Abstract
Background
The Hospital Acquired Condition Strategy (HACS) denies payment for venous thromboembolism (VTE) after total knee arthroplasty (TKA). The intention is to reduce complications and associated costs, while improving the quality of care by mandating VTE prophylaxis. We applied a system dynamics model to estimate the impact of HACS on VTE rates, and potential unintended consequences such as increased rates of bleeding and infection and decreased access for patients who might benefit from TKA.
Methods and Findings
The system dynamics model uses a series of patient stocks including the number needing TKA, deemed ineligible, receiving TKA, and harmed due to surgical complication. The flow of patients between stocks is determined by a series of causal elements such as rates of exclusion, surgery and complications. The number of patients harmed due to VTE, bleeding or exclusion were modeled by year by comparing patient stocks that results in scenarios with and without HACS. The percentage of TKA patients experiencing VTE decreased approximately 3-fold with HACS. This decrease in VTE was offset by an increased rate of bleeding and infection. Moreover, results from the model suggest HACS could exclude 1.5% or half a million patients who might benefit from knee replacement through 2020.
Conclusion
System dynamics modeling indicates HACS will have the intended consequence of reducing VTE rates. However, an unintended consequence of the policy might be increased potential harm resulting from over administration of prophylaxis, as well as exclusion of a large population of patients who might benefit from TKA.
Introduction
Recent public debate on health care reform has renewed focus on patient safety and cost containment [1]. A value-based health care strategy strives to ‘bend the healthcare cost curve’ by publically reporting rates of post-operative complications and adherence to quality of care and patient safety measures [2]. Value-based systems assume institutions will minimize complication rates thereby costs will be reduced while improving the quality of care. Value-based mandates, however, may have unintended consequences on overall complications, unanticipated costs, or reduced access for individuals who may benefit from a treatment.
As a part of the Deficit Reduction Act of 2005, the Secretary of Health and Human Services (USA) identified high cost and high volume procedures where preventable hospital acquired conditions were reimbursed at higher rates [3]. For example, total knee arthroplasty (TKA) patients who develop venous thromboembolism (VTE), a term used to collectively describe deep vein thrombosis (DVT) and pulmonary embolism (PE), accrue higher costs than those who do not experience this condition. Evidence-based guidelines suggest administering prophylaxis medications (anti-clotting blood thinners) reduces VTE rates [4], [5], [6]. To encourage adherence to these guidelines, beginning on October 1, 2008, the “hospital acquired condition” strategy (HACS) no longer reimburses inpatient costs after a newly diagnosed VTE if recommended prophylaxis was not administered prior to TKA procedures [7].
Every year in the U.S., over 185,000 VTE cases are diagnosed in patients aged 45 years or older [8]. More than 35% occur after surgical events [9], frequently leading to long-term disability or death [10], [11]. Certain clinical conditions, such as malignant neoplasm, trauma, congestive heart failure, obesity, multiparity, advancing age and hypercoagulability increase VTE risk [12], [13], [14]. Despite evidence suggesting its efficacy to reduce complication risk, 41 to 84% of the patients do not receive recommended VTE prophylaxis [15], [16]. Patients receiving TKA have increased risk of VTE, and recommended prophylaxis reduces this risk [17]. Therefore, a policy that encourages anti-thrombotic prophylaxis seems reasonable.
System Dynamics [18] has been used to model the consequences of national smoking cessation initiatives [19], smallpox vaccination [20], and prevention vs. management of chronic disease, [21]. Changes in health care policy can affect access, reimbursement and clinical outcomes. Complex interrelationships have multiple clinical ramifications on patients, payors, providers, and hospital administrators. System Dynamics uses stock and flow diagrams, feedback loops and various ‘what if scenarios’ to portray the casual relationships among these elements and predict long term implications of these complex interactions [22]. For example, improving adherence to prophylaxis guidelines may decrease VTE rates, but this may also increase the rates of bleeding which in turn may increase infection rates. Mandating VTE prophylaxis may reduce access to TKA for patients who have increased risk of bleeding.
This study applies system dynamics to estimate the impact of HACS on rates of VTE and other surgical complications (such as bleeding and infection). We hypothesize that the increased rates of VTE prophylaxis will actually increase rates of bleeding and infection [23]. Additionally, patient subgroups who might benefit from a knee replacement but have increased risk of bleeding will accumulate.
Methods
Modeling Context
The Hospital Acquired Condition Strategy (HACS) enacted by federal legislation applied to Medicare reimbursement for VTE [7]. The majority of patients eligible for Medicare are older than age 65, and VTE risk varies considerably by age [24], so patients younger than age 65 were excluded when developing parameters for the model. Typical Medicare reimbursement to hospitals is $10,000, $13,000 for patients with a major comorbidity or those who experience a complication [25]. The rationale for implementing the HACS were the low rates of guideline-recommended proplylaxis [26]. Some argue is neither preventable nor accurately measurable [27]. Nearly half of patients with a DVT detectable by ultrasound do not experience clinical symptoms [28]. Patients with DVT risk factors will more likely receive prophylaxis and can be excluded from HACS policy by indicating “presence on admission can not be determined” [29]. On the other hand, clinicians may be hesitant to administer DVT prophylaxis to patients with increased risk of bleeding so these patients might be affected by the policy change.
