Abstract
Aim
We recently reported that glucose-dependent insulinotropic polypeptide (GIP) prevents the development of atherosclerosis in apolipoprotein E-null (Apoe −/−) mice. GIP receptors (GIPRs) are found to be severely down-regulated in diabetic animals. We examined whether GIP can exert anti-atherogenic effects in diabetes.
Methods
Nondiabetic Apoe −/− mice, streptozotocin-induced diabetic Apoe −/− mice, and db/db mice were administered GIP (25 nmol/kg/day) or saline (vehicle) through osmotic mini-pumps for 4 weeks. The animals were assessed for aortic atherosclerosis and for oxidized low-density lipoprotein-induced foam cell formation in exudate peritoneal macrophages.
Results
Diabetic Apoe −/− mice of 21 weeks of age exhibited more advanced atherosclerosis than nondiabetic Apoe −/− mice of the same age. GIP infusion in diabetic Apoe −/− mice increased plasma total GIP levels by 4-fold without improving plasma insulin, glucose, or lipid profiles. GIP infusion significantly suppressed macrophage-driven atherosclerotic lesions, but this effect was abolished by co-infusions with [Pro3]GIP, a GIPR antagonist. Foam cell formation was stimulated by 3-fold in diabetic Apoe −/− mice compared with their nondiabetic counterparts, but this effect was halved by GIP infusion. GIP infusion also attenuated the foam cell formation in db/db mice. In vitro treatment with GIP (1 nM) reduced foam cell formation by 15% in macrophages from diabetic Apoe −/− mice, and this attenuating effect was weaker than that attained by the same treatment of macrophages from nondiabetic counterparts (35%). While GIPR expression was reduced by only about a half in macrophages from diabetic mice, it was reduced much more dramatically in pancreatic islets from the same animals. Incubation with high glucose (500 mg/dl) for 9–10 days markedly reduced GIPR expression in pancreatic islet cells, but not in macrophages.
Conclusions
Long-term infusion of GIP conferred significant anti-atherogenic effects in diabetic mice even though the GIPR expression in macrophages was mildly down-regulated in the diabetic state.
Introduction
Type 2 diabetes is well known to accelerate the course of atherosclerosis, a condition associated with arterial endothelial dysfunction, macrophage foam cell formation, and vascular smooth muscle cell (VSMC) proliferation. New treatments based on incretins provide a novel approach to mediate parts of the complex pathophysiology of type 2 diabetes [1]–[2]. Incretin-based therapies have been reported to improve vascular inflammation and endothelial dysfunction beyond glucose normalization [3]. Indeed, several experimental studies have revealed that incretin-based treatments, such as glucagon-like peptide-1 (GLP-1) receptor agonists and dipeptidyl peptidase-4 (DPP-4) inhibitors, significantly suppress the development of atherosclerosis in animal models [4]–[8]. In 2011, our group reported that GLP-1 and glucose-dependent insulinotropic polypeptide (GIP), two native incretins, elicited anti-atherogenic effects in apolipoprotein-E-null (Apoe −/−) mice, an animal model of atherosclerosis [9]. Then, more recently, we found that a DPP-4 inhibitor, an enhancer of endogenous GLP-1 and GIP, prevented the development of atherosclerosis in the same animal model [10].
It is well known that GLP-1 exerts insulinotropic action whereas GIP looses this action in diabetes [11], [12]. Several lines of experimental evidence have revealed that GIP receptors (GIPRs) in pancreatic islets are substantially down-regulated under a persistent hyperglycemic condition [13]–[17]. This may explain the inability of GIP to induce insulin secretion in diabetes. If GIPRs turn out to be down-regulated in other tissues besides the pancreatic islets, the extra-pancreatic effects of GIP will presumably be attenuated or lost. Thinking along these lines, we decided to investigate whether GIP exerts anti-atherogenic effects in diabetic Apoe −/− mice as well as nondiabetic Apoe −/− mice.
Materials and Methods
Animals
Animal experiments were performed in accordance with the NIH guidelines for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Showa University.
Experiment 1: Ninety 8-week-old Apoe −/− mice were purchased from Sankyo Labo Service (Tokyo, Japan) and kept on a normal chow until the start of the GIP infusion described below. When the Apoe −/− mice reached the age of 15 weeks, the animals were given multiple peritoneal injections of streptozotocin (STZ, 50 mg/kg/day) for 5 consecutive days to induce diabetes, according to the method described by Takeda et al. [18]. Two weeks later, animals with a 6-hour fasting blood glucose (Glutest Sensor, Sanwa Chemical, Tokyo) of greater than 200 mg/dl were selected as diabetic. These 17-week-old diabetic Apoe −/− mice were then infused with human GIP(1–42) (25 nmol/kg/day, Abgent, San Diego, CA) or saline (vehicle) for 4 weeks through osmotic mini-pumps (Alzet Model 1007D, Durect, Cupertino, CA) [9]. In a subset of diabetic Apoe −/− mice, a GIPR antagonist called [Pro3]GIP (25 nmol/kg/day, Abgent) was co-infused with the GIP through other mini-pumps [9]. As nondiabetic controls, 17-week-old Apoe −/− mice without the STZ pretreatment were infused with saline for 4 weeks through osmotic mini-pumps. When the continuous GIP infusion was commenced, the animals were also started on an atherogenic diet containing 30% fat, 20% sucrose, 8% NaCl, and 500 mg/g cholesterol (Oriental Yeast, Tokyo) [9], [19].
