×/1.4 oil objective. The fluorescence of GFP was analyzed by excitation of the cells with a 488 nm argon/krypton laser, and signal was detected by a BP 500–530 Photo Multiplier Tube (PMT). DsRed fluorescence was analyzed by excitation of the cells with a 543 nm argon/krypton laser, and fluorescence was detected by a BP 565–615 PMT.
Wide-field fluorescence microscopy was performed using a Zeiss Axio Observer Z1 fluorescence microscope. Images were taken using an EC-Plan-Neofluar 100×/1.3 objective and a coolsnap HQ2 Camera (Roper scientific Inc). GFP signal was visualized with a 470/40 nm bandpass excitation filter, a 495 nm dichromatic mirror, and a 525/50 nm bandpass emission filter. DsRed and mCherry fluorescence were analyzed with a 545/25 nm bandpass excitation filter, a 570 nm dichromatic mirror, and a 605/70 nm bandpass emission filter, respectively. Z-stack images were made using an interval of 0.5 µm. Image analysis was carried out using ImageJ (http://rsb.info.nih.gov/nih-image/) and Adobe Photoshop.
For electron microscopy P. chrysogenum cells were fixed in 1.5% KMnO4 and prepared as described previously [39].
Results
P. chrysogenum Pex11 and Pex11C, but not Pex11B, are peroxisomal proteins
We recently demonstrated that the P. chrysogenum Pex11 protein family consists of three members, namely Pex11, Pex11B and Pex11C [27]. In a phylogenetic tree, Pex11B clusters with Pex11 in the same clade, while Pex11C is clearly part of a separate clade together with other fungal Pex11C members (Fig. S1). Despite the relatively low level of sequence identity between Pex11B and Pex11 (22% identity, 35% similarity), both proteins are structurally highly similar, suggesting an identical topology and an analogous function. Both proteins have two N-terminally located amphipathic helices (AMPH) as well as three C-terminally located hydrophobic regions (HR). In Pex11, the second AMPH is essential for membrane remodeling [16], while the first and third of the HRs are thought to represent membrane spanning domains. Pex11C appears both on sequence level (only 17% identity to Pex11) as well as structurally more distinct. Although AMPH and HR regions seem to be present, these deviate significantly from those found in Pex11 and Pex11B (Fig. S1). In fact, Pex11C members seem more related to mammalian Pex11γ [27], of which the function is unknown. Remarkably, filamentous ascomycetes like P. chrysogenum lack a Pex25 ortholog, which was shown to be essential in the yeast Hansenula polymorpha for de novo peroxisome formation from the ER [7]. This implies that the principles of peroxisome formation from the ER in filamentous ascomycetes may deviate from the processes identified in yeast.
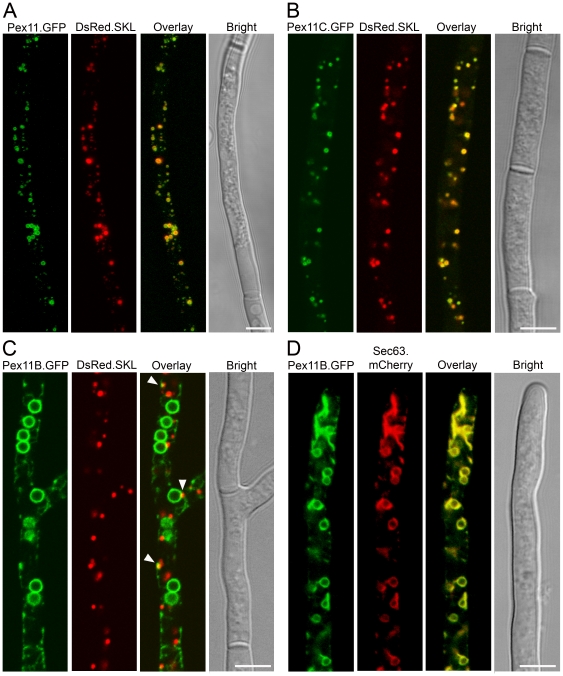
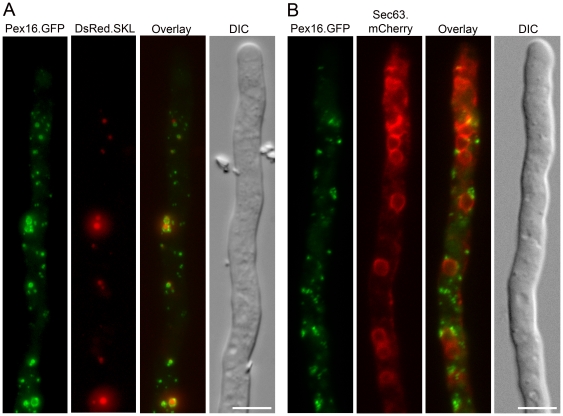
We first analyzed the subcellular localization of the Pex11 family members in P. chrysogenum, using monomeric GFP tagged versions and a derivative of the high PEN producing strain DS17690 that also produced DsRed.SKL protein to mark peroxisomes. Cells were cultivated for two days in PPM and subsequently analyzed by confocal laser scanning microscopy (CLSM). Both Pex11.mGFP and Pex11C.mGFP formed fluorescent ring-like structures encompassing DsRed fluorescent spots, suggesting that these proteins are components of the peroxisome membrane ( Fig. 1A and B ). Pex11B.mGFP fluorescence however did not co-localize with DsRed labeled peroxisomes ( Fig. 1C ). Instead, Pex11B.mGFP was found to fully colocalize with Sec63.mCherry in a strain producing this fusion protein to mark the ER membrane ( Fig. 1D ). Nevertheless, occasionally GFP fluorescence was found to overlap with DsRed fluorescence (arrowheads in Fig. 1C ). We showed before that the hyphal tip represents the main site of organelle proliferation [24]. DsRed.SKL Pex11B.mGFP cells were grown for 40 h on PPM supplemented with 0.1% oleate as a carbon source to stimulate massive peroxisome induction and analyzed by CLSM. Under these conditions, the co-localization between DsRed fluorescent peroxisomes and Pex11B.mGFP labeled ER was much more pronounced especially at the site of organelle development in the hyphal tips ( Fig. 2 ) and suggests a role for the ER in formation of new peroxisomes. This co-localization was much less evident in sub-apical cells.
Figure 1. Subcellular localization of Pex11 family members.
P. chrysogenum cells producing C-terminal mGFP fusions of Pex11 (A), Pex11C (B) or Pex11B (C) with DsRed.SKL as peroxisome marker were grown for 40 h in PPM and analyzed by CLSM. D. CLSM analysis of P. chrysogenum hyphae producing Pex11B.mGFP and Sec63.mCherry as marker of the ER, grown for 40 h in PPM. Scale bars represent 5 µm. Arrowheads (in C) indicate the sites of overlap between Pex11B.mGFP and Sec63.mCherry fluorescence.
