Abstract
Counting cells is often a necessary but tedious step for in vitro cell culture. Consistent cell concentrations ensure experimental reproducibility and accuracy. Cell counts are important for monitoring cell health and proliferation rate, assessing immortalization or transformation, seeding cells for subsequent experiments, transfection or infection, and preparing for cell-based assays. It is important that cell counts be accurate, consistent, and fast, particularly for quantitative measurements of cellular responses.
Despite this need for speed and accuracy in cell counting, 71% of 400 researchers surveyed1 who count cells using a hemocytometer. While hemocytometry is inexpensive, it is laborious and subject to user bias and misuse, which results in inaccurate counts. Hemocytometers are made of special optical glass on which cell suspensions are loaded in specified volumes and counted under a microscope. Sources of errors in hemocytometry include: uneven cell distribution in the sample, too many or too few cells in the sample, subjective decisions as to whether a given cell falls within the defined counting area, contamination of the hemocytometer, user-to-user variation, and variation of hemocytometer filling rate2.
To alleviate the tedium associated with manual counting, 29% of researchers count cells using automated cell counting devices; these include vision-based counters, systems that detect cells using the Coulter principle, or flow cytometry1. For most researchers, the main barrier to using an automated system is the price associated with these large benchtop instruments1.
The Scepter cell counter is an automated handheld device that offers the automation and accuracy of Coulter counting at a relatively low cost. The system employs the Coulter principle of impedance-based particle detection3 in a miniaturized format using a combination of analog and digital hardware for sensing, signal processing, data storage, and graphical display. The disposable tip is engineered with a microfabricated, cell- sensing zone that enables discrimination by cell size and cell volume at sub-micron and sub-picoliter resolution. Enhanced with precision liquid-handling channels and electronics, the Scepter cell counter reports cell population statistics graphically displayed as a histogram.
Keywords: Cellular Biology, Issue 45, Scepter, cell counting, cell culture, hemocytometer, Coulter, Impedance-based particle detection
Protocol
1. The Importance of Cell Counting
Counting cells is a necessary but tedious step for in vitro cell culture.
Consistent cell concentrations ensure experimental reproducibility and accuracy.
Cell counts are important for monitoring cell health and proliferation rate, assessing immortalization or transformation, seeding cells for subsequent experiments, transfection or infection, and preparing for cell-based assays.
Despite the need for speed and accuracy in cell counting,, 71% of 400 researchers surveyed count cells using a hemocytometer. While hemocytometry is inexpensive, it is laborious and subject to user bias and misuse, which results in inaccurate counts.
Sources of error in hemocytometry include: uneven cell distribution in the sample, too many or too few cells in the sample, subjective decisions as to whether a given cell falls within the defined counting area, contamination of the hemocytometer, user-to-user variation, and variation of hemocytometer filling rate.
To alleviate the tedium associated with manual counting, 29% of researchers count cells using automated cell counting devices. However, for most researchers, the price associated with these large bench-top instruments is a barrier to their use.
The Scepter cell counter is an automated handheld device that offers the automation and accuracy of Coulter counting at a relatively low cost.
The system employs the Coulter principle of impedance-based particle detection in a miniaturized format using a combination of analog and digital hardware for sensing, signal processing, data storage, and graphical display.
The disposable sensor is engineered with a microfabricated, cell- sensing zone that enables discrimination by cell size and cell volume at sub-micron and sub-picoliter resolution.
Enhanced with precision liquid-handling channels and electronics, the Scepter cell counter graphically displays cell population statistics as a histogram.
2. Preparation of a Single-cell Suspension for Counting
To use the Scepter cell counter, begin by diluting a single cell suspension of cells, in phosphate buffered saline. A concentration of 10,000-500,000 cells per mL is optimal.
Transfer 100 μL of diluted cells to a 1.5 mL microfuge tube.
3. Performing Cell Counts using the Scepter
Turn on the Scepter cell counter by pressing and holding the toggle on the back of the instrument and wait for the on-screen instructions to appear.
When prompted, attach a sensor to the end of the Scepter unit with the electrode sensing panel facing toward the front of the instrument.
Scepter sensors consist of a precision molded sampling chamber, with an electronic sensing zone and integrated cell-sensing electrodes. The sensors make it possible to discriminate cell size at sub-micron resolution, and cell volume at sub-picoliter resolution.
Consistent sensor engineering, ensures that equal sample volumes are counted and accurate size measurements are made.
Once the sensor is attached, perform each step of the counting process according to the on-screen instructions as follows:
When the screen reads "Hold down the plunger to begin," depress the plunger. The screen will then read "submerge the sensor." Submerge the sensor into the cell suspension solution and release the plunger to aspirate the cell suspension into the sensor.
