Abstract
Background
Several small diffusible molecules are involved in bacterial quorum sensing and virulence. The production of autoinducers-1 and -2, quinolone, indole and γ-amino butyrate signaling molecules was investigated in a set of soft-rot bacteria belonging to six Dickeya or Pectobacterium species including recent or emerging potato isolates.
Methodology/Principal Findings
Using bacterial biosensors, immunoassay, and chromatographic analysis, we showed that soft-rot bacteria have the common ability to produce transiently during their exponential phase of growth the N-3-oxo-hexanoyl- or the N-3-oxo-octanoyl-l-homoserine lactones and a molecule of the autoinducer-2 family. Dickeya spp. produced in addition the indole-3-acetic acid in tryptophan-rich conditions. All these signaling molecules have been identified for the first time in the novel Dickeya solani species. In contrast, quinolone and γ-amino butyrate signals were not identified and the corresponding synthases are not present in the available genomes of soft-rot bacteria. To determine if the variations of signal production according to growth phase could result from expression modifications of the corresponding synthase gene, the respective mRNA levels were estimated by reverse transcriptase-PCR. While the N-acyl-homoserine lactone production is systematically correlated to the synthase expression, that of the autoinducer-2 follows the expression of an enzyme upstream in the activated methyl cycle and providing its precursor, rather than the expression of its own synthase.
Conclusions/Significance
Despite sharing the S-adenosylmethionine precursor, no strong link was detected between the production kinetics or metabolic pathways of autoinducers-1 and -2. In contrast, the signaling pathway of autoinducer-2 seems to be switched off by the indole-3-acetic acid pathway under tryptophan control. It therefore appears that the two genera of soft-rot bacteria have similarities but also differences in the mechanisms of communication via the diffusible molecules. Our results designate autoinducer-1 lactones as the main targets for a global biocontrol of soft-rot bacteria communications, including those of emerging isolates.
Introduction
An important aspect of bacterial communication is based on the release in the microenvironment of small signaling molecules that can diffuse through cell membranes. The most studied model relies on both synthesis and perception of autoinducer-1 (AI-1). AI-1 signaling molecules are N-acyl-homoserine lactones (NAHSLs) that serve for cell-to-cell communication called quorum sensing (QS) [1]. Such regulatory systems allow bacteria to sense cell density and containment within a microenvironment according to which they synchronize the functions of the entire population [2]–[5].
Within soft-rot bacteria, the AI-1 communication system is involved in plant virulence with a considerable importance according to the bacterial species. Among them, phytopathogens belonging to Dickeya and Pectobacterium genera (formerly grouped in the Erwinia genus) are responsible for important diseases affecting plant health and compromising the quality of harvested vegetables and other plant products [6]–[10]. In the psychrotolerant Pectobacterium atrosepticum, species adapted to temperate regions and specific to the potato host Solanum tuberosum L., AI-1 based-QS controls virulence factors involved in a typical stem infection (blackleg) and tuber maceration. Twenty-six percent of the P. atrosepticum SCRI1043 genes are under QS control, including genes coding for plant cell wall degrading enzymes and their secretion systems [11]. AI-1 based-QS can also regulate mechanisms involved in the manipulation of plant cell defenses, like harpin synthesis, leading to the hypersensitive response (HR) in non host plant [12]. Generating signaling molecules is therefore a crucial step within plant-P. atrosepticum interactions. Indeed, a single mutation of the NAHSL synthase gene or the degradation of signaling molecules before their release in the microenvironment is sufficient to remove all symptoms on potato and to prevent an HR reaction [12], [13]. In Pectobacterium carotovorum species, which are found worldwide in a large range of hosts, AI-1 QS controls similar bacterial traits and is necessary for full virulence and antibiotic synthesis [14]–[16]. As a result of these observations, Pectobacterium and its AI-1 QS system has become the target for a novel biocontrol approach, which aims at reducing the expression of virulence systems rather than eradicating the pathogen [17]. This biocontrol strategy is based on the biostimulation of NAHSL-degradative microflora using structural analogs of AI-1 signaling molecules [18]. First evaluated on S. tuberosum cultures to control blackleg in Western Europe, it could be extended to other crops and diseases [7], [19], [20].
NAHSL-dependent QS appears less important in virulence of other soft-rot bacteria such as Dickeya dadantii 3937 and Dickeya chrysanthemi EC16 [21], [22], suggesting a possible failure when using the biocontrol method mentioned above [23]. Moreover, data on the environmental distribution of Pectobacterium model strains on which signal studies were conducted are often lacking [14], [16], [24], [25]. Furthermore, many recent epidemiologic reports on devastating potato diseases with worldwide distribution, show emergence of new pectinolytic agents, in which communication systems have not yet been identified [26]–[32]. It therefore appears necessary to screen QS systems encountered in a larger panel of strains comprising both type strains from the current taxonomical described species and emergent virulent isolates. The discovery of the autoinducer-2 (AI-2) QS in three Pectobacterium strains [33], [34] as well as assumptions regarding the role of signaling molecules synthesized by both plants and Proteobacteria [35]–[37] guided our research and has opened up new prospects for biocontrol, provided that such signals are frequently used by pathogenic bacteria. Particular attention will be given to the indole-3-acetic acid (IAA), a compound that harbors all traits of QS signals and whose production can be autoinduced [38]–[39]. Finally, another point that needs to be updated is the possible interconnections between metabolic pathways involved in signals production.
The aims of this work are (i) to characterize the nature and production patterns of AI-1, AI-2, quinolone, indole and γ-amino butyric acid (GABA) signaling molecules synthesized by potato soft-rot bacteria, (ii) to evaluate their representation within a collection of Dickeya and Pectobacterium including type and reference strains with a selection of recent isolates belonging to species described on the potato in European growing areas, and (iii) to investigate the possible links between the anabolic pathways of signaling molecules by comparing the kinetics of signal generation and the expression of key genes involved in signal syntheses. To our knowledge, this is the first screening evaluating with comparable parameters the potential of a wide range of pectinolytic bacteria to synthesize various signaling molecules.
Materials and Methods
Bacterial strains and plasmids
The characteristics of the bacterial sensors and controls used in this work are presented in Table 1. The strains Agrobacterium tumefaciens NT1(pZNLR4) and Escherichia coli DH5α(pSB401) were used as biosensors for NAHSL detection. Two Vibrio harveyi strains, BB170 and BB120, were used as biosensors of the AI-2 signal and as AI-2 producer for the positive control, respectively. Pseudomonas aeruginosa H103 was used as a positive control for the detection of 4-hydroxy-2-heptylquinoline (HHQ) and Pseudomonas quinolone signal (PQS) molecules. The characteristics of the soft-rot bacterial panel used in this work are presented in Table 2.
Table 1. Bacterial QS signal producers or biosensors, and plasmids.
| Strains/Plasmids | Relevant characteristics | Reference |
| Agrobacterium tumefaciens | ||
| NT1(pZNLR4) | NT1 derivative of strain C58, carrying pZLR4 plasmid, NAHSL biosensor, Gmr | [43] |
| Escherichia coli | ||
| E. coli DH5α | φ80lacZ ΔM15 Δ(lacZYA-argF) U169 hsdR17 recA1 endA1 thi-1 | [85] |
| E. coli DH5α(pSB401) | Derivative of strain DH5α, carrying pSB401 plasmid, NAHSL biosensor, Tetr | [45] |
| Pseudomonas aeruginosa | ||
| P. aeruginosa H103 | HHQ and PQS producer | [86] |
| Vibrio harveyi | ||
| V. harveyi BB120 | Wild-type strain, AI-2 producer | [87] |
| V. harveyi BB170 | luxN::Tn5, Dysfunctional AI-2 receptor, AI-2 biosensor | [88] |
| Plasmids | ||
| pZLR4 | traG::lacZ/traR reporter system, Gmr | [43] |
| pSB401 | NAHSL biosensor luxRI'::luxCDABE fusion; pACYC184 derived, Tetr | [45] |
Gmr and Tetr indicate resistance to gentamicin and tetracycline, respectively.
NAHSL, N-acyl homoserine lactones; AI-2, Autoinducer-2; HHQ, 4-hydroxy-2-heptylquinoline; PQS, Pseudomonas quinolone signal.
Table 2. Virulence traits and origin of potato soft-rot pathogens.
| Strain | Host of origin | Year of isolation | Tuber* soft-rot | HR# | Reference or source |
| Pectobacterium atrosepticum | |||||
| P. atrosepticum CFBP 1526T | Solanum tuberosum | 1957 | + | − | CFBP, species type strain |
| P. atrosepticum CFBP 6276 | Solanum tuberosum | 1999 | + | + | [40] |
| P. atrosepticum 100T | Solanum tuberosum | 2003 | + | − | [27] |
| P. atrosepticum RNS 08.30.1A | Solanum tuberosum | 2008 | + | − | V. Hélias' collection |
| Pectobacterium carotovorum | |||||
| P. carotovorum CFBP 2046T | Solanum tuberosum | 1952 | + | + | CFBP, species type strain |
| P. carotovorum EC153 | Capsicum annuum | 1951 | + | − | [24] |
| P. carotovorum 98.1 | Solanum tuberosum | 1998 | + | + | [27] |
| P. carotovorum RNS 08.42.1A | Solanum tuberosum | 2008 | + | − | V. Hélias' collection |
| Dickeya spp. | |||||
| D.chrysanthemi CFBP 2048T | Chrysanthemum morifolium | 1956 | + | + | CFBP, type strain |
| D. dadantii 3937 | Saintpaulia ionantha | 1981 | + | + | [89] |
| D. dianthicola RNS 04.9 | Solanum tuberosum | 2004 | + | + | V. Hélias' collection |
| D. solani RNS 08.23.3.1A | Solanum tuberosum | 2008 | + | + | [27] |
The potential of each strain to induce a tuber soft-rot was evaluated in potato host plant seven days after infection by intra-medulla injection.
The potential of each strain to induce a hypersensitive response (HR) was evaluated in tobacco non host plant 24 hours after leaves infiltration.
CFBP, Collection Française de Bactéries Phytopathogènes, Institut National de la Recherche Agronomique, Angers, France.