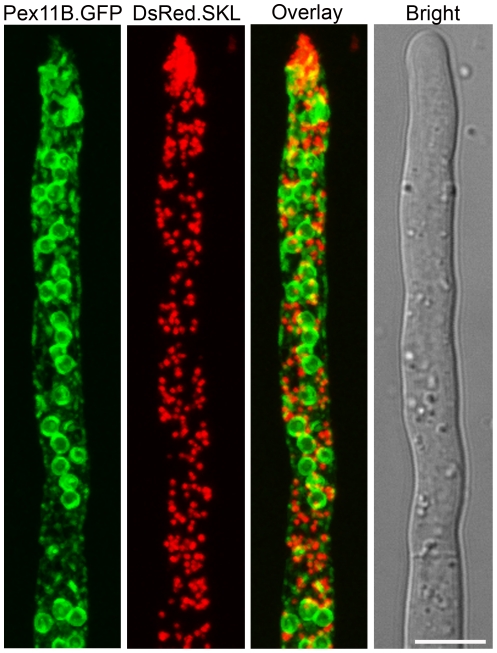
Figure 2. DsRed.SKL labeled peroxisomes co-localize with Pex11B.mGFP tagged ER at peroxisome inducing conditions in P. chrysogenum apical cells.
P. chrysogenum cells producing Pex11B.mGFP and DsRed.SKL were grown in PPM with addition of oleic acid (0.1%) and analyzed by CLSM. Co-localization of red fluorescent peroxisomes with Pex11B.mGFP labeled ER was observed in hyphal tips. The frequency of these events decreased towards the older subapical cells. The scale bar represents 5 µm.
Pex11 is a key component of peroxisome proliferation in P. chrysogenum
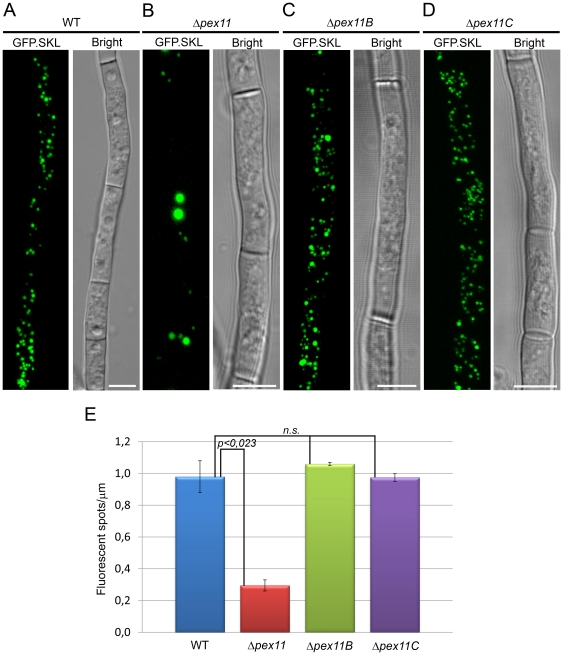
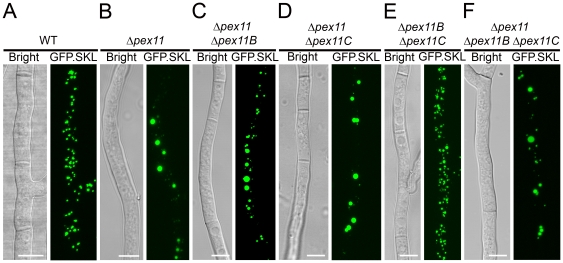
To study the role of the Pex11 family members in peroxisome development, strains carrying a deletion of either pex11, pex11B or pex11C were constructed. Deletion of pex11 resulted in a strong reduction of peroxisome numbers. In addition to low numbers of organelles of increased size also organelles of highly reduced size, relative to those in the parental strain, were observed ( Fig. 3A, B and E ). Possibly, the latter organelles represent newly formed peroxisomes in an early stage of their development. In contrast, in cells lacking either pex11B or pex11C peroxisome number and size were not significantly affected ( Fig. 3C, D and E ).
Figure 3. Impact of deletion of individual Pex11 family genes on peroxisome numbers.
P. chrysogenum hdfA GFP.SKL (A), Δpex11 GFP.SKL (B), Δpex11B GFP.SKL (C), Δpex11C GFP.SKL (D) cells were grown for 40 h on PPM and analyzed by CLSM. Scale bars represent 5 µm. E. Quantification of peroxisome numbers in P. chrysogenum cells depleted of individual pex11 family genes; n.s.- statistically not significant based on student t test. Error bars represent SEM.
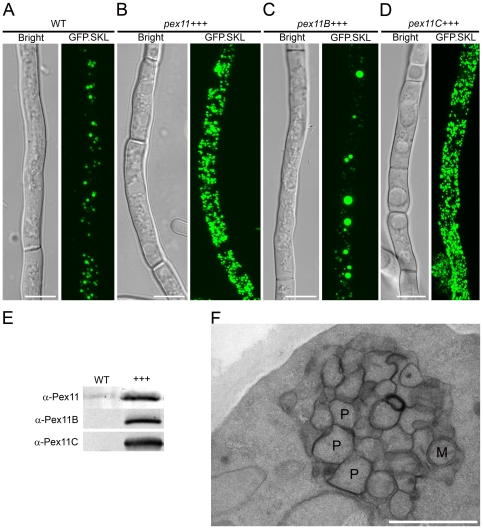
To further analyze the contribution of Pex11 family members in peroxisome proliferation, strains overproducing Pex11, Pex11B or Pex11C were constructed ( Fig. 4E ). CLSM analysis revealed that overproduction of Pex11 or Pex11C strongly stimulated peroxisome proliferation ( Fig. 4A, B and D ). Surprisingly, cells overproducing Pex11B contained relatively few, strongly enlarged GFP fluorescent spots in conjunction with small spots ( Fig. 4C ). Electron microscopy (EM) analysis of Pex11B overproducing cells revealed that the enlarged GFP.SKL containing structures did not represent normal peroxisomes, but rather represented large membranous clusters of peroxisomes of reduced size occasionally also including mitochondrial-like profiles ( Fig. 4F ). These data suggest that of the P. chrysogenum Pex11 protein family members, Pex11 is the main component controlling peroxisome numbers. When overproduced, Pex11C also can induce peroxisome multiplication, whereas the ER-located Pex11B cannot.
Figure 4. The effect of overproduction of Pex11 family members on peroxisome proliferation.