Literature Review
Citations from the Federal Register “hospital acquired condition” policy [7] and the Surgeon General’s Call to Action to prevent DVT and PE [30] were used to define an initial set of risk factors and rates of DVT, PE, and bleeding in various populations as well as potential risk factors. Additional studies were identified through PubMed database by taking the intersection of each risk factor and the terms venous thrombosis, DVT, PE, or DVT prophylaxis.
Expert Interview
Certain model inputs such as willingness to treat patients with increased risk of DVT or bleeding, rates of surgical site infection in patients with excessive bleeding, and those harmed by DVT and bleeding were not clearly described in the literature. Three arthroplasty surgeons at the University of California, San Francisco (UCSF) were queried to obtain a range for these values through mutual agreement. (Refer Information S1 -Questions asked to expert panel) All three surgeons are board certified knee specialists performing a large volume of total knee replacement procedures in a University hospital setting.
Model Parameters
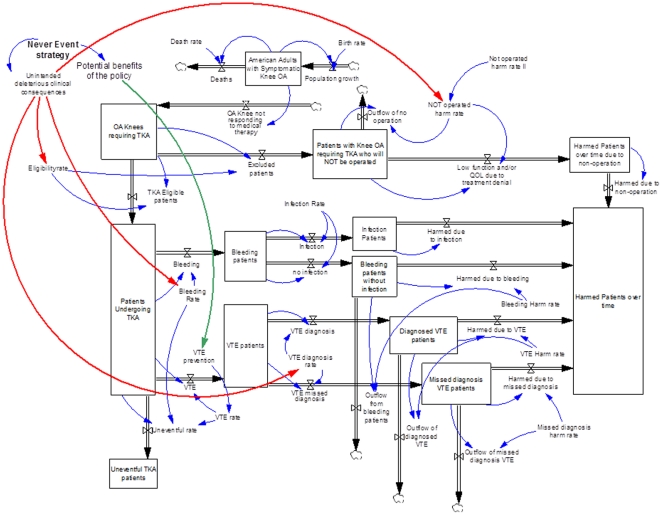
The parameters included in the model are either stocks, represented as boxes in Figure 1 or causal elements represented at the ends of arrows. Stocks, accumulate over time with the quantity regulated by influx and efflux rates. For example, the stock of patients who can benefit with TKA increase as the number of patients with osteoarthritis in the overall population increases. Meanwhile, patients deemed ineligible and those who receive TKA decrease this stock. The causal elements affect results derived from the model but do not accumulate over time. An example of a causal element is the percentage of patients receiving TKA or fraction of patients with postoperative bleeding who later developed a surgical site infection.
Figure 1. An illustration of the stocks, causal elements, relationships and impact of HACS in the system dynamics model.
Model Relationships
The movement of patients through the system is regulated by the relationships between the stocks and causal elements. A flow is represented by a thick, double-lined arrow emerging out of or entering into a stock. Causal links illustrating the effect of the causal variables are represented by thin arrows. Blue arrows represent causal effects that may increase or decrease flows, red and green arrows indicate the impact of HACS.
Policy Intervention
We compare two simulation scenarios, with and without HACS. The HACS scenario represents the potential result of withholding reimbursement for hospital care associated with the treatment of VTE when VTE prophylaxis is not administered prior to TKA. VTE rates are those indicated when Enoxaparin, the prophylaxis recommened by both the American College of Chest Physicians (ACCP) [5] and the American Academy of Orthopaedic Surgeons (AAOS) [6], is or is not administered. We assume VTE rates reported in clinical trials would be achieved with increased adherence to VTE prophylaxis guidelines.
Dynamics Hypothesis
The ideal scenario as envisioned by those who implemented HACS policy is a hypothesis that rates of VTE will decrease over time, while rates of surgical complications and disparity remain stable.
The Systems Dynamics Model
Vensim software was used to design and simulate the System Dynamics model [18]. The first parameter illustrated in Figure 1 is the stock representing the prevalence (9.7 million) of patients over age 65 suffering with symptomatic knee osteoarthritis (OA) [24]. As the model cycles from 2008 through 2020, the number of patients in this stock is dependent on birth and death rates. The number of patients requiring TKA are those with OA who do not respond to non-operative therapy. These patients could either undergo TKA or be excluded, depending on eligibility requirements. The stock entitled “Patients with Knee OA requiring TKA who will NOT be operated” represents patients with clinical conditions (such as malignant neoplasm, morbid obesity, immobile standing position, hypercoagulable state, etc) who are not eligible for TKA. Patients undergoing TKA could have an uneventful course, a VTE, or a bleeding complication. Patients who experience excess bleeding may develop a surgical site infection resulting from hematoma, evacuation or prolonged wound drainage [31], [32]. Of those experiencing VTE, a fraction will die due to fatal PE, some have to undergo at least 6 months of oral anticoagulation, and those who will not be diagnosed with VTE may experience chronic venous insufficiency requiring long term mechanical compression therapy. We modeled four complication scenarios (bleeding, infection, diagnosed and undiagnosed VTE), with fractions of harmed patients.