Experiment 2: 17 db/db mice, a mouse model of type 2 diabetes, and 10 db/misty mice, a control group, were purchased from Sankyo Labo Service at the age of 6 weeks and kept on normal chow. From the age of 8 weeks, a point at which diabetes is established to be active in db/db mice, 10 db/misty mice and 11 diabetic db/db mice were infused with saline (vehicle) through osmotic mini-pumps for 4 weeks, and the other 6 db/db mice were infused with GIP (25 nmol/kg/day) under the same conditions.
Measurements
Four weeks after infusion, systolic blood pressure (SBP) was measured using indirect tail-cuff equipment (MK-2000, Muromachi Kikai, Tokyo) [9], [20]. Blood samples were collected after a 6-hour fast. Plasma concentrations of glucose, total-cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and triglyceride were measured by enzymatic methods using an autoanalyzer (Hitachi 7020, Hitachi, Tokyo) [20]. Non-HDL-C was calculated as TC minus HDL-C. Non-esterified fatty acid (NEFA) was measured by an NEFA C-test (Wako, Osaka, Japan). Plasma concentrations of total GIP, total GLP-1 and insulin were determined by ELISA (Millipore, Billerica, MA; Morinaga, Yokohama, Japan) [9], [10].
Assessment of atherosclerotic lesions
Four weeks after infusion, the mice were anesthetized with diethyl ether. The whole aorta was washed with perfused PBS and fixed with 4% paraformaldehyde [20], [21]. The aorta was excised from the root to the abdominal area and the connective and adipose tissues were carefully removed. The entire aorta and cross-sections of the aortic root were stained with oil red O for the assessment of atherosclerotic lesions [20], [21]. Monocyte/macrophage infiltration into atherosclerotic lesions in the aortic roots was visualized by staining with anti-mouse MOMA-2 antibody (Abcam, Tokyo) [9], [10], [20], [21]. The atherosclerotic lesions and areas with monocyte/macrophage migration were traced by an investigator blind to the treatment and measured by an image analyzer (Adobe Photoshop, San Jose, CA and NIH Scion Image, Frederick, MD) [9], [10], [20], [21].
Cholesterol esterification assay
The mice were given intraperitoneal injections of 4 ml of aged-autoclaved thioglycolate broth just after the 4-week infusion period, and the exudate peritoneal cells were isolated by peritoneal lavage with 8 ml of ice-cold PBS 4 days later [19], [20]. The cells were suspended in culture medium (RPMI-1640 containing 200 mg/dl glucose and supplemented with 10% FCS, 0.1 mg/ml streptomycin, and 100 U/ml penicillin) and seeded onto 6-cm dishes (4×106 cells/2 ml/dish) for real-time reverse transcription polymerase chain reaction (RT-PCR) and 3.5-cm dishes (3×106 cells/1 ml/dish) for cholesterol esterification assay, a standard procedure for assessing foam cell formation. After a 1-hour incubation at 37°C in 5% CO2 to allow adhesion, the medium was discarded to remove non-adherent cells. Adherent macrophages were incubated for 18 hours with culture medium containing 10 µg/ml human oxidized low-density lipoprotein (oxLDL) in the presence of 0.1 mM [3H]oleate conjugated with BSA [19], [20]. Cellular lipids were extracted and the radioactivity of the cholesterol [3H]oleate was determined by thin-layer chromatography [19], [20].
In vitro effect of GIP on cholesterol esterification in macrophages
GIP (1 nM) was added to the cultured mediums of exudate peritoneal macrophages from nondiabetic and diabetic Apoe −/− mice. After 24-hour incubation with or without GIP, the cells were used for cholesterol esterification (foam cell formation) assay [9], [10], [19], [20].
Measurements of GIPR expression
Exudate peritoneal macrophages from mice, THP-1 cells (ATCC, Manassas, VA), J774A.1 monocyte-macrophages (JCRB9108, Human Science, Osaka), and 1.2B4 pancreatic β-cells (DS Pharma Biomedical, Osaka) were suspended in culture medium and seeded onto dishes. Each type of cell was cultured under each standard culture condition and then incubated for 9 to 10 days in high-glucose medium (500 mg/dl). Total RNA was extracted from the cells using ISOGEN reagent (Nippon-Gene, Tokyo) before and after the incubation with the high-glucose medium. The cDNAs were synthesized from isolated RNA templates with a High-Capacity cDNA Archive Kit (Applied Biosystems, Carlsbad, CA). Real-time RT-PCR was performed using TaqMan Gene Expression Assays (Applied Biosystems). Pre-designed TaqMan probes for GIPR (Mm01316350_g1,Hs00164732_m1) and 18S rRNA were purchased from Applied Biosystems [9]. Amplification and fluorescent measurements were carried out during the elongation step with an ABI PRISM 7900 Sequence Detection System (Applied Biosystems).