Growth media and conditions
Liquid cultures were grown in polygalacturonic acid (PGA) mineral salt medium [40] the composition of which has been modified as follows: K2HPO4, 16.266 g/l; KH2PO4, 899 mg/l; (NH4)2SO4, 1.2 g/l; MgSO4.6H2O, 818 mg/l; CaCl2, 75 mg/l (pH 8.0) and polygalacturonic acid 4.0 g/l (Potassium salt, Sigma-Aldrich, France). For production of quinolones and auxins, bacteria were also grown in King B and M9 minimal media supplemented with l-tryptophan at 500 µg/ml [36]. Batch cultures were performed under gyratory agitation (180 rpm) at 25°C in Erlenmeyer flasks in which the liquid medium is 10% of the total flask volume. Batch precultures and cultures were made in the same experimental conditions. Bacterial growth was monitored by measuring optical density (OD) at 580 nm. The initial OD580 of the cultures was usually 0.05. Each experiment using triplicate independent cultures were repeated at least three times. The filtered supernatants and cells obtained were stored at −20°C. E. coli carrying pSB401 strains were grown in LB medium supplemented with tetracycline (10 µg/ml), and maintained on LB agar supplemented with tetracycline.
Soft-rot test in potato tuber
NaCl-washed Dickeya and Pectobacterium cell suspensions (cell density ca. 108 cfu/ml in 0.9% NaCl) were prepared from stationary-phase cultures grown in PGA minimal medium. S. tuberosum cv. Allians tubers were surface-sterilized and infected by intra-medulla injection (1-cm depth with 10 µl of the above bacterial suspension). The inoculated tubers were incubated in a Minitron incubator (Infors, Massy, France) at 25°C under a relative humidity of 65%±2%. For each strain, five tubers were analyzed and the development of the symptoms was evaluated after 7 days.
Hypersensitive Response assays in tobacco plants
Nicotinia tabacum cv. Xanthi XHFD8 was cultivated for 8 weeks under greenhouse conditions (T = 28°C, photoperiod = 16 h). Bacteria were cultivated in PGA minimal medium at 25°C, washed twice with 0.9% NaCl, and resuspended at 2×108 cfu/ml. Tobacco leaves were infiltrated with ca. 100 µl of cell suspensions. The margins of the water-soaked infiltrated areas were marked, and the plants were inspected for HR development at 24 h.
NAHSL standards, extraction of supernatants and NAHSL assays
NAHSLs were extracted and analyzed as described previously [12], [41]. Briefly, the synthetic standards (Sigma-Aldrich, France) and stock solutions prepared in high performance liquid chromatography (HPLC)-grade ethyl acetate (Fisher Scientific, France) were stored at −20°C. The supernatants (1 ml) were extracted twice with equal volumes of ethyl acetate. The combined extracts were dried over anhydrous magnesium sulfate, evaporated to dryness, dissolved in 500 µl of HPLC-grade ethyl acetate and stored at −20°C until analysis. Components in the ethyl acetate extracts were spotted onto C18 reversed-phase thin-layer chromatography (TLC) Silicagel (Whatman, Maidstone, U.K.) plates and separated with methanol-water (60∶40, v/v), as described by Shaw and associates [42]. NAHSL were detected by overlaying the TLC with the indicator strains essentially as indicated by Cha et al. [43].
Concentrated extracts were analyzed by on-line liquid chromatography mass spectrometry (LC-MS-MS). They were applied to a C18 reverse-phase HPLC column (Agilent Hypersyl ODS, 250×4.6 mm, particle size 5 µm, Interchim, France) using an Agilent Technologies Series 1100 vacuum degasser, LC pump and autosampler (Hewlett Packard, Germany). The elution procedure consisted of an isocratic profile of methanol-water (50∶50, v/v) (Fisher Scientific, France) for 10 min at a flow rate of 0.4 ml/min, followed by a linear gradient from 50% to 90% methanol in water over 15 min, and a isocratic profile over 25 min. The post-column flow was split (1/10) by a micro-splitter valve (Upchurch Scientific, USA) and a mixture of 5 mM ammonium acetate and 0.05% trifluoroacetic acid (Sigma-Aldrich, France) in methanol-water (50∶50, v/v, 150 µl/h) was added using a Cole-Parmer syringe pump. Detection was performed by electrospray ionisation-ion trap mass spectrometry (ESI-MS) using a Bruker Esquire-LC spectrometer (Bruker Daltonic, Germany) under positive-ion conditions. The identification of NAHSLs from supernatant extracts was done by comparison with synthetic standards, based on three criteria: HPLC retention times, MS-MS fragment ions of the molecular [M+H]+ ions (four product ions: the lactone ring m/z 102, [M+H−101]+ ion corresponding to the acyl chain, [M+H−H2O]+ and [M+H−CO]+ ions) and on their relative intensities. Chromatographic peak area of the m/z 102 ion was measured for quantification [41].
AI-1 and AI-2 bioluminescence assays
Potato soft-rot strains were grown in PGA minimal medium at 25°C, whereas E. coli and V. harveyi were cultivated in LB at 37°C and in autoinducer bioassay (AB) medium at 30°C, respectively. Bacterial growth was monitored by measuring OD at 580 nm. After the appropriate incubation period, bacterial cells were removed by centrifugation at 10 000× g for 10 min and the resulting supernatant was subsequently filter sterilized through a 0.22 µm pore size filter (Millipore, Billerica, MA) and stored at −20°C. The filtered samples were then analyzed using the biosensor strains E. coli DH5α(pSB401) and V. harveyi BB170 as previously described [44], [45].
Detection of the 2-alkyl-4-(1H)-quinolones
Potato soft-rot strains were grown at 25°C in PGA minimal medium or M9 minimal medium supplemented with l-tryptophan at 500 µg/ml. Aliquot of stationary phase cell-free supernatants (10 ml) were twice extracted with 10 ml acidified ethyl acetate, vortexed vigorously and centrifuged 10 000× g for 5 min. The organic phase was transferred to a fresh tube and dried to completion under a stream of nitrogen gas. The solute residue was resuspended in 500 µl methanol. Samples of ethyl-acetate extracted culture supernatant were spotted onto a normal phase silica 60F254 (Merck) TLC plate that had been previously soaked for 30 min in 5% w/v KH2PO4 and activated at 90°C for 1 h. HHQ and PQS produced by P. aeruginosa H103 strain were used as positive controls. After drying the spots, TLC plates were developed by using dichloromethane∶methanol (95∶5 v/v) as the mobile phase then visualized with a UV transilluminator and photographed.
Auxins quantification, extraction and characterization
Auxin quantification was performed with a Fe-H2SO4 reagent [46]. Briefly, bacterial strains were propagated overnight into 5 ml of King B medium and transferred in PGA minimal medium or M9 minimal medium supplemented with l-tryptophan at 500 µg/ml. Samples were taken at different points of the growth curves, 1 ml aliquot of culture supernatant was mixed vigorously with 4 ml of Salkowski's reagent (150 ml of concentrated H2SO4, 250 ml of distilled H2O, 7.5 ml of 0.5 M FeCL3·6H2O), and the absorbance at 535 nm was measured. Auxin production was measured as IAA equivalents using a standard curve of IAA.
Aliquots of sterilized supernatants (8.5 ml) were acidified to reach a pH 3 using HCl (37%). Supernatants were extracted with 4 ml acidified ethyl acetate, vortexed vigorously and incubated overnight at 4°C. Supernatants were extracted twice with 3 ml acidified ethyl acetate during 2 hours at 4°C. The organic phase was transferred to a fresh tube after centrifugation at 10 000× g for 5 min, then was dried to completion under a stream of nitrogen gas. The solute residue was resuspended in 1 ml methanol/water (v/v, 80/20). Concentrated extracts were analyzed by HPLC. They were applied to a C18 reverse-phase HPLC column (C18 Atlantis, 150×4.6 mm, particle size 3 µm, Waters, France) using an Agilent Technologies Series 1100 vacuum degasser, LC pump and autosampler (Hewlett Packard, Germany). Mobile phase contained purified water 18 MΩ/H3PO4 (1/1000) (A) and acetonitrile (B). The gradient elution was as follows: 5–90% B at 0–25 min at a flow rate of 0.5 ml/min; 90% B at 33–37 min at a flow rate of 1 ml/min; 90–5% B at 33–37 min at a flow rate of 0.5 ml/min; re-equilibrium was 20 min; the total run time was 57 min. Detection was performed by Chromeleon. The identification of the putative auxins in supernatant extracts was done by comparison with synthetic standards, based on HPLC retention times at 325 nm. For quantification, chromatographic peak area was measured and compared to standards. Seven indolic compounds were studied: IAA, indole-3-pyruvic acid (IPyA), indole-3-acetamide (IAM), indole-3-butyric acid (IBA), indole-3-propionic acid (IPA), kynurenic acid (KA) and l-tryptophan. All this compounds were purchased from Sigma-Aldrich (France).
GABA detection by immunoassay
Soft-rot strains were grown in PGA minimal medium at 25°C. After the appropriate incubation period, bacterial cells were removed by centrifugation at 10 000× g for 10 min and the resulting supernatant was subsequently filter sterilized through a 0.22 µm pore size filter (Millipore, Billerica, MA) and stored at −20°C. The filtered samples were analyzed by GABA Elisa test in accordance with instructions from GABA Elisa kit (Labor Diagnostika Nord & Co., KG).
RNA extraction and reverse transcription-PCR (RT-PCR)
Total RNA was isolated from ∼2×109 cells of bacterial cultures using hot acidic phenol. A lysis buffer (0.02 M sodium acetate, pH 5.5, 0.5% (w/v) SDS, 1 mM EDTA) allowed the obtaining of cell lysates subjected to three consecutive phenol extractions, followed by a chloroform extraction. Total RNAs were precipitated with 100% ethanol (2∶1, v/v) and 1 M sodium acetate (1∶10, v/v), and resuspended in RNase-free water. DNase I treatment using RNAse-free DNase I (Ozyme, Saint-Quentin en Yvelines, France) allowed removal of contaminating DNA. Quality and concentration of RNA samples were checked by agarose gel electrophoresis, and using a Nanodrop spectrophotometer (Bio-Rad Laboratories). Absence of genomic DNA contamination was confirmed by PCR. RT-PCR was performed with 5 ng RNA as template using Transcriptor one step RT-PCR kit (Roche, Meylan, France) according to the manufacturer's recommendations. Because of the high abundance of 16S rRNA, RNA samples were diluted 100 fold more when analyzing 16S rRNA reference gene expression. After optimization of the RT-PCR protocol according to the abundance of the respective mRNAs, 35 and 40 cycles were used for all genes of Pectobacterium and Dickeya strains, respectively.