The plunger acts as an electrical signal and will draw the sample plus dead volume into the sensor. The speed or accuracy of plunging does not matter as it does with pipetting. This process will take several seconds. When ready, the instrument will beep, and "Sample loaded" will be displayed on the screen.
Once the sample is loaded into the sensor it is drawn through the sensing zone to assess cell size and cell volume, then through the microchannels. Once 50 μL of sample is counted the Sensor tells the instrument to stop counting.
After the count is complete, the instrument's screen will read "Count Complete. Please remove sensor and discard." Follow the prompt, and remove and discard the sensor.
The instrument will then display a histogram of cell size or diameter on its screen as well as the cell concentration per mL.
4. Data Analysis
After the count is complete and the histogram is displayed on the instrument, apply gates by pushing the toggle button. Select "auto gating" or "use last" to either automatically set the gate based on the histogram profile, or to use the gates from the previous count.
Regardless of the selection, the gates can be manually moved to fine-tune the data. Push the toggle button again to select the left gate and scroll the toggle to move the gate as desired.
Then press the toggle again to activate the right gate and scroll the toggle to place the right gate. Click again; a new cell concentration, average cell volume and diameter will be displayed.
The toggle can also be moved to either side to switch the display between date, time, mean cell size, and mean cell volume.
Up to 72 histograms can be stored on the instrument itself. To upload the data to a personal computer, attach the instrument via the USB cable port on the display screen.
On the PC, double click on the Scepter Software Application to launch the software then click on "connect". All counts will automatically port to the software.
The Scepter Application Software displays the histogram detailing the population distribution of the culture in either volume or diameter. Downloaded files can then be exported to Microsoft Excel, where the raw data will be displayed in bins of specified cell diameters or cell volumes.
5. Representative Results
Nineteen different cell lines were prepared for counting and theoretical starting concentrations were determined using a Coulter Counter. These cell suspensions were then further diluted to obtain cell suspensions at theoretical cell concentrations.
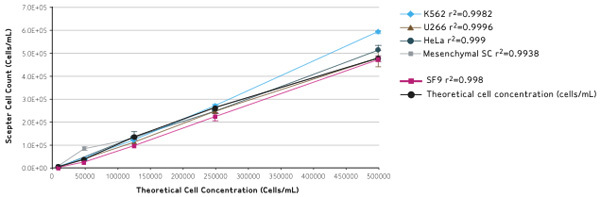
The Scepter cell counter was then used to determine cell concentrations. Figure 1 shows 5 dilutions that were measured for 5 representative cell lines along with the theoretical cell concentration.
The high degree of linearity as indicated by the R2 values, demonstrates that Scepter counting is a reliable method for the cell lines tested, across a wide linear operating range.
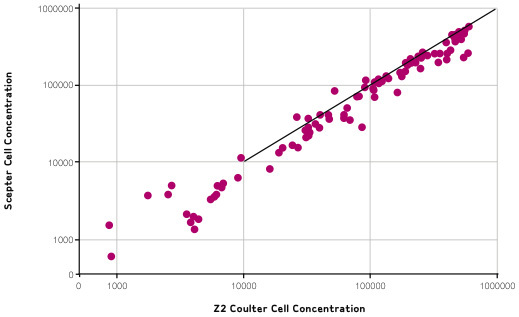
The overall relative accuracy of Scepter counting is demonstrated in Figure 2, in which data obtained from Scepter counting are compared with counts obtained from Coulter Counting.
The log of Scepter cell counts is plotted against the log of Coulter counts, and shows that, across all cell lines tested, Scepter counts match Coulter counts.
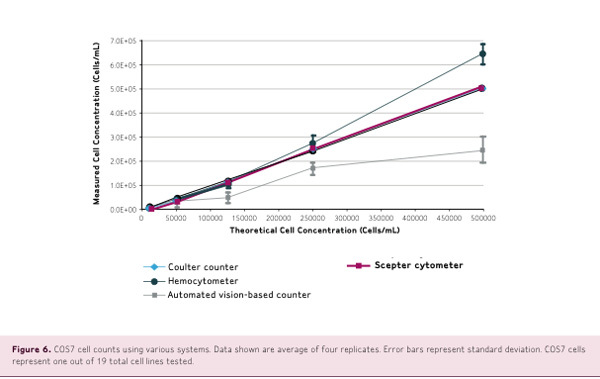
Counts of 19 cell lines were performed using a Z2 Coulter Counter, the Scepter cell counter, an automated vision-based counter such as Vi-Cell or Countless system, and a hemocytemeter.