Thin-sliced pancreases and peritoneal macrophages from mice seeded onto Chamber Slides (Iwaki Asahi Glass, Funabashi, Japan) were fixed with 4% paraformaldehyde and stained with goat polyclonal anti-GIPR antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Next, the macrophages were stained with anti-goat Alexa Fluor 568 (Invitrogen, Carlsbad, CA). Nuclei and F-actin cytoskeleton were visualized using DAPI and Phalloidin Alexa Fluor 488 (Invitrogen). Fluorescence-stained cells were examined by microscope (Olympus IX71, Olympus, Tokyo).
Statistical analysis
All values are expressed as mean ± SEM. Comparisons between 2 groups were performed by the 2-tailed unpaired Student's t test. Comparisons among 3 or more groups were performed by 1-way ANOVA followed by Bonferroni's post hoc test. Paired data were compared by the paired Student's t test. Statistics were performed using Statview-J 5.0 (SAS Institute, Cary, NC) and differences were considered statistically significant at P<0.05.
Results
Characteristics and laboratory data
Table 1 lists the blood glucose levels and other parameters in Apoe −/− mice. Before infusion (17 weeks of age), glucose levels were significantly higher in the vehicle-infused, GIP-infused, and GIP+[Pro3]GIP-infused diabetic Apoe −/− mice than in the nondiabetic Apoe −/− mice. Four weeks after infusion (21 weeks of age), glucose levels were significantly higher in the GIP-infused diabetic group than in the vehicle-infused diabetic group and GIP+[Pro3]GIP-infused diabetic group. Food intake in the diabetic groups was significantly higher than that in the nondiabetic group. Body weight was significantly decreased in the vehicle-infused diabetic group and improved in the GIP-infused and GIP+[Pro3]GIP-infused diabetic groups. There were no significant differences in the SBP or heart rate among the four groups. Plasma insulin levels in the diabetic groups were significantly lower than the plasma insulin level in the nondiabetic group. Plasma levels of TC and non-HDL-C were significantly higher in the diabetic groups than in the nondiabetic group. GIP infusion had no significant effect on TC or non-HDL-C levels. TC and non-HDL-C levels were both significantly lower in the GIP+[Pro3]GIP-infused diabetic group than in the vehicle- and GIP-infused diabetic groups. HDL-C levels in the diabetic groups were significantly lower than the HDL-C level in the nondiabetic group. Triglyceride and NEFA levels were both comparable among the four groups. Total GIP levels were 4-fold higher in the GIP-infused and GIP+[Pro3]GIP-infused diabetic groups than in the vehicle-infused diabetic group and nondiabetic group. The total GLP-1 level in each diabetic group was increased by more than 3-fold compared with that in the nondiabetic group.
Table 1. General characteristics and plasma measurements.
| Nondiabetic Vehicle (n = 14) | Diabetic Vehicle (n = 14) | Diabetic GIP (n = 14) | Diabetic GIP+[Pro3]GIP (n = 7) | |
| Glucose (Pre-Infusion) (mg/dl) | 138±9 | 358±24a | 369±22a | 368±41a |
| Glucose (Post-Infusion) (mg/dl) | 104±6 | 246±26a | 437±33a , b | 255±28a , c |
| Food Intake (g/day) | 2.5±0.2 | 3.8±0.3a | 4.4±0.3a | 4.0±0.2a |
| Body Weight (g) | 31±0.6 | 22±1.1a | 28±0.7b | 29±0.7b |
| SBP (mm Hg) | 108±3 | 106±3 | 108±3 | 109±5 |
| Heart Rate | 605±13 | 606±17 | 615±14 | 665±18 |
| Insulin (ng/ml) | 1.15±0.4 | 0.25±0.04a | 0.27±0.03a | 0.47±0.13a |
| TC (mg/dl) | 431±35 | 759±88a | 611±63 | 442±24b , c |
| HDL-C (mg/dl) | 18±1.0 | 13±2.4 | 10±0.8a | 8±0.6a |
| Non-HDL-C (mg/dl) | 413±35 | 746±86a | 600±63 | 434±24b |
| Triglyceride (mg/dl) | 90±10 | 52±10 | 88±15 | 34±7a , c |
| NEFA (mEq/l) | 1.8±0.2 | 2.1±0.3 | 1.5±0.1 | 1.0±0.1b |
| Total GIP (pM) | 44.1±4.4 | 38.5±3.1 | 176.7±53.3a , b | 150.4±39.1a , b |
| Total GLP-1 (pM) | 4.4±1.4 | 16.1±2.8a | 13.4±1.9a | 15.2±2.9a |
= vs. Nondiabetic,
= vs. Diabetic,
= Diabetic GIP at P<0.001–0.05.
In comparison with the db/misty mice (n = 10), the db/db mice infused with vehicle (n = 11) exhibited marked hyperglycemia (371±5 vs. 110±6 mg/dl, P<0.0001), obesity (46±1.2 vs. 24±0.2 g, P<0.0001), and hyperinsulinemia (0.98±0.26 vs. 0.26±0.06 ng/ml, P<0.0001). In comparison with the vehicle-infused db/db mice, the GIP-infused db/db mice (n = 6) showed a significant increase in the plasma total GIP level (135±64 vs. 6.6±0.7 pM, P<0.05) but no change in the glucose level (374±14 vs. 371±5 mg/dl, P = NS).