The set of primers used for amplification of mRNA encoding the synthases from S-adenosylmethionine (SAM), AI-1, AI-2 and IAA precursor are listed in Supporting Information (Table S1). The corresponding genes are respectively named metK, luxI, luxS and iaaM. The primer sequences were deduced from the P. atrosepticum SCRI1043, P. carotovorum PC1, D. dadantii 3937 and D. zeae Ech586 genomes (GenBank accession number NC_004547, NC_012917, NC_014500, and NC_013592, respectively). For calibration, 16S rRNA and recA were used as references [47],[48]. The corresponding primers were designed from a multiple sequence alignment and allowed to amplify a unique 591-bp and 974-bp fragment, respectively. All amplified fragments displayed the expected size.
Results
Characterization of signal molecules produced by potato soft-rot bacteria
In this study, the potato was chosen as the model host-plant because of its sensitivity to a broad range of soft-rot bacteria and its key role in the world global food system as it is the world's fourth most produced food commodity [23]. Moreover, the large-scale cultivation of this plant facilitates the recurrent isolation of many isolates including isolates used in this study. A panel of twelve bacteria was compared for signal production (Tables 2 and 3). It was composed of three type strains which are very ancient isolates used as international taxonomic references of P. atrosepticum, P. carotovorum and Dickeya chrysanthemi (formerly Erwinia chrysanthemi) species [49]. Three reference strains which are commonly used for virulence studies were also included in this panel: (i) the psychrotroph strain P. atrosepticum CFBP 6276 known both for its virulence on the potato and for the unusual ability among this species to induce HR in non host plant [12], [40], (ii) the strain P. carotovorum EC153, isolated from rotted bell pepper fruit shipped from Mexico and which NAHSL production and virulence were exceptionally enabled at elevated temperatures above 34°C [24], and (iii) D. dadantii 3937, a mesophile strain isolated from the African violet, widely used as a model system for research on the molecular biology and pathogenicity of soft-rot bacteria and which genome has recently been published [50]. Finally, six recent isolates from potato blackleg wounds were added to this sampling. They represent the different soft-rot potato species currently encountered in European soils, including an emerging Dickeya clade, provisionally called D. solani [26], [27], [31]. All P. carotovorum strains of this collection belong to carotovorum subspecies division.
Table 3. Characterization of signaling molecules produced by potato soft-rot pathogens.
| Strains | AI-1 (NAHSL)*(ng/OD580) | AI-2# | Quinolones° | Auxins† (ng/OD580) | GABA§ | ||||||||
| 3-oxo-C6-HSL | 3-oxo-C8-HSL | 3-oxo-C10-HSL | C6-HSL | C8-HSL | Activity | HHQ | PQS | IAA | IPA | IBA | KA | ||
| Pectobacterium atrosepticum | |||||||||||||
| P. atrosepticum CFBP 1526T | 59±5 | 1475±125 | ≤5±2 | ≤5±2 | 67±10 | + | − | − | − | − | − | − | − |
| P. atrosepticum CFBP 6276 | 92±10 | 2300±250 | 10±3 | ≤5±2 | 100±14 | + | − | − | − | − | − | − | − |
| P. atrosepticum 100T | 26±6 | 650±100 | − | ≤5±2 | 34±4 | + | − | − | − | − | − | − | − |
| P. atrosepticum RNS 08.30.1A | 24±4 | 600±150 | − | ≤5±2 | 41±6 | + | − | − | − | − | − | − | − |
| Pectobacterium carotovorum | |||||||||||||
| P. carotovorum CFBP 2046T | 2500±125 | 100±5 | − | ≤5±2 | − | + | − | − | − | − | − | − | − |
| P. carotovorum EC153 | 25±4 | 625±100 | − | 36±3 | − | + | − | − | − | − | − | − | − |
| P. carotovorum 98.1 | 2225±200 | 89±8 | − | ≤5±2 | − | + | − | − | − | − | − | − | − |
| P. carotovorum RNS 08.42.1A | 950±225 | 38±9 | − | ≤5±2 | − | + | − | − | − | − | − | − | − |
| Dickeya spp. | |||||||||||||
| D. chrysanthemiCFBP 2048T | 950±75 | 23±3 | − | ≤5±2 | − | + | − | − | 11 990±1000 | − | 106±33 | 78±5 | − |
| D. dadantii3937 | 300±25 | 12±1 | − | ≤5±2 | − | + | − | − | 12 910±1440 | 364±71 | 78±7 | 54±20 | − |
| D. dianthicolaRNS 04.9 | 125±25 | 5±1 | − | ≤5±2 | − | + | − | − | 35 950±2350 | 22±10 | 90±30 | 67±20 | − |
| D. solaniRNS 08.23.3.1A | 50±5 | ≤5±2 | − | ≤5±2 | − | + | − | − | 876±190 | − | 82±2 | 66±10 | − |
Production of different N-acyl homoserine lactones (NAHSL) was determined by thin-layer chromatography (TLC) and HPLC-MS/MS. For quantification, NAHSL were extracted from PGA minimal medium culture at late exponential phase (optimal production).
Autoinducer-2 (AI-2) activity was determined using biosensor V. harveyi BB170.
Production of 4-hydroxy-2-heptylquinoline (HHQ) and Pseudomonas quinolone signal (PQS) were determined by TLC.
Production of indole-3-acetic acid (IAA), indole-3-propionic acid (IPA), indole-3-butyric acid (IBA) and kynurenic acid (KA) were determined by HPLC-UV. For quantification, indolic compounds were extracted from M9 minimal medium supplemented with l-tryptophan culture at stationary growth phase (optimal production).
Production of γ-amino butyric acid (GABA) was determined by ELISA test.(+) positive detection by the biosensor; (−) not detected or below threshold.
All strains were able to induce tissue maceration seven days after potato tuber inoculation. In addition, all the Dickeya strains were able to induce an HR in tobacco, but only half of Pectobacterium strains had this capacity (Table 2). This result is consistent with recent work published by Kim et al. [51] reporting that deletions can occur in the locus encoding the type III secretion system in Pectobacterium strains. However, no difference on potato virulence was observed between HR-negative and HR-positive Pectobacterium strains (Table 2).
Characterization of quorum sensing signal molecules was done on bacterial cells cultivated at 25°C. Two minimal media were used for that purpose. The mineral salt medium supplemented with PGA was used in each assay. This vegetable compound induces the synthesis of virulence factors involved in plant disease or resistance, which were shown to be under AI-1 QS control [12]. The M9 medium was supplemented with l-tryptophan, a precursor of numerous auxins and quinolones [52], [53]. The tryptophan was used as the sole source of carbon for bacterial growth during auxin and 2-alkyl-4-(1H)-quinolone detection assays. Produced NAHSLs were identified and quantified by TLC and HPLC coupled with mass spectrometry. In these conditions, all P. atrosepticum strains and the P. carotovorum EC153 strain produced mainly N-3-oxo-octanoyl-l-HSL (3-oxo-C8-HSL) and minor quantities of N-octanoyl-l-HSL (C8-HSL) and N-3-oxo-hexanoyl-l-HSL (3-oxo-C6-HSL) (Table 3). They also synthesized traces of N-hexanoyl-l-HSL (C6-HSL) and N-3-oxo-decanoyl-l-HSL (3-oxo-C10-HSL). The P. atrosepticum CFBP 6276 and 1526T strains were the largest producers of 3-oxo-C8-HSL with amounts superior to 1 µg/OD580. The other P. atrosepticum strains and P. carotovorum EC153 displayed lower production of NAHSL with about 600 ng/OD580 of 3-oxo-C8 plus amounts generally twenty-fold lower of C8-HSL. The three other P. carotovorum strains and all Dickeya spp. strains produced mainly 3-oxo-C6-HSL. Comparing these two bacterial genera revealed that the NAHSL production is lower in Dickeya than in Pectobacterium (Table 3).
The production of AI-2 was indirectly assessed by detecting AI-2 signal activity. Supernatants from all soft-rot pathogens studied here were tested for their ability to induce luminescence in V. harveyi BB170. In each case, the addition of 10% cell-free culture induced a luminescence emission within the reporter strain. As the positive control V. harveyi BB120, all strains are able to produce extracellular AI-2 activity (Table 3). In contrast, the negative control (E. coli DH5α) did not induce luminescence of the reporter strain.
Salkowski's reaction showed that only Dickeya. spp. can produce indole compounds. This production only occurred when bacteria were grown in the minimal medium supplemented with l-tryptophan. The characterization of the different auxins was carried out by HPLC-UV and compared to standards (Table 3). IAA was always the major auxin extracted from culture supernatants. Among all tested supernatants, the highest IAA amount was observed in the supernatant of isolate D. dianthicola RNS 04.9, while the lowest was found in the supernatant of isolate D. solani RNS 08.23.3.1A. All the Dickeya strains synthesized IBA and KA in amounts 10- to 700-fold lower than the IAA ones. Finally, traces of IPA are also detected in D. dadantii and D. dianthicola strains (Table 3).
The ability of soft-rot pathogens to produce 2-alkyl-4-(1H)-quinolones and GABA signaling molecules was also assessed using TLC visualized under UV and ELISA, respectively. We failed to detect HHQ, PQS or GABA in culture supernatants of all soft-rot pathogens, indicating that either these molecules were not produced or that they were produced at amounts lower than the detection limits estimated to be about 20 nmol for quinolones and 75 ng for GABA in our conditions (data not shown).
