Abstract
Traumatic brain injury (TBI) contributes to morbidity in children, and boys are disproportionately represented. Endothelin-1 (ET-1) contributes to impaired autoregulation via oxygen (O2-) after TBI in piglets, but its relative role in males compared with females has not been previously investigated. Increased cerebral perfusion pressure (CPP) via phenylephrine (Phe) sex dependently improves impairment of autoregulation after TBI through modulation of extracellular signal-related kinase (ERK) mitogen-activated protein kinase (MAPK) upregulation, aggravated in males, but blocked in females. Activation of adenosine-5'-triphosphate (ATP) and Ca sensitive K channels produce vasodilation, contributing to autoregulation. We hypothesized that ET-1 upregulation is greater in males after TBI and that disturbed autoregulation will be prevented by Phe in a sex-dependent manner through modulation of ET-1, O2-, and ERK. Results show that ET-1 release was greater in males after fluid percussion injury (FPI), blunted by Phe in females, but aggravated in males. K channel vasodilation was impaired more in males than in females after TBI. Phe prevented reductions in K channel vasodilation in females, but further reduced dilation in males after TBI. Co-administration of BQ-123, U0126, or PEG-SOD (ET-1, ERK antagonist, and O2-scavenger) with Phe restored dilation to K agonists and hypotension in males after TBI. ERK upregulation was blocked by BQ-123 and PEG-SOD. These data indicate that TBI upregulates ET-1 more in males than in females. Elevation of CPP with Phe sex dependently prevents impairment of cerebral autoregulation after TBI through modulation of ET-1, O2-, and ERK mediated impairment of K channel vasodilation. These observations advocate for the consideration of development of sex-based therapies for the treatment of hemodynamic sequelae of pediatric TBI.
Key words: cerebral autoregulation, endothelin, K channels, signal transduction, TBI
Introduction
Hypotension and low cerebral perfusion pressure (CPP) are associated with poor outcomes after traumatic brain injury (TBI) in children (Català-Temprano et al., 2007). After TBI, boys of all ages and children<4 years of age have particularly devastating outcomes (Langlois et al., 2005). Cerebral autoregulation is often impaired after TBI (Freeman et al., 2008) and with concomitant hypotension, cerebral ischemia may ensue, leading to poor outcome.
Because ethical considerations constrain mechanistic studies in children with TBI, we have used an established porcine model of fluid percussion injury (FPI) that mimics TBI, to corroborate clinical observations regarding cerebral autoregulation and hypotension after TBI (Armstead, 2000). Like humans, piglets have gyrencephalic brains, are sensitive to FPI, and newborn and juvenile pigs mimic young (<4 years) and older (≥4 years) children. Autoregulation is impaired to a greater extent in newborn compared with juvenile pigs, which parallels that observed clinically in humans (DiGennaro et al., 2011; Freeman et al., 2008). Upregulation of the spasmogen endothelin-1 (ET-1) is greater and contributes to the more robust impairment of autoregulation in younger compared with older pigs (Armstead, 1999b). However, at the time that this study was performed, the potential for differences in magnitude of autoregulatory impairment in males and females was not considered.
Activation of K+ channels, particularly adenosine-5'-triphosphate (ATP) and calcium sensitive (KATP and KCa) channels, increases K+ efflux, produces hyperpolarization of vascular muscle, and is an important mechanism for cerebral vasodilation, including hypotension (Faraci and Heistad, 1998). Cerebrovasodilation mediated by K channel agonists and autoregulation during hypotension were observed to be impaired more in males than females after FPI (Armstead, 1997; Armstead and Vavilala, 2007; Armstead et al., 2011). Administration of an endogenous K channel agonist, adrenomedullin (ADM), prevents sex-dependent impairment of autoregulation through blockade of the upregulation of the extracellular signal-related kinase (ERK) isoform of mitogen-activated protein kinase (MAPK), a family of at least three kinases (ERK, p38, and JNK) that are critically important in hemodynamics after TBI (Armstead, 2000, 2005; Armstead and Vavilala, 2007; Armstead et al., 2010a; Laher and Zhang, 2001). ET-1 also contributes to blunted K channel agonist mediated dilation after FPI, via release of activated oxygen (O2-) (Armstead, 2005).
