Abstract
Voluntary exercise increases levels of brain-derived neurotrophic factor (BDNF) after traumatic brain injury (TBI) when it occurs during a delayed time window. In contrast, acute post-TBI exercise does not increase BDNF. It is well known that increases in glucocorticoids suppress levels of BDNF. Moreover, recent work from our laboratory showed that there is a heightened stress response after fluid percussion injury (FPI). In order to determine if a heightened stress response is also observed with acute exercise, at post-injury days 0–4 and 7–11, corticosterone (CORT) and adrenocorticotropic hormone (ACTH) release were measured in rats running voluntarily or exposed to two daily 20-min periods of forced running wheel exercise. Forced, but not voluntary exercise, continuously elevated CORT. ACTH levels were initially elevated with forced exercise, but decreased by post-injury day 7 in the control, but not the FPI animals. As previously reported, voluntary exercise did not increase BDNF in the FPI group as it did in the control animals. Forced exercise did not increase levels of BDNF in any group. It did, however, decrease hippocampal glucocorticoid receptors in the control group. The results suggest that exercise regimens with strong stress responses may not be beneficial during the early post-injury period.
Key Words: adrenocorticotropic hormone, corticosterone, brain-derived neurotrophic factor, exercise, glucocorticoid receptors
Introduction
Traumatic brain injury (TBI) is a serious health concern that is not limited to those cases categorized as severe. Mild TBI is associated with long-term affective and cognitive impairments, along with a significant decrease in quality of life (Bazarian et al., 2006; McAllister et al., 2006; Niogi et al, 2008; Rapoport et al., 2002). Both human and animal studies have demonstrated that mild TBI results in axonal damage (MacDonald et al., 2011), changes in neuronal excitability (Avramescu and Timofeev, 2008; Tran et al., 2006), and metabolic alterations (Golding et al., 1999; Vagnozzi et al., 2008). These diverse pathophysiological effects are likely to influence activation responses to different stimuli. In addition, we have recently shown, in an animal model of TBI, that the regulation of the hypothalamic-pituitary-adrenal (HPA) axis is altered in that the response to stress is heightened during the subacute post-injury period (Griesbach et al., 2011). It is during the first post-injury weeks that the effects of mild TBI are most noticeable. During this period the brain is more vulnerable to sustaining further damage (Tavazzi et al., 2007). Along these lines, earlier studies indicated that acute post-traumatic physical exertion performed in a voluntary running wheel worsens cognitive outcome and decreases proteins associated with synaptic plasticity (Griesbach et al., 2004a,2004b). Normally exercise increases proteins such as brain-derived neurotrophic factor (BDNF). BDNF facilitates the synapse (Tyler and Pozzo-Miller, 2001), and enhances neurotransmitter release (Levine et al, 1995). An improvement in cognitive capability is also associated with exercise. In contrast to acute post-traumatic exercise, the beneficial effects of exercise are best seen when it is delayed following experimental brain injury (Griesbach et al., 2004b,2009).
Given that increases in glucocorticoids are known to suppress levels of BDNF (Hansson et al., 2006), it is plausible that injury-induced disruptions in glucocorticoid regulation contribute to the negative effects of acute post-injury exercise. This raises the possibility that acute exercise elicits a pronounced stress response. Here we set out to determine if a heightened stress response is also observed with acute exercise. In addition we also explored if the type of exercise (forced versus voluntary running wheel) would have differential effects on the stress and BDNF responses. The glucocorticoid receptor (GR) was also measured. Different motivational factors are involved in forced exercise compared to voluntary exercise (Leasure and Jones, 2008); thus it is predicted that the stress response will also differ. We utilized the fluid percussion injury (FPI) model for these studies, a type of TBI that in our hands results in no gross neuronal death (Gurkoff et al., 2006; Osteen et al., 2001).
Methods
Subjects
A total of 108 male Sprague-Dawley rats (mean weight 289±3.37 standard error or the mean [SEM]) from Charles River Breeding Labs (Hollister, CA) were utilized in these experiments. The animals were handled daily and habituated to a reversed lighting schedule (lights off 09:30–21:30 h). During the experiments, the rats were single-housed in opaque plastic bins (50.8×25.4×25.4 cm), which were lined with bedding material. Rats had ad libitum access to water and rat chow. All procedures were performed in accordance with the United States National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals, and were approved by the UCLA Chancellor's Animal Research Committee.
Transmitter implantation
One week prior to brain injury, the rats were implanted with telemetry devices (Mini Mitter; Respironics, Bend, OR) to monitor heart rate, core body temperature, and gross motor activity (results to be reported separately). The rats were anesthetized with isofluorane (4% for induction and 2.0% for maintenance in 100% oxygen) via a nose mask, and transmitters were implanted according to the manufacturer's protocol.