Kinetics of AI-1, AI-2 and IAA signal molecules production
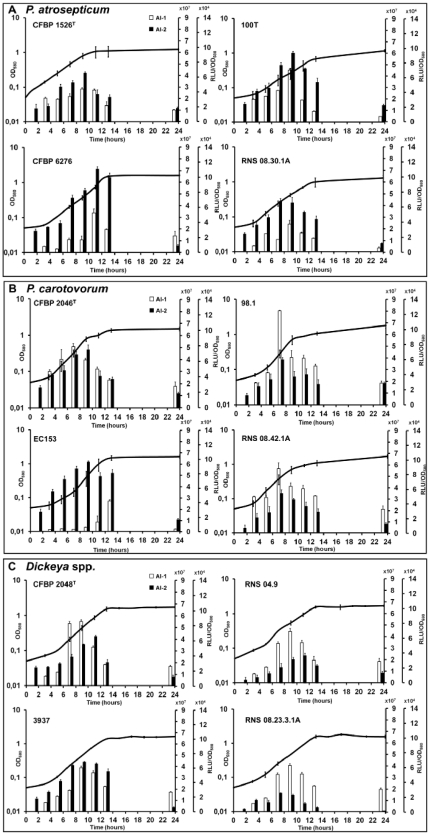
Free-cell supernatants were prepared from cultures at different ODs in PGA minimal medium. The kinetics of AI-1 and AI-2 production were then determined by bioluminescence assay using as reporters E. coli DH5α(pSB401) and V. harveyi BB170 strains, respectively. Results show a concomitant AI-1 and AI-2 production by all P. atrosepticum strains (Fig. 1A). AI-1 and AI-2 activities increased from the second half of the logarithmic phase with a peak between 8 and 12 hours, and then declined during the stationary phase, which started between 10 and 14 h in this culture medium (Fig. 1A). This shows the transient activities of AI-1 and AI-2 in all soft-rot bacteria whatever their origin. The two P. carotovorum isolates 98.1 and RNS 08.42.1A (Fig. 1B), the D. dadantii 3937 strain and the D. solani RNS 08.23.3.1A isolate (Fig. 1C) displayed production profiles similar to the ones of P. atrosepticum (Fig. 1A). On the other hand, P. carotovorum CFBP 2046T and EC153 presented atypical patterns with an early or a delayed AI-1 activity, respectively (Fig 1B). For Dickeya spp. strains, while AI-1 activity was always optimal at 9 h, AI-2 activity was more or less offset in time relative to that of AI-1 (Fig. 1C).
Figure 1. Kinetics of AI-1 and AI-2 activities measured in the supernatant of potato soft-rot.
Pectobacterium atrosepticum (A), Pectobacterium carotovorum (B) and Dickeya spp. (C) using a bioluminescence assay with Escherichia coli DH5α(pSB401) and Vibrio harveyi BB170 reporter strains, respectively. Bacterial growth was monitored by measuring optical density (OD) at 580 nm. For each point, at least 3 independent cultures in PGA minimal medium were analyzed, with standard deviation shown. Legend: white bars, AI-1 activity measurable with the first right Y-axis; black bars, AI-2 activity measurable with the second right Y-axis; RLU, relative luminescence unit.
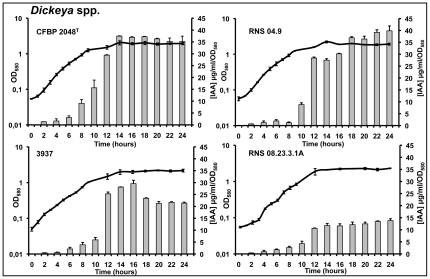
The production of auxins only occurred in Dickeya spp. strains grown in minimal medium supplemented with l-tryptophan. It was studied by a rapid colorimetric method using Salkowski's reagent. Among indolic compounds detected by this method, IAA is one of the most reactive [46]. According to our HPLC-UV results, the recorded concentrations of indolic compounds reflect essentially IAA concentrations found in culture supernatants. IAA-like concentrations increased from the middle of the exponential phase to reach their maximum values in the stationary phase (Fig. 2). Unlike AI-1 and AI-2 molecules, indole compounds persist at high concentrations throughout the stationary phase (Fig. 2), suggesting a considerable stability of the IAA-like molecules and/or the absence of a degradation mechanism.
Figure 2. Kinetics of IAA-like production measured in the supernatant of potato soft-rot Dickeya spp.
Indolic compounds were quantified by a colorimetric method with Salkowski's reagent. Bacterial growth was monitored by measuring optical density (OD) at 580 nm. For each point, at least 3 independent cultures in M9 minimal medium supplemented with l-tryptophan (500 µg/ml) were analyzed, with standard deviation shown.
Expression of AI-1, AI-2 and IAA synthase genes in soft-rot bacteria
To determine if the variations of signal production according to growth phase could result from expression modifications of the corresponding synthase genes, levels of specific transcripts have been estimated by semi-quantitative RT-PCR. Among them, luxI and luxS genes were chosen because they encode the enzyme catalyzing the last step in the pathway for AI-1 and AI-2 biosynthesis, respectively. The gene metK was added to this study because its product is responsible for the intracellular accumulation of SAM, a precursor common to AI-1 and AI-2 anabolic pathways (Fig. 3A). In the IAA synthesis pathway, only present in Dickeya strains (Fig. 2), tryptophan is first converted to indole-3-acetamine (IAM) by the IaaM tryptophan-2-monooxygenase, and IAM is then converted to IAA by the IaaH hydrolase [36], [54]. iaaM expression was followed during the bacterial growth, because it has been previously demonstrated that a iaaM gene disruption, but not a iaaH gene mutation, reduces the production of pectinolytic enzymes and effectors in strain D. dadantii 3937 [36].
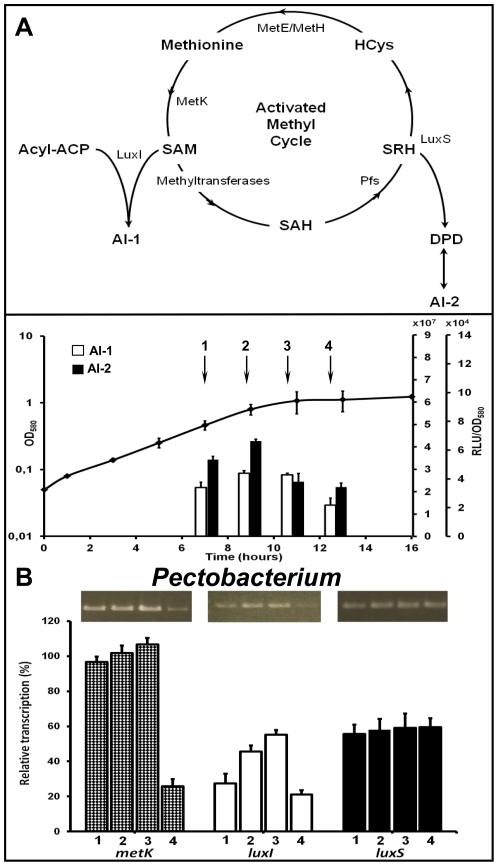
Figure 3. Kinetics of metK, luxI and luxS gene expression involved in AI-1 and AI-2 production by P. atrosepticum strain CFBP 1526T.
(A) AI-1 and AI-2 biosynthetic pathways are schematized from references [2], [67], [81]. The synthesis of S-adenosylmethionine (SAM), a key molecule precursor of both AI-1 and AI-2 signals, is catalyzed by the SAM synthase (MetK), which activates the methyl group of methionine after ATP hydrolysis. AI-1 signal is generated by LuxI enzyme from SAM and various acyls-ACP that determine the structural traits of each N-acyl homoserine lactone (NAHSL). AI-2 signal is generated as a by-product of the activated methyl cycle: S-adenosylhomocysteine (SAH) is produced by the action of methyltransferases, and is then converted to S-ribosylhomocysteine (SRH) by the nucleosidase Pfs. The enzyme LuxS converts SRH to homocysteine (HCys) and 4,5-dihydroxy 2,3-pentanedione (DPD), which spontaneously cyclizes into several furanones, including AI-2. Homocysteine is then recycled back to methionine using methionine synthases (MetH or MetE). (B) Abundances of metK (checkered bars), luxI (white bars) and luxS (black bars) mRNAs were determined by RT-PCR experiments on RNA extracts from cells grown in PGA minimal medium culture and harvested at mid-exponential phase (1), late exponential phase (2), during the transition from exponential to stationary phases (3) or early stationary growth phase (4) followed by electrophoresis on 1% (m/v) agarose gels. Results were expressed as a ratio: synthase transcripts vs. 16S transcripts. The corresponding kinetics of AI-1 and AI-2 activities in the supernatant of potato soft-rot Pectobacterium strain are shown at the top.
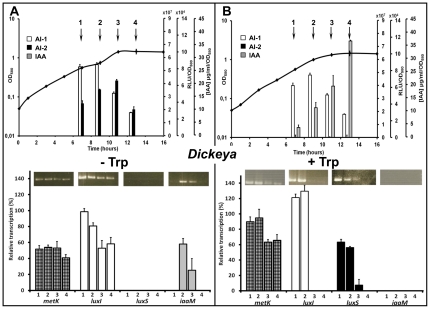
The type strains were chosen as models for this study. As the two Pectobacterium type strains yielded similar results, only P. atrosepticum CFBP 1526T and D. chrysanthemi CFBP 2048T data are presented here. RNAs were extracted from cells under the same culture conditions as those used for signal molecule extractions, i.e. in PGA or l-tryptophan supplemented media and at four different points during the bacterial growth (mid-exponential phase, late exponential phase, transition to stationary phase and early stationary phase). In these conditions, the expression of the two reference genes (16S rRNA and recA) was stable under all culture conditions whatever the growth phase. The variation in metK and luxI mRNA abundances is consistent with that of AI-1 activity levels throughout the bacterial growths. For both strains and in each culture condition, metK and luxI transcript amounts are generally more abundant at the end of the exponential phase of growth (Fig. 3B and 4). In contrast, the luxS expression varied depending on the bacterial genus. In Pectobacterium, luxS was expressed constitutively throughout the bacterial growth (Fig. 3B). Because they vary simultaneously in the same way, the kinetic of the AI-2 activity seems to be under the influence of metK transcription responsible for the SAM synthesis that occurs upstream of the metabolic pathway of AI-2 production. In Dickeya grown in PGA minimal medium, luxS expression is transient and at its most intense in the middle of exponential phase (Fig. 4A). Our results showed a variation of signals and synthases generation according to the carbon source available for the bacteria. In the absence of tryptophan (Fig. 4A), iaaM mRNA was not detected, explaining the lack of IAA production, whereas AI-1 and AI-2 activities showed the same transient profile as that of metK, luxI and luxS transcripts. In the presence of tryptophan (Fig. 4B), iaaM expression and IAA synthesis rose while the luxS expression and the AI-2 production were inhibited. If the production of iaaM transcript amount is transient, that of IAA continues to increase in culture until reaching a plateau, showing that this substance is accumulated in the extracellular medium (Fig. 2 and 4B).