The model examined hypothetical simulations over a period of 12 years from 2008 continued through 2020. The stocks examined were the number of patients experiencing VTE, bleeding, infection, or were deemed ineligible for surgery individually, as well as the cumulative number of harmed patients per year. Inputs for the model are indicated in Table 1. The parameters assume a 1.5% decrease (from 14.4–12.9%) in TKA eligibility among patients with OA with HACS. Higher rates of bleeding (9.6% vs. 1.4%) and lower rates of VTE (2 vs. 5%) due to increased administrating VTE prophylaxis with HACS. We also speculate that a fraction of VTE diagnoses (25 vs. 15%) will be missed because of a disincentive with HACS to identify the condition, and that 10% of patients with a missed diagnosis will be harmed. The range of values for each parameter that were modeled in the sensitivity analyses are also indicated in Table 1.
Table 1. Model parameters, minimum and maximum range, and reference sources.
| Baseline (%) | Sensitivity Analysis (minimum - maximum value) | Reference | |||
| Without HACS | With HACS | Without HACS | With HACS | ||
| Eligibility rate | 14.4% | 12.9% | 13.4–15.4% | 11.9–13.9% | [49], [59], Expert panel |
| Bleeding rate | 1.4% | 9.6% | 0.4–2.4% | 7.6–10.6% | [60] |
| VTE rate | 5% | 2% | 3–7% | 0.5–4% | [21, 61, 62] |
| VTE diagnosis rate | 85% | 75% | 75–90% | 65–85% | Expert panel |
| Infection rate | 10% | 10% | 5–20% | 5–20% | [32], [33], [38] Expert panel, |
| Bleeding harm rate | 58% | 58% | 46–70% | 46–70% | [62] |
| VTE harm rate | 75% | 75% | 65–85% | 65–85% | [63], Expert panel |
| Missed diagnosis harm rate | 10% | 10% | 5–20% | 5–20% | [64], Expert panel |
Results
Model stock outputs indicate HACS results in a 3-fold decrease in VTE rates (Table 2). However, the fraction of HACS with bleeding complications associated is 6-fold higher, and 6-fold more patients are potentially ineligible for TKA per year with HACS in place.
Table 2. Model outputs indicating the number of harmed patients per year.
| Time (Year) | 2008 | 2011 | 2014 | 2017 | 2020 |
| VTE without HACS | 19,500 | 20,560 | 21,279 | 21,679 | 22,040 |
| VTE with HACS | 19,500 | 8,050 | 7,661 | 7,770 | 7,898 |
| Diagnosed VTE without HACS | 16,575 | 17,151 | 17,913 | 18,316 | 18,631 |
| Diagnosed VTE with HACS | 16,575 | 7,428 | 5,834 | 5,803 | 5,892 |
| Missed VTE without HACS | 2,925 | 3,027 | 3,161 | 3,232 | 3,288 |
| Missed VTE with HACS | 2,925 | 2,359 | 1,939 | 1,934 | 1,964 |
| Bleeding patients without HACS | 5,460 | 5,757 | 5,958 | 6,070 | 6,171 |
| Bleeding patients with HACS | 5,460 | 34,659 | 36,593 | 37,288 | 37,909 |
| Bleeding without infection without HACS | 4,914 | 5,085 | 5,311 | 5,430 | 5,524 |
| Bleeding without infectionwith HACS | 4,914 | 27,238 | 32,387 | 33,347 | 33,931 |
| Infection without HACS | 546 | 565 | 590 | 603 | 614 |
| Infection with HACS | 546 | 3,026 | 3,602 | 4,705 | 3,770 |
| Ineligible patients without HACS | 0 | 0 | 0 | 0 | 0 |
| Ineligible patients with HACS | 0 | 35,751 | 38,543 | 39,335 | 39,993 |
| Total harmed without HACS | 0 | 15,698 | 17,175 | 17,689 | 18,018 |
| Total harmed with HACS | 0 | 51,387 | 64,038 | 66,454 | 67,680 |
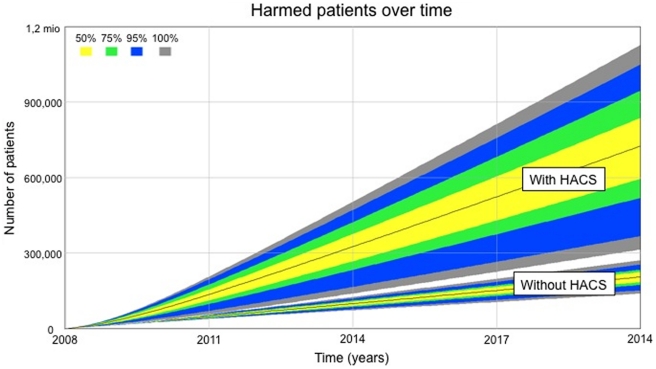
The cumulative potential harm over time due to VTE, bleeding, infection and denied access that could be attributed to HACS is illustrated in Figure 2. The model indicates the fraction harmed with HACS will be 2.8 times higher in 2011, 3.3 times in 2014, 3.4 times in 2017 and 3.5 times in 2020. The increase in the total number of adults harmed by the HACS reaches half a million people by year 2020. Sensitivity analysis indicates this increase might be as small as 43,000 and as high as 980,000.
