Abstract
CASTLE was a randomized 96-week study that demonstrated that atazanavir/ritonavir (ATV/r) was noninferior to lopinavir/ritonavir (LPV/r) in treatment-naïve HIV-infected patients. Analyses were carried out among patients who received ATV/r in the CASTLE study to better understand the clinical significance of unconjugated hyperbilirubinemia associated with administration of boosted ATV. Hyperbilirubinemia was defined as total bilirubin (conjugated and unconjugated) elevation greater than 2.5 times the upper limit of normal (grade 3–4). Patients in the ATV/r arm were assessed based on the presence or absence of hyperbilirubinemia through week 96. Analyses included number of confirmed virologic responders (CVR; HIV RNA <50 copies per milliliter), impact of hyperbilirubinemia on symptoms, elevations in liver enzymes, patient quality of life, and medication adherence. Through 96 weeks in the CASTLE study, 44% of patients who received ATV/r had hyperbilirubinemia at any time point, and between 12.5% and 21.6% had hyperbilirubinemia at any single study visit. At 96 weeks, 74% of patients overall and 84% and 69% of patients with and without hyperbilirubinemia, respectively, achieved CVR. Symptoms of jaundice or scleral icterus occurred in 5% of patients overall and in 11% with hyperbilirubinemia and 0% without hyperbilirubinemia. Four percent of patients with and 3% of patients without hyperbilirubinemia had grade 3–4 elevations in liver transaminases. Less than 1% of patients discontinued treatment due to hyperbilirubinemia. There were no differences in quality of life or adherence between patients with or without hyperbilirubinemia. In the CASTLE study, hyperbilirubinemia observed in the ATV/r group did not negatively impact clinical outcomes in HIV-infected patients.
Introduction
Atazanavir (ATV) is a once-daily HIV-1 protease inhibitor (PI) with proven antiviral efficacy in both treatment-naïve and treatment-experienced patients.1,2 Use of ATV, and another PI, indinavir, is associated with unconjugated hyperbilirubinemia as a result of inhibition of the UGT1A1 enzyme.3,4 Elevated levels of bilirubin are best characterized among individuals with Gilbert syndrome, which is the most common inherited cause of unconjugated hyperbilirubinemia, present in 3–10% of the general population.5 Gilbert syndrome arises through variants in the UGT1A1 enzyme; thus, these PIs induce a biochemical picture similar to Gilbert syndrome. Although elevations of bilirubin may occasionally lead to scleral icterus or jaundice, cohort studies of individuals with Gilbert syndrome indicate that bilirubin elevations may have antioxidant and anti-inflammatory properties and that they are associated with reduced risk of cardiovascular events.6,7
In previously reported studies involving ATV, the prevalence of bilirubin elevations greater than 2.5 and 5 times the upper limit of normal (ULN) range (grades 3 and 4) was 33–41% among patients receiving the unboosted ATV dose of 400 mg daily8,9 and 40% to 49% in those on once daily 300 mg of ATV boosted with 100 mg of ritonavir (ATV/r).10 Unless clinical signs of jaundice and scleral icterus, which may impact a patient's appearance and quality of life,11 are observed, hyperbilirubinemia does not require management and is both reversible and independent of hepatocellular toxicity. Data from the BMS study AI424-089 indicated that bilirubin levels correlated with ATV plasma exposure and thus may indirectly inform physicians about drug exposure and adherence.12 However, the trends of ATV-related hyperbilirubinemia over time and its clinical significance have not been well characterized in clinical studies.
This analysis aims to assess the presence or absence of hyperbilirubinemia among patients who received ATV/r in the CASTLE study and includes the proportion of patients achieving a complete virologic response, defined as HIV RNA less than 50 copies per milliliter. CASTLE was a randomized study that demonstrated that ATV/r was noninferior to lopinavir/ritonavir (LPV/r) in treatment-naïve HIV-infected patients through 96 weeks.13,14 The objectives of the current analysis are to better understand the patterns of hyperbilirubinemia, defined as laboratory abnormalities of grade 3–4 total bilirubin adverse events associated with administration of ATV/r, and to investigate the clinical impact of hyperbilirubinemia on treatment outcomes, patient quality of life, and medication adherence.