Figure 4. Kinetics of metK, luxI, luxS and iaaM gene expression involved in AI-1, AI-2 and IAA production by D. chrysanthemi strain CFBP 2048T.
AI-1 and AI-2 biosynthetic pathways are described in the legend to Fig. 3. A third signal, the indole-3-acetic acid (IAA) is synthesized by the indole-3-acetamine (IAM) pathway, in which l-tryptophan is first converted to IAM by the key enzyme of this pathway, the IaaM tryptophan-2-monooxygenase. IAM is then converted to IAA by IaaM hydrolase [36], [54]. Abundances of metK (checkered bars), luxI (white bars), luxS (black bars) and iaaM (grey bars) mRNAs were determined by RT-PCR experiments on RNA extracts from cells grown in PGA minimal medium (A) or M9 minimal medium supplemented with l-tryptophan (500 µg/ml) (B) and harvested at mid-exponential phase (1), late exponential phase (2), during the transition from exponential to stationary phases (3) or early stationary growth phase (4) followed by electrophoresis on 1% (m/v) agarose gels. Results were expressed as a ratio: synthase transcripts vs. 16S transcripts. The corresponding kinetics of AI-1 and AI-2 activities and IAA production in the supernatant of potato soft-rot Dickeya strain are shown at the top.
Discussion
Communication systems play an important role throughout bacterial interactions with the host (virulence, symbiosis) and the environment (biofilm development). Fundamental knowledge on these communication systems are therefore of particular interest to the community of microbiologists and could find application in improving human health, food safety and crop yields [2]–[5]. As an example, novel biocontrol strategies based on the disruption of bacterial communication are being developed [23]. One of them consists in characterizing the signaling molecules used by the target pathogen, and then using harmless structural analogs to stimulate microflora able to degrade both molecule families [18]. Because the steps between the transfer of laboratory knowledge to the registration of biocontrol formulations are time- and money-consuming, the identification of the signals involved in host virulence and the study of their representation in the current and emerging pathogens are prerequisite for a global success of this approach. Unfortunately, there are random limited numbers of Pectobacterium and Dickeya genomes available: they do not reflect the diversity of the current fields isolates nor correspond to emergent strains. The signaling molecule production was therefore checked in a significant number of strains rather than relying on an in silico study only.
NAHSL-based QS is the best known autoinducer system, including in soft-rot bacteria [15]. The production of signal molecules involved in this QS system has indeed been observed very early in P. carotovorum [14], [16] and then in D. dadantii [22]. More recently, Chatterjee et al. [55] defined two classes of QS systems, using mainly 3-oxo-C8-HSL (Class I) or 3-oxo-C6-HSL (Class II) as autoinducer. This duality is linked to the structural characteristics of the NAHSL synthase (LuxI-type protein) encountered in each Pectobacterium strain [56], [57]. It is also related to the existence in Pectobacterium of two types of NAHSL receptors (LuxR-type proteins) that have a different affinity for each of the two NAHSLs [58]–[60]. Our results show that NAHSLs production is widespread within both old and emerging soft-rot bacteria isolates. Minor species of NAHSLs, likely to be less specific products of LuxI synthase or catabolites resulting from NAHSLs turnover, coexist with 3-oxo-C6-HSL and 3-oxo-C8-HSL, the two molecules identified as true QS signals [13], [56], [57]. However, the nature of these signals seems to be dependent on the bacterial genus or species. P. atrosepticum strains produce 3-oxo-C8-HSL while Dickeya spp. produce 3-oxo-C6-HSL as signal molecules. These findings are consistent with previous studies made on other soft-rot strains [21], [43], [55], [61]. There is however an exception to this rule: to our knowledge, P. atrosepticum SCRI1043 is the only studied strain harboring the QS signaling system of class II and unfortunately it is today the only strain with a sequenced genome [62], [63]. The class I and II distinction is not easy for P. carotovorum strains. Although a majority of strains produce 3-oxo-C6-HSL, at least P. carotovorum EC153 and SCC3193 strains produce 3-oxo-C8-HSL as signaling molecules [24], [56], [64]. These differences could be explained by the strong heterogeneity of the P. carotovorum species and the diversity of their hosts and environmental niches [65], [66].
AI-2 is another type of autoinducer described as a mediator of interspecies communication in Gram-positive and Gram-negative bacteria [5], [67]. Today, the fine structure of AI-2 has been only resolved in V. harveyi (as furanosyl borate diester) and Salmonella enterica ser. typhimurium (as 2R,4S tetrahydroxy-tetrahydrofurane) after co-crystallization of AI-2 with their binding protein [68], [69]. Nevertheless, the ‘AI-2’ term refers more generally to furanones derived from the spontaneous cyclization of 4,5-dihydroxy 2,3-pentanedione (DPD) with or without boron, and which induce bioluminescence in the V. harveyi bioassay [67]. The production of AI-2 and the presence of the AI-2 synthase (LuxS-type protein) have been demonstrated in three Pectobacterium strains in 2006. In two of them, ATCC39048 and SCC3193, a luxS mutation affects bacterial motility, virulence and the progression of disease symptoms during early stages of infection through the modulation of the expression of pectinolytic enzymes [33], [34]. In contrast, virulence does not seem to be affected in a luxS derivative of P. atrosepticum SCRI1043 [33]. For the first time, we are reporting AI-2 synthesis within four Dickeya species and, more consequently, the ubiquitous production of this signaling molecule by potato soft-rot pathogens. Provided that physiological function is confirmed, this autoinducer therefore can be a novel target for overall pathogen control in plants.
The 2-alkyl-4-(1H)-quinolones form a third family of QS signaling molecules recently reviewed by Heeb et al. [70], in which PQS and HHQ are the best known members. These autoinducers were involved in regulation and expression of multiple virulence genes in P. aeruginosa and related γ-Proteobacteria [71]–[73]. In addition, the production of quinolones and NAHSL are closely linked to each other within P. aeruginosa. In this species, the effectiveness of the two QS systems depends on synchronous interactions of these molecules [74]. As soft-rot bacteria are also γ-Proteobacteria which synthesize NAHSL, we attempted to detect, in vain, the production of HHQ and PQS with or without l-tryptophan precursor in the culture medium. It is unlikely that these signals are used by the six bacterial species studied. Consistently, the available Pectobacterium and Dickeya genomes are devoid of genes for enzymes similar to known HHQ and PQS synthases (data not shown).
Auxins are well known phytohormones, among which the most famous member is IAA [54]. IAA is also produced by bacteria and its synthesis can be autoinduced [38], [52]. Several recent reports indicate that IAA and some other auxins can function as signal molecules with a direct effect on bacterial physiology for example in the transition from a low- to a high-cell-density state [39], [53], [54]. Indole compounds have therefore the potential to be QS molecules although their perception and transport mechanisms have not yet been elucidated in bacteria [39], [54]. IAA synthesis has been shown to be required for full virulence of D. dadantii 3937 in Saintpaulia host plant, influencing the local maceration symptoms in leaves but not the bacterial growth in plants [36]. As IAA controls virulence gene expression via the RsmA/rsmB pathway in a similar manner to NAHSL in Pectobacterium, IAA has been suggested to play an analogous role in D. dadantii [75]. Our results show that all tested Dickeya species produce IAA as the major auxin. On the contrary, in our conditions, the fact that none of the Pectobacterium strains is able to synthesize a detectable indolic compound means an important distinction between the two bacterial genera responsible for potato soft-rot. To date, six IAA anabolic pathways were identified in bacteria [54]. Among them, only the IAM pathway was identified in D. dadantii 3937 [36]. In this pathway, tryptophan is first converted by iaaM gene product, which plays a key role. The iaaM expression is transient, whereas IAA accumulates in the culture supernatant during the stationary phase. Furthermore, iaaM transcription and IAA production occur only in the presence of l-tryptophan. This suggests that tryptophan can act both as a precursor and as an inducer of durable signal molecule synthesis.
GABA is also an extracellular signal molecule involved in multiple plant-microorganism interactions [76]. GABA is rapidly accumulated by plants in response to a variety of stresses including bacterial or fungal infection at wounding sites [77]. In numerous γ-Proteobacteria, GABA is synthesized by glutamate decarboxylase that catalyses the irreversible α-decarboxylation of glutamate [78]. This molecule can affect the behavior and the QS system of the pathogen. For example, GABA and IAA signaling pathways are suspected to interfere with the virulence program of the plant pathogen Agrobacterium tumefaciens [37]. GABA also may modulate bacterial QS, thereby affecting the virulence of Agrobacterium by controlling the level of intracellular NAHSL [35], [79]. In our test conditions, Pectobacterium and Dickeya spp. do not produce detectable amounts of GABA. Moreover, the absence of glutamate decarboxylase-like enzymes among the proteins encoded by the known genomes of Pectobacterium and Dickeya strengthens the notion that this amino acid does not play a role in the soft-rot bacteria communication (data not shown).