Current 2003 pediatric guidelines recommend maintaining CPP>40 mm Hg (Adelson et al., 2003). Despite these therapeutic targets, there are no guidelines regarding how this should be achieved other than therapies to lower intracranial pressure (ICP) by mannitol or hypertonic saline (Bratton et al., 2007), the latter of which may be desirable because of the added benefit of increasing CPP beyond what would be expected because of the drop in ICP (Keenan et al., 2005). In addition to decreasing ICP, one common strategy to increase CPP is to use vasopressors to increase mean arterial pressure (MAP). However, CPP-directed therapy has remained somewhat controversial because it has been observed to either have no effect or to worsen outcome (Coles et al., 2004). Additionally, CPP has been considered to be a poor surrogate for cerebral blood flow (CBF) (Cremer et al., 2005), because regional or local CBF may be markedly reduced even if CPP is normal (Coles et al., 2004). Because ICP monitors are not universally used to guide CPP therapy, especially in young children, clinicians often rely on MAP to estimate CBF. A vasopressor commonly used to elevate MAP is phenylephrine (Phe) (Ishikawa et al., 2009). We recently observed that Phe prevented in females but exacerbated in males the impairment of cerebral autoregulation (Armstead et al., 2010a). In these studies, there was a Phe-mediated aggravation of ERK MAPK upregulation and concomitant decrease of adrenomedullin in males compared to the diminished ERK MAPK upregulation and alternate elevation of adrenomedullin in females after FPI (Armstead et al., 2010a).
In the present study, we hypothesized that ET-1 upregulation is greater in males than in females after TBI, and that disturbed autoregulation will be prevented by Phe in a sex-dependent manner through modulation of ET-1, O2-, and ERK.
Methods
Closed cranial window technique and TBI
Newborn pigs (1–5 days old, 1.0–1.5 Kg) of either sex were studied. All protocols were approved by the Institutional Animal Care and Use Committee. Animals were anesthetized with isoflurane (1–2 minimum alveolar concentration [MAC]). Anesthesia was maintained with a-chloralose (30–50 mg/kg. supplemented with 5 mg /kg/h i.v.). A catheter was inserted into a femoral artery to monitor blood pressure and to sample for blood gas tensions and pH. Drugs to maintain anesthesia were administered through a second catheter placed in a femoral vein. The trachea was cannulated, and the animals were ventilated with room air. A heating pad was used to maintain the animals at 37–39°C, monitored rectally.
A cranial window was placed in the parietal skull of these anesthetized animals. This window consisted of three parts: a stainless steel ring, a circular glass cover-slip, and three ports consisting of 17-gauge hypodermic needles attached to three pre-cut holes in the stainless steel ring. For placement, the dura was cut and retracted over the cut bone edge. The cranial window was placed in the opening and cemented in place with dental acrylic. The volume under the window was filled with a solution, similar to cerebrospinal fluid (CSF), of the following composition (in mM): 3.0 KCl, 1.5 MgCl2, 1.5 CaCl2, 132 NaCl, 6.6 urea, 3.7 dextrose, and 24.6 NaHCO3. This artificial CSF was warmed to 370C and had the following chemistry: pH 7.33, pCO2 46 mm Hg, and pO2 43 mm Hg, which was similar to that of endogenous CSF. Pial arterial vessel diameter was measured with a microscope, a camera, a video output screen and a video microscaler. An Integra Camino monitor was used to measure ICP.
The method used to induce brain FPI has been described previously (Armstead, 1997). A device designed by the Medical College of Virginia was used. A small opening was made in the parietal skull contralateral to the cranial window. A metal shaft was sealed into the opening on top of intact dura and fluid coupled to the brain injury device, that is, the shaft was connected to the transducer housing, which was in turn connected to the fluid percussion device. The device itself consisted of an acrylic plastic cylindrical reservoir 60 cm long, 4.5 cm in diameter, and 0.5 cm thick. One end of the device was connected to the transducer housing, whereas the other end had an acrylic plastic piston mounted on O-rings. The exposed end of the piston was covered with a rubber pad. The entire system was filled with 0.9 % saline. The percussion device was supported by two brackets mounted on a platform. FPI was induced by striking the piston with a 4.8 kg pendulum. The intensity of the injury (usually 1.9–2.3 atm with a constant duration of 19–23 ms) was controlled by varying the height from which the pendulum was allowed to fall. The pressure pulse of the injury was recorded on a storage oscilloscope triggered photoelectrically by the fall of the pendulum. The amplitude of the pressure pulse was used to determine the intensity of the injury.