Methods
CASTLE was a randomized, open-label, multicenter 96-week study to assess the efficacy and safety of ATV/r (300/100 mg once daily) compared with lopinavir/r (LPV/r; 400/100 mg twice daily) administered with fixed-dose tenofovir/emtricitabine (300/200 mg once daily) in treatment-naïve patients. Full methodology and primary end points have been reported.13,14
This study was performed in accordance with Good Clinical Practice and the ethical principles of the Declaration of Helsinki. The protocol was approved by the institutional review board at each study site, and patients provided written informed consent before participation in the study. The trial is registered with ClinicalTrials.gov, number NCT00272779.
Hyperbilirubinemia was defined as total bilirubin (conjugated and unconjugated) elevation greater than 2.5×ULN (grade 3) and greater than 5×ULN (grade 4). The numbers of patients taking ATV/r who had hyperbilirubinemia were assessed at each study visit. Hyperbilirubinemia (grades 3 and 4) and total bilirubin through week 96 were reported in patients receiving ATV/r (as-treated patients).
For this analysis, patients in the ATV/r arm were assessed based on the presence or absence of hyperbilirubinemia through week 96. Analyses included proportion of patients achieving a confirmed virologic response (CVR, modified intent-to-treat [ITT], noncompleter=failure [NC=F]), defined as HIV RNA less than 50 copies per milliliter. The impact of hyperbilirubinemia on symptoms of jaundice or scleral icterus, elevations in liver enzymes (aspartate transaminase [AST] and alanine transaminase [ALT] greater than 5×ULN), patient quality of life, medication adherence, and reasons for nonadherence was also assessed. Quality of life was assessed at baseline and weeks 12, 24, 48, and 96 using the Medical Outcomes Study HIV Health Survey (MOS-HIV), a 35-item questionnaire to assess physical and mental well-being.15 The summary scores of physical and mental health range from 0 to 100, with higher scores indicating better health. Adherence was measured and reasons for nonadherence were assessed at every visit from week 4 through week 96 using the Multicenter AIDS Cohort Study (MACS) adherence questionnaire.16,17 Adherence was determined by comparing study medications received with those reported on the MACS questionnaire at the same visit. Adherent was defined as taking all doses and number of pills as prescribed for each medication.
Statistical analysis
Statistical methods for the 96-week analysis have been published14 and are summarized briefly below. Efficacy results are presented by the as-randomized treatment regimen (ITT). Safety results are presented by the as-treated regimen (i.e., by the treatment regimen actually received). The hyperbilirubinemia analysis was not powered to detect statistical differences, and no statistical comparisons were carried out for this report. The proportions of patients with hyperbilirubinemia (grade 3–4), the median total bilirubin, and the proportions of patients who were responders (CVR NC=F, HIV RNA <50 copies per milliliter) through week 96 in patients receiving ATV/r (as-treated patients) were demonstrated with plots. Scatter plots of total bilirubin versus AST and ALT were produced for patients with hyperbilirubinemia at weeks 24, 48, and 96. The impact of hyperbilirubinemia on symptoms (jaundice or scleral icterus), grade 3–4 liver transaminase elevations, quality of life (MOS-HIV physical and mental summary scores), and adherence (MACS adherence questionnaire) were summarized. Adherence was assessed in the NC=missing (NC=M) analysis.
Results
A total of 441 treatment-naïve HIV-infected patients were randomized to and received at least one dose of ATV/r (as-treated patients). Full details of patient disposition and primary and secondary end points have been reported previously.13,14
Patients with hyperbilirubinemia in the CASTLE study
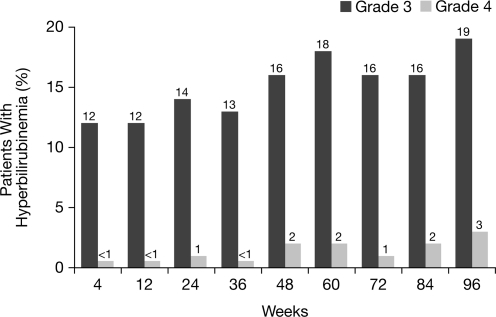
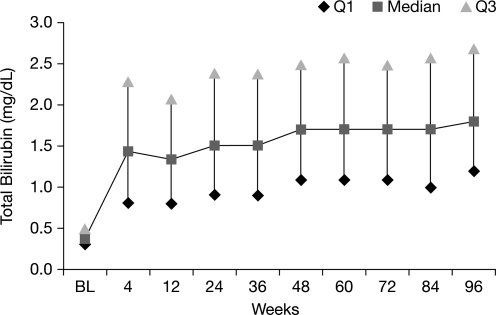
Overall, 44% of patients receiving ATV/r had hyperbilirubinemia (grade 3–4) at any time point during the 96-week study, compared with 12.5–21.6% of patients with hyperbilirubinemia at any single study visit (Fig. 1). At baseline, median total bilirubin was 0.4 mg/dL (6.8 μmol/L) among patients receiving ATV/r. During weeks 4 through 96, median total bilirubin ranged between 1.3 mg/dL (22.2 μmol/L) and 1.8 mg/dL (30.8 μmol/L; Fig. 2).