Atherosclerotic lesions
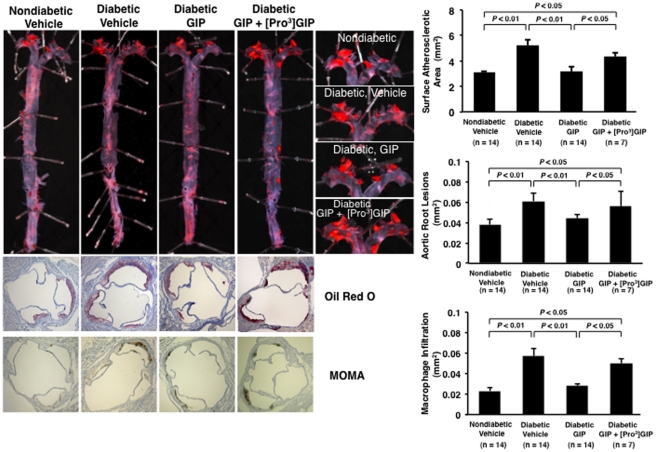
Atherosclerotic lesions were observed in 21-week-old nondiabetic Apoe −/− mice (Fig. 1), and further progressed atherosclerotic lesions were observed in diabetic Apoe −/− mice of the same age. GIP infusion remarkably reduced the surface areas of the atherosclerotic lesions and suppressed the atheromatous plaque size and macrophage infiltration in the aortic root, as compared with vehicle-infused counterparts (Fig. 1). These suppressive effects of GIP were abolished by the simultaneous infusion with [Pro3]GIP (Fig. 1).
Figure 1. Suppressive effects of GIP on the progression of atherosclerotic lesions in diabetic Apoe −/− mice.
Thirty-five mice at 15 weeks of age were made diabetes by peritoneal injection of STZ (50 mg/kg/day) for 5 days and 14 mice were untreated. The 17-week-old diabetic Apoe −/− mice were infused for 4 weeks with vehicle (control), GIP (25 nmol/kg/day), or GIP+[Pro3]GIP (both 25 nmol/kg/day) by osmotic mini-pumps. The aortic surface was stained with oil red O. Cross-sections of the aortic root were stained with oil red O or anti-MOMA-2 antibody. Hematoxylin was used for nuclear staining. The areas occupied by atherosclerotic lesions and by macrophage infiltration in the aortic wall were determined.
The atherosclerotic lesions in the 12-week-old diabetic db/db mice were not remarkably developed than those in the db/misty mice (data not shown). When administered to the db/db mice, GIP failed to induce any conspicuous suppressive effects against the development of atherosclerotic lesions (data not shown).
Foam cell formation in exudate peritoneal macrophages
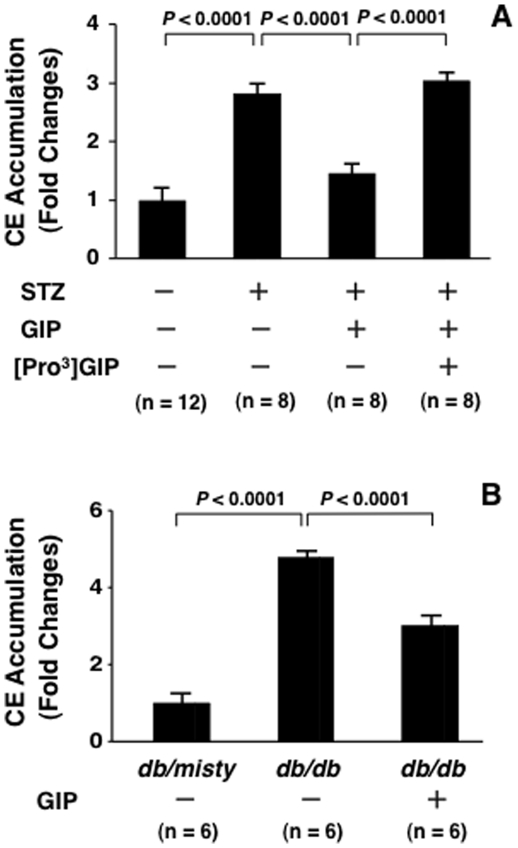
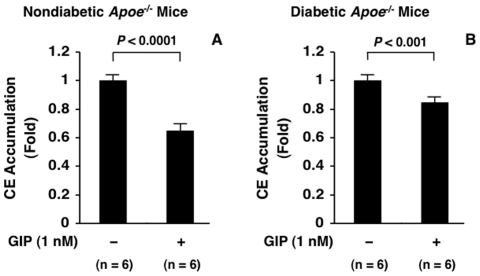
As shown in Fig. 2A, oxLDL-induced cholesterol ester (CE) accumulation in macrophages was 3-fold higher in STZ-induced diabetic Apoe −/− mice than in nondiabetic Apoe −/− mice. Foam cell formation in macrophages from GIP-infused diabetic Apoe −/− mice was suppressed significantly, by as much as one half, in comparison with that in the vehicle-infused counterpart. The GIP-induced suppression of foam cell formation was significantly abolished by the co-infusion with [Pro3]GIP.
Figure 2. Foam cell formation in exudate peritoneal mouse macrophages.