Protocol
Two types of pial vessels, small arteries (resting diameter, 120–160 μm) and arterioles (resting diameter, 50–70 μm) were examined to determine whether segmental differences in the effects of FPI could be identified. Typically, 2–3 mL of artificial CSF were flushed through the window over a 30 sec period, and excess CSF was allowed to run off through one of the needle ports. For sample collection, 300 μL of the total cranial window volume of 500 μL was collected by slowly infusing artificial CSF into one side of the window and allowing the CSF to drip freely into a collection tube on the opposite side.
Twenty-two experimental groups were studied (all n=5) (Table 1). Phe was administered at a dose of 1 μg/Kg/min, i.v., the BQ-123 dose was 1 mg/kg i.v., SODCAT (polyethylene glycol superoxide dismutase and catalase) was 1000 and 10,000 μg/kg i.v., respectively, and U0126 was given at a dose of 1 mg/kg i.v. Hypotension was induced by the rapid withdrawal of either 5–8 or 10–15 mL blood/Kg to induce moderate or severe hypotension (decreases in MAP of 25 and 45%, respectively). Such decreases in blood pressure were maintained constant for 10 min by titration of additional blood withdrawal or blood reinfusion. The vehicle for all agents was 0.9% saline, except for the MAPK inhibitor, which used dimethyl sulfoxide (100 μL) diluted with 9.9 mL 0.9% saline. In sham control animals, responses to hypotension (moderate, severe), papaverine (10−8, 10−6 M), cromakalim, calcitonin gene-related peptide (CGRP) (KATP channel agonists), and NS 1619 (KCa channel agonist) (all 10−8,10−6 M) were obtained initially and then again 1 h later in the presence of the agent vehicle. In drug pre-treated FPI animals, drugs were administered 30 min before FPI, and the responses to hypotension, papaverine, cromakalim, CGRP, and NS 1619 were obtained at 1 h after injury. In drug post-treated animals, drugs were administered 30 min after FPI, and responses to hypotension, papaverine, cromakalim, CGRP, NS 1619, and CSF samples collected at 1 h post- insult. The order of agonist administration was randomized within animal drug treatment groups.
Table 1.
Experimental Groups
|
Pre-treatment | |||||
|---|---|---|---|---|---|
| Sham | FPI-veh | FPI-Phe | FPI+Phe+BQ 123 | FPI+Phe+SODCAT | FPI+Phe+U 0126 |
| M 5 | 5 | 5 | 5 | 5 | 5 |
| F 5 | 5 | 5 | 5 | 5 | 5 |
|
Post-treatment | |||||
|---|---|---|---|---|---|
| Sham | FPI-veh | FPI-Phe | FPI+Phe+BQ 123 | FPI+Phe+SODCAT | FPI+Phe+U 0126 |
| M | 5 | 5 | 5 | 5 | 5 |
| F | 5 | 5 | 5 | 5 | 5 |
n=number of animals per group.
Enzyme-linked immunosorbent assay (ELISA) and radioimmunoassay (RIA)
Commercially available ELISA Kits were used to quantity CSF ERK MAPK (Assay Designs) concentration, whereas RIA kits were used to quantify ET-1 (Phoenix Pharmaceuticals). Phosphorylated ERK MAPK enzyme values were normalized to total form and then expressed as percent of the control condition.
Statistical analysis
Pial artery diameter, CSF ERK MAPK and ET-1 values were analyzed using ANOVA for repeated measures. If the value was significant, the data were then analyzed by Fishers protected least significant difference test. An α level of p<0.05 was considered significant in all statistical tests. Values are represented as mean±SEM of the absolute value or as percentage changes from control value.