FIG. 1.
Hyperbilirubinemia through 96 weeks in patients receiving atazanavir/ritonavir (ATV/r; as-treated patients). Note: hyperbilirubinemia was defined as total bilirubin (conjugated and unconjugated) elevation >2.5×upper limit of normal (ULN) (grade 3) and >5×ULN (grade 4).
FIG. 2.
Median total bilirubin through 96 weeks in patients receiving atazanavir/ritonavir (ATV/r; as-treated patients).
ATV/r efficacy among patients with and without hyperbilirubinemia
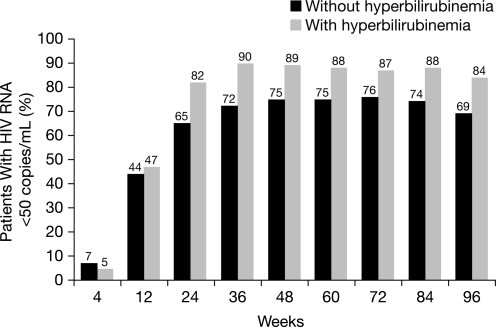
The percentages of patients in the ATV/r treatment group with and without hyperbilirubinemia achieving CVR (HIV RNA <50 copies per milliliter) at any point during the study are illustrated in Fig. 3 and Table 1. At 96 weeks, 74% (327/440) of patients overall and 84% (162/192) and 69% (167/243) of patients with and without hyperbilirubinemia had achieved CVR. Virologic failures occurred in 9% of the patients with and in 9% without hyperbilirubinemia (Table 1).
FIG. 3.
Percentage of patients with (n=192) and without (n=243) hyperbilirubinemia and HIV RNA <50 copies per milliliter receiving atazanavir/ritonavir (ATV/r) through 96 weeks (CVR, NC=F) (as-treated patients). Note: hyperbilirubinemia was defined as total bilirubin (conjugated and unconjugated) elevation >2.5×upper limit of normal (grade 3–4).
Table 1.
Demographics and Treatment Outcomes at Week 96 (HIV RNA <50 Copies per Milliliter)
| Without hyperbilirubinemia (n=243) | With hyperbilirubinemia (n=192) | |
|---|---|---|
| Age (years), median | 34 | 34 |
| Gender n (%) | ||
| Male | 154 (63) | 146 (76) |
| Female | 89 (37) | 46 (24) |
| Treatment outcomes at week 96 | ||
| Responder | 167 (69) | 162 (84) |
| Virologic failure | 23 (9) | 17 (9) |
| Rebound | 21 (9) | 12 (6) |
| Never suppressed through week 96 and on study at Week 96 | 2 (<1) | 5 (3) |
| Death | 4 (2) | 0 |
| Discontinued due to AE | 7 (3) | 5 (3) |
| Discontinued due to other | 42 (17) | 8 (4) |
AE, adverse event.