A, 12 Apoe −/− mice at 15 weeks of age were made diabetes by peritoneal injection of STZ (50 mg/kg/day) for 5 days and 6 Apoe −/− mice were untreated. The 17-week-old diabetic Apoe −/− mice were infused for 4 weeks with vehicle, GIP (25 nmol/kg/day), or GIP+[Pro3]GIP (both 25 nmol/kg/day) by osmotic mini-pumps. B, 6 db/misty mice and 6 db/db mice at 8 weeks of age were started to be infused with saline and 6 db/db mice were ifused with GIP (25 nmol/kg/day). Four weeks after infusions, exudate peritoneal macrophages were obtained from these mice by intraperitoneal injection of thioglycolate, and incubated with oxLDL (10 µg/ml) for 18 hours. Foam cell formation was evaluated by oxLDL-induced CE accumulation in macrophages. Assays were performed in duplicate or single. 1 fold = 17.5±1.0 nmol/mg cell protein (A) and 14.7±1.2 nmol/mg cell protein (B).
As shown in Fig. 2B, foam cell formation was 5-fold higher in diabetic db/db mice than in db/misty mice. The GIP infusion, however, attenuated the foam cell formation significantly, by 40% in diabetic db/db mice.
Expression of GIPR in exudate peritoneal macrophages and pancreatic islets
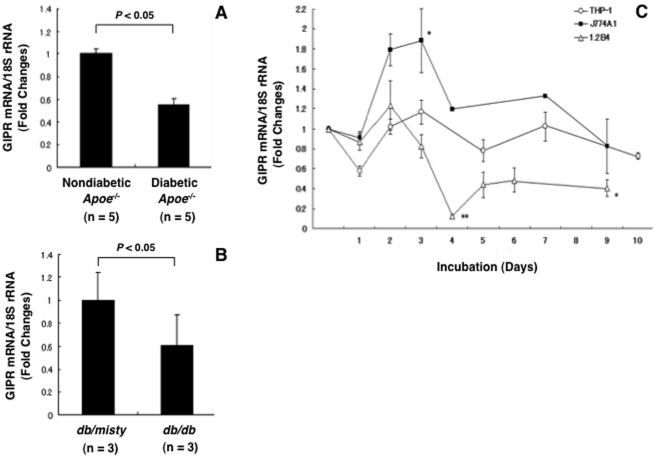
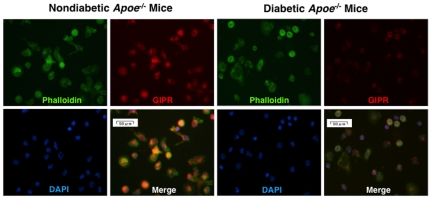
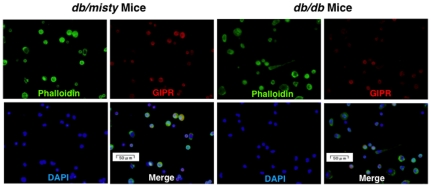
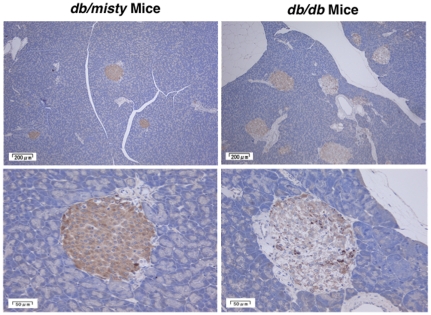
According to real-time RT-PCR, the GIPR mRNA levels in the exudate peritoneal macrophages of diabetic Apoe −/− mice were about half of those in nondiabetic Apoe −/− mice (Fig. 3A). GIPR mRNA levels in the exudate peritoneal macrophages from db/db mice were reduced to the same extent (Fig. 3B). Immunostaining for GIPR confirmed that the GIPR expressions were lower in the peritoneal macrophages of diabetic Apoe −/− mice and db/db mice than in those of counterpart mice (Figs. 4 and 5). Since the pancreatic islets were destroyed in STZ-treated Apoe −/− mice, only the islets of db/db mice were used for GIPR staining. The GIPR staining in the islets was much fainter in the db/db mice than in the db/misty controls (Fig. 6).
Figure 3. Changes in GIPR mRNA levels in macrophages and pancreatic β-cells ex vivo and in vitro.
A, Exudate peritoneal macrophages were obtained from 5 nondiabetic mice and 5 STZ-induced diabetic Apoe −/− mice by intraperitoneal injection of thioglycolate. B, Exudate peritoneal macrophages were obtained from 3 db/misty mice and 3 db/db mice at 12 weeks of age. C, THP-1 macrophages (human), J774A1 macrophages (mouse), and 1.2B4 pancreatic β-cells (human) were incubated with RPMI-1640 containing high glucose (500 mg/dl) for 9–10 days. Changes in GIPR mRNA levels were determined by real-time RT-PCR. Three independent experiments were performed. *P<0.05, **P<0.01 vs. each baseline of day 0.
Figure 4. Expression of GIPR in exudate peritoneal macrophages from nondiabetic and diabetic Apoe −/− mice.
GIPR was stained with goat polyclonal anti-GIPR antibody followed by anti-goat Alexa Fluor 568. Phalloidin/DAPI staining shows F-actin cytoskeleton and nuclear morphology of mouse macrophages. These images were merged. Representative results are shown.
Figure 5. Expression of GIPR in exudate peritoneal macrophages from db/misty and db/db mice.