Results
Phe aggravates upregulation of ET-1, contributing to worsened impairment of autoregulation in the male, but blunts ET-1 upregulation, contributing to protection of autoregulation in the female pig after FPI during hypotension
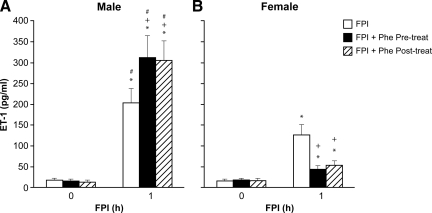
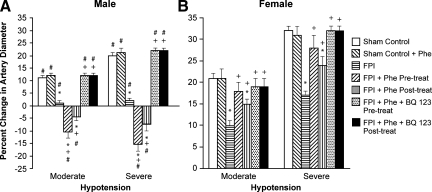
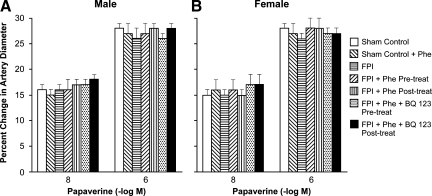
CSF ET-1 concentration was elevated more in the male than in the female within 1 h after equivalent levels of FPI (1.9±0.1 and 2.0±0.1 atm) (Fig. 1). Phe (1 μg/kg/min i.v.) administered either 30 min before or 30 min after FPI aggravated ET-1 release in the male but blunted release in the female after FPI (Fig. 1). Moderate and severe hypotension (24±1 and 45±2% decrease in MAP, respectively) produced reproducible increases in pial artery diameter. Pial artery dilation during hypotension was blunted more in the male than in the female after FPI (Fig. 2). Pre- and post-injury treatment with Phe reversed hypotension-induced pial artery vasodilation to vasoconstriction in the male but protected autoregulatory pial artery dilation in the female (Fig. 2). Combined administration of Phe+the ET-1 antagonist BQ 123 (1 mg/kg i.v.) either pre- or post-injury protected autoregulatory pial artery dilation during hypotension in both males and females (Fig. 2). In contrast, papaverine-induced pial artery dilation was unchanged by FPI or administration of Phe and BQ 123 (Fig. 3). Pial artery dilation during hypotension was unchanged by Phe administered to sham control male and female pigs. Similar observations to all of the above were made in pial arterioles (data not shown).
FIG. 1.
Influence of fluid percussion injury (FPI) on cerebrospinal fluid (CSF) Endothelin-1 (ET-1) in (A) male and (B) female pigs. Conditions are: before (0 time) and 1 h after FPI; in vehicle (FPI), phenylephrine (Phe) pre- and post-treated animals. Pre-treatment is 30 min before whereas post-treatment is 30 min after FPI, n=5. *p<0.05 compared with corresponding before FPI value; +p<0.05 compared with corresponding untreated FPI value; #p<0.05 compared with corresponding female value.
FIG. 2.
Influence of fluid percussion injury (FPI) on pial artery diameter during hypotension (moderate, severe) in (A) male and (B) female pigs. Conditions are: before (sham control) and 1 h after FPI; in vehicle (FPI), phenylephrine (Phe), and Phe+BQ-123 (1 mg/kg i.v.) pre- and post-treated animals. Pre-treatment is 30 min before whereas post-treatment is 30 min after FPI, n=5. *p<0.05 compared with corresponding before FPI value; +p<0.05 compared with corresponding untreated FPI value; #p<0.05 compared with corresponding female value.
FIG. 3.
Influence of papaverine (10−8, 10−6 M) on pial artery diameter in the absence and presence of fluid percussion injury (FPI) in (A) male and (B) female pigs. Conditions are: before (sham control) and 1 h after FPI; in vehicle (FPI), phenylephrine (Phe), and Phe+BQ-123 (1 mg/kg i.v.) pre- and post-treated animals. Pre-treatment is 30 min before whereas post-treatment is 30 min after FPI, n=5. *p<0.05 compared with corresponding before FPI value; +p<0.05 compared with corresponding untreated FPI value; #p<0.05 compared with corresponding female value.
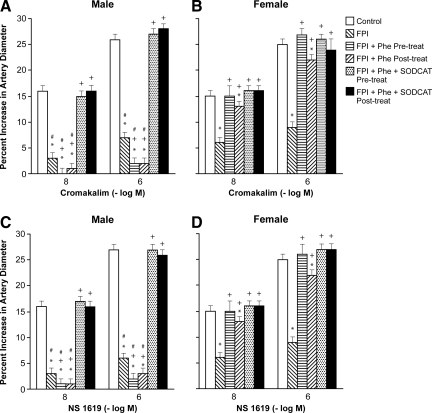
Phe aggravates impairment of cromakalim, CGRP, and NS 1619 induced pial artery dilation after FPI in the male, but prevents such impairment in the female in an ET-1 and superoxide-dependent manner
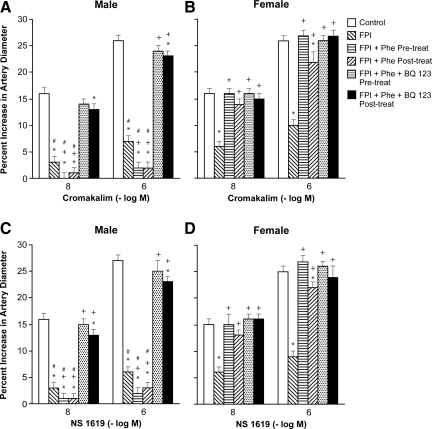
Cromakalim and NS 1619 (10−8, 10−6 M), KATP and KCa channel agonists, elicited pial artery dilation, which was blunted more in the male than in the female after FPI (Figs. 4 and 5). Pre-and post-injury treatment with Phe aggravated impairment of cromakalim and NS 1619 induced pial artery dilation in the male, but prevented such impairment in the female after FPI (Figs. 4 and 5). Co-administration of either the ET-1 antagonist BQ 123 or the free radical scavenger SODCAT with Phe also prevented impairment of cromakalim and NS 1619 induced pial artery dilation in both males and females after FPI (Figs. 4 and 5). Similar observations were made for the endogenous KATP channel agonist CGRP.