Impact of hyperbilirubinemia on bilirubin-associated adverse events and laboratory values
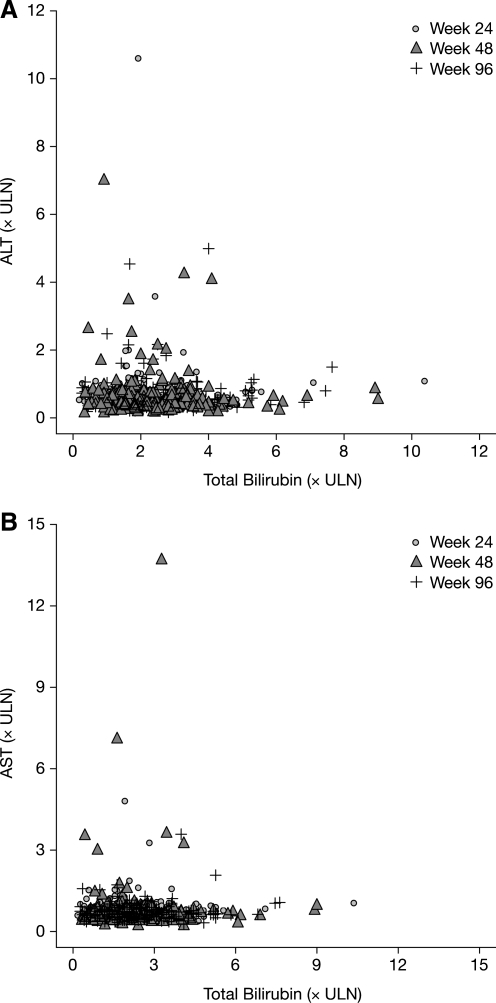
Through 96 weeks in the CASTLE study, 5% (21/441) of patients overall and 11% (21/192) of patients with hyperbilirubinemia (compared with 0% patients without hyperbilirubinemia) had grade 2–4 treatment-related adverse events of jaundice or scleral icterus at any time during the study (Table 2). Four percent (8/192) of patients with and 3% (8/243) of patients without hyperbilirubinemia had grade 3–4 elevations in liver transaminases. Only four patients had concurrent (within 1 week) hyperbilirubinemia and grade 3-4 ALT or AST abnormalities when receiving ATV/r during the study. Figure 4, a scatter plot of ALT, AST, and bilirubin levels, suggests that there is no relationship between hyperbilirubinemia and elevated liver transaminases at any time point during the study. Less than 1% (3/441) of patients discontinued treatment as a result of hyperbilirubinemia, jaundice, or scleral icterus before week 48, and no patients discontinued after week 48. Discontinuations due to adverse events occurred in 3% of the patients with and without hyperbilirubinemia (Table 1).
Table 2.
Grade 2–4 Treatment-Related Adverse Events and Grade 3–4 Liver Enzyme Elevations in Patients With and Without Hyperbilirubinemia (Grade 3–4) at Any Time Point (as-Treated Patients)
| Without hyperbilirubinemia (n=243) | With hyperbilirubinemia (n=192) | |
|---|---|---|
| Grade 2–4 treatment-related adverse events, n (%) | ||
| Hyperbilirubinemia,a reported as an adverse event | 9 (4) | 34 (18) |
| Jaundice or scleral icterus | 0 | 21 (11) |
| Grade 3–4 laboratory abnormalities, n (%) | ||
| ALT | 4 (2) | 7 (4) |
| AST | 7 (3) | 4 (2) |
| ALT or AST | 8 (3) | 8 (4) |
Hyperbilirubinemia reported as an adverse event (not a laboratory elevation), including the terms “blood bilirubin abnormal, blood bilirubin increased, blood bilirubin unconjugated, and blood bilirubin unconjugated increased.”
ALT, alanine transaminase; AST, aspartate transaminase.
FIG. 4.
Total bilirubin versus (A) alanine transaminase (ALT) and (B) aspartate transaminase (AST) in patients with hyperbilirubinemia (n=192) at weeks 24, 48, and 96 (as-treated patients). ULN, upper limit of normal. Note: hyperbilirubinemia was defined as total bilirubin (conjugated and unconjugated) elevation >2.5×ULN (grade 3–4).
Impact of hyperbilirubinemia on quality of life and adherence
There was no difference in change of quality of life among patients with and without hyperbilirubinemia throughout the study. Results at 96 weeks of the MOS-HIV survey are presented in Table 3. In the MACS adherence questionnaire, adherence to the regimen, 84% (147/176) versus 83% (154/186), and to ATV, 87% (153/176) versus 85% (159/186), was also similar among patients with and without hyperbilirubinemia (NC=M) at week 96. Reasons for nonadherence to the regimen reported at weeks 4 and 96 included: didn't want others to notice, fell asleep, felt depressed, felt sick or ill, were away from home or busy with other things, too many pills to take, ran out of pills, or simply forgot. There was no common pattern of reasons for nonadherence reported by patients with hyperbilirubinemia. None of the patients with hyperbilirubinemia and two patients without hyperbilirubinemia selected “feeling like the drug was too toxic” as a reason for nonadherence during the study.
Table 3.
MOS-HIV Summary Score Categories for Patients Receiving ATV/r With and Without Hyperbilirubinemia at Week 96 (as-Treated Patients)
| Without hyperbilirubinemia n=243 | With hyperbilirubinemia n=192 | |
|---|---|---|
| Physical, n/N (%) | ||
| Improvement | 76/138 (55) | 70/128 (55) |
| No change | 35/138 (25) | 29/128 (23) |
| Worsening | 27/138 (20) | 29/128 (23) |
| Mental, n/N (%) | ||
| Improvement | 97/138 (70) | 92/128 (72) |
| No change | 25/138 (18) | 18/128 (14) |
| Worsening | 16/138 (12) | 18/128 (14) |
ATV/r, atazanavir/ritonavir; MOS-HIV, Medical Outcomes Study HIV Health Survey.