Lateral fluid percussion injury
As previously done (Griesbach et al., 2004a,2007), the rats were anesthetized with isoflurane via nose mask. The level of anesthesia was monitored by level of respiration, muscular relaxation, and pedal reflexes. After loss of pedal reflexes, the scalp and scapular regions were shaved, the animal was secured in a stereotaxic head frame, and the scalp was cleansed with ethanol and povidone-iodine. Rectal temperature was monitored and maintained between 36.5 and 38.0°C with a thermostatically-controlled heating pad (Braintree Scientific Inc., Braintree, MA). The scalp and temporal muscle were reflected and a 3-mm-diameter circular craniotomy was made over the left parietal cortex, centered 3 mm posterior to the bregma and 6 mm lateral to the midline. The bone flap was removed and the dura left intact in all animals to receive FPI (n=42). The dura was inspected with the aid of a microscope in order to assure that it was intact and thus allow for animal inclusion. A plastic injury cap was placed over the craniotomy with silicone adhesive, cyanoacrylate, and dental cement. When the dental cement hardened, the cap was filled with 0.9% NaCl solution. Anesthesia was discontinued and the animal was removed from the stereotaxic device. The injury cap was attached to the fluid percussion device. At the first sign of hindlimb withdrawal to a paw pinch, a mild/moderate fluid percussion pulse (1.5 atm) was administered. Apnea times were determined as the time from injury to the return of spontaneous breathing. The time of unconsciousness was determined from the time of injury until the return of the hindlimb withdrawal reflex. Immediately upon responding to a paw pinch, anesthesia was restored, the injury cap removed, and the scalp was sutured. Anesthesia control and sham animals (n=24 and 38 respectively) underwent an identical preparation with the exception of the FPI. Anesthesia controls were placed under anesthesia for a period of time similar to that of the rats that underwent FPI or sham surgery. A skin incision was also made, as described above, to assure that there would be experimenter blindness for the following procedures. After suturing, bupivacaine (0.25 mg) was injected into the margins of the scalp incision and triple antibiotic ointment was applied over the incision. The rat was placed in a recovery chamber for approximately 1 h before being returned to its cage. All injuries were performed before 12:00 h.
Forced and voluntary wheel exercise
Animals were randomly assigned to either voluntary exercise (vRW; FPI: n=18, sham: n=15, anesthesia control: n=8), forced exercise (fRW; FPI: n=9, sham: n=8, anesthesia control: n=8), or sedentary (SED; FPI: n=15, sham: n=15, anesthesia control: n=8) conditions. Exposure to exercise commenced 3–5 h after the FPI. Rats in the vRW condition were placed in cages equipped with a running wheel (RW, diameter=31.8 cm, width=10 cm; Nalge Nunc International, Rochester, NY) that rotated against a resistance of 100 g. These animals were allowed to exercise from post-injury days (PIDs) 0–4 and PIDs 7–11. Exercise was quantified by recording the number of wheel revolutions per hour using VitalView Data Acquisition System software (Respironics). Rats under the fRW condition were exposed to similar wheels as those utilized for the vRW, with the exception that these had a motor attached (Pittman, Harleysville, PA), that allowed for speed to be individually controlled. Rats under the fRW condition received two daily 20-min exercise sessions (10:00 h and 14:00 h) at PIDs 0–4 and PIDs 7–11.
Corticosterone and ACTH radioimmunoassays
Blood was collected by tail venipuncture prior to the injury in order to obtain baseline values. Following injury, collections were made on PID 0, 4, 7, and 11. All blood samples were obtained between 14:00 and 16:00 h during the active dark phase. For those animals under the forced exercise condition (fRW), samples were obtained 5 min after exercise. Additional samples were obtained 1 h after exercise at PIDs 4 and 7. Rats under the voluntary exercise condition (vRW) were exercising when removed from their cage to obtain blood samples. After the last blood collection on PID 11, the rats were sacrificed and the hippocampus was dissected for analysis. The thymus and adrenal glands were removed and weighed.
Blood samples (250 μL/sample) were collected in EDTA-micro collection tubes with added trasylol (200 kIU/mL aprotinin; Sigma-Aldrich, St. Louis). Blood samples were then centrifuged at 2000 rpm for 20 min at 4°C, and plasma was separated, aliquotted, and stored at −80°C until assayed for CORT and ACTH. Plasma CORT was assayed with a commercial rat CORT 125I-RIA kit (MP Biomedicals, Inc., Orangeburg, NY), according to the vendor's instructions, as done previously (Griesbach et al., 2011). The reported detection limit for the CORT assay is 8 ng/mL, and intra- and inter-assay coefficients of variation are lower than 10.3% and 7.2%, respectively. The results are expressed as nanograms per milliliter of plasma. Plasma ACTH levels were assessed with a human ACTH 125I-RIA kit (DiaSorin Corp., Stillwater, MN), according to the vendor's instructions, as done previously (Griesbach et al., 2011). The reported detection limit of this assay is 15 g/mL, and intra- and inter-assay coefficients of variation are lower than 10.7% and 5.7%, respectively. The results are expressed as picograms per milliliter of plasma.
Western blots for BDNF and GR
Hippocampal tissue within the injured hemisphere was dissected and immediately placed on dry ice. Tissue samples were weighed and homogenized in lysis buffer (100 mM Tris/HCL [pH 7], 1 M NaCl, 4 mM EDTA, 2% Triton X-100, 0.1% sodium azide, and protease inhibitor cocktail tablets ([Roche Applied Science, Indianapolis, IN]). Homogenates were centrifuged at 14,000g for 30 min at 4°C. The resulting supernatants were collected and immediately processed for total protein concentration according to the Micro BCA procedure (Pierce Biotechnology, Rockford, IL) using bovine serum albumin as the standard. All chemicals were obtained from Sigma-Aldrich unless otherwise noted.