To our knowledge, the interactions between signal production pathways of soft-rot bacteria were not previously studied. We investigated the expression of key genes involved in metabolic pathways of AI-1, AI-2 and IAA, including the expression of SAM synthase, because AI-1 and AI-2 productions are dependent on the available SAM pool. In the cell, the primary roles of SAM are to serve as a precursor for the membrane phospholipid phosphatidyl-choline and as methyl group donor for methylation of nucleic acids and other molecules. In addition, SAM is a substrate for the synthetic reactions leading to AI-1 and AI-2 molecules [80]. In the bacterial cytosol, the SAM synthase (MetK) is responsible for intracellular generation of SAM, while the NAHSL synthase (LuxI) is directly responsible for the net consumption of a part of the SAM pool. Another part of this pool enters in the activated methyl cycle in which the AI-2 synthase (LuxS) yields the 4,5-dihydroxy 2,3-pentanedione (DPD), which then spontaneously cyclizes to yield AI-2. Here, there is no SAM consumption since the second product of this reaction, the homocysteine, is recycled to favor SAM regeneration [67], [81], [82]. Our results show that only 7 of 12 soft-rot strains have concomitant kinetics of AI-1 and AI-2 activity. Therefore there is no obligate link between the production kinetics of these two signals throughout the bacterial growth. These observations are confirmed by differences in AI-1 and AI-2 synthase gene transcription in Pectobacterium. If the NAHSL production appears to be directly linked to the NAHSL synthase expression, the AI-2 one seems rather under the control of the activated methyl cycle and the SAM synthase expression. Nevertheless, we confirm the hypothesis that one of the mechanisms involved in the regulation of Pectobacterium AI-1 QS consists of controlling NAHSL synthase production by modulating the amounts of luxI transcripts [13]. Another outcome of the present work is that AI-2 and IAA signaling pathways do not seem to act simultaneously. The switch occurs in Dickeya bacteria according to tryptophan amino acid availability in the microenvironment. Under conditions with sufficient amount of tryptophan, luxS mRNA is undetectable (whereas the enzyme MetK remains active to provide the necessary SAM) while iaaM expression and IAA production are induced. At low tryptophan concentration, only AI-2 production takes place. Tryptophan is exuded from plants in large quantities within the rhizosphere [83]. It is also involved in IAA synthesis that occurs in plant cells during lateral root initiation. Therefore it is widely released by older root sections resulting from lateral root perforation of the root epidermis [84]. The release of significant amounts of indole compounds (i.e. plant tryptophan or IAA) may therefore induce a change in the type of QS-based communication, thereby revealing to Dickeya the proximity of its privileged infection areas, such as root hair emergence or wounded roots. This could partly explain why Dickeya isolates appear to be better root invaders than Pectobacterium isolates (V. Hélias, unpublished data).
In conclusion, we showed that potato soft-rot bacteria possess the common ability to produce a substituted NAHSL and a DPD-derived furanone as signal molecules with a transient activity. In addition, Dickeya spp. can produce stable quantities of IAA instead of the furanone autoinducer in tryptophan rich condition. All these signaling molecules have been identified for the first time in an isolate belonging to the novel D. solani species. To this step of our knowledge, results designate 3-oxo-C6/8-HSL as the main targets for a global biocontrol of soft-rot bacteria communications, including those of emerging isolates. Even if the production of signaling molecules was shown in media that mimic plant wound conditions and promote the synthesis of induced virulence factors, the next stage of this work will be to assess the role of each bacterial signaling pathway in planta virulence.
Supporting Information
Primers used for RT-PCR of potato soft-rot pathogens.
(DOC)
Acknowledgments
These works are related to COST 631 action-Understanding and Modelling Plant-Soil Interactions in the Rhizosphere Environment. The authors thank Dr Bonnie Bassler and Dr Arun Chatterjee for kindly providing the V. harveyi BB120 and BB170, and the P. carotovorum EC153 strains respectively. They also thank Sandra Leblanc from Biogalenys society, Asma Ouchiha and Raphaël Vanleynseele for their technical assistance, and Christine Farmer for linguistic support.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by: European Union grants: Fonds Européens de DEveloppement Régional (FEDER); National grants: the National Association of Technical Research through the Compte d'Affectation Spéciale pour le Développement Agricole et Rural, (CAS-DAR AAP 7124 and the Conventions Industrielles de Formation par la Recherche (CIFRE) carried by the Association Nationale de la Recherche et de la Technologie (ANRT); Regional grants: Conseil Régional de Haute-Normandie & Ministère délégué à l'Enseignement Supérieur et à la Recherche through the Grand Réseau de recherche Régional Végétal, Agronomie, Sols et Innovations (GRR VASI, ex-GRR VATA). All for financial supports of researches. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Fuqua WC, Winans SC, Greenberg EP. Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J Bacteriol. 1994;176:269–275. doi: 10.1128/jb.176.2.269-275.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keller L, Surette MG. Communication in bacteria: an ecological and evolutionary perspective. Nat Rev. 2006;4:249–258. doi: 10.1038/nrmicro1383. [DOI] [PubMed] [Google Scholar]
- 3.Reading NC, Sperandio V. Quorum sensing: the many languages of bacteria. FEMS Microbiol Lett. 2006;254:1–11. doi: 10.1111/j.1574-6968.2005.00001.x. [DOI] [PubMed] [Google Scholar]
- 4.Ryan RP, Dow JM. Diffusible signals and interspecies communication in bacteria. Microbiology. 2008;154:1845–1858. doi: 10.1099/mic.0.2008/017871-0. [DOI] [PubMed] [Google Scholar]
- 5.Waters CM, Bassler BL. Quorum-sensing: cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol. 2005;21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- 6.Charkowsky AO. The Soft Rot Erwinia. In: Gnanamanickam SS, editor. Plant-Associated Bacteria. Netherlands: Springer; 2006. pp. 423–505. [Google Scholar]
- 7.Czajkowski R, Pérombelon MCM, van Veen JA, van der Wolf JM. Control of blackleg and tuber soft rot of potato caused by Pectobacterium and Dickeya species: a review. Plant Pathol. 2011;60:999–1013. [Google Scholar]
- 8.Diallo S, Latour X, Groboillot A, Copin P, Smadja B, et al. Simultaneous and selective detection of two major soft rot pathogens of potato: Pectobacterium atrosepticum (Erwinia carotovora subsp. atrosepticum) and Dickeya spp. (Erwinia chrysanthemi). Eur J Plant Pathol. 2009;125:349–354. [Google Scholar]
- 9.Latour X, Faure D, Diallo S, Cirou A, Smadja B, et al. Control of bacterial diseases of potato caused by Pectobacterium spp. (Erwinia carotovora). Cah Agric. 2008;17:355–360. [Google Scholar]
- 10.Pérombelon MCM. Potato diseases caused by soft rot erwinias: an overview of pathogenesis. Plant Pathol. 2002;51:1–12. [Google Scholar]
- 11.Liu H, Coulthurst SJ, Pritchard L, Hedley PE, Ravensdale M, et al. Quorum sensing coordinates brute force and stealth modes of infection in the plant pathogen Pectobacterium atrosepticum. PloS Pathogens. 2008;20:e1000093. doi: 10.1371/journal.ppat.1000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smadja B, Latour X, Faure D, Chevalier S, Dessaux Y, et al. Involvement of N-acylhomoserine lactones throughout the plant infection by Erwinia carotovora subsp. atroseptica (Pectobacterium atrosepticum). Mol Plant-Microbe Interact. 2004;17:1269–1278. doi: 10.1094/MPMI.2004.17.11.1269. [DOI] [PubMed] [Google Scholar]
- 13.Latour X, Diallo S, Chevalier S, Morin D, Smadja B, et al. Thermoregulation of N-acyl homoserine lactones-based quorum sensing in the soft rot bacterium Pectobacterium atrosepticum. Appl Environ Microbiol. 2007;73:4078–4081. doi: 10.1128/AEM.02681-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bainton NJ, Stead P, Chhabra SR, Bycroft BW, Salmond GPC, et al. N-(3-oxohexanoyl)-L-homoserine lactone regulates carbapenem antibiotic production in Erwinia carotovora. Biochem J. 1992;288:997–1004. doi: 10.1042/bj2880997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barnard AM, Salmond GP. Quorum sensing in Erwinia species. Anal Bioanal Chem. 2007;387:415–423. doi: 10.1007/s00216-006-0701-1. [DOI] [PubMed] [Google Scholar]
- 16.Pirhonen M, Flego D, Heikinheimo R, Palva ET. A small diffusible signal molecule is responsible for the global control of virulence and exoenzyme production in plant pathogen Erwinia carotovora. EMBO J. 1993;12:2467–2476. doi: 10.1002/j.1460-2075.1993.tb05901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faure D, Dessaux Y. Quorum sensing as a target for developing control strategies for the plant pathogen Pectobacterium. Eur J Plant Pathol. 2007;119:353–365. [Google Scholar]
- 18.Crépin A, Barbey C, Cirou A, Tannières M, Orange N, et al. Biological control of pathogen communication in the rhizosphere: a novel approach applied to potato soft rot due to Pectobacterium atrosepticum. Plant Soil. 2011 In press. DOI: 10.1007/s11104-011-1030-5. [Google Scholar]