Discussion
Although elevated levels of bilirubin are associated with the use of some PIs, including atazanavir, the effect of these elevations on clinical outcomes in HIV-infected patients is not well characterized in clinical studies.11 In the CASTLE study,14 hyperbilirubinemia occurred in 44% of patients receiving ATV/r at any time through 96 weeks, and this prevalence rate is similar to the rates reported in other studies.8–10,18 The mechanism by which some patients experience hyperbilirubinemia and others do not is not fully understood. Genetic polymorphisms of the UGT1A1 gene impact UGT activity and have been previously reported to contribute to hyperbilirubinemia. Haplotype variants of UGT1A1, UGT1A3, and UGT1A7 genes have previously been associated with occurrence of unconjugated hyperbilirubinemia among patients receiving ATV/r.19–23
Our study showed similar low rates of hepatotoxicity in patients with and without hyperbilirubinemia. Hepatotoxicity, chronic liver disease, and cirrhosis are common with HIV/HCV co-infected individuals, and HIV infection may accelerate liver damage caused by hepatitis C virus.24 In addition to liver toxicity, people with HIV infection have been suggested to be at greater risk of cardiovascular disease (CVD) than the general population.25,26 Several cohort studies have identified an inverse relationship between bilirubin and CVD risk.7,27–33
Overall, patients receiving ATV/r responded well to treatment, with 74% achieving a CVR14; a greater percentage of patients with hyperbilirubinemia than without achieved a CVR. Previous studies have reported that bilirubin levels correlate with ATV plasma concentrations.23,34–36 It is possible that a higher number of patients with hyperbilirubinemia than without achieved a CVR due to higher ATV plasma concentrations, but supporting pharmacokinetic sampling and data were not available in this population.
Quality of life and adherence were similar in patients with and without hyperbilirubinemia, and were similar to values reported in previous studies.37,38 Hyperbilirubinemia was not associated with any increase in abnormalities in liver transaminases compared with absence of hyperbilirubinemia, which supports the results of previous reports that ATV-associated hyperbilirubinemia does not impact hepatic function.3,9–11,39 Most antiretroviral regimens are safe in HIV/HCV-coinfected individuals, including those patients with cirrhosis.35,40 The prevalence of symptoms of jaundice or scleral icterus among patients receiving ATV/r was low overall in the CASTLE study (5%) and in patients with hyperbilirubinemia (11%). Furthermore, few patients discontinued treatment as a result of hyperbilirubinemia, jaundice, or icterus, and none of the discontinuations were between weeks 48 and 96.14 Similarly low rates of discontinuations because of hyperbilirubinemia were also reported in other ATV studies.9,10
The limitations of this study are that it is a post hoc analysis of a subset of patients and is therefore not powered to make robust conclusions. Additionally, CVD was not assessed. However, the study provides a valuable opportunity to assess the general trends of hyperbilirubinemia and its clinical impact in patients treated with ATV/r in the CASTLE study.
Although hyperbilirubinemia was common among patients receiving ATV/r at any time through 96 weeks in the CASTLE study, it was less frequent at specific time points and was not associated with related symptoms in most patients. The majority of patients responded well to treatment overall, with numerically more patients suppressing HIV RNA (<50 copies per milliliter) with hyperbilirubinemia than without. The presence of hyperbilirubinemia did not affect AST/ALT elevations, quality of life, or adherence. These data suggest that hyperbilirubinemia observed with ATV/r does not negatively impact clinical outcomes in HIV-infected patients.
Acknowledgments
Editorial support was provided by Cheryl Jenkins and Jean Turner of PAREXEL and Emily Cullinan of Bristol-Myers Squibb and was funded by Bristol-Myers Squibb.
Author Disclosure Statement
C.M. has served on speakers' bureaus for Bristol-Myers Squibb, Gilead, and ViiV Healthcare, and has received research funding from Bristol-Myers Squibb, Gilead, GlaxoSmithKline/ViiV Healthcare, Boehringer-Ingelheim, Merck, and Pfizer. J.U., W.H., V.W., S.J., D.B., D.M., and A.F. are employees and stockholders of Bristol-Myers Squibb. G.M. has received research funding from Abbott, Ardea Biosciences, Bionor, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck, Pfizer, Theratechnologies, and Tibotec. He has from received honoraria as a speaker and/or advisor from Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck, Pfizer, Theratechnologies, Tibotec, and ViiV Healthcare.