Figure 2. The fraction of patients harmed by HACS over time including sensitivity analysis adjustments.
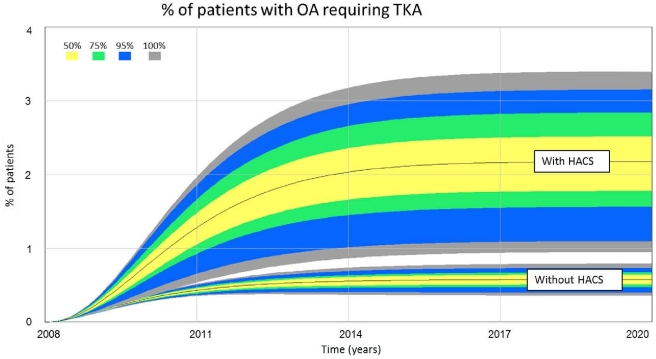
The fraction of patients affected by policy stabilizes as the model reaches equilibrium (Figure 3). HACS increased the percentage of patients suffering from OA pain who could benefit from, but do not receive TKA by 1.6% (2.2% with HACS, 0.6% without). Sensitivity analysis indicates the percentage of patient denied TKA with HACS may be as high as 3.4% and as low as 0.95%, and 0.79–0.36% without.
Figure 3. The fraction % of patients with osteoarthritis who might benefit from, but not receive TKA.
Discussion
Several interventions [33], including physician alerts [34], decision support informatics [35], and regular audits [36] are shown to increase the rates of VTE prophylaxis. Despite these efforts, only 60% of TKA patients receive enoxaparin as recommended by ACCP guidelines [16]. The monetary incentive mandated by HACS, not reimbursing costs resulting from VTE when VTE prophylaxis was not administered, was effective for decreasing VTE rates, but our model suggests HACS will result in an overall 6-fold increase in complication rates. While others have suggested the possibility of unintended consequences [37], this study indicates half a million people might be harmed by HACS by the year 2020. Furthermore, the fraction of Americans who could benefit, but are denied for TKA is increased 1.6% with HACS because of their risk of developing a VTE complication which would place the providers and hospitals at financial risk for the episode of care.
Mandating VTE prophylaxis increases the risk of prolonged wound drainage, extended hospital stay, and surgical site infection [31], [32], [38]. Surgical site infection measureably reduces health-related quality of life [39]. We did not attempt to estimate whether savings to Medicare by refusing to reimburse care for VTE complications with HACS is offset by the cost of prophylaxis, extended hospitalization and readmission resulting from bleeding and infection complications. Other potential model parameters were not studied as well. The efficacy of recommended prophylaxis to reduce the risk of death due to a PE when compared to aspirin in TKA patients remains controversial [6]. Other prophylaxis regimens may result in a different impact of HACS. Bleeding rates for this study were based on published clinical experience with low molecular weight heparin.
A policy that penalizes the occurrence of adverse outcomes will likely decrease access to at-risk patients. The potential for inequity may be greater than estimated in this model. Kahneman’s Prospect Theory suggests aversion of loss is psychologically twice as powerful as the potential for gain [40]. The desire to avoid HACS consequences could result in overly aggressive VTE prophylaxis, under reporting of VTE, and exclusion of patients who could benefit from TKA. Our model estimates the policy will exclude over 35,000 patients/year. Access to care is driven by perceptions of both the surgeon and patient. Only a third of surveyed patients with painful osteoarthritis were willing to consider TKA as a treatment option [41]. TKA significantly improves the quality of life of patients with osteoarthritis [42], [43], [44]. Typical patients experience a gain of more than one quality adjusted life year (QALY from 6.8 to 8.0 with TKA) [45]. Elderly patients with comorbidity and those living in poverty might be comparable to those who are excluded by HACS. These patients experienced a similar QALY gain, (0.8, 5.8 to 6.6 with TKA) [45].