Representative CLSM images of P. chrysogenum GFP.SKL cells: WT (A); overproducing Pex11 (B), Pex11B (C) or Pex11C (D). Cells were grown for 40 h in PPM. Scale bars represent 5 µm. E. Western blot showing overproduction of Pex11 (top), Pex11B, (center) or Pex11C (bottom). Crude extracts of DS17690 and strains overproducing Pex11 proteins were used for SDS-PAGE and western blotting and probed with specific antisera. Equal amounts of protein were loaded per lane. F. Electron micrograph of P. chrysogenum cells overproducing Pex11B. P-peroxisome; M-mitochondrion. Scale bar represents 1 µm.
A P. chrysogenum triple Δpex11 Δpex11B Δpex11C mutant still forms peroxisomes
To analyze the contribution of each single member of the Pex11 family in peroxisome development, double deletion strains were constructed in all three possible combinations. After two days of cultivation on PPM, CLSM analysis revealed that peroxisome numbers in cells of the Δpex11B Δpex11C GFP.SKL strain were comparable to the parental strain ( Fig. 5A and E ). In contrast, Δpex11 Δpex11C GFP.SKL cells and Δpex11 Δpex11B GFP.SKL contained reduced numbers of peroxisomes of enhanced size in conjunction with very small organelles akin to the phenotype of Δpex11 GFP.SKL cells ( Fig. 5B, C and D ).
Figure 5. P. chrysogenum cells devoid of all Pex11 family members still contain peroxisomes.
Double and triple deletion mutants of pex11 family genes were prepared and analyzed by CLSM after growth for 40 h in PPM: WT (A), Δpex11 (B), Δpex11 Δpex11B GFP.SKL (C), Δpex11 Δpex11C GFP.SKL (D), Δpex11B Δpex11C GFP.SKL (E), Δpex11 Δpex11B Δpex11C GFP.SKL (F). Scale bars represent 5 µm.
We have recently reported that simultaneous inhibition of the two routes of peroxisome formation in the yeast H. polymorpha i.e. fission and de novo formation (by deletion of both Hp-PEX11 and Hp-PEX25) results in a peroxisome-deficient phenotype [7]. To study whether P. chrysogenum cells lacking all Pex11 family members are peroxisome deficient, a Δpex11 Δpex11B Δpex11C GFP.SKL triple deletion strain was constructed. This mutant did not show any specific abnormal phenotypic features relative to the parental strain (not shown). CLSM analysis of this strain revealed the presence of few enlarged peroxisomes together with some small organelles, which is similar to the phenotype of Δpex11 GFP.SKL cells ( Fig. 5B and F ). This implies that in the absence of all Pex11 family proteins peroxisome formation still proceeds in P. chrysogenum.
It has been suggested that, unlike its paralog Dnm1, the DRP Vps1 is involved in peroxisome proliferation in a route that is independent of Pex11 family members [40]. To study if peroxisomes in Δpex11 Δpex11B Δpex11C GFP.SKL cells are the result of residual Vps1 dependent organelle fission, a quadruple deletion mutant Δpex11 Δpex11B Δpex11C Δvps1 GFP.SKL was constructed. Fluorescence microscopy (FM) analysis of these cells revealed that the additional absence of Vps1 did not influence peroxisome formation significantly, since the peroxisome numbers and sizes remained comparable to those observed in Δpex11 GFP.SKL and Δpex11 Δpex11B Δpex11C GFP.SKL cells (Fig. S2). These data suggest that, in comparison to yeast, (an) additional route(s) of peroxisome formation may be operative in P. chrysogenum and that peroxisomes present in cells devoid of the peroxisome fission machinery most likely originate from a de novo pathway, which is independent of the Pex11 family members and Vps1.
Penicillium chrysogenum cells lacking Pex16 still have peroxisomes
Filamentous ascomycetes like P. chrysogenum do not contain a Pex25 protein but instead possess a Pex16 ortholog. In plants and mammals, but not in the yeast Yarrowia lipolytica (which contains both Pex16 and a Pex25-related protein), Pex16 is essential for peroxisome formation [41]. It has been suggested that Pex16 may be involved in the initial stages of peroxisome formation from the ER [33], [34]. This led us to investigate whether P. chrysogenum pex16 is essential for peroxisome development.
We first analyzed the subcellular localization of the P. chrysogenum pex16 gene product using an mGFP fusion protein. FM analysis of cells that produce Pex16.mGFP together with DsRed.SKL as peroxisome marker showed a co-localization of the two fluorescent proteins, indicating that P. chrysogenum Pex16 is a bona fide peroxisomal membrane protein ( Fig. 6A ). Occasionally, Pex16.GFP fluorescence was also observed as very small punctuate structures that lacked DsRed.SKL fluorescence. These structures may represent early/small peroxisomes that have not yet imported sufficient amounts of DsRed.SKL to allow visualization in fluorescence microscopy. In contrast to the situation in plants, Pex16.mGFP was not observed at the ER [31]. However, we frequently observed Pex16.mGFP labeled peroxisomes in close proximity to the Sec63.mCherry tagged ER ( Fig. 6B ).
Figure 6. P. chrysogenum Pex16 is a peroxisomal membrane protein.
P. chrysogenum cells producing Pex16.mGFP and either DsRed.SKL (A) or Sec63.mCherry (B) were grown for 40 h in PPM and analyzed by fluorescence microscopy. Scale bars represent 5 µm.
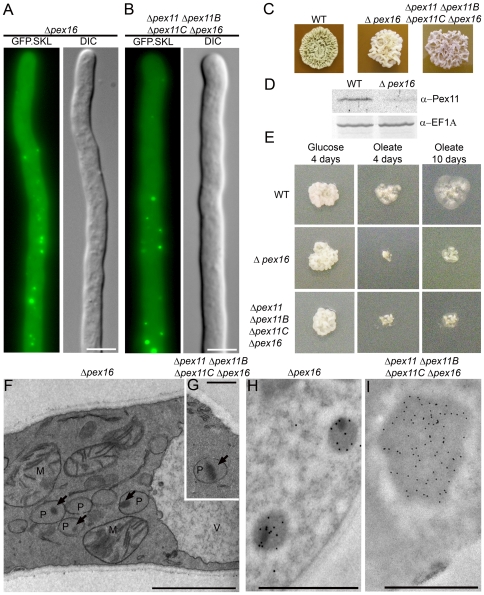
Subsequently, the effect of deletion of pex16 on peroxisome development in P. chrysogenum was analyzed. FM analyses of Δpex16 GFP.SKL cells revealed that the fluorescent protein was largely mislocalized to the cytosol. Interestingly, also significant numbers of green fluorescent spots were present, suggesting that these mutant cells may still contain peroxisomes ( Fig. 7A ). EM analyses confirmed that Δpex16 GFP.SKL cells indeed contain peroxisomes of decreased size which often harbor electron dense protein aggregates in the organelle matrix ( Fig. 7F ). These organelles still contained significant amounts of IAT protein ( Fig. 7H ), Indeed, peroxisomes in Δpex16 GFP.SKL cells are still partially functional, since these cells were able to grow on oleic acid, however at a highly decreased rate ( Fig. 7E ). Cells devoid of pex16 were affected in their ability to produce conidiospores ( Fig. 7C ), a phenomenon shown to be related with P. chrysogenum pex mutants [23], [42]. Remarkably, Δpex16 GFP.SKL had strongly decreased levels of Pex11 ( Fig. 7D ). Combined, these data demonstrate that in P. chrysogenum Pex16 is involved in peroxisome development, but not essential for this process.