- 19.Cirou A, Raffoux A, Diallo S, Latour X, Dessaux Y, et al. Gamma-caprolactone stimulates growth of quorum-quenching Rhodococcus populations in a large-scale hydroponic system for culturing Solanum tuberosum. Res Microbiol. 2011;162:945–950. doi: 10.1016/j.resmic.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 20.Cirou A, Uroz S, Chapelle E, Latour X, Orange N, et al. Quorum sensing as a target for novel biocontrol strategies. In: Gisi U, Chet I, Gullino ML, editors. Recent developments in disease management. Plant Pathology in the 21st Century. Netherlands: Springer; 2009. pp. 121–132. [Google Scholar]
- 21.Ham JH, Cui Y, Alfano JR, Rodriguez-Palenzuela P, Rojas CM, et al. Analysis of Erwinia chrysanthemi EC16 pelE::uidA, pel::uidA, and hrpN::uidA mutants reveals strain-specific atypical regulation of the Hrp type III secretion system. Mol Plante-Microbe Interact. 2004;17:184–194. doi: 10.1094/MPMI.2004.17.2.184. [DOI] [PubMed] [Google Scholar]
- 22.Nasser W, Bouillant ML, Salmond GPC, Reverchon S. Characterization of the Erwinia chrysanthemi expI-expR locus directing the synthesis of two N-acyl-homoserine lactone signal molecules. Mol Microbiol. 1998;29:1391–1405. doi: 10.1046/j.1365-2958.1998.01022.x. [DOI] [PubMed] [Google Scholar]
- 23.Diallo S, Crépin A, Barbey C, Orange N, Burini J-F, et al. Mechanisms and recent advances in biological control mediated through the potato rhizosphere. FEMS Microbiol Ecol. 2011;75:351–364. doi: 10.1111/j.1574-6941.2010.01023.x. [DOI] [PubMed] [Google Scholar]
- 24.Hasegawa H, Chatterjee A, Cui Y, Chatterjee AK. Elevated temperature enhances virulence of Erwinia carotovora subsp. carotovora strain EC153 to plants and stimulates production of the quorum-sensing signal, N-acyl homoserine lactone, and extracellular proteins. Appl Environ Microbiol. 2005;71:4655–4663. doi: 10.1128/AEM.71.8.4655-4663.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zink RT, Kemble RJ, Chatterjee AK. Transposon Tn5 mutagenesis in Erwinia carotovora subsp. carotovora and Erwinia carotovora subsp. atroseptica. J Bacteriol. 1984;157:809–814. doi: 10.1128/jb.157.3.809-814.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Czajkowski R, Grabe GJ, van der Wolf JM. Distribution of Dickeya spp. and Pectobacterium carotovorum subsp. carotovorum in naturally infected seed potatoes. Eur J Plant Pathol. 2009;125:263–275. [Google Scholar]
- 27.Hélias V, Hamon P, Huchet E, van der Wolf J, Andrivon D. Two new effective semiselective crystal violet pectate media for isolation of Pectobacterium and Dickeya. Plant Pathol. 2011 In press. Doi: 10.1111/j.1365-3059.2011.02508.x. [Google Scholar]
- 28.Ma B, Hibbing ME, Kim H-S, Reedy RM, Yedidia I, et al. Host range and molecular phylogenies of the soft rot enterobacterial genera Pectobacterium and Dickeya. Phytopathology. 2007;97:1150–1163. doi: 10.1094/PHYTO-97-9-1150. [DOI] [PubMed] [Google Scholar]
- 29.Pitman AR, Harrow SA, Visnovsky SB. Genetic characterization of Pectobacterium wasabiae causing soft rot disease of potato in New Zealand. Eur J Plant Pathol. 2010;126:423–435. [Google Scholar]
- 30.Slawiak M, Van Beckhoven JRCM, Specksnijder AGCL, Czajkowski R, Grabe G, et al. Biochemical and genetical analysis reveal a new clade of biovar 3 Dickeya spp. strains isolated from potato in Europe. Eur J Plant Pathol. 2009;125:245–261. [Google Scholar]
- 31.Toth IK, van der Wolf JM, Saddler G, Lojkowska E, Hélias V, et al. Dickeya species: an emerging problem for potato production in Europe. Plant Pathol. 2011;60:385–399. [Google Scholar]
- 32.Tsor (Lahkim) L, Erlich O, Lebiush S, Hazanovsky M, Zig U. Assessment of recent outbreaks of Dickeya sp. (syn. Erwinia chrysanthemi) slow wilt in potato crops in Israel. Eur J Plant Pathol. 2009;123:311–320. [Google Scholar]
- 33.Coulthurst SJ, Lilley KS, Salmond GPC. Genetic and proteomic analysis of the role of luxS in the enteric phytopathogen, Erwinia carotovora. Mol Plant Pathol. 2006;7:31–45. doi: 10.1111/j.1364-3703.2005.00314.x. [DOI] [PubMed] [Google Scholar]
- 34.Laasik E, Andresen L, Maë A. Type II quorum sensing regulates virulence in Erwinia carotovora ssp. carotovora. FEMS Microbiol Lett. 2006;258:227–234. doi: 10.1111/j.1574-6968.2006.00222.x. [DOI] [PubMed] [Google Scholar]
- 35.Chevrot R, Rosen R, Haudecoeur E, Cirou A, Shelp BJ. GABA controls the level of quorum-sensing signal in Agrobacterium tumefaciens. Proc Natl Acad Sci USA. 2006;103:7460–7464. doi: 10.1073/pnas.0600313103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang S, Zhang Q, Guo J, Charkowsky AO, Glick BR, et al. Global effect of indole-3-acetic acid biosynthesis on multiple virulence factors of Erwinia chrysanthemi 3937. Appl Environ Microbiol. 2007;73:1079–1088. doi: 10.1128/AEM.01770-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yuan ZC, Haudecoeur E, Faure D, Kerr KF, Nester EW. Comparative transcriptome analysis of Agrobacterium tumefaciens in response to plant signal salicylic acid, indole-3-acetic acid and gamma-amino butyric acid reveals signalling cross-talk and Agrobacterium–plant co-evolution. Cell Microbiol. 2008;10:339–354. doi: 10.1111/j.1462-5822.2008.01215.x. [DOI] [PubMed] [Google Scholar]
- 38.Vande Broek A, Lambrecht M, Eggermont K, Vanderleyden J. Auxins upregulate expression of the indole-3-pyruvate decarboxylase gene in Azospirillum brasilense. J Bacteriol. 1999;181:1338–1342. doi: 10.1128/jb.181.4.1338-1342.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee J-H, Lee J. Indole as an intracellular signal in microbial communities. FEMS Microbiol Rev. 2010;34:426–444. doi: 10.1111/j.1574-6976.2009.00204.x. [DOI] [PubMed] [Google Scholar]
- 40.Smadja B, Latour X, Trigui S, Burini J-F, Chevalier S, et al. Thermodependence of growth and enzymatic activities implicated in pathogenicity of two Erwinia carotovora subspecies (Pectobacterium spp.). Can J Microbiol. 2004;50:19–27. doi: 10.1139/w03-099. [DOI] [PubMed] [Google Scholar]
- 41.Morin D, Grasland B, Vallée-Réhel K, Dufau C, Haras D. On-line high-performance liquid chromatography-mass spectrometric detection and quantification of N-acyl homoserine lactones, quorum-sensing signal molecules, in the presence of biological matrices. J Chromatogr A. 2003;1002:79–92. doi: 10.1016/s0021-9673(03)00730-1. [DOI] [PubMed] [Google Scholar]
- 42.Shaw PD, Ping G, Daly SL, Cha C, Cronan JE, Jr, et al. Detecting and characterization N-acyl-homoserine lactone signal molecules by thin layer chromatography. Proc Natl Acad Sci USA. 1997;94:6036–6041. doi: 10.1073/pnas.94.12.6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cha C, Gao P, Chen Y-C, Shaw P, Farrand SK. Production of acyl-homoserine lactone quorum-sensing signals by Gram-negative plant-associated bacteria. Mol Plant-Microbe Interact. 1998;11:1119–1129. doi: 10.1094/MPMI.1998.11.11.1119. [DOI] [PubMed] [Google Scholar]
- 44.Surette MG, Bassler BL. Quorum sensing in Escherichia coli and Salmonella typhimurium. Proc Natl Acad Sc USA. 1998;95:7046–7050. doi: 10.1073/pnas.95.12.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Winson MK, Swift S, Fish L, Throup JP, Jorgensen F. Construction and analysis of luxCDABE-based plasmid sensors for investigating N-acyl homoserine lactone-mediated quorum sensing. FEMS Microbiol Lett. 1998;163:185–192. doi: 10.1111/j.1574-6968.1998.tb13044.x. [DOI] [PubMed] [Google Scholar]
- 46.Glickman E, Dessaux Y. A Critical examination of the specificity of the Salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Appl Environ Microbiol. 1995;61:793–796. doi: 10.1128/aem.61.2.793-796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fessehaie A, De Boer SH, Levesque CA. Molecular characterization of DNA encoding 16S–23S rRNA intergenic spacer regions and 16S rRNA of pectinolytic Erwinia species. Can J Microbiol. 2002;48:387–398. doi: 10.1139/w02-026. [DOI] [PubMed] [Google Scholar]
- 48.Takle GW, Toth IK, Brurberg MB. Evaluation of reference genes for real-time RT-PCR expression studies in the plant pathogen Pectobacterium atrosepticum. BMC Plant Biol. 2007;7:50. doi: 10.1186/1471-2229-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Samson R, Legendre JB, Christen R, Fisher-Le Saux M, Achouak W, et al. Transfer of Pectobacterium chrysanthemi (Burkholder et al. 1953) Brenner et al. 1973 and Brenneria paradisiaca to the genus Dickeya gen. nov. as Dickeya chrysanthemi comb. nov. and Dickeya paradisiaca comb. nov. and delineation of four novel species: Dickeya dadantii sp. nov., Dickeya dianthicola sp. nov., Dickeya dieffenbachiae sp. nov. and Dickeya zeae sp. nov. Int J Syst Evol Microbiol. 2005;55:1415–1427. doi: 10.1099/ijs.0.02791-0. [DOI] [PubMed] [Google Scholar]
- 50.Glasner JD, Yang C-H, Reverchon S, Hugouvieux-Cotte-Pattat N, Condemine G, et al. Genome sequence of the plant-pathogenic bacterium Dickeya dadantii 3937. J Bacteriol. 2011;193:2076–2077. doi: 10.1128/JB.01513-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim H-S, Ma B, Perna NT, Charkowski A. Phylogeny and virulence of naturally occurring type III secretion system-deficient Pectobacterium strains. Appl Environ Microbiol. 2009;75:4539–4549. doi: 10.1128/AEM.01336-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patten CL, Glick BR. Bacterial biosynthesis of indole-3-acetic acid. Can J Microbiol. 1996;42:207–220. doi: 10.1139/m96-032. [DOI] [PubMed] [Google Scholar]
- 53.Shen D-K, Filopon D, Chaker H, Boullanger S, Derouazi M, et al. High-cell-density regulation of the Pseudomonas aeruginosa type III secretion system: implications for tryptophan catabolites. Microbiology. 2008;154:2195–2208. doi: 10.1099/mic.0.2007/013680-0. [DOI] [PubMed] [Google Scholar]