GIPR was stained with goat polyclonal anti-GIPR antibody followed by anti-goat Alexa Fluor 568. Phalloidin/DAPI staining shows F-actin cytoskeleton and nuclear morphology of mouse macrophages. These images were merged. Representative results are shown.
Figure 6. Expression of GIPR in pancreatic islets from db/misty and db/db mice.
GIPR was stained with goat polyclonal anti-GIPR antibody. Hematoxylin was used for nuclear staining. Representative results are shown.
Changes in GIPR mRNA levels in cultured macrophages and pancreatic islet cells
To determine whether the down-regulation of GIPR in diabetic animals is attributable to hyperglycemia per se, cultured THP-1 (human) and J774A1 (mouse) macrophages were incubated with high-glucose medium for approximately 10 days. Long-term incubation with high-glucose medium conferred no suppressive effect against GIPR mRNA levels in THP-1 macrophages or J774A1 macrophages, but it did significantly reduce GIPR mRNA levels in 1.2B4 pancreatic β-cells (human) 4 days after the start of the high-glucose incubation (Fig. 3C).
In vitro effect of GIP on macrophage foam cell formation
The 24-hour incubation with GIP (1 nM) suppressed foam cell formation significantly, by 15%, in macrophages from diabetic Apoe −/− mice (Fig. 7B). This was weaker than the GIP-induced suppression of foam formation in macrophages from the nondiabetic counterpart mice (35%) (Fig. 7A).
Figure 7. In vitro suppressive effects of GIP on foam cell formation in exudate peritoneal macrophages from nondiabetic and diabetic Apoe −/− mice.
Exudate peritoneal macrophages were obtained from 6 nondiabetic Apoe −/− mice (A) and 6 STZ-induced diabetic Apoe −/− mice (B) by intraperitoneal injection of thioglycolate, and were incubated with or without GIP (1 nM) for 24 hours followed by addition of oxLDL (10 µg/ml) for 18 hours. Foam cell formation was evaluated by oxLDL-induced CE accumulation in macrophages. Assays were performed in duplicate. 1 fold = 15.6±1.5 nmol/mg cell protein (A) and 13.8±1.0 nmol/mg cell protein (B).
Discussion
The atherosclerotic lesions were more severe in diabetic Apoe −/− mice than in nondiabetic Apoe −/− mice. The most prominent changes in the atherosclerotic lesions were an accumulation of macrophage foam cells in atheromatous plaques. High glucose accelerates atherosclerotic lesions and macrophage foam cell formation associated with increased uptake of oxLDL into macrophages via the scavenger receptor CD36 in STZ-induced diabetic Apoe −/− mice [22]. High glucose also down-regulates ATP-binding cassette transporters A1 and G1, key molecules for cholesterol efflux in macrophages, contributing to foam cell formation [23].
Consistent with our experience in an earlier study with nondiabetic Apoe −/− mice [9], our current result confirmed that chronic GIP infusion suppresses the progression of atherosclerosis in diabetic Apoe −/− mice. We know that GIPR mediated this anti-atherogenic effect, because co-infusion of GIPR antagonist abolished the GIP-induced suppression of atherosclerosis. GIP has a potent stimulatory effect on insulin release from the pancreas under conditions of normal glucose tolerance. Its insulinotropic action, however, is reduced or even entirely absent in a diabetic state [11], [12]. While the exact mechanisms behind the loss of GIP's insulinotropic action in diabetes remains obscure, several lines of experimental evidence suggest that GIPR is substantially down-regulated in pancreatic β-cells under a persistent hyperglycemic condition [13]–[17]. If GIPR is severely down-regulated in extrapancreatic cells in a fashion similar to pancreatic β-cells, the direct anti-atherogenic action of GIP could be largely diminished or lost. We thus anticipated that the down-regulation of GIPR may blunt the anti-atherogenic effect of GIP in diabetic animals.
GIPR gene expression in monocyte-derived macrophages from diabetic Apoe −/− mice and db/db mice, a spontaneous diabetic animal, was about half of that in nondiabetic counterparts. The in vitro suppressive effect of GIP on foam cell formation was blunted in macrophages from diabetic mice vs. nondiabetic mice (15% vs. 35%), probably because of the reduced GIPR in the macrophages in diabetes. As such, we were confident in surmising that the suppressive effect of GIP against atherosclerosis weakens in a diabetic condition. Yet contrary to our expectation, chronic administration of GIP in vivo conferred a satisfactorily anti-atherogenic effect in the diabetic Apoe −/− mice. The total plasma GIP concentration following subcutaneous infusion of human active GIP at a dose 25 nmol/kg/day was 130–170 pM. This was 4-times higher than the concentration in vehicle-infused mice, but not a super-physiological concentration [24]. These results suggest that GIP infusion at a level several-fold higher is sufficient to confer an anti-atherogenic effect via residual GIPR that eluded the diabetes-induced down-regulation. Contrary to our finding in pancreatic β-cells, high glucose left GIPR gene expression unaffected in cultured macrophages in vitro. We can thus infer that the down-regulation of GIPR in macrophages requires a long time, or that the suppression of GIPR expression is association with other metabolic alterations induced by diabetes. Gupta et al. [17], for instance, reported that peroxisome proliferator-activated receptor-γ (PPAR-γ) was reduced in the β-cells of remnant pancreas in partial pancreatectomized-diabetic rats, and that this suppression of PPAR-γ was closely associated with the down-regulation of GIPR. Plasma total GLP-1 levels were increased 3- to 4-fold in every diabetic group. Hyperphagia in STZ-induced diabetic animals may stimulate GLP-1 production in the intestines.