Figure 7. Pex16 is not essential for peroxisome biogenesis.
Fluorescence microscopy analysis of P. chrysogenum Δpex16 GFP.SKL (A) and Δpex11 Δpex11B Δpex11C Δpex16 GFP.SKL (B) cells. Cells were grown for 40 h in PPM. For corresponding WT see Fig. 3A. Scale bars represent 5 µm. C. Cells devoid of pex16 are sporulation deficient. Colonies of WT and both Δpex16 GFP.SKL and Δpex11 Δpex11B Δpex11C Δpex16 GFP.SKL strains were grown for 7 days on sporulation inducing R-agar plates. D. Δpex16 cells are characterized by decreased levels of Pex11. Western blots of WT and Δpex16 GFP.SKL cell extracts were prepared and decorated with α-Pex11 antibodies; translation elongation factor 1- (eEF1A) was used as a loading control. E. P. chrysogenum cells lacking pex16 are able to grow on oleic acid although at decreased rates. WT, Δpex16 GFP.SKL and Δpex11 Δpex11B Δpex11C Δpex16 GFP.SKL strains were grown for 10 days on mineral medium containing 0.5% glucose or 0.1% oleic acid as a sole carbon source. Electron micrographs of Δpex16 GFP.SKL (F) and Δpex11 Δpex11B Δpex11C Δpex16 GFP.SKL (G) cells grown for 40 h in PPM; P – peroxisome; M – mitochondrion; V – vacuole; arrows indicate protein dense inclusions. Scale bars represent 1 µm. Electron micrographs representing α-IAT immunolabelling of sections of Δpex16 GFP.SKL (H) and Δpex11 Δpex11B Δpex11C Δpex16 GFP.SKL (I) cells. Scale bars represent 1 µm.
(eEF1A) was used as a loading control. E. P. chrysogenum cells lacking pex16 are able to grow on oleic acid although at decreased rates. WT, Δpex16 GFP.SKL and Δpex11 Δpex11B Δpex11C Δpex16 GFP.SKL strains were grown for 10 days on mineral medium containing 0.5% glucose or 0.1% oleic acid as a sole carbon source. Electron micrographs of Δpex16 GFP.SKL (F) and Δpex11 Δpex11B Δpex11C Δpex16 GFP.SKL (G) cells grown for 40 h in PPM; P – peroxisome; M – mitochondrion; V – vacuole; arrows indicate protein dense inclusions. Scale bars represent 1 µm. Electron micrographs representing α-IAT immunolabelling of sections of Δpex16 GFP.SKL (H) and Δpex11 Δpex11B Δpex11C Δpex16 GFP.SKL (I) cells. Scale bars represent 1 µm.
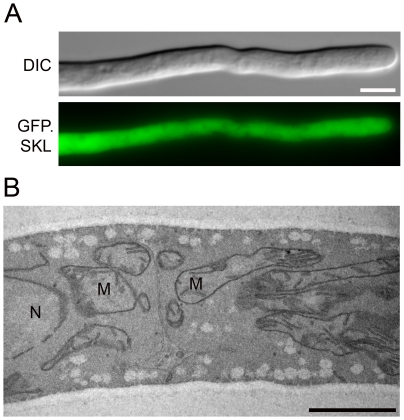
As a control, we also deleted pex3 in P. chrysogenum. In all eukaryotes studied so far, deletion of this gene leads to a complete absence of peroxisomal structures [43]. Indeed, P. chrysogenum Δpex3 GFP.SKL cells were devoid of peroxisomes as concluded from the mislocalization of GFP.SKL to the cytosol ( Fig. 8A ) and the complete absence of peroxisomes in EM analyses ( Fig. 8B ). To analyze if Pex16 is involved in de novo biogenesis of peroxisomes, the quadruple mutant Δpex11 Δpex11B Δpex11C Δpex16 GFP.SKL was constructed. FM and EM analyses of cells of this mutant revealed that this strain was not peroxisome deficient ( Fig. 7B, G and I ) and showed a phenotype largely comparable to that observed for the pex16 mutant ( Fig. 7C and E ). Thus, also P. chrysogenum Pex16 is not essential for de novo peroxisome biogenesis.
Figure 8. Deletion of pex3 in P. chrysogenum results in cells completely devoid of peroxisomes.
P. chrysogenum Δpex3 cells, producing GFP.SKL were grown for 40 h in PPM medium and analyzed by fluorescence (A) and electron (B) microscopy. Scale bars represent 5 µm in A and 1 µm in B; M-mitochondrion, N – nucleus.
Absence of Pex16 and Pex3, but not manipulation of the levels of Pex11 family proteins, affects PEN production
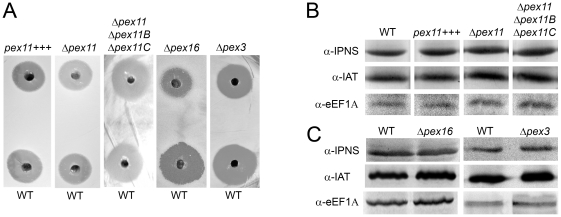
Previously, we demonstrated that overproduction of Pex11 in two laboratory strains, promoted PEN production approximately two-fold [25]. To analyze the effects of adapting peroxisome numbers or creating dysfunctional organelles on antibiotic production in the high PEN producer DS17690, various mutant strains were grown on PPM for 6 days using WT as control. Spent media were collected and analyzed for PEN levels, using a plate bioassay. The results ( Fig. 9A and Fig. S3) show that in the DS17690 background manipulation of the protein levels of any member of the Pex11 family did not influence the antibiotic activity present in the culture supernatants, judged from the size of the clearing halo. In contrast, in case of Δpex16 and Δpex3 cells a reduction in anti-bacterial activity of approximately 50% was observed, which was confirmed by quantification of the PEN-G levels in culture supernatants. In agreement with the plate bioassay, Δpex16 and Δpex3 cells produced 51% (±1.5%) and 33% (±0.4%) less PEN-G than corresponding WT cells. The observed decrease in PEN production was not related to a reduction in the levels of the enzymes of the PEN biosynthetic pathway as IAT and IPNS levels were unaltered relative to those in the WT control, as was evident from western blotting ( Fig. 9B and C ). These data suggest that in a high PEN producing strain the presence of functional peroxisomes rather than a high number of these organelles is important for efficient antibiotic biosynthesis.