Homogenates were separated by SDS-page on 15% and 10% Tris-HCl Criterion Precast Gels (Bio-Rad Laboratories Inc., Hercules, CA). Proteins were transferred to PVDF membranes for Western blot analysis and stained for total protein using Sypro Ruby protein blot stain (Invitrogen Molecular Probes, Eugene, OR) according to the manufacturer's instructions. Non-specific binding sites were blocked in TBS with 0.1% Tween-20 and 5% milk for 1 h at room temperature. The membranes were incubated at 4°C overnight with rabbit polyclonal anti-BDNF N-20 (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA), or 2 h at room temperature with rabbit polyclonal anti-GR M-20 (1:500; Santa Cruz Biotechnology), followed by 1-h incubation at room temperature with anti-rabbit IgG horseradish peroxidase-conjugate secondary antibody (1:10,000; Pierce Biotechnology). All washing steps were carried out with TBS with 0.1% Tween-20. The blots were developed using a chemiluminescence detection method with the Super Signal West Femto Maximum Sensitivity Substrate kit (Thermo-Pierce Biotechnology, Rockford, IL) according to the manufacturer's instructions. The blots were processed on the Chemi Doc XRS Imaging System (Bio-Rad Laboratories Inc.). Optical densities for all blots were standardized against Sypro values, and each experimental group was normalized to Control-SED values within the same gel. The final data were expressed as the percent change from the mean Control-SED values.
ELISA for BDNF
BDNF protein was quantified using an enzyme-linked immunosorbent assay (ELISA) kit (Chemikine BDNF; Millipore, Billerica, MA) according to the manufacturer's instructions. Absorbance at 450 nm was measured using an automated microplate reader (Model 3550; Bio-Rad Laboratories Inc.). Standards were assayed with the samples on each plate. Duplicate measures were averaged and values were corrected for the total amount of protein (as described above) in the sample to derive the picograms of BDNF protein per milligram of total protein.
Statistical analysis
Mixed models analyses were conducted on all dependent variables involving repeated assessments over time (Pinheiro and Bates, 2000). These models incorporate both fixed and random effects and are calculable even with missing entries (e.g., a case with a missing data point is not deleted from the analysis as happens in older statistical routines). Conventional two-factor analyses of variance (ANOVA) were conducted for dependent measures obtained only once. Tukey's HSD test was used to assess significant main or interaction effects. The threshold for statistical significance was set at p<0.05. All analyses were conducted using R version 2.13.0 (R Development Core Team, 2011).
Results
Subjects
Injured rats had a mean (±standard error of the mean [SEM]) unconsciousness duration of 145±10 sec, and a mean apnea duration of 41±10 sec. Four animals were deleted from the study after FPI because the severity of injury was determined to be too high (over 300 sec unconsciousness), and it was the purpose of these experiments to study mild/moderate TBI. Additionally, severely-injured animals have an increased risk of cardiac arrest (Neigh et al., 2009). Although we did not measure cardiac output in our animals, by eliminating severely-injured subjects we eliminated cardiac arrest-induced increases in CORT. No gross motor impairments of ambulatory ability were observed in any of the injured rats. No significant differences in mobility, as detected by telemetry, were observed during the dark (active) phase of the diurnal cycle. No significant differences were observed between anesthesia controls and sham-injured rats in any of the assessment measures. Therefore these groups were pooled and will be referred to as the control group.
A mixed-models analysis of the percent change in body weight of each animal during the first 11 days post-FPI indicated no effect of surgery or exercise, and no significant interactions except for the exercise×day interaction. Percent weight gain of the FPI groups tended to lag behind that of the controls on PID 4 and PID 7, and on PID 4, vRW produced a significantly (p<0.05) greater effect in the controls. By PID 11 weight gain was significantly (p<0.01) reduced by fRW in both the FPI and control groups compared to their respective SED conditions.
Forced and voluntary exercise
No group differences were observed between groups when fRW speed was analyzed. A significant time (i.e., PID) effect was observed across all groups [F(1,15)=44.05, p<0.01]. The number of wheel revolutions during the 12-h dark phase was analyzed. Results indicated that the FPI-vRW rats ran significantly less compared to the control-vRW group [F(1,15)=5.66, p<0.05]. Post-hoc comparisons indicated significant differences at PIDs 0, 1, 2, and 3. The amount of running increased over time across groups, as indicated by a significant time effect [F(1,15)=14.76, p<0.01].
Effect of 20-min forced exercise or voluntary exercise on CORT and ACTH in control and FPI rats
Tail vein samples were collected at 5 and 60 min after 20-min forced exercise, and randomly during the same 1400–1600 h period in voluntary exercising and sedentary rats. Figures 1 and 2 show the CORT and ACTH responses at the 5-min time point. All 60-min values had returned to pre-surgery levels by this time (data not shown).
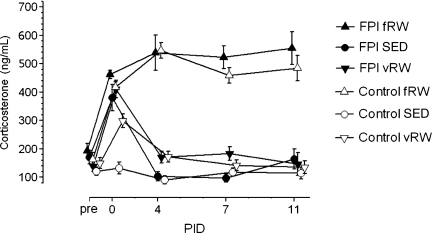
FIG. 1.
Levels of plasma corticosterone (CORT) in tail vein samples collected pre-surgery and on post-injury days (PIDs) 0, 4, 7, and 11, at 5 min after 20-min forced exercise (fRW), and randomly during the same 1400–1600 h period in voluntary exercising (vRW) and sedentary (SED) control and fluid percussion injury (FPI) rats. The symbols and bars represent means±standard error of the mean (n=9–22 per group).
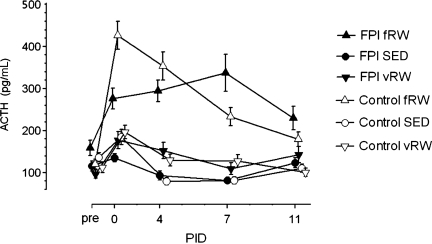
FIG. 2.