References
- 1.Johnson M. Grinsztejn B. Rodriguez C, et al. 96-week comparison of once-daily atazanavir/ritonavir and twice-daily lopinavir/ritonavir in patients with multiple virologic failures. AIDS. 2006;20:711–718. doi: 10.1097/01.aids.0000216371.76689.63. [DOI] [PubMed] [Google Scholar]
- 2.Malan DR. Krantz E. David N. Wirtz V. Hammond J. McGrath D. Efficacy and safety of atazanavir, with or without ritonavir, as part of once-daily highly active antiretroviral therapy regimens in antiretroviral-naive patients. J Acquir Immune Defic Syndr. 2008;47:161–167. doi: 10.1097/QAI.0b013e31815ace6a. [DOI] [PubMed] [Google Scholar]
- 3.Bentue-Ferrer D. Arvieux C. Tribut O. Ruffault A. Bellissant E. Clinical pharmacology, efficacy and safety of atazanavir: A review. Expert Opin Drug Metab Toxicol. 2009;5:1455–1468. doi: 10.1517/17425250903321514. [DOI] [PubMed] [Google Scholar]
- 4.Satija P. Parikh F. Aggarwal V. Sharma B. Hakim A. Pai-Dhungat JV. Indirect hyperbilirubinemia with indinavir. J Assoc Physicians India. 2002;50:1316–1317. [PubMed] [Google Scholar]
- 5.Bosma PJ. Chowdhury JR. Bakker C, et al. The genetic basis of the reduced expression of bilirubin UDP-glucuronosyltransferase 1 in Gilbert's syndrome. N Engl J Med. 1995;333:1171–1175. doi: 10.1056/NEJM199511023331802. [DOI] [PubMed] [Google Scholar]
- 6.Keshavan P. Deem TL. Schwemberger SJ. Babcock GF. Cook-Mills JM. Zucker SD. Unconjugated bilirubin inhibits VCAM-1-mediated transendothelial leukocyte migration. J Immunol. 2005;174:3709–3718. doi: 10.4049/jimmunol.174.6.3709. [DOI] [PubMed] [Google Scholar]
- 7.Perlstein TS. Pande RL. Creager MA. Weuve J. Beckman JA. Serum total bilirubin level, prevalent stroke, and stroke outcomes: NHANES 1999–2004. Am J Med. 2008;121:781–788. doi: 10.1016/j.amjmed.2008.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanne I. Piliero P. Squires K. Thiry A. Schnittman S. Results of a phase 2 clinical trial at 48 weeks (AI424-007): A dose-ranging, safety, and efficacy comparative trial of atazanavir at three doses in combination with didanosine and stavudine in antiretroviral-naive subjects. J Acquir Immune Defic Syndr. 2003;32:18–29. doi: 10.1097/00126334-200301010-00004. [DOI] [PubMed] [Google Scholar]
- 9.Squires K. Lazzarin A. Gatell JM, et al. Comparison of once-daily atazanavir with efavirenz, each in combination with fixed-dose zidovudine and lamivudine, as initial therapy for patients infected with HIV. J Acquir Immune Defic Syndr. 2004;36:1011–1019. doi: 10.1097/00126334-200408150-00003. [DOI] [PubMed] [Google Scholar]
- 10.Johnson M. Grinsztejn B. Rodriguez C, et al. Atazanavir plus ritonavir or saquinavir, and lopinavir/ritonavir in patients experiencing multiple virological failures. AIDS. 2005;19:685–694. doi: 10.1097/01.aids.0000166091.39317.99. [DOI] [PubMed] [Google Scholar]
- 11.Korenblat KM. Berk PD. Hyperbilirubinemia in the setting of antiviral therapy. Clin Gastroenterol Hepatol. 2005;3:303–310. doi: 10.1016/s1542-3565(05)00083-2. [DOI] [PubMed] [Google Scholar]
- 12.Efficacy, safety of atazanavir-based therapy in antiretroviral-naive HIV-1-infected subjects, both with, without ritonavir: 96-week results from AI424-089. 4th International AIDS Conference on HIV Pathogenesis and Treatment; Sydney, Australia. Jul 22–25;. [Google Scholar]
- 13.Molina JM. Andrade-Villanueva J. Echevarria J, et al. Once-daily atazanavir/ritonavir versus twice-daily lopinavir/ritonavir, each in combination with tenofovir and emtricitabine, for management of antiretroviral-naive HIV-1-infected patients: 48 week efficacy and safety results of the CASTLE study. Lancet. 2008;372:646–655. doi: 10.1016/S0140-6736(08)61081-8. [DOI] [PubMed] [Google Scholar]