Figure 9. Impact of deletion of pex11 family genes, pex16 or pex3 on penicillin production.
A. Analysis of the production of antibacterial compounds by selected strains using plate bioassays with M. luteus as an indicator strain. Clarified culture supernatants were diluted 3200 times before analysis. During each experiment a corresponding WT supernatant at the same dilution was tested on the same plate as the supernatants of the analyzed mutants. B and C. Western blot analysis of the levels of penicillin biosynthetic enzymes IPNS and IAT in strains with manipulated levels of Pex11 family proteins (B), Pex3 or Pex16 (C). eEF1A was used as a loading control.
Discussion
We have analyzed the function of the members of the Pex11 protein family Pex11, Pex11B and Pex11C as well as Pex16 in peroxisome formation and penicillin production in the fungus P. chrysogenum. In general, the number of peroxisomes present in a eukaryotic cell is the resultant of organelle formation (biogenesis) versus degradation (autophagy) processes. The biogenesis of peroxisomes may occur via organelle fission and de novo formation from the ER [7]. As an initial step of fission, Pex11 induces membrane curvature that causes organelle tubulation, which is followed by recruitment of a large GTPase of the DRP superfamily that performs scission [13], [16], [17]. Elongated peroxisomes can effectively recruit DRP, suggesting an interplay between Pex11 and DRP [44]. Moreover, other proteins involved in direct association of DRP with peroxisomes have been identified (Fis1 in all eukaryotes and in mammals MFF) [45], [46]. In agreement with these findings, P. chrysogenum Pex11 is a prominent peroxisomal membrane protein that has a major role in peroxisome fission. Remarkably, both in Δpex11 GFP.SKL and Δpex11 Δpex11B Δpex11C GFP.SKL cells, organelles of highly reduced size were present, which may represent newly formed immature organelles. This implies the presence of a Pex11 family independent peroxisome formation process. In addition to Pex11, the P. chrysogenum membrane also contains Pex11C, the absence of which does not affect the peroxisomal profile in the cell significantly. However, when this protein is present at elevated levels, this also leads to massive organelle proliferation. The same observation was made in the yeast H. polymorpha, suggesting that also Pex11C has the capability to function as a general peroxisome proliferation factor (R. Saraya et al., unpublished data). Previously, transcriptome data were collected for various P. chrysogenum strains cultured in chemostats at PEN producing conditions, analysis of which showed that the pex11 and pex11C genes are both expressed at significant levels, although the pex11 gene is transcribed to a much higher extent than pex11C [26]. Thus, of the peroxisomally located Pex11 family proteins, Pex11 is the key component of the fission machinery, while the function of Pex11C in WT cells remains unknown.
In yeast species a third member of the Pex11 family, Pex25, was demonstrated to be involved in de novo biogenesis of peroxisomes from the ER [7], [8]. P. chrysogenum lacks a Pex25 ortholog [27]. Instead, filamentous ascomycetes like P. chrysogenum contain Pex11B, which we demonstrate to represent a component of the ER. This is the first Pex11 protein that is uniquely localized to an organelle other than the peroxisome. Deletion of pex11B did not affect peroxisomal profiles significantly. However, upon overproduction of this ER protein, a massive clustering of peroxisomes of reduced size was observed. Thus, although Pex11B is not essential for de novo formation of peroxisomes, we conclude that the protein is nevertheless involved in peroxisome biogenesis. Clearly, unraveling the precise role of Pex11B in P. chrysogenum requires further investigation.
Our data demonstrate that, in contrast to the yeast H. polymorpha, the de novo peroxisome biogenesis pathway in P. chrysogenum is independent of all Pex11 family members as well as Pex16 and the DRP Vps1. Pex16 is a peroxisomal membrane protein that is essential for peroxisome development in higher eukaryotes. It was proposed that mammalian Pex16 may function at a very early stage of peroxisome biogenesis by recruiting Pex3 to the ER during peroxisome formation [33], [34]. Our findings demonstrate that Pex16 is not essential for peroxisome development in P. chrysogenum, since partially functional peroxisomes are still present in pex16 deletion cells. Pex16 is also not essential for de novo formation of peroxisomes, as peroxisomes were observed in cells lacking all Pex11 family members as well as Pex16. In contrast, P. chrysogenum cells depleted of Pex3 are devoid of peroxisomes. These data may suggest that, in contrast to findings in higher eukaryotes, in P. chrysogenum Pex16 is not required for targeting of Pex3 to the ER and that the ER targeting of Pex3 for de novo peroxisome formation is possibly an intrinsic property of the fungal Pex3 protein itself or is mediated by (an)other, yet unknown factor(s). This hypothesis is supported by the fact that Pex16 was never seen at the ER in P. chrysogenum, a feature that was reported for plants and mammals [31], [33], [34]. Our observation that Pex16 is also present on punctuate structures lacking a peroxisomal matrix marker suggest that Pex16 may be a component of early, young peroxisomes which have not yet, or to only a very low extent, accumulated matrix proteins. The drastically reduced levels of Pex11 in the pex16 deletion strain suggest that Pex16 may be involved in membrane protein insertion/assembly in peroxisomes [29]. Thus, all our data suggest that the de novo peroxisome biogenesis pathway in P. chrysogenum differs from yeast species and mammals in that it is independent of all Pex11 proteins as well as Pex16 and may be performed by Pex3 alone and by other, yet unidentified factors.