Levels of plasma adrenocorticotropic hormone ACTH in tail vein samples collected pre-surgery and on post-injury days (PIDs) 0, 4, 7, and 11 at 5 min after 20-min forced exercise (fRW), and randomly during the same 1400–1600 h period in voluntary exercising (vRW) and sedentary (SED) control and fluid percussion injury (FPI) rats. The symbols and bars present means±standard error of the mean (n=11–23 per group).
A mixed-models analysis of CORT (Fig. 1) shows no main effect of group and no main effect of exercise, but strong (p<0.01) effects due to time (PID), and strong effects due to the exercise×time interaction. Tukey's post-hoc comparisons indicated that pre-surgery levels of CORT did not differ among the groups. On PID 0, within 3–5 h after FPI or control surgeries, significant (p<0.05) increases in CORT levels compared to pre-surgery levels occurred in all groups except the control-SED group; among the controls, CORT was significantly (p<0.01) increased by fRW and vRW compared to the SED condition, while in the FPI groups, there were no differences among the elevated CORT levels regardless of condition (SED, vRW, or fRW). From PID 0 to PID 4, CORT levels increased significantly (p<0.05) in the control-fRW group, and decreased significantly (p<0.05) in the control-vRW, FPI-SED, and FPI-vRW groups, with no further changes on PIDs 7 and 11. On PIDs 4, 7, and 11, CORT levels were significantly (p<0.01) higher in the control-fRW and FPI-fRW groups than in their respective vRW and SED groups, without any differences in the elevated CORT levels between the two fRW groups.
A mixed-models analysis of plasma ACTH levels (Fig. 2) showed no main effect of group, but significant effects of exercise (p<0.05), and time (i.e., PID, p<0.01), along with strong (p<0.01) effects due to the exercise×time interaction, and to the three-way interaction between group, exercise, and time. Tukey's post-hoc comparisons indicated that on PID 0, within 3–5 h after FPI or control surgeries, significant (p<0.05) increases in ACTH levels compared to pre-surgery levels occurred in all groups except the FPI-SED group; among the controls, ACTH was significantly (p<0.01) increased by fRW compared to the vRW and SED conditions, resulting in significantly (p<0.01) higher ACTH in the control-fRW group than in the FPI-fRW group. On PIDs 4, 7, and 11, ACTH levels were significantly (p<0.05) higher in the control-fRW and FPI-fRW groups than in their respective vRW and SED groups. From PID 4 to PID 7, control-fRW ACTH decreased significantly (p<0.05), resulting in significantly (p<0.01) lower ACTH levels in the control-fRW group than in the FPI-fRW group on PID 7.
Effect of 20-min forced exercise or voluntary exercise on hippocampal BDNF in control and FPI rats
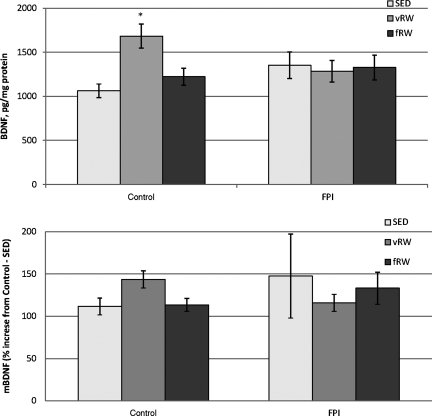
Two-way ANOVA of BDNF on PID 11 obtained with an ELISA indicated no significant effects of surgery or exercise, but a significant interaction between the two factors [F(2,54)=3.82, p<0.05]. Tukey's post-hoc analysis indicated significantly higher BDNF content in the control-vRW group than in either the control-fRW (p<0.05) or control-SED (p<0.01) groups (Fig. 3A). However, there were no significant differences among the three conditions in the FPI rats, or between each FPI group and its corresponding control condition.
FIG. 3.
Hippocampal brain-derived neurotrophic factor (BDNF) levels in control and fluid percussion injured (FPI) rats at post-injury day (PID) 11 after forced (fRW) or voluntary (vRW) exercise. Each histogram represents the mean±standard error of the mean for 6–16 samples per condition. (A) Total BDNF as detected by an enzyme-linked immunosorbent assay expressed as pg/mg protein (*p<0.05 versus the control-SED and control-fRW groups). (B) Mature BDNF (mBDNF) detected with Western blot, expressed as percent increase over control-SED content (SED, sedentary).
Values for PID 11 mature BDNF (mBDNF) that were obtained with Western blot showed a similar pattern; however, they did not attain statistical significance (Fig. 3B). No significant effects were found when proBDNF was analyzed.
Effect of 20-min forced exercise or voluntary exercise on hippocampal glucocorticoid receptors in control and FPI rats
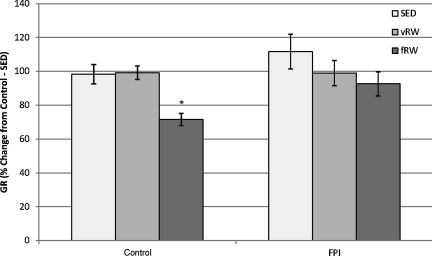
Two-way ANOVA of GR as the percent of the control-SED value indicated significant effects of surgery [F(1,55)=5.41, p<0.05], and exercise [F(2,55)=8.57, p<0.01], but the interaction of these two factors was not significant. Tukey's post-hoc analysis indicated that GR of the control-fRW rats was significantly (p<0.01) lower than that of the control-vRW or control-SED groups, and significantly lower than the FPI-vRW (p<0.05) and FPI-SED (p<0.01) groups, but only tended to be lower (p<0.1) that that of the FPI-fRW group (Fig. 4).