FIG. 4.
Influence of fluid percussion injury (FPI) on pial artery diameter in response to cromakalim (10−8, 10−6 M) in (A) male and (B) female pigs, and NS 1619 (10−8, 10−6 M), also in (C) male and (D) female pigs. Conditions are: before (control) and 1 h after FPI; in vehicle (FPI), phenylephrine (Phe), and Phe+BQ-123 (1 mg/kg i.v.) pre- and post-treated animals. Pre-treatment is 30 min before whereas post-treatment is 30 min after FPI, n=5. *p<0.05 compared with corresponding before FPI value; +p<0.05 compared with corresponding untreated FPI value; #p<0.05 compared with corresponding female value.
FIG. 5.
Influence of fluid percussion injury (FPI) on pial artery diameter in response to cromakalim (10−8, 10−6 M) in (A) male and (B) female pigs, and NS 1619 (10−8, 10−6 M), also in (C) male and (D) female pigs. Conditions are: before (control) and 1 h after FPI; in vehicle (FPI), phenylephrine (Phe), and SODCAT pre- and post-treated animals. Pre-treatment is 30 min before whereas post-treatment is 30 min after FPI, n=5. *p<0.05 compared with corresponding before FPI value; +p<0.05 compared with corresponding untreated FPI value; #p<0.05 compared with corresponding female value.
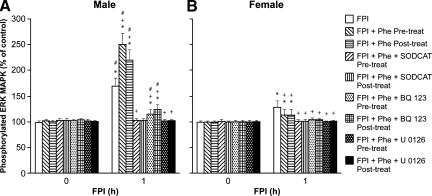
Phe aggravates increases in CSF ERK MAPK after FPI in the male but blocks such elevation in the female in an ET-1 and superoxide-dependent manner
CSF ERK MAPK was increased more in the male than in the female after FPI (Fig. 6). Pre-and post-injury treatment with Phe aggravated elevations of CSF ERK MAPK in the male, but blunted such increases in the female (Fig. 6). Co-administration of either SODCAT or BQ 123+Phe blunted elevations of ERK MAPK after FPI in males and females (Fig. 6). The housekeeping ERK MAPK antagonist U0126 (1 mg/kg i.v.) blocked elevations of CSF ERK MAPK after FPI in males and females (Fig. 6).
FIG. 6.
Influence of fluid percussion injury (FPI) on cerebrospinal fluid (CSF) extracellular signal-related kinase (ERK) mitogen-activated protein kinase (MAPK) (expressed as percent of total) in (A) male and (B) female pigs. Conditions are: before (0 time) and 1 h after FPI; in vehicle (FPI), phenylephrine (Phe), SODCAT, BQ-123, and UO126 pre- and post-treated animals. Pre-treatment is 30 min before whereas post-treatment is 30 min after FPI, n=5. *p<0.05 compared with corresponding before FPI value; +p<0.05 compared with corresponding untreated FPI value; #p<0.05 compared with corresponding female value.
Blood chemistry
Blood chemistry values were collected before and after all experiments. There were no statistically significant differences in pH, pCO2, or pO2 between sham control, FPI, and FPI and drug-treated animals.