Glucose levels fell in our vehicle-infused diabetic mice, whereas GIP infusion worsened the hyperglycemia in diabetes. Intriguingly, co-infusion with [Pro3]GIP nullified GIP's pro-hyperglycemic effect. GIP infusion might exacerbate insulin resistance in the diabetic mice by increasing adiposity [25]. In contrast to our finding in STZ-diabetic mice, GIP infusion left glucose levels unchanged in spontaneous diabetic db/db mice. This suggests that GIP may modulate the processes of STZ-induced diabetes. The GIP infusion worsened the hyperglycemia in Apoe −/− mice, but atherosclerosis in these animals was milder and less developed. Meanwhile, GIPR antagonist abolished the anti-atherogenic effects of GIP in spite of its effect in reducing glucose levels. These results strongly suggest that the anti-atherogenic effects of GIP derive from mechanisms with no direct connection to the amelioration of glucose metabolism. TC and non-HDL-C levels were both higher in diabetic Apoe −/− mice than in nondiabetic Apoe −/− mice, and the HDL-C level was lower. These lipoprotein changes may accelerate atherosclerosis in diabetic mice. Plasma cholesterol levels were unaffected by GIP infusion, but they decreased when GIP+[Pro3]GIP was co-infused. It remains unclear why the co-infusion with [Pro3]GIP lowered the plasma cholesterol, but the anti-atherogenic property of GIP is probably unrelated to the improved atherogenic lipoprotein profile.
Unlike STZ-induced diabetic Apoe −/− mice, diabetic db/db mice do not develop visible atherosclerosis in the aorta. Thus, we investigated foam cell formation in exudate peritoneal macrophage as a surrogate marker for the atherosclerosis process in this animal model of diabetes. Similar to what we found in STZ-induced diabetic Apoe −/− mice, foam cell formation was substantially stimulated in db/db diabetic mice, and the GIP infusion significantly attenuated foam cell formation without affecting glucose levels. GIPR in pancreatic islets was remarkably diminished in db/db diabetic mice, whereas that in peritoneal macrophages was only marginally reduced. This down-regulation of GIPR in macrophages may have been limited enough to permit the suppression of foam cell formation by the several-fold increase of circulating GIP levels.
Conclusions
Chronic administration of GIP remarkably suppressed the progression of atherosclerosis in STZ-induced diabetic Apoe −/− mice and suppressed macrophage foam cell formation in both diabetic Apoe −/− mice and diabetic db/db mice, even though GIPR expression in macrophages was mildly down-regulated in the diabetic state.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The authors have no funding or support to report.
References
- 1.Kim W, Egan JM. The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol Rev. 2008;60:470–512. doi: 10.1124/pr.108.000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;23:153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Ban K, Hui S, Drucker DJ, Husain M. Cardiovascular consequences of drugs used for the treatment of diabetes: potential promise of incretin-based therapies. J Am Soc Hypertens. 2009;3:245–259. doi: 10.1016/j.jash.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Arakawa M, Mita T, Azuma K, Ebato C, Goto H, et al. Inhibition of monocyte adhesion to endothelial cells and attenuation of atherosclerotic lesion by a glucagon-like peptide-1 receptor agonist, exendin-4. Diabetes. 2010;59:1030–1037. doi: 10.2337/db09-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goto H, Nomiyama T, Mita T, Yasunari E, Azuma K, et al. Exendin-4, a glucagon-like peptide-1 receptor agonist, reduces intimal thickening after vascular injury. Biochem Biophys Res Commun. 2011;405:79–84. doi: 10.1016/j.bbrc.2010.12.131. [DOI] [PubMed] [Google Scholar]
- 6.Matsui T, Nishino Y, Takeuchi M, Yamagishi S. Vildagliptin blocks vascular injury in the thoracic aorta of diabetic rats by suppressing advanced glycation end product-receptor axis. Pharmacol Res. 2011;63:383–388. doi: 10.1016/j.phrs.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Shah Z, Kampfrath T, Deiuliis JA, Zhong J, Pineda C, et al. Long-term dipeptidyl-peptidase 4 inhibition reduces atherosclerosis and inflammation via effects on monocyte recruitment and chemotaxis. Circulation. 2011;124:2338–2349. doi: 10.1161/CIRCULATIONAHA.111.041418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsubara J, Sugiyama S, Sugamura K, Nakamura T, Fujiwara Y, et al. A dipeptidyl peptidase-4 inhibitor, des-fluoro-sitagliptin, improves endothelial function and reduces atherosclerotic lesion formation in apolipoprotein E-deficient mice. J Am Coll Cardiol. 2012;59:265–276. doi: 10.1016/j.jacc.2011.07.053. [DOI] [PubMed] [Google Scholar]