FIG. 4.
Hippocampal glucocorticoid receptor (GR) levels in control and fluid percussion injured (FPI) rats at post-injury day (PID) 11 after forced (fRW) or voluntary (vRW) exercise. Each histogram represents the mean±standard error of the mean percent change from control-SED GR content in 6–16 samples per condition (*p<0.05 versus control-SED and FPI-SED, and control-vRW and FPI-vRW groups). Results are expressed as percent change from control-SED GR content (SED, sedentary).
Discussion
The current study demonstrates that mild/moderate FPI affects HPA responses to fRW during the first 11 days post-injury. Daily fRW is a potent stimulus for the HPA axis, unlike vRW, which is only a stimulus on its first presentation. In contrast to the significant elevation of ACTH on PID 0, and the subsequently lower response to daily fRW in the controls, ACTH levels continued to be elevated by daily fRW exposure in the FPI rats. It therefore appears that control rats habituate to the daily stressor of fRW, whereas FPI rats are slower to habituate. Plasma CORT levels were elevated in both control and FPI rats in response to daily fRW, suggesting that fRW increased adrenocortical sensitivity to ACTH. Two further differential effects of FPI emerged when the hippocampus ipsilateral to the FPI was analyzed for BDNF and GR content on PID 11. FPI rats, regardless of whether they were sedentary or in the vRW or fRW condition on PID 11, did not show any changes in BDNF or GR, while in control rats, vRW resulted in a significant increase in BDNF, and fRW caused a significant decline in GR. These findings suggest that the apparent delay of habituation in the ACTH response to fRW during the first 11 days after mild/moderate FPI may be mediated in part by an FPI-induced lack of responsiveness of hippocampal BDNF and GR.
HPA responsiveness to physiological stimulation by exercise is altered after FPI
An acute activation of the HPA axis occurs initially after brain injury as a protective response that modulates the immune/inflammatory response and increases metabolic substrate availability (Johnson, 2006). Clinical studies suggest that plasma cortisol levels are dependent on injury severity during the subacute period (Woolf et al., 1990). Increases in CORT and ACTH in the first 1.5–6 h have also been observed with other experimental models (Gottesfeld et al., 2002; McCullers et al., 2002; Shohami et al., 1995). Likewise, elevations in CORT, which in the rodent is equivalent to cortisol, were found 3–5 h after FPI in the SED rats in the present experiments, although they were not accompanied by increases of ACTH (Figs. 1 and 2). Whereas this might suggest discordance between plasma ACTH and CORT levels at this time point, the well-characterized temporal lag between stimulus-induced changes in ACTH and CORT levels very likely underlies this dissociation (Engeland et al., 1977). However, subsequently at PIDs 4, 7, and 11, discordance between levels of the two HPA hormones is apparent, as discussed below.
Several key distinctions between the forced and voluntary exercise paradigms should be noted. Although the amount of exercise increased over time for both the forced and voluntary forms, the pattern of exercise was notably distinct. Rats under the vRW condition exercised ad libitum, mostly during the dark phase of PIDs 0–4 and PIDs 7–11, whereas rats under the fRW condition were exposed to exercise in two 20-min daily dark-phase sessions on PIDs 0–4 and again on PIDs 7–11. Important distinctions between these forms of exercise were also found when the pattern of weight gain and the HPA response were examined (Figs. 1 and 2).
By PID 4, the percent weight gain of the control-vRW and all FPI groups was significantly less than that of the control-SED group. By PID 11, weight gain was significantly less in both the control-fRW and FPI-fRW groups than their respective SED groups. Overall, these results indicate that FPI in and of itself, as well as twice-daily exposure to 20-min fRW, suppresses weight gain by 11 days of exposure in both control and FPI rats.
On PIDs 4, 7, and 11, fRW was a significantly stronger stressful stimulus than vRW, for both CORT and ACTH release in the controls. On PID 0, ACTH in the control-fRW group was significantly higher than in the FPI-fRW group, suggesting that FPI blunts the stress-induced ACTH response on the day of injury. The ACTH response of the FPI animals to fRW recovered by PID 4, with fRW becoming a stronger stressor than vRW on PIDs 4, 7, and 11. ACTH levels began to decline from the first to the second week of fRW in the controls, but not in the FPI animals. In fact, on PID 7, ACTH in the FPI-fRW group was significantly higher than in the control-fRW group, reminiscent of our previous finding of hyper-responsiveness to restraint-induced stress following a mild FPI during the first post-injury week (Griesbach et al., 2011). Interestingly, despite the time-dependent alterations in ACTH, CORT levels remained significantly elevated after fRW in both the control and FPI groups. This apparent dissociation of pituitary ACTH release and adrenal glucocorticoid production may be due to an altered sensitivity of the adrenal cortex to ACTH or ACTH-independent effects of stimuli, such as exercise, directly on the adrenal gland (Droste et al., 2007). It remains to be determined whether levels of ACTH and CORT in response to fRW would decline to those of the SED animals at later time points post-FPI. Despite the high levels of CORT after fRW on PID 11 in both the control and FPI groups, there were no differences in adrenal or thymus weights of either group on PID 11, regardless of exercise condition, again suggesting that 11 days of exposure to vRW or fRW was insufficient to affect regulation of the HPA axis.