Either a “carrot” or “stick” approach can be used to provide monetary incentives to improve adherence to recommended care guidelines. “Pay for performance” (P4P) rewards increased adherence to quality metrics [46] while “hospital acquired condition” penalizes undesirable outcomes. The amount of increased guideline adherence varies across studies [47], , and these programs may either exacerbate [50], [51], [52] or reduce racial disparity [46], [49]. Underserved patients may experience significant out-of-pocket costs so they may delay seeking of care, both for the OA leading to TKA, but also for post-surgical monitoring of emerging complications. An alternative to the current approaches for rewarding guideline-based care might be to reward those who provide high quality and equitable access to underserved patients [48], [53].
Although our study quantifies the relative impact of intended and unintended consequences of the HACS policy, the model has some limitations. First, model inputs were based on assumptions drawn from publications. In cases where the data could not be directly extracted from the literature and only approximations were available, expert opinions from three surgeons in an academic healthcare setting were obtained. Surgeons in other settings may have opinions that differ, resulting in a greater or lesser likelihood to treat patients with risk factors. However, the dynamic nature of the model allows changing model parameters whenever desired. Second, the relationship between age and complication rates, or the effectiveness of VTE prophylaxis by risk profile is not well documented in the literature. Consequences of aggressive prophylaxis and the tendency to deny surgery to subgroups of underserved patients were disregarded and would need special attention. Third, we did not stratify complications by severity. So potentially lethal and less harmful complications were included in the same stock.
Our objective was to provide a range of potential outcomes resulting from the Hospital Acquired Condition Strategy. A logical extension could determine how HACS differently impacts various at-risk populations. While it seems logical to propose process variables such as VTE prophylaxis administration for measuring quality of care, it is also clear that VTE is a problematic outcome because it can occur even with proper prophylaxis [54], [55], [56], [57]. Enforcing policies to prevent VTE can decrease access to care and pose a theoretical risk of increasing overall complication rates.
Supporting Information
Questions asked to expert panel.
(DOC)
Acknowledgments
Team “Research on Research” for 1. templates for writing introduction and discussion sections of the manuscript [58] and 2. templates for literature review [59].
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The authors have no funding or support to report.
References
- 1.Kohn LT, Corrigan J, Donaldson MS. To err is human : building a safer health system. Washington, D.C.: National Academy Press. 2000;xxi:287. [PubMed] [Google Scholar]
- 2.Porter ME, Teisberg EO. 2006 (2006) Redefining Health Care: Creating Value-Based Competition on Results: Harvard Business Press %@ 1591397782 %7 1. [Google Scholar]
- 3.Centers for Medicare & Medicaid Services; 2010. Hospital-Acquired Conditions (Present on Admission Indicator). [Google Scholar]
- 4.Kakkar AK, Cohen AT, Tapson VF, Bergmann J-F, Goldhaber SZ, et al. 2010. Venous thromboembolism risk and prophylaxis in the acute care hospital setting (ENDORSE survey): findings in surgical patients. Annals of Surgery 251: 330–338 %U http://www.ncbi.nlm.nih.gov/pubmed/20054273.
- 5.Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, et al. 2008. Prevention of Venous Thromboembolism*. Chest 133: 381S–453S %U http://chestjournal.chestpubs.org/content/133/386_suppl/381S.abstract. [DOI] [PubMed]
- 6.Johanson NA, Lachiewicz PF, Lieberman JR, Lotke PA, Parvizi J, et al. 2009. Prevention of symptomatic pulmonary embolism in patients undergoing total hip or knee arthroplasty. The Journal of the American Academy of Orthopaedic Surgeons 17: 183–196 %U http://www.ncbi.nlm.nih.gov/pubmed/19264711. [DOI] [PubMed]
- 7.Medicare program: changes to the hospital inpatient prospective payment systems and fiscal year 2009 rates; payments for graduate medical education in certain emergency situations; changes to disclosure of physician ownership in hospitals and physician self-referral rules; updates to the long-term care prospective payment system; updates to certain IPPS-excluded hospitals; and collection of information regarding financial relationships between hospitals. Final rules. : Fed Regist. 2008;73(161)) [PubMed] [Google Scholar]
- 8.Cushman M, Tsai AW, White RH, Heckbert SR, Rosamond WD, et al. 2004. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. The American Journal of Medicine 117: 19–25 %U http://www.ncbi.nlm.nih.gov/pubmed/15210384. [DOI] [PubMed]
- 9.Goldhaber SZ, Tapson VF. 2004. (2004) A prospective registry of 5,451 patients with ultrasound-confirmed deep vein thrombosis. The American Journal of Cardiology 93: 259–262 %U http://www.ncbi.nlm.nih.gov/pubmed/14715365.
- 10.Heit JA. 2006. (2006) The epidemiology of venous thromboembolism in the community: implications for prevention and management. Journal of Thrombosis and Thrombolysis 21: 23–29 %U http://www.ncbi.nlm.nih.gov/pubmed/16475038.