- 54.Spaepen S, Vanderleyden J, Remans R. Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol Rev. 2007;31:425–448. doi: 10.1111/j.1574-6976.2007.00072.x. [DOI] [PubMed] [Google Scholar]
- 55.Chatterjee A, Cui Y, Hasegawa H, Leigh N, Dixit V, et al. Comparative analysis of two classes of quorum-sensing signaling systems that control production of extracellular proteins and secondary metabolites in Erwinia carotovora subspecies. J Bacteriol. 2005;187:8026–8038. doi: 10.1128/JB.187.23.8026-8038.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brader G, Sjöblom S, Hyytiäinen H, Sims-Huopaniemi K, Palva ET. Altering substrate chain length specificity of an acylhomoserine lactone synthase in bacterial communication. J Biol Chem. 2005;280:10403–10409. doi: 10.1074/jbc.M408603200. [DOI] [PubMed] [Google Scholar]
- 57.Welch M, Dutton JM, Glansdorp FG, Thomas GL, Smith DS, et al. Structure-activity relationships of Erwinia carotovora quorum sensing signaling molecules. Bioorg Med Chem Lett. 2005;15:4235–4238. doi: 10.1016/j.bmcl.2005.06.066. [DOI] [PubMed] [Google Scholar]
- 58.Burr T, Barnard AML, Corbett MJ, Pemberton CL, Simpson NJL, et al. Identification of the central quorum sensing regulator of virulence in the enteric phytopathogen, Erwinia carotovora; the VirR repressor. Mol Microbiol. 2006;59:113–125. doi: 10.1111/j.1365-2958.2005.04939.x. [DOI] [PubMed] [Google Scholar]
- 59.Cui Y, Chatterjee A, Hasegawa H, Chatterjee AK. Erwinia carotovora subspecies produce duplicate variants of ExpR, LuxR homologs that activate rsmA transcription but differ in their interactions with N-acylhomoserine lactone signals. J Bacteriol. 2006;188:4715–4726. doi: 10.1128/JB.00351-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sjöblom S, Brader G, Koch G, Palva ET. Cooperation of two distinct ExpR regulators controls quorum sensing specificity and virulence in the plant pathogen Erwinia carotovora. Mol Microbiol. 2006;60:1474–1489. doi: 10.1111/j.1365-2958.2006.05210.x. [DOI] [PubMed] [Google Scholar]
- 61.Hussain MBB, Zhang H-B, Xu J-L, Liu Q, Jiang Z, et al. The acyl-homoserine lactone-type quorum sensing system modulates cell motility and virulence of Erwinia chrysanthemi pv. zeae. J Bacteriol. 2008;190:1045–1053. doi: 10.1128/JB.01472-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bell KS, Sebaihia M, Pritchard L, Holden MT, Hyman LJ, et al. Genome sequence of the enterobacterial phytopathogen Erwinia carotovora subsp. atroseptica and characterization of virulence factors. Proc Natl Acad Sci USA. 2004;101:11105–11110. doi: 10.1073/pnas.0402424101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Toth IK, Pritchard L, Birch PRJ. Comparative genomics reveals what makes an enterobacterial plant pathogen. Annu Rev Phytopathol. 2006;44:305–336. doi: 10.1146/annurev.phyto.44.070505.143444. [DOI] [PubMed] [Google Scholar]
- 64.Jafra S, Jalink H, van der Schoor R, van der Wolf JM. Pectobacterium carotovorum subsp. carotovorum strains show diversity in production of response to N-acyl homoserine lactones. J Phytopathol. 2006;154:729–739. [Google Scholar]
- 65.Gardan L, Gouy C, Christen R, Samson R. Elevation of three subspecies of Pectobacterium carotovorum to species level: Pectobacterium atrosepticum sp. nov., Pectobacterium betavasculorum sp. nov. and Pectobacterium wasabiae sp. nov. Int J Syst Evol Microbiol. 2003;53:381–391. doi: 10.1099/ijs.0.02423-0. [DOI] [PubMed] [Google Scholar]
- 66.Glasner JD, Marquez-Villavicencio M, Kim H-S, Jahn CE, Ma B, et al. Niche-specificity and the variable fraction of the Pectobacterium Pan-genome. Mol Plant-Microbe Interact. 2008;21:1549–1560. doi: 10.1094/MPMI-21-12-1549. [DOI] [PubMed] [Google Scholar]
- 67.Vendeville A, Winzer K, Heurlier K, Tang CM, Hardie KR. Making ‘sense’ of metabolism: autoinducer-2, LuxS and pathogenic bacteria. Nat Rev. 2005;3:1–16. doi: 10.1038/nrmicro1146. [DOI] [PubMed] [Google Scholar]
- 68.Chen X, Schauder S, Potier N, Van Dorsselaer A, Pelczer I, et al. Structural identification of a bacterial quorum-sensing signal containing boron. Nature. 2002;415:545–549. doi: 10.1038/415545a. [DOI] [PubMed] [Google Scholar]
- 69.Miller ST, Xavier KB, Campagna SR, Tagal ME, Semmelhack MF, et al. Salmonella typhimurium recognizes a chemically distinct form of the bacterial quorum-sensing signal AI-2. Mol Cell. 2004;15:677–687. doi: 10.1016/j.molcel.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 70.Heeb S, Fletcher MP, Chhabra SR, Diggle SP, Williams P, et al. Quinolones: from antibiotics to autoinducers. FEMS Microbiol Rev. 2011;35:247–274. doi: 10.1111/j.1574-6976.2010.00247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Déziel E, Lépine F, Milot S, He J, Mindrinos MN, et al. Analysis of Pseudomonas aeruginosa 4-hydroxy-2-alkylquinolines (HAQs) reveals a role for 4-hydroxy-2-heptylquinoline in cell-to-cell communication. Proc Natl Acad USA. 2004;101:1339–1344. doi: 10.1073/pnas.0307694100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fernandez-Piñar R, Cámara M, Dubern J-F, Ramos JL, Espinosa-Urgel ME. The Pseudomonas aeruginosa quinolone quorum sensing signal alters the multicellular behaviour of Pseudomonas putida KT2440. Res Microbiol. 2011;162:773–781. doi: 10.1016/j.resmic.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 73.Kim K, Kim YU, Koh BH, Hwang SS, Kim S-H, et al. HHQ and PQS, two Pseudomonas aeruginosa quorum-sensing molecules, down-regulate the innate responses through the nuclear factor-κB pathway. Immunology. 2010;129:578–588. doi: 10.1111/j.1365-2567.2009.03160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ortori CA, Dubern J-F, Chhabra SR, Cámara M, Hardie K, et al. Simultaneous quantitative profiling of N-acyl-l-homoserine lactone and 2-alkyl-4(1H)-quinolone families of quorum sensing signaling molecules using LC-MS/MS. Anal Bioanal Chem. 2011;399:839–850. doi: 10.1007/s00216-010-4341-0. [DOI] [PubMed] [Google Scholar]
- 75.Charkowsky AO. Decaying signals: will understanding bacterial-plant communications lead to control of soft rot. Curr Opin Biotechnol. 2009;20:1–7. doi: 10.1016/j.copbio.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 76.Shelp BJ, Bown AW, Faure D. Extracellular gamma-aminobutyrate mediates communication between plants and other organisms. Plant Physiol. 2006;142:1350–1352. doi: 10.1104/pp.106.088955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bolton MD. Primary metabolism and plant defense-Fuel for the fire. Mol Plant-Microbe Interact. 2009;22:487–497. doi: 10.1094/MPMI-22-5-0487. [DOI] [PubMed] [Google Scholar]
- 78.Ueno H. Enzymatic and structural aspects on glutamate decarboxylase. J Mol Catal. 2000;10:5820–5826. [Google Scholar]
- 79.Wang C, Zhang H-B, Wang L-H, Zhang L-H. Succinic semialdehyde couples stress response to quorum-sensing signal decay in Agrobacterium tumefaciens. Mol Microbiol. 2006;62:45–56. doi: 10.1111/j.1365-2958.2006.05351.x. [DOI] [PubMed] [Google Scholar]
- 80.Parveen N, Cornell KA. Methylthioadenosine/S-adenosylhomocysteine nucleosidase, a critical enzyme for bacterial metabolism. Mol Microbiol. 2011;79:7–20. doi: 10.1111/j.1365-2958.2010.07455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fuqua C, Eberhard A. Signal generation in autoinduction systems: synthesis of acylated homoserine lactones by LuxI-type protein. In: Dunny GM, Winans SC, editors. Cell-cell signaling in bacteria. Washington DC: ASM Press; 1999. pp. 211–230. [Google Scholar]
- 82.Hardie KR, Heurlier K. Establishing bacterial communities by ‘word of mouth’: LuxS and autoinducer 2 in biofilm development. Nat Rev Microbiol. 2008;6:635–643. doi: 10.1038/nrmicro1916. [DOI] [PubMed] [Google Scholar]
- 83.Kamilova F, Kravchenko LV, Shaposhnikov AI, Azarova T, Makarova N, et al. Organic acids, sugars, and l-tryptophan in exudates of vegetables growing on stonewool and their effect on activities of rhizosphere bacteria. Mol Plant-Microbe Interact. 2006;19:250–256. doi: 10.1094/MPMI-19-0250. [DOI] [PubMed] [Google Scholar]
- 84.Jaeger CH, III, Lindow SE, Miller W, Clark E, Firestone MK. Mapping of sugar and amino acid availability in soil around roots with bacterial sensors of sucrose and tryptophan. Appl Environ Microbiol. 1999;65:2685–2690. doi: 10.1128/aem.65.6.2685-2690.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sambroock J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual, 2nd ed. New York: Cold Spring Harbor Laboratory Press; 1989. 2344 [Google Scholar]
- 86.Hancock REW, Carey AM. Outer membrane of Pseudomonas aeruginosa: heat and 2-mercaptoethanol-modifiable proteins. J Bacteriol. 1979;140:902–910. doi: 10.1128/jb.140.3.902-910.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bassler BL, Greenberg EP, Stevens AM. Cross-species induction of luminescence in the quorum-sensing bacterium Vibrio harveyi. J Bacteriol. 1997;179:4043–4045. doi: 10.1128/jb.179.12.4043-4045.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bassler BL, Wright M, Showalter RE, Silverman MR. Intercellular signaling in Vibrio harveyi: sequence and function of genes regulating expression of luminescence. Mol Microbiol. 1993;9:773–786. doi: 10.1111/j.1365-2958.1993.tb01737.x. [DOI] [PubMed] [Google Scholar]
- 89.Kotoujansky A, Kotoujansky M, Boistard P. Utilization of a thermosensitive episome bearing transposon Tn10 to isolate Hfr donor strains of Erwinia carotovora subsp. chrysanthemi. J Bacteriol. 1982;150:122–131. doi: 10.1128/jb.150.1.122-131.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primers used for RT-PCR of potato soft-rot pathogens.
(DOC)