Molecular mediators of exercise are affected by FPI
Exposure to vRW on PID 11 resulted in elevations of BDNF in the control but not in the FPI animals. These findings are in accordance with earlier studies, in which acute post-injury vRW exercise did not lead to increases in certain molecular markers of plasticity (Griesbach et al., 2004b). It should be noted that the total distance run (or number of wheel revolutions) by FPI rats in these experiments was equivalent to that of previous studies in which vRW increased levels of hippocampal BDNF when it was delayed after injury (Griesbach et al., 2004b,2007).
Although it is still unknown why the injured brain does not respond to acute voluntary exercise, it is plausible that an array of injury-induced alterations interfere with the effects of exercise. Exercise introduces an increase in metabolic demand at a time when the brain is energetically compromised, thus diverting cerebral metabolism from needed functions such as energy restoration and production of synaptic plasticity molecules. Moreover, experimental findings have shown that proteins associated with plasticity that were increased as a result of TBI are reduced when FPI rats are acutely exercised (Griesbach et al., 2004a). These disruptions, along with disruptions in neural activation and axonal integrity (Niogi et al., 2008; Topolnik et al., 2003), will make the injured brain less responsive to exercise.
Forced exercise did not increase BDNF levels in the control and FPI animals. The lack of an increase in BDNF was unexpected in the control group. Although to our knowledge there are no studies measuring BDNF after fRW exposure in intact rodents, there have been studies that have utilized a treadmill (O'Callaghan et al., 2009). However, these studies required pre-training and had more days of exposure. It is still questionable if an increase of BDNF would have been observed if the fRW exposure continued for more days. It is also likely that BDNF was not increased due to CORT elevations. Glucocorticoid inhibition of hippocampal BDNF has been observed both at the protein and mRNA level (Gronli et al., 2006; Schaaf et al., 1998). If BDNF were being suppressed due to glucocorticoid increases, it is possible that increases of BDNF with fRW would be present after more days of exposure, given that the ACTH response to fRW in the control animals appeared to diminish by PID 11.
Decreases in hippocampal GR were observed in the control animals exposed to fRW. These animals also showed pronounced elevations in CORT, thus suggesting that the downregulation of GR was due to enhanced negative feedback. Interestingly, although the FPI-fRW rats had equivalent increases in CORT, there were no decreases in hippocampal GRs. GRs are widespread throughout the brain and are predominantly expressed within the hippocampus (McEwen, 1999; McEwen and Magarinos, 2001). Given that forebrain GR is of particular importance in limiting CORT responses to psychogenic stressors (Furay et al., 2008), our results suggest that the downregulation of hippocampal GR may cause stressor-specific feedback impairment. However, the reduction of hippocampal GR did not appear to affect fRW-induced facilitation of the HPA axis response in the controls, suggesting that GR in other brain regions or other regulatory mechanisms, such as different circuits or cell populations or metabolic signaling (Dallman et al., 2004; Kwon et al., 2006), are differentially affected by FPI.
Conclusions
Glucocorticoids, which are known to suppress levels of BDNF and other key proteins, are elevated acutely after injury, and may contribute to the undesired effects of early post-injury exercise. In addition, a hyper-response to exercise is likely to magnify exercise's normal metabolic effects and enhance injury-induced disruptions in neuronal activation. These studies indicate that some forms of exercise elicit a stronger stress response. Thus certain exercise regimens with stronger stress responses may be particularly counterproductive during the early post-injury time period.
Acknowledgments
We thank David Garfinkel and Shyama Nair for their excellent technical assistance. This research was supported by NIH grant NS06190 to G.S.G., and the UCLA Brain Injury Research Center.
Author Disclosure Statement
No competing financial interests exist.
References
- Avramescu S. Timofeev I. Synaptic strength modulation after cortical trauma: a role in epileptogenesis. J. Neurosci. 2008;28:6760–6772. doi: 10.1523/JNEUROSCI.0643-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazarian J.J. Blyth B. Cimpello L. Bench to bedside: evidence for brain injury after concussion—looking beyond the computed tomography scan. Acad. Emerg. Med. 2006;13:199–214. doi: 10.1197/j.aem.2005.07.031. [DOI] [PubMed] [Google Scholar]
- Dallman M.F. Akana S.F. Strack A.M. Scribner K.S. Pecoraro N. La Fleur S.E. Houshyar H. Gomez F. Chronic stress-induced effects of corticosterone on brain: direct and indirect. Ann. NY Acad. Sci. 2004;1018:141–150. doi: 10.1196/annals.1296.017. [DOI] [PubMed] [Google Scholar]