- 11.Sørensen HT, Horvath-Puho E, Pedersen L, Baron JA, Prandoni P. 2007. (2007) Venous thromboembolism and subsequent hospitalisation due to acute arterial cardiovascular events: a 20-year cohort study. Lancet 370: 1773–1779 %U http://www.ncbi.nlm.nih.gov/pubmed/18037081.
- 12.White RH, Gettner S, Newman JM, Trauner KB, Romano PS. 2000. (2002) Predictors of rehospitalization for symptomatic venous thromboembolism after total hip arthroplasty. The New England Journal of Medicine 343: 1758–1764 %U http://www.ncbi.nlm.nih.gov/pubmed/11114314. [DOI] [PubMed]
- 13.Heit JA, O’Fallon WM, Petterson TM, Lohse CM, Silverstein MD, et al. 2002. Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population-based study. Archives of Internal Medicine 162: 1245–1248 %U http://www.ncbi.nlm.nih.gov/pubmed/12038942.
- 14.Rogers SO, Kilaru RK, Hosokawa P, Henderson WG, Zinner MJ, et al. 2007. Multivariable predictors of postoperative venous thromboembolic events after general and vascular surgery: results from the patient safety in surgery study. Journal of the American College of Surgeons 204: 1211–1221 %U http://www.ncbi.nlm.nih.gov/pubmed/17544079. [DOI] [PubMed]
- 15.Kahn SR, Panju A, Geerts W, Pineo GF, Desjardins L, et al. 2007. Multicenter evaluation of the use of venous thromboembolism prophylaxis in acutely ill medical patients in Canada. Thrombosis Research 119: 145–155 %U http://www.ncbi.nlm.nih.gov/pubmed/16516275. [DOI] [PubMed]
- 16.Cohen AT, Tapson VF, Bergmann J-F, Goldhaber SZ, Kakkar AK, et al. 2008. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross-sectional study. Lancet 371: 387–394 %U http://www.ncbi.nlm.nih.gov/pubmed/18242412. [DOI] [PubMed]
- 17.Turpie AGG, Lassen MR, Davidson BL, Bauer KA, Gent M, et al. 2009. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD4): a randomised trial. Lancet 373: 1673–1680 %U http://www.ncbi.nlm.nih.gov/pubmed/19411100.
- 18.Sterman J, Sterman JD. 2000 (2000) Business Dynamics: Systems Thinking and Modeling for a Complex World with CD-ROM: McGraw-Hill/Irwin %@ 007238915X. [Google Scholar]
- 19.Abrams DB, Graham AL, Levy DT, Mabry PL, Orleans CT. 2010. (2010) Boosting population quits through evidence-based cessation treatment and policy. American Journal of Preventive Medicine 38: S351–363%U http://www.ncbi.nlm.nih.gov/pubmed/20176308.
- 20.Bozzette SA, Boer R, Bhatnagar V, Brower JL, Keeler EB, et al. 2003. A model for a smallpox-vaccination policy. The New England Journal of Medicine 348: 416–425 %U http://www.ncbi.nlm.nih.gov/pubmed/12496353. [DOI] [PubMed]
- 21.Homer JB, Hirsch GB. System dynamics modeling for public health: background and opportunities. Am J Public Health. 2006;96:452–458. doi: 10.2105/AJPH.2005.062059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sterman JD. Learning from evidence in a complex world. Am J Public Health. 2006;96:505–514. doi: 10.2105/AJPH.2005.066043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Streiff MB, Haut ER. The CMS ruling on venous thromboembolism after total knee or hip arthroplasty: weighing risks and benefits. JAMA. 2009;301:1063–1065. doi: 10.1001/jama.301.10.1063. [DOI] [PubMed] [Google Scholar]
- 24.Jordan JM, Helmick CG, Renner JB, Luta G, Dragomir AD, et al. 2007. Prevalence of knee symptoms and radiographic and symptomatic knee osteoarthritis in African Americans and Caucasians: the Johnston County Osteoarthritis Project. The Journal of Rheumatology 34: 172–180 %U http://www.ncbi.nlm.nih.gov/pubmed/17216685.
- 25.Wachter RM, Foster NE, Dudley RA. 2008. Medicare’s decision to withhold payment for hospital errors: the devil is in the det. Joint Commission Journal on Quality and Patient Safety/Joint Commission Resources 34: 116–123 %U http://www.ncbi.nlm.nih.gov/pubmed/18351196. [DOI] [PubMed]
- 26.Nutescu EA, Shorr AF, Farrelly E, Horblyuk R, Happe LE, et al. 2008. Burden of deep vein thrombosis in the outpatient setting following major orthopedic surgery. The Annals of Pharmacotherapy 42: 1216–1221 %U http://www.ncbi.nlm.nih.gov/pubmed/18611992. [DOI] [PubMed]
- 27.Pronovost PJ, Goeschel CA, Wachter RM. 2008. (2008) The wisdom and justice of not paying for “preventable complications”. JAMA: The Journal of the American Medical Association 299: 2197–2199 %U http://www.ncbi.nlm.nih.gov/pubmed/18477787.