Counts were performed according to the manufacturer's instructions, using the same cell starting suspension and identical dilutions. The results shown in Figure 3 demonstrate that cell concentrations measured by Scepter counting closely match theoretical cell concentrations with high linearity.
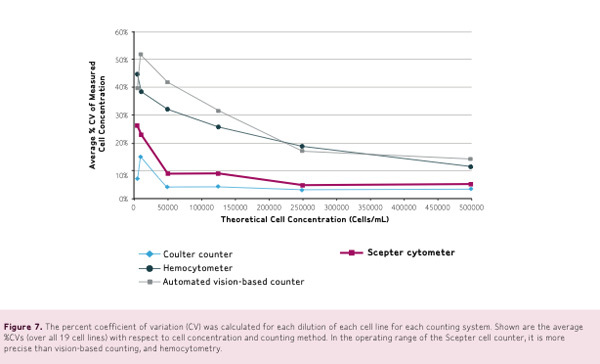
The percent coefficient of variation, or CV, was calculated for each dilution of each cell line for each counting system. Figure 4 shows the average %CVs over 19 cell lines with respect to cell concentration and counting method.
Within the operating range, the Scepter cell counter is more precise than vision based hemocytometer counting, displaying smaller standard deviations and smaller average coefficients of variation.
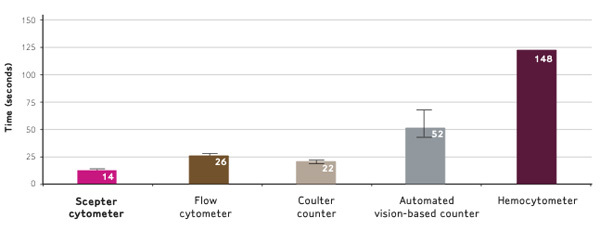
Scepter counting is ten times faster than hemocytometry and faster than other automated counters too. In Figure 5, SF9, MCF7, and HEK293 cells were counted using the methods described in this video.
The average time required to measure cell concentration was recorded. Note that hemocytometry was performed only once per sample.
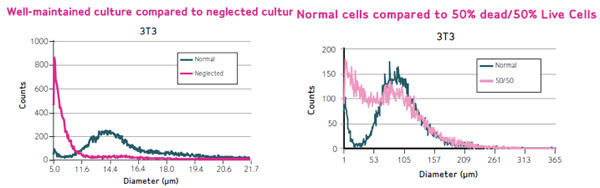
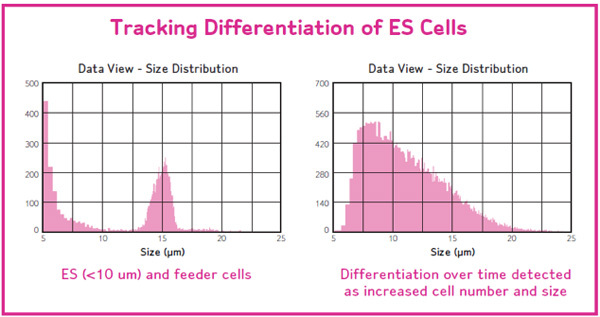
Scepter makes measurements based on cell size. Thus any changes in cellular morphology can be captured in the histogram. Healthy cultures display a nice bell shaped Gaussian distribution, while unhealthy cells, lyse, and cause a shift in the histogram to the left (Figure 6, left panel).
Similarly, when a population of 50% dead cells were mixed with 50% live cells, histogram shifts were also observed (Figure 6, right panel).
Discussion
Comparing the performance of the Scepter cell counter to results from other counting methods, we conclude that this new handheld, automated cell counter delivers precise, fast, and reliable cell counts over a wide operating range. The functionality of Scepter counting is a result of the precision engineered technology embedded into the sensor tip and the sophisticated counting instrumentation based upon the Coulter principle. The performance quality suggests Scepter counting as method to increase the speed and improve the reproducibility of cell-based assays. The handheld format allows quick tedium free counting right at the cell culture hood.
Disclosures
Kathleen Ongena, Chandreyee Das, Janet L. Smith, Sónia Gil, and Grace Johnston are employed by Millipore, which produces the instruments used in this article.
References
- Barghshoon S. Cell Counting Survey. Millipore; 2009. [Google Scholar]
- Tucker KG, Chalder S, al-Rubeai M, Thomas CR. Measurement of hybridoma cell number, viability, and morphology using fully automated image analysis. Enzyme Microb Technol. 1994;16(1):29–35. doi: 10.1016/0141-0229(94)90106-6. [DOI] [PubMed] [Google Scholar]
- Houwen B. Fifty years of hematology innovation: the Coulter principle. Medical Laboratory Observer; 2003. [Google Scholar]