- Droste S.K. Chandramohan Y. Hill L.E. Linthorst A.C. Reul J.M. Voluntary exercise impacts on the rat hypothalamic-pituitary-adrenocortical axis mainly at the adrenal level. Neuroendocrinology. 2007;86:26–37. doi: 10.1159/000104770. [DOI] [PubMed] [Google Scholar]
- Engeland W.C. Shinsako J. Winget C.M. Vernikos-Danellis J. Dallman M.F. Circadian patterns of stress-induced ACTH secretion are modified by corticosterone responses. Endocrinology. 1977;100:138–147. doi: 10.1210/endo-100-1-138. [DOI] [PubMed] [Google Scholar]
- Furay A.R. Bruestle A.E. Herman J.P. The role of the forebrain glucocorticoid receptor in acute and chronic stress. Endocrinology. 2008;149:5482–5490. doi: 10.1210/en.2008-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding E.M. Steenberg M.L. Contant C.F., Jr. Krishnappa I. Robertson C.S. Bryan R.M., Jr. Cerebrovascular reactivity to CO(2) and hypotension after mild cortical impact injury. Am. J. Physiol. 1999;277:H1457–H1466. doi: 10.1152/ajpheart.1999.277.4.H1457. [DOI] [PubMed] [Google Scholar]
- Gottesfeld Z. Moore A.N. Dash P.K. Acute ethanol intake attenuates inflammatory cytokines after brain injury in rats: a possible role for corticosterone. J. Neurotrauma. 2002;19:317–326. doi: 10.1089/089771502753594882. [DOI] [PubMed] [Google Scholar]
- Griesbach G.S. Gomez-Pinilla F. Hovda D.A. The upregulation of plasticity-related proteins following TBI is disrupted with acute voluntary exercise. Brain Res. 2004a;1016:154–162. doi: 10.1016/j.brainres.2004.04.079. [DOI] [PubMed] [Google Scholar]
- Griesbach G.S. Gomez-Pinilla F. Hovda D.A. Time window for voluntary exercise-induced increases in hippocampal neuroplasticity molecules after traumatic brain injury is severity dependent. J. Neurotrauma. 2007;24:1161–1171. doi: 10.1089/neu.2006.0255. [DOI] [PubMed] [Google Scholar]
- Griesbach G.S. Hovda D.A. Gomez-Pinilla F. Exercise-induced improvement in cognitive performance after traumatic brain injury in rats is dependent on BDNF activation. Brain Res. 2009;1288:105–115. doi: 10.1016/j.brainres.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesbach G.S. Hovda D.A. Molteni R. Wu A. Gomez-Pinilla F. Voluntary exercise following traumatic brain injury: brain-derived neurotrophic factor upregulation and recovery of function. Neuroscience. 2004b;125:129–139. doi: 10.1016/j.neuroscience.2004.01.030. [DOI] [PubMed] [Google Scholar]
- Griesbach G.S. Hovda D.A. Tio D.L. Taylor A.N. Heightening of the stress response during the first weeks after a mild traumatic brain injury. Neuroscience. 2011;178:147–158. doi: 10.1016/j.neuroscience.2011.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronli J. Bramham C. Murison R. Kanhema T. Fiske E. Bjorvatn B. Ursin R. Portas C.M. Chronic mild stress inhibits BDNF protein expression and CREB activation in the dentate gyrus but not in the hippocampus proper. Pharmacol. Biochem. Behav. 2006;85:842–849. doi: 10.1016/j.pbb.2006.11.021. [DOI] [PubMed] [Google Scholar]
- Gurkoff G.G. Giza C.C. Hovda D.A. Lateral fluid percussion injury in the developing rat causes an acute, mild behavioral dysfunction in the absence of significant cell death. Brain Res. 2006;1077:24–36. doi: 10.1016/j.brainres.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Hansson A.C. Sommer W.H. Metsis M. Stromberg I. Agnati L.F. Fuxe K. Corticosterone actions on the hippocampal brain-derived neurotrophic factor expression are mediated by exon IV promoter. J. Neuroendocrinol. 2006;18:104–114. doi: 10.1111/j.1365-2826.2005.01390.x. [DOI] [PubMed] [Google Scholar]
- Johnson J.A. The hypothalmic-pituitary-adrenal axis in critical illness. AACN Clin. Issues. 2006;17:39–49. doi: 10.1097/00044067-200601000-00006. [DOI] [PubMed] [Google Scholar]
- Kwon M.S. Seo Y.J. Shim E.J. Choi S.S. Lee J.Y. Suh H.W. The effect of single or repeated restraint stress on several signal molecules in paraventricular nucleus, arcuate nucleus and locus coeruleus. Neuroscience. 2006;142:1281–1292. doi: 10.1016/j.neuroscience.2006.07.027. [DOI] [PubMed] [Google Scholar]
- Leasure J.L. Jones M. Forced and voluntary exercise differentially affect brain and behavior. Neuroscience. 2008;156:456–465. doi: 10.1016/j.neuroscience.2008.07.041. [DOI] [PubMed] [Google Scholar]
- Levine E.S. Dreyfus C.F. Black I.B. Plummer M.R. Brain-derived neurotrophic factor rapidly enhances synaptic transmission in hippocampal neurons via postsynaptic tyrosine kinase receptors. Proc. Natl. Acad. Sci. USA. 1995;92:8074–8077. doi: 10.1073/pnas.92.17.8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Donald C.L. Johnson A.M. Cooper D. Nelson E.C. Werner N.J. Shimony J.S. Snyder A.Z. Raichle M.E. Witherow J.R. Fang R. Flaherty S.F. Brody D.L. Detection of blast-related traumatic brain injury in U.S. military personnel. N. Engl. J. Med. 2011;364:2091–2100. doi: 10.1056/NEJMoa1008069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister T.W. Flashman L.A. McDonald B.C. Saykin A.J. Mechanisms of working memory dysfunction after mild and moderate TBI: evidence from functional MRI and neurogenetics. J. Neurotrauma. 2006;23:1450–1467. doi: 10.1089/neu.2006.23.1450. [DOI] [PubMed] [Google Scholar]