- 28.Anderson FA, Wheeler HB, Goldberg RJ, Hosmer DW, Patwardhan NA, et al. 1991. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Archives of Internal Medicine 151: 933–938 %U http://www.ncbi.nlm.nih.gov/pubmed/2025141. [DOI] [PubMed]
- 29.Prandoni P. 2005. (2005) Acquired risk factors for venous thromboembolism in medical patients. Hematology/the Education Program of the American Society of Hematology American Society of Hematology Education Program: 458–461 %U http://www.ncbi.nlm.nih.gov/pubmed/16304420.
- 30.Services UDoHaH., editor. The Surgeon General’s Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. Washington DC. 2008. [PubMed]
- 31.Patel VP, Walsh M, Sehgal B, Preston C, DeWal H, et al. 2007. Factors associated with prolonged wound drainage after primary total hip and knee arthroplasty. The Journal of Bone and Joint Surgery American Volume 89: 33–38 %U http://www.ncbi.nlm.nih.gov/pubmed/17200307. [DOI] [PubMed]
- 32.Galat DD, McGovern SC, Hanssen AD, Larson DR, Harrington JR, et al. 2008. Early return to surgery for evacuation of a postoperative hematoma after primary total knee arthroplasty. The Journal of Bone and Joint Surgery American Volume 90: 2331–2336 %U http://www.ncbi.nlm.nih.gov/pubmed/18978401.
- 33.Tooher R, Middleton P, Pham C, Fitridge R, Rowe S, et al. 2005. A systematic review of strategies to improve prophylaxis for venous thromboembolism in hospitals. Annals of Surgery 241: 397–415 %U http://www.ncbi.nlm.nih.gov/pubmed/15729062. [DOI] [PMC free article] [PubMed]
- 34.Piazza G, Rosenbaum EJ, Pendergast W, Jacobson JO, Pendleton RC, et al. 2009. Physician alerts to prevent symptomatic venous thromboembolism in hospitalized patients. Circulation 119: 2196–2201 %U http://www.ncbi.nlm.nih.gov/pubmed/19364975. [DOI] [PMC free article] [PubMed]
- 35.Durieux P, Nizard R, Ravaud P, Mounier N, Lepage E. 2000. (2000) A clinical decision support system for prevention of venous thromboembolism: effect on physician behavior. JAMA: The Journal of the American Medical Association 283: 2816–2821 %U http://www.ncbi.nlm.nih.gov/pubmed/10838650. [DOI] [PubMed]
- 36.Byrne GJ, McCarthy MJ, Silverman SH. 1996. (1996) Improving uptake of prophylaxis for venous thromboembolism in general surgical patients using prospective audit. BMJ (Clinical Research Ed) 313: 917 %U http://www.ncbi.nlm.nih.gov/pubmed/8876095.
- 37.Streiff MB, Haut ER. 2009. (2009) The CMS ruling on venous thromboembolism after total knee or hip arthroplasty: weighing risks and benefits. JAMA: The Journal of the American Medical Association 301: 1063–1065 %U http://www.ncbi.nlm.nih.gov/pubmed/19278950. [DOI] [PubMed]
- 38.Saleh K, Olson M, Resig S, Bershadsky B, Kuskowski M, et al. 2002. Predictors of wound infection in hip and knee joint replacement: results from a 20 year surveillance program. Journal of Orthopaedic Research: Official Publication of the Orthopaedic Research Society 20: 506–515 %U http://www.ncbi.nlm.nih.gov/pubmed/12038624. [DOI] [PubMed]
- 39.Whitehouse JD, Friedman ND, Kirkland KB, Richardson WJ, Sexton DJ. 2002. (2002) The impact of surgical-site infections following orthopedic surgery at a community hospital and a university hospital: adverse quality of life, excess length of stay, and extra cost. Infection Control and Hospital Epidemiology: The Official Journal of the Society of Hospital Epidemiologists of America 23: 183–189 %U http://www.ncbi.nlm.nih.gov/pubmed/12002232.
- 40.Kahneman D, Tversky A. 1979. (1979) Prospect Theory: An Analysis of Decision under Risk. Econometrica 47: 263–291 %U http://www.jstor.org/stable/1914185.
- 41.Hawker GA, Wright JG, Badley EM, Coyte PC. 2004. (2004) Perceptions of, and willingness to consider, total joint arthroplasty in a population-based cohort of individuals with disabling hip and knee arthritis. Arthritis and Rheumatism 51: 635–641 %U http://www.ncbi.nlm.nih.gov/pubmed/15334438.