- 9.Nagashima M, Watanabe T, Terasaki M, Tomoyasu M, Nohtomi K, et al. Native incretins prevent the development of atherosclerotic lesions in apolipoprotein E knockout mice. Diabetologia. 2011;54:2649–2659. doi: 10.1007/s00125-011-2241-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Terasaki M, Nagashima M, Watanabe T, Nohtomi K, Mori Y, et al. Effects of PKF275-055, a dipeptidyl peptidase-4 inhibitor, on the development of atherosclerotic lesions in apolipoprotein E-null mice. Metabolism. 2012 doi: 10.1016/j.metabol.2011.11.011. in press. [DOI] [PubMed] [Google Scholar]
- 11.Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, et al. Preserved incretin activity of glucagon-like peptide 1 [7–36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest. 1993;91:301–307. doi: 10.1172/JCI116186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elahi D, McAloon-Dyke M, Fukagawa NK, Meneilly GS, Sclater AL, et al. The insulinotropic actions of glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (7–37) in normal and diabetic subjects. Regul Pept. 1994;51:63–74. doi: 10.1016/0167-0115(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 13.Lynn FC, Pamir N, Ng EH, McIntosh CH, Kieffer TJ, et al. Defective glucose-dependent insulinotropic polypeptide receptor expression in diabetic fatty Zucker rats. Diabetes. 2001;50:1004–1011. doi: 10.2337/diabetes.50.5.1004. [DOI] [PubMed] [Google Scholar]
- 14.Lynn FC, Thompson SA, Pospisilik JA, Ehses JA, Hinke SA, et al. A novel pathway for regulation of glucose-dependent insulinotropic polypeptide (GIP) receptor expression in β cells. FASEB J. 2003;17:91–93. doi: 10.1096/fj.02-0243fje. [DOI] [PubMed] [Google Scholar]
- 15.Zhou J, Livak MF, Bernier M, Muller DC, Carlson OD, et al. Ubiquitination is involved in glucose-mediated downregulation of GIP receptors in islets. Am J Physiol Endocrinol Metab. 2007;293:E538–E547. doi: 10.1152/ajpendo.00070.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu G, Kaneto H, Laybutt DR, Duvivier-Kali VF, Trivedi N, et al. Downregulation of GLP-1 and GIP receptor expression by hyperglycemia: possible contribution to impaired incretin effects in diabetes. Diabetes. 2007;56:1551–1558. doi: 10.2337/db06-1033. [DOI] [PubMed] [Google Scholar]
- 17.Gupta D, Peshavaria M, Monga N, Jetton TL, Leahy JL. Physiologic and pharmacologic modulation of glucose-dependent insulinotropic polypeptide (GIP) receptor expression in β-cells by peroxisome proliferator-activated receptor (PPAR)-γ signaling: possible mechanism for the GIP resistance in type 2 diabetes. Diabetes. 2010;59:1445–1450. doi: 10.2337/db09-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takeda Y, Fujita Y, Honjo J, Yanagimachi T, Sakagami H, et al. Reduction of both beta cell death and alpha cell proliferation by dipeptidyl peptidase-4 inhibition in a streptozotocin-induced model of diabetes in mice. Diabetologia. 2012;55:404–412. doi: 10.1007/s00125-011-2365-4. [DOI] [PubMed] [Google Scholar]
- 19.Shiraishi Y, Watanabe T, Suguro T, Nagashima M, Kato R, et al. Chronic urotensin II infusion enhances macrophage foam cell formation and atherosclerosis in apolipoprotein E-knockout mice. J Hypertens. 2008;26:1955–1965. doi: 10.1097/HJH.0b013e32830b61d8. [DOI] [PubMed] [Google Scholar]
- 20.Nagashima M, Watanabe T, Shichiri M, Morita R, Terasaki M, et al. Chronic infusion of salusin-α and -βexerts opposite effects on macrophage foam cell formation and atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis. 2010;212:70–77. doi: 10.1016/j.atherosclerosis.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 21.Xu G, Watanabe T, Iso Y, Koba S, Sakai T, et al. Preventive effects of heregulin-β1 on macrophage foam cell formation and atherosclerosis. Circ Res. 2009;105:500–510. doi: 10.1161/CIRCRESAHA.109.193870. [DOI] [PubMed] [Google Scholar]
- 22.Hayek T, Hussein K, Aviram M, Coleman R, Keidar S, et al. Macrophage foam-cell formation in streptozotocin-induced diabetic mice: stimulatory effect of glucose. Atherosclerosis. 2005;183:25–33. doi: 10.1016/j.atherosclerosis.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 23.Mauerer R, Ebert S, Langmann T. High glucose, unsaturated and saturated fatty acids differentially regulate expression of ATP-binding cassette transporters ABCA1 and ABCG1 in human macrophages. Exp Mol Med. 2009;41:126–132. doi: 10.3858/emm.2009.41.2.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee S, Yabe D, Nohtomi K, Takada M, Morita R, et al. Intact glucagon-like peptide-1 levels are not decreased in Japanese patients with type 2 diabetes. Endocr J. 2010;57:119–126. doi: 10.1507/endocrj.k09e-269. [DOI] [PubMed] [Google Scholar]
- 25.Zhou H, Yamada Y, Tsukiyama K, Miyawaki K, Hosokawa M, et al. Gastric inhibitory polypeptide modulates adiposity and fat oxidation under diminished insulin action. Biochem Biophys Res Commun. 2005;335:937–942. doi: 10.1016/j.bbrc.2005.07.164. [DOI] [PubMed] [Google Scholar]