- 14.Molina JM. Andrade-Villaneuva J. Echevarria J, et al. Once-daily atazanavir/ritonavir compared with twice-daily lopinavir/ritonavir, each in combination with tenofovir and emtricitabine, for management of antiretroviral-naive HIV-1-infected patients: 96-week efficacy and safety results of the CASTLE study. J Acquir Immune Defic Syndr. 2010;53:323–332. doi: 10.1097/QAI.0b013e3181c990bf. [DOI] [PubMed] [Google Scholar]
- 15.Wu AW. Revicki DA. Jacobson D. Malitz FE. Evidence for reliability, validity and usefulness of the Medical Outcomes Study HIV Health Survey (MOS-HIV) Qual Life Res. 1997;6:481–493. doi: 10.1023/a:1018451930750. [DOI] [PubMed] [Google Scholar]
- 16.Chesney MA. Ickovics JR. Chambers DB, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: The CTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG) AIDS Care. 2000;12:255–266. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- 17.Kleeberger CA. Phair JP. Strathdee SA. Detels R. Kingsley L. Jacobson LP. Determinants of heterogeneous adherence to HIV-antiretroviral therapies in the Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr. 2001;26:82–92. doi: 10.1097/00126334-200101010-00012. [DOI] [PubMed] [Google Scholar]
- 18.Johnson M. Grinsztejn B. Rodriguez C, et al. 96-week comparison of once-daily atazanavir/ritonavir and twice-daily lopinavir/ritonavir in patients with multiple virologic failures. AIDS. 2006;20:711–718. doi: 10.1097/01.aids.0000216371.76689.63. [DOI] [PubMed] [Google Scholar]
- 19.Lankisch TO. Behrens G. Ehmer U, et al. Gilbert's syndrome and hyperbilirubinemia in protease inhibitor therapy—An extended haplotype of genetic variants increases risk in indinavir treatment. J Hepatol. 2009;50:1010–1018. doi: 10.1016/j.jhep.2008.12.030. [DOI] [PubMed] [Google Scholar]
- 20.Park WB. Choe PG. Song KH, et al. Genetic factors influencing severe atazanavir-associated hyperbilirubinemia in a population with low UDP-glucuronosyltransferase 1A1*28 allele frequency. Clin Infect Dis. 2010;51:101–106. doi: 10.1086/653427. [DOI] [PubMed] [Google Scholar]
- 21.Rotger M. Taffe P. Bleiber G, et al. Gilbert syndrome and the development Sydney, Australia: Of antiretroviral therapy-associated hyperbilirubinemia. J Infect Dis. 2005;192:1381–1386. doi: 10.1086/466531. [DOI] [PubMed] [Google Scholar]
- 22.Strassburg CP. Lankisch TO. Manns MP. Ehmer U. Family 1 uridine-5'-diphosphate glucuronosyltransferases (UGT1A): From Gilbert's syndrome to genetic organization and variability. Arch Toxicol. 2008;82:415–433. doi: 10.1007/s00204-008-0314-x. [DOI] [PubMed] [Google Scholar]
- 23.Tozzi V. Pharmacogenetics of antiretrovirals. Antiviral Res. 2010;85:190–200. doi: 10.1016/j.antiviral.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Bica I. McGovern B. Dhar R, et al. Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clin Infect Dis. 2001;32:492–497. doi: 10.1086/318501. [DOI] [PubMed] [Google Scholar]
- 25.Holmberg SD. Moorman AC. Greenberg AE. Trends in rates of myocardial infarction among patients with HIV. N Engl J Med. 2004;350:730–732. doi: 10.1056/NEJM200402123500719. [DOI] [PubMed] [Google Scholar]
- 26.Obel N. Thomsen HF. Kronborg G, et al. Ischemic heart disease in HIV-infected and HIV-uninfected individuals: A population-based cohort study. Clin Infect Dis. 2007;44:1625–1631. doi: 10.1086/518285. [DOI] [PubMed] [Google Scholar]
- 27.Clark JE. Foresti R. Sarathchandra P. Kaur H. Green CJ. Motterlini R. Heme oxygenase-1-derived bilirubin ameliorates postischemic myocardial dysfunction. Am J Physiol Heart Circ Physiol. 2000;278:H643–H651. doi: 10.1152/ajpheart.2000.278.2.H643. [DOI] [PubMed] [Google Scholar]