The filamentous fungus P. chrysogenum is used in industry as an efficient cell factory for production of β-lactam antibiotics. The biosynthetic pathway of PEN production in this fungus is partially compartmentalized to peroxisomes, where the final stages of this process occur [47]. Many studies have demonstrated that the presence of functional peroxisomes is important for the efficiency of PEN biosynthesis in this filamentous fungus (reviewed in [2]). During P. chrysogenum strain improvement, the peroxisome volume/fraction has increased significantly in the strain lineage, confirming the importance of peroxisomes in this process [26]. Previously, we showed that the overproduction of Pex11 in low PEN producing strains led to peroxisome proliferation and increased penicillin production levels [25]. We speculated that the positive effect of Pex11 overproduction on PEN biosynthesis was related to an increased transport of PEN and/or its precursors (e.g., isopenicillin N (IPN) across the peroxisomal membrane. We show here that, surprisingly, manipulation of the number and size of peroxisomes (by deleting or overexpressing pex11 family genes) has no significant influence on antibiotic production in a high PEN producing strain. It must be noted that all these strains still contain fully functional peroxisomes as demonstrated by the complete import of GFP.SKL protein. In contrast, in pex mutant strains, where most or all matrix protein was mislocalized to the cytosol (Δpex16 and Δpex3), PEN production is significantly reduced. These observations suggest that during a late stage of P. chrysogenum strain improvement the product/precursor flux over the peroxisomal membrane is no longer a limiting factor for antibiotic production like it was in low PEN producing strains. So far, it is unknown whether PEN/IPN transport over the peroxisomal membrane requires active transport or utilizes peroxisomal pore proteins [48]. Apparently, in the high PEN producing strains the efficiency of this transport step has become more independent of the size/structure of the peroxisomal membrane surface.
Supporting Information
Sequence properties of P. chrysogenum Pex11 family members. A. Sequence alignment of Pex11 proteins from H. polymorpha (Hp; Genbank accession number ABG36520), S. cerevisiae (Sc; NP_014494) and P. chrysogenum (Pc; AAQ08763) and Pex11B from P. chrysogenum (ABH11428). Protein sequences were aligned using Clustal-X [49] and depicted by Genedoc (http://www.nrbsc.org/downloads/). The one letter code is shown. Conserved residues are shaded. B. Phylogenetic tree of Pex11 family members. Protein sequences used were S. cerevisiae (Sc) Pex11 (NP_014494), Pex25 (NP_015213) and Pex27 (NP_014836); H. polymorpha (Hp) Pex11(ABG36520), Pex11C (ABG36521) and Pex25 (ABG36525) and P. chrysogenum (Pc) Pex11 (AAQ08763), Pex11B (ABH11428) and Pex11C (ABH11429). The tree was constructed with TREECON for Windows [50] using protein sequences aligned with Clustal-X. The distance scale represents the number of differences between the sequences with 0.1 indicating a 10% difference. C. Schematic representation of Pex11 family members from filamentous fungi. The scheme was build based on a sequence alignment of at least 5 protein sequences from filamentous ascomycetes. Conserved motifs are indicated (red - putative amphipathic helices (AMPH); black -hydrophobic regions (HR)).
(TIF)
P. chrysogenum cells lacking all Pex11 family proteins and Vps1 still contain peroxisomes. P. chrysogenum Δpex11 Δpex11B Δpex11C Δvps1 cells, producing GFP.SKL were grown for 40 h in PPM and analyzed by FM. Mutant hyphae still contain peroxisomes and largely resemble hyphae of the Δpex11 Δpex11B Δpex11C GFP.SKL mutant. The scale bar represents 5 µm.
(TIF)
Impact of the manipulation of protein levels of Pex11 family members on penicillin production in a high PEN producing derivative of P. chrysogenum . The indicated P. chrysogenum mutant strains (+, overexpression; Δ, deletion; WT, wild type) were grown for 6 days in PPM and clarified culture supernatants (1600× diluted) were used in plate bioassays using M. luteus as the indicator strain. In all cases, the clearance zones are not significantly different than those obtained with spent medium of WT cells.
(TIF)
P. chrysogenum strains used in this study.
(PDF)
Plasmids used in this study.
(PDF)
Oligonucleotides used in this study (5′ to 3′).
(PDF)
(PDF)
Acknowledgments
We thank Charlotte Roux and Valerie Ott for excellent technical assistance and Stefan Weber for quantitative determination of PEN-G by HPLC.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: Ł.O. and M.B. were financially supported by the research programme of the Kluyver Centre for Genomics of Industrial Fermentation, which is part of the Netherlands Genomics Initiative/Netherlands Organization for Scientific Research. J.A.K.W.K. and S.F. were financially supported by the Netherlands Ministry of Economic Affairs and the B-Basic partner organizations (www.b-basic.nl) through B-Basic, a public-private NWO-ACTS programme (ACTS = Advanced Chemical Technologies for Sustainability). R.d.B. was supported by the IBOS Programme (Integration of Biosynthesis and Organic Synthesis), which is part of Advanced Chemical Technologies for Sustainability (ACTS). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Aksam EB, de Vries B, van der Klei IJ, Kiel JA. Preserving organelle vitality: peroxisomal quality control mechanisms in yeast. FEMS Yeast Res. 2009;9:808–820. doi: 10.1111/j.1567-1364.2009.00534.x. [DOI] [PubMed] [Google Scholar]
- 2.Bartoszewska M, Opalinski L, Veenhuis M, van der Klei IJ. The significance of peroxisomes in secondary metabolite biosynthesis in filamentous fungi. Biotechnol Lett. 2011;33:1921–1931. doi: 10.1007/s10529-011-0664-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Klei IJ, Veenhuis M. Yeast and filamentous fungi as model organisms in microbody research. Biochim Biophys Acta. 2006;1763:1364–1373. doi: 10.1016/j.bbamcr.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 4.Martin JF, Ullan RV, Garcia-Estrada C. Role of peroxisomes in the biosynthesis and secretion of beta-lactams and other secondary metabolites. J Ind Microbiol Biotechnol. 2012;39:367–382. doi: 10.1007/s10295-011-1063-z. [DOI] [PubMed] [Google Scholar]
- 5.Nuttall JM, Motley A, Hettema EH. Peroxisome biogenesis: recent advances. Curr Opin Cell Biol. 2011;23:421–426. doi: 10.1016/j.ceb.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Saraya R, Veenhuis M, van der Klei IJ. Peroxisomes as dynamic organelles: peroxisome abundance in yeast. FEBS J. 2010;277:3279–3288. doi: 10.1111/j.1742-4658.2010.07740.x. [DOI] [PubMed] [Google Scholar]