- McCullers D.L. Sullivan P.G. Scheff S.W. Herman J.P. Traumatic brain injury regulates adrenocorticosteroid receptor mRNA levels in rat hippocampus. Brain Res. 2002;947:41–49. doi: 10.1016/s0006-8993(02)02904-9. [DOI] [PubMed] [Google Scholar]
- McEwen B.S. Magarinos A.M. Stress and hippocampal plasticity: implications for the pathophysiology of affective disorders. Hum. Psychopharmacol. 2001;16:S7–S19. doi: 10.1002/hup.266. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Ann Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- Neigh G.N. Karelina K. Zhang N. Glasper E.R. Owens M.J. Plotsky P.M. Nemeroff C.B. Devries A.C. Cardiac arrest and cardiopulmonary resuscitation dysregulates the hypothalamic-pituitary-adrenal axis. J. Cereb. Blood Flow Metab. 2009;29:1673–1682. doi: 10.1038/jcbfm.2009.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niogi S.N. Mukherjee P. Ghajar J. Johnson C. Kolster R.A. Sarkar R. Lee H. Meeker M. Zimmerman R.D. Manley G.T. McCandliss B.D. Extent of microstructural white matter Injury in postconcussive syndrome correlates with impaired cognitive reaction time: A 3T diffusion tensor imaging study of mild traumatic brain injury. Am. J. Neuroradiol. 2008;29:967–973. doi: 10.3174/ajnr.A0970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan R.M. Griffin E.W. Kelly A.M. Long-term treadmill exposure protects against age-related neurodegenerative change in the rat hippocampus. Hippocampus. 2009;19:1019–1029. doi: 10.1002/hipo.20591. [DOI] [PubMed] [Google Scholar]
- Osteen C.L. Moore A.H. Prins M.L. Hovda D.A. Age-dependency of 45calcium accumulation following lateral fluid percussion: acute and delayed patterns. J. Neurotrauma. 2001;18:141–162. doi: 10.1089/08977150150502587. [DOI] [PubMed] [Google Scholar]
- Pinheiro J.C. Bates D.M. Mixed Effects Models in S and S-Plus. Springer-Verlag; New York: 2000. [Google Scholar]
- Rapoport M. McCauley S. Levin H. Song J. Feinstein A. The role of injury severity in neurobehavioral outcome 3 months after traumatic brain injury. Neuropsychiatry Neuropsychol. Behav. Neurol. 2002;15:123–132. [PubMed] [Google Scholar]
- R Development Core Team. R Foundation for Statistical Computing; Vienna, Austria: 2011. R: A language and environment for statistical computing. ISBN 3-900051-07-0, URL. [Google Scholar]
- Schaaf M.J. de Jong J. de Kloet E.R. Vreugdenhil E. Downregulation of BDNF mRNA and protein in the rat hippocampus by corticosterone. Brain Res. 1998;813:112–120. doi: 10.1016/s0006-8993(98)01010-5. [DOI] [PubMed] [Google Scholar]
- Shohami E. Bass R. Trembovler V. Weidenfeld J. The effect of the adrenocortical axis upon recovery from closed head injury. J. Neurotrauma. 1995;12:1069–1077. doi: 10.1089/neu.1995.12.1069. [DOI] [PubMed] [Google Scholar]
- Tavazzi B. Vagnozzi R. Signoretti S. Amorini A.M. Belli A. Cimatti M. Delfini R. Di Pietro V. Finocchiaro A. Lazzarino G. Temporal window of metabolic brain vulnerability to concussions: oxidative and nitrosative stresses—part II. Neurosurgery. 2007;61:390–395. doi: 10.1227/01.neu.0000255525.34956.3f. discussion 395x–396. [DOI] [PubMed] [Google Scholar]
- Topolnik L. Steriade M. Timofeev I. Hyperexcitability of intact neurons underlies acute development of trauma-related electrographic seizures in cats in vivo. Eur. J. Neurosci. 2003;18:486–496. doi: 10.1046/j.1460-9568.2003.02742.x. [DOI] [PubMed] [Google Scholar]
- Tran L.D. Lifshitz J. Witgen B.M. Schwarzbach E. Cohen A.S. Grady M.S. Response of the contralateral hippocampus to lateral fluid percussion brain injury. J. Neurotrauma. 2006;23:1330–1342. doi: 10.1089/neu.2006.23.1330. [DOI] [PubMed] [Google Scholar]
- Tyler W.J. Pozzo-Miller L.D. BDNF enhances quantal neurotransmitter release and increases the number of docked vesicles at the active zones of hippocampal excitatory synapses. J. Neurosci. 2001;21:4249–4258. doi: 10.1523/JNEUROSCI.21-12-04249.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagnozzi R. Signoretti S. Tavazzi B. Floris R. Ludovici A. Marziali S. Tarascio G. Amorini A.M. Di Pietro V. Delfini R. Lazzarino G. Temporal window of metabolic brain vulnerability to concussion: a pilot 1H-magnetic resonance spectroscopic study in concussed athletes—part III. Neurosurgery. 2008;62:1286–1295. doi: 10.1227/01.neu.0000333300.34189.74. discussion 1295–1286. [DOI] [PubMed] [Google Scholar]
- Woolf P.D. Cox C. Kelly M. Nichols D. McDonald J.V. Hamill R.W. The adrenocortical response to brain injury: correlation with the severity of neurologic dysfunction, effects of intoxication, and patient outcome. Alcohol Clin. Exp. Res. 1990;14:917–921. doi: 10.1111/j.1530-0277.1990.tb01838.x. [DOI] [PubMed] [Google Scholar]