- 28.Erdogan D. Gullu H. Yildirim E, et al. Low serum bilirubin levels are independently and inversely related to impaired flow-mediated vasodilation and increased carotid intima-media thickness in both men and women. Atherosclerosis. 2006;184:431–437. doi: 10.1016/j.atherosclerosis.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 29.Kawamura K. Ishikawa K. Wada Y, et al. Bilirubin from heme oxygenase-1 attenuates vascular endothelial activation and dysfunction. Arterioscler Thromb Vasc Biol. 2005;25:155–160. doi: 10.1161/01.ATV.0000148405.18071.6a. [DOI] [PubMed] [Google Scholar]
- 30.Lin JP. Vitek L. Schwertner HA. Serum bilirubin and genes controlling bilirubin concentrations as biomarkers for cardiovascular disease. Clin Chem. 2010;56:1535–1543. doi: 10.1373/clinchem.2010.151043. [DOI] [PubMed] [Google Scholar]
- 31.Schwertner HA. Vitek L. Gilbert syndrome, UGT1A1*28 allele, and cardiovascular disease risk: Possible protective effects and therapeutic applications of bilirubin. Atherosclerosis. 2008;198:1–11. doi: 10.1016/j.atherosclerosis.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 32.Vitek L. Jirsa M. Brodanova M, et al. Gilbert syndrome and ischemic heart disease: A protective effect of elevated bilirubin levels. Atherosclerosis. 2002;160:449–456. doi: 10.1016/s0021-9150(01)00601-3. [DOI] [PubMed] [Google Scholar]
- 33.Yoshino S. Hamasai S. Ishida S, et al. Effect of serum concentration of bilirubin in the obese patients: The action for coronary endothelial function and inflammatory stress. J Am Coll Cardiol. 2010;55:E1434. [Google Scholar]
- 34.Rodriguez-Novoa S. Martin-Carbonero L. Barreiro P, et al. Genetic factors influencing atazanavir plasma concentrations and the risk of severe hyperbilirubinemia. AIDS. 2007;21:41–46. doi: 10.1097/QAD.0b013e328011d7c1. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez JM. Hermida JM. Casado JL, et al. The use of atazanavir in HIV-infected patients with liver cirrhosis: Lack of hepatotoxicity and no significant changes in bilirubin values or model for end-stage liver disease score. AIDS. 2011;25:1006–1009. doi: 10.1097/QAD.0b013e3283466f85. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez NS. Barreiro P. Rendon A, et al. Plasma levels of atazanavir and the risk of hyperbilirubinemia are predicted by the 3435C–>T polymorphism at the multidrug resistance gene 1. Clin Infect Dis. 2006;42:291–295. doi: 10.1086/499056. [DOI] [PubMed] [Google Scholar]
- 37.Malan DR. Krantz E. David N, et al. 96-week efficacy and safety of atazanavir, with and without ritonavir, in a HAART regimen in treatment-naive patients. J Int Assoc Physicians AIDS Care (Chic Ill) 2010;9:34–42. doi: 10.1177/1545109709355828. [DOI] [PubMed] [Google Scholar]
- 38.Malan N. Su J. Mancini M, et al. Gastrointestinal tolerability and quality of life in antiretroviral-naive HIV-1-infected patients: Data from the CASTLE study. AIDS Care. 2010;22:677–686. doi: 10.1080/09540120903334641. [DOI] [PubMed] [Google Scholar]
- 39.Lankisch TO. Moebius U. Wehmeier M, et al. Gilbert's disease and atazanavir: From phenotype to UDP-glucuronosyltransferase haplotype. Hepatology. 2006;44:1324–1332. doi: 10.1002/hep.21361. [DOI] [PubMed] [Google Scholar]
- 40.Merchante N. Lopez-Cortes LF. Delgado-Fernandez M, et al. Liver toxicity of antiretroviral combinations including fosamprenavir plus ritonavir 1400/100 mg once daily in HIV/hepatitis C virus-coinfected patients. AIDS Patient Care STDs. 2011;25:395–402. doi: 10.1089/apc.2011.0109. [DOI] [PubMed] [Google Scholar]