- 7.Saraya R, Krikken AM, Veenhuis M, van der Klei IJ. Peroxisome reintroduction in Hansenula polymorpha requires Pex25 and Rho1. J Cell Biol. 2011;193:885–900. doi: 10.1083/jcb.201012083. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Huber A, Koch J, Kragler F, Brocard C, Hartig A. A Subtle Interplay Between Three Pex11 Proteins Shapes De Novo Formation and Fission of Peroxisomes. Traffic. 2012;13:157–167. doi: 10.1111/j.1600-0854.2011.01290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lam SK, Yoda N, Schekman R. A vesicle carrier that mediates peroxisome protein traffic from the endoplasmic reticulum. Proc Natl Acad Sci U S A. 2011;108:E51–52. doi: 10.1073/pnas.1103526108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agrawal G, Joshi S, Subramani S. Cell-free sorting of peroxisomal membrane proteins from the endoplasmic reticulum. Proc Natl Acad Sci U S A. 2011;108:9113–9118. doi: 10.1073/pnas.1018749108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagotu S, Saraya R, Otzen M, Veenhuis M, van der Klei IJ. Peroxisome proliferation in Hansenula polymorpha requires Dnm1p which mediates fission but not de novo formation. Biochim Biophys Acta. 2008;1783:760–769. doi: 10.1016/j.bbamcr.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 12.Motley AM, Hettema EH. Yeast peroxisomes multiply by growth and division. J Cell Biol. 2007;178:399–410. doi: 10.1083/jcb.200702167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koch J, Brocard C. Membrane elongation factors in organelle maintenance: the case of peroxisome proliferation. Biomol Concepts. 2011;2:353–364. doi: 10.1515/BMC.2011.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koch J, Pranjic K, Huber A, Ellinger A, Hartig A, et al. PEX11 family members are membrane elongation factors that coordinate peroxisome proliferation and maintenance. J Cell Sci. 2010;123:3389–3400. doi: 10.1242/jcs.064907. [DOI] [PubMed] [Google Scholar]
- 15.Lingard MJ, Trelease RN. Five Arabidopsis peroxin 11 homologs individually promote peroxisome elongation, duplication or aggregation. J Cell Sci. 2006;119:1961–1972. doi: 10.1242/jcs.02904. [DOI] [PubMed] [Google Scholar]
- 16.Opalinski L, Kiel JA, Williams C, Veenhuis M, van der Klei IJ. Membrane curvature during peroxisome fission requires Pex11. EMBO J. 2011;30:5–16. doi: 10.1038/emboj.2010.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Opalinski L, Veenhuis M, van der Klei IJ. Peroxisomes: membrane events accompanying peroxisome proliferation. Int J Biochem Cell Biol. 2011;43:847–851. doi: 10.1016/j.biocel.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Zhang X, Hu J. The Arabidopsis chloroplast division protein DYNAMIN-RELATED PROTEIN5B also mediates peroxisome division. Plant Cell. 2010;22:431–442. doi: 10.1105/tpc.109.071324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lingard MJ, Gidda SK, Bingham S, Rothstein SJ, Mullen RT, et al. Arabidopsis PEROXIN11c-e, FISSION1b, and DYNAMIN-RELATED PROTEIN3A cooperate in cell cycle-associated replication of peroxisomes. Plant Cell. 2008;20:1567–1585. doi: 10.1105/tpc.107.057679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Motley AM, Ward GP, Hettema EH. Dnm1p-dependent peroxisome fission requires Caf4p, Mdv1p and Fis1p. J Cell Sci. 2008;121:1633–1640. doi: 10.1242/jcs.026344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuravi K, Nagotu S, Krikken AM, Sjollema K, Deckers M, et al. Dynamin-related proteins Vps1p and Dnm1p control peroxisome abundance in Saccharomyces cerevisiae. J Cell Sci. 2006;119:3994–4001. doi: 10.1242/jcs.03166. [DOI] [PubMed] [Google Scholar]
- 22.Hoepfner D, van den Berg M, Philippsen P, Tabak HF, Hettema EH. A role for Vps1p, actin, and the Myo2p motor in peroxisome abundance and inheritance in Saccharomyces cerevisiae. J Cell Biol. 2001;155:979–990. doi: 10.1083/jcb.200107028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Opalinski L, Kiel JA, Homan TG, Veenhuis M, van der Klei IJ. Penicillium chrysogenum Pex14/17p–a novel component of the peroxisomal membrane that is important for penicillin production. FEBS J. 2010;277:3203–3218. doi: 10.1111/j.1742-4658.2010.07726.x. [DOI] [PubMed] [Google Scholar]
- 24.Meijer WH, Gidijala L, Fekken S, Kiel JA, van den Berg MA, et al. Peroxisomes are required for efficient penicillin biosynthesis in Penicillium chrysogenum. Appl Environ Microbiol. 2010;76:5702–5709. doi: 10.1128/AEM.02327-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiel JA, van der Klei IJ, van den Berg MA, Bovenberg RA, Veenhuis M. Overproduction of a single protein, Pc-Pex11p, results in 2-fold enhanced penicillin production by Penicillium chrysogenum. Fungal Genet Biol. 2005;42:154–164. doi: 10.1016/j.fgb.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 26.van den Berg MA, Albang R, Albermann K, Badger JH, Daran JM, et al. Genome sequencing and analysis of the filamentous fungus Penicillium chrysogenum. Nat Biotechnol. 2008;26:1161–1168. doi: 10.1038/nbt.1498. [DOI] [PubMed] [Google Scholar]
- 27.Kiel JA, Veenhuis M, van der Klei IJ. PEX genes in fungal genomes: common, rare or redundant. Traffic. 2006;7:1291–1303. doi: 10.1111/j.1600-0854.2006.00479.x. [DOI] [PubMed] [Google Scholar]
- 28.Honsho M, Tamura S, Shimozawa N, Suzuki Y, Kondo N, et al. Mutation in PEX16 is causal in the peroxisome-deficient Zellweger syndrome of complementation group D. Am J Hum Genet. 1998;63:1622–1630. doi: 10.1086/302161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Honsho M, Hiroshige T, Fujiki Y. The membrane biogenesis peroxin Pex16p. Topogenesis and functional roles in peroxisomal membrane assembly. J Biol Chem. 2002;277:44513–44524. doi: 10.1074/jbc.M206139200. [DOI] [PubMed] [Google Scholar]
- 30.Karnik SK, Trelease RN. Arabidopsis peroxin 16 trafficks through the ER and an intermediate compartment to pre-existing peroxisomes via overlapping molecular targeting signals. J Exp Bot. 2007;58:1677–1693. doi: 10.1093/jxb/erm018. [DOI] [PubMed] [Google Scholar]
- 31.Karnik SK, Trelease RN. Arabidopsis peroxin 16 coexists at steady state in peroxisomes and endoplasmic reticulum. Plant Physiol. 2005;138:1967–1981. doi: 10.1104/pp.105.061291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim PK, Mullen RT, Schumann U, Lippincott-Schwartz J. The origin and maintenance of mammalian peroxisomes involves a de novo PEX16-dependent pathway from the ER. J Cell Biol. 2006;173:521–532. doi: 10.1083/jcb.200601036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsuzaki T, Fujiki Y. The peroxisomal membrane protein import receptor Pex3p is directly transported to peroxisomes by a novel Pex19p- and Pex16p-dependent pathway. J Cell Biol. 2008;183:1275–1286. doi: 10.1083/jcb.200806062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toro AA, Araya CA, Cordova GJ, Arredondo CA, Cardenas HG, et al. Pex3p-dependent peroxisomal biogenesis initiates in the endoplasmic reticulum of human fibroblasts. J Cell Biochem. 2009;107:1083–1096. doi: 10.1002/jcb.22210. [DOI] [PubMed] [Google Scholar]