- 42.Norman-Taylor FH, Palmer CR, Villar RN. 1996. (1996) Quality-of-life improvement compared after hip and knee replacement. The Journal of Bone and Joint Surgery British Volume 78: 74–77 %U http://www.ncbi.nlm.nih.gov/pubmed/8898131. [PubMed]
- 43.Räsänen P, Paavolainen P, Sintonen H, Koivisto A-M, Blom M, et al. 2007. Effectiveness of hip or knee replacement surgery in terms of quality-adjusted life years and costs. Acta Orthopaedica 78: 108–115 %U http://www.ncbi.nlm.nih.gov/pubmed/17453401. [DOI] [PubMed]
- 44.Ballal RD, Botteman MF, Foley I, Stephens JM, Wilke CT, et al. 2008. Economic evaluation of major knee surgery with recombinant activated factor VII in hemophilia patients with high titer inhibitors and advanced knee arthropathy: exploratory results via literature-based modeling. Current Medical Research and Opinion 24: 753–768 %U http://www.ncbi.nlm.nih.gov/pubmed/18234151. [DOI] [PubMed]
- 45.Losina E, Walensky RP, Kessler CL, Emrani PS, Reichmann WM, et al. 2009. Cost-effectiveness of total knee arthroplasty in the United States: patient risk and hospital volume. Archives of Internal Medicine 169: 1113–1121; discussion 1121–1122 %U http://www.ncbi.nlm.nih.gov/pubmed/19546411.
- 46.Rosenthal MB, Dudley RA. Pay-for-performance: will the latest payment trend improve care? JAMA. 2007;297:740–744. doi: 10.1001/jama.297.7.740. [DOI] [PubMed] [Google Scholar]
- 47.McDonald R, White J, Marmor TR. 2009. (2009) Paying for performance in primary medical care: learning about and learning from “success” and “failure” in England and California. Journal of Health Politics, Policy and Law 34: 747–776 %U http://www.ncbi.nlm.nih.gov/pubmed/19778931.
- 48.Rosenthal MB, Dudley RA. 2007. (2007) Pay-for-Performance. JAMA: The Journal of the American Medical Association 297: 740–744 %U http://jama.ama-assn.org/content/297/747/740.short.
- 49.Millett C, Netuveli G, Saxena S, Majeed A. Impact of pay for performance on ethnic disparities in intermediate outcomes for diabetes: a longitudinal study. Diabetes Care. 2009;32:404–409. doi: 10.2337/dc08-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Werner RM, Asch DA, Polsky D. 2005. (2005) Racial profiling: the unintended consequences of coronary artery bypass graft report cards. Circulation 111: 1257–1263 %U http://www.ncbi.nlm.nih.gov/pubmed/15769766. [DOI] [PubMed]
- 51.Millett C, Gray J, Saxena S, Netuveli G, Khunti K, et al. Ethnic disparities in diabetes management and pay-for-performance in the UK: the Wandsworth Prospective Diabetes Study. PLoS Med. 2007;4:e191. doi: 10.1371/journal.pmed.0040191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Millett C, Gray J, Bottle A, Majeed A. Ethnic disparities in blood pressure management in patients with hypertension after the introduction of pay for performance. Ann Fam Med. 2008;6:490–496. doi: 10.1370/afm.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chien AT, Chin MH, Davis AM, Casalino LP. 2007. (2007) Pay for performance, public reporting, and racial disparities in health care: how are programs being designed? Medical Care Research and Review: MCRR 64: 283S–304S %U http://www.ncbi.nlm.nih.gov/pubmed/17881629.
- 54.Brown J, Doloresco F, Iii, Mylotte JM. 2009. (2009) “Never events”: not every hospital-acquired infection is preventable. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America 49: 743–746 %U http://www.ncbi.nlm.nih.gov/pubmed/19624274.
- 55.Kirsch M. 2009. (2009) Medical quality: never events or never never land? The American Journal of Gastroenterology 104: 2671–2672 %U http://www.ncbi.nlm.nih.gov/pubmed/19888233.
- 56.Duska LR, Garrett L, Henretta M, Ferriss JS, Lee L, et al. 2010. When ‘never-events’ occur despite adherence to clinical guidelines: the case of venous thromboembolism in clear cell cancer of the ovary compared with other epithelial histologic subtypes. Gynecologic Oncology 116: 374–377 %U http://www.ncbi.nlm.nih.gov/pubmed/19922988. [DOI] [PubMed]
- 57.Fry DE, Pine M, Jones BL, Meimban RJ. 2010. (2010) Patient characteristics and the occurrence of never events. Archives of Surgery (Chicago, Ill: 1960) 145: 148–151 %U http://www.ncbi.nlm.nih.gov/pubmed/20157082. [DOI] [PubMed]
- 58.Shah J, Shah A, Pietrobon R. Scientific writing of novice researchers: what difficulties and encouragements do they encounter? Acad Med. 2009;84:511–516. doi: 10.1097/ACM.0b013e31819a8c3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pietrobon R, Taylor M, Guller U, Higgins LD, Jacobs DO, et al. Predicting gender differences as latent variables: summed scores, and individual item responses: a methods case study. Health Qual Life Outcomes. 2004;2:59. doi: 10.1186/1477-7525-2-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Questions asked to expert panel.
(DOC)