- 35.Gidijala L, Bovenberg RA, Klaassen P, van der Klei IJ, Veenhuis M, et al. Production of functionally active Penicillium chrysogenum isopenicillin N synthase in the yeast Hansenula polymorpha. BMC Biotechnol. 2008;8:29. doi: 10.1186/1472-6750-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning, a Laboratory Manual, 2nd edition. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY; 1989. [Google Scholar]
- 37.Cantoral JM, Díez B, Barredo JL, Alvarez E, Martín JF. High-frequency transformation of Penicillium chrysogenum. Biotechnology. 1987;5:494–497. [Google Scholar]
- 38.Kiel JA, van den Berg MA, Fusetti F, Poolman B, Bovenberg RA, et al. Matching the proteome to the genome: the microbody of penicillin-producing Penicillium chrysogenum cells. Funct Integr Genomics. 2009;9:167–184. doi: 10.1007/s10142-009-0110-6. [DOI] [PubMed] [Google Scholar]
- 39.Waterham HR, Titorenko VI, Haima P, Cregg JM, Harder W, et al. The Hansenula polymorpha PER1 gene is essential for peroxisome biogenesis and encodes a peroxisomal matrix protein with both carboxy- and amino-terminal targeting signals. J Cell Biol. 1994;127:737–749. doi: 10.1083/jcb.127.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo T, Gregg C, Boukh-Viner T, Kyryakov P, Goldberg A, et al. A signal from inside the peroxisome initiates its division by promoting the remodeling of the peroxisomal membrane. J Cell Biol. 2007;177:289–303. doi: 10.1083/jcb.200609072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eitzen GA, Szilard RK, Rachubinski RA. Enlarged peroxisomes are present in oleic acid-grown Yarrowia lipolytica overexpressing the PEX16 gene encoding an intraperoxisomal peripheral membrane peroxin. J Cell Biol. 1997;137:1265–1278. doi: 10.1083/jcb.137.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kiel JA, van den Berg M, Bovenberg RA, van der Klei IJ, Veenhuis M. Penicillium chrysogenum Pex5p mediates differential sorting of PTS1 proteins to microbodies of the methylotrophic yeast Hansenula polymorpha. Fungal Genet Biol. 2004;41:708–720. doi: 10.1016/j.fgb.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 43.Schliebs W, Kunau WH. Peroxisome membrane biogenesis: the stage is set. Curr Biol. 2004;14:R397–399. doi: 10.1016/j.cub.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 44.Li X, Gould SJ. The dynamin-like GTPase DLP1 is essential for peroxisome division and is recruited to peroxisomes in part by PEX11. J Biol Chem. 2003;278:17012–17020. doi: 10.1074/jbc.M212031200. [DOI] [PubMed] [Google Scholar]
- 45.Gandre-Babbe S, van der Bliek AM. The novel tail-anchored membrane protein Mff controls mitochondrial and peroxisomal fission in mammalian cells. Mol Biol Cell. 2008;19:2402–2412. doi: 10.1091/mbc.E07-12-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koch A, Yoon Y, Bonekamp NA, McNiven MA, Schrader M. A role for Fis1 in both mitochondrial and peroxisomal fission in mammalian cells. Mol Biol Cell. 2005;16:5077–5086. doi: 10.1091/mbc.E05-02-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muller WH, van der Krift TP, Krouwer AJ, Wosten HA, van der Voort LH, et al. Localization of the pathway of the penicillin biosynthesis in Penicillium chrysogenum. EMBO J. 1991;10:489–495. doi: 10.1002/j.1460-2075.1991.tb07971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Antonenkov VD, Hiltunen JK. Transfer of metabolites across the peroxisomal membrane. Biochim Biophys Acta. 2011 doi: 10.1016/j.bbadis.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 49.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van de Peer Y, De Wachter R. TREECON for Windows: a software package for the construction and drawing of evolutionary trees for the Microsoft Windows environment. Comput Appl Biosci. 1994;10:569–570. doi: 10.1093/bioinformatics/10.5.569. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sequence properties of P. chrysogenum Pex11 family members. A. Sequence alignment of Pex11 proteins from H. polymorpha (Hp; Genbank accession number ABG36520), S. cerevisiae (Sc; NP_014494) and P. chrysogenum (Pc; AAQ08763) and Pex11B from P. chrysogenum (ABH11428). Protein sequences were aligned using Clustal-X [49] and depicted by Genedoc (http://www.nrbsc.org/downloads/). The one letter code is shown. Conserved residues are shaded. B. Phylogenetic tree of Pex11 family members. Protein sequences used were S. cerevisiae (Sc) Pex11 (NP_014494), Pex25 (NP_015213) and Pex27 (NP_014836); H. polymorpha (Hp) Pex11(ABG36520), Pex11C (ABG36521) and Pex25 (ABG36525) and P. chrysogenum (Pc) Pex11 (AAQ08763), Pex11B (ABH11428) and Pex11C (ABH11429). The tree was constructed with TREECON for Windows [50] using protein sequences aligned with Clustal-X. The distance scale represents the number of differences between the sequences with 0.1 indicating a 10% difference. C. Schematic representation of Pex11 family members from filamentous fungi. The scheme was build based on a sequence alignment of at least 5 protein sequences from filamentous ascomycetes. Conserved motifs are indicated (red - putative amphipathic helices (AMPH); black -hydrophobic regions (HR)).
(TIF)
P. chrysogenum cells lacking all Pex11 family proteins and Vps1 still contain peroxisomes. P. chrysogenum Δpex11 Δpex11B Δpex11C Δvps1 cells, producing GFP.SKL were grown for 40 h in PPM and analyzed by FM. Mutant hyphae still contain peroxisomes and largely resemble hyphae of the Δpex11 Δpex11B Δpex11C GFP.SKL mutant. The scale bar represents 5 µm.
(TIF)
Impact of the manipulation of protein levels of Pex11 family members on penicillin production in a high PEN producing derivative of P. chrysogenum . The indicated P. chrysogenum mutant strains (+, overexpression; Δ, deletion; WT, wild type) were grown for 6 days in PPM and clarified culture supernatants (1600× diluted) were used in plate bioassays using M. luteus as the indicator strain. In all cases, the clearance zones are not significantly different than those obtained with spent medium of WT cells.
(TIF)
P. chrysogenum strains used in this study.
(PDF)
Plasmids used in this study.
(PDF)
Oligonucleotides used in this study (5′ to 3′).
(PDF)
(PDF)