Discussion
An important new finding in this study is that the increase in the CSF concentration of the spasmogen ET-1 is greater in males than in females after equivalent FPI. Previously, we had observed that ET-1 was released after FPI and that it contributed to impaired autoregulation during hypotension more in younger than in older pigs (Armstead, 1999b). What was uninvestigated at that time was whether release was similarly different as a function of sex. Interestingly, autoregulation during hypotension, similarly more impaired in the male than in the female after FPI (Armstead and Vanilla, 2007; Armstead et al., 2010a,b), paralleled the sex-dependent release of this spasmogen, suggesting that ET-1 may be an important contributor to sex-based differences in dysregulation in TBI.
Elevation of MAP is often used as a clinical intervention to limit hypoperfusion after TBI. Whereas several different pressors can be used, the pressor of choice often may be Phe because of its longer duration of action and peak elevation of MAP (DiGennaro et al., 2011). Because of our previous observation that an infusion of Phe that equipotently elevates MAP in male and female piglets aggravates dysregulation during hypotension after FPI in male pigs but prevents such derangements in female pigs (Armstead et al., 2010a), we have more recently designed our studies to reveal mechanism(s) that could explain this differential sex-dependent hemodynamic outcome to use of a well-accepted critical care pathway for treatment. Our first follow-up study observed that ERK MAPK was elevated after FPI more in the male than in the female pig and that Phe aggravated ERK MAPK upregulation in males but abrogated such elevation in female pigs (Armstead et al., 2010b). ERK MAPK upregulation is known to contribute to hypoperfusion after FPI (Armstead et al., 2009). Because years earlier we had noted a series of observations suggestive that ET-1 could elevate CSF ERK MAPK in FPI (Armstead, 2005), we first wanted to directly confirm this in the present study. New results now show that the ET-1 antagonist BQ 123 blocks elevation of CSF ERK MAPK and the aggravation of such elevation by Phe after FPI. Co-administered BQ 123 with Phe also prevented impairment of autoregulatory pial artery dilation during hypotension after FPI, supportive of the intermediary role for ET-1 in sex-dependent Phe-mediated hemodynamic dysregulation. In contrast, papaverine-induced pial artery dilation was unchanged after FPI and Phe in male and female piglets, indicating the specificity of the Phe effect. BQ 123 has been shown to be an efficacious ET-1 antagonist in the piglet cerebral circulation (Armstead and Raghupathi, 2011).
Because pial artery dilation during hypotension is blocked by glibenclamide and iberiotoxin, KATP and KCa channel antagonists, autoregulation is dependent upon activation of these K channels (Armstead, 1999a). Cerebrovasodilation in response to K channel openers can be used as an index of intactness of K channel function. Because pial artery dilation in response to the KATP channel openers cromakalim and CGRP and the KCa channel agonist NS 1619 is blunted after FPI (Armstead, 1997, 2005), K channel impairment is a mechanistic explanation for impaired cerebral autoregulation during hypotension post-insult in the piglet. We recently observed that Phe infusion aggravates impairment of K channel agonist-induced pial artery dilation in male piglets after FPI, but protects against such impairment in female piglets through modulation of ERK MAPK upregulation (Armstead et al., 2010b). Results of the present study using BQ 123 and the activated oxygen radical scavenger SODCAT extend these observations to indicate that ET-1 and superoxide also contribute to sex-dependent impairment of K channel induced cerebrovasodilation by Phe in FPI. Because CGRP is an endogenous activator of the KATP channel (Edvinsson, 1987), these observations have physiological functional significance.
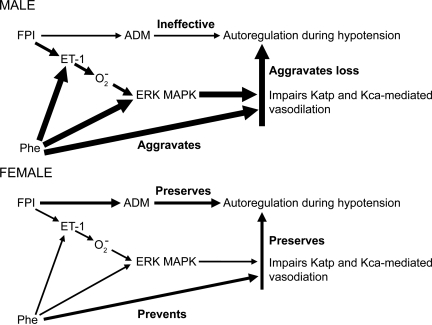
Final experiments in the present study were designed to elucidate the sequential relationships between ET-1, ERK MAPK, activated oxygen, and K channels in impairment of cerebral autoregulation, and its aggravation by Phe, as a function of sex in FPI. In a prior study, we observed that generation of activated oxygen on the brain in a concentration roughly similar to that observed endogenously after FPI blocked pial artery dilation in response to KATP and KCa channel agonists, which was prevented by the ERK MAPK antagonist U0126 (Armstead, 1999c), indicating that superoxide was upstream of ERK MAPK (Fig. 7). Results of the present study show that the ET-1 antagonist BQ 123 blunted, whereas the free radical scavenger SODCAT blocked, ERK MAPK release after FPI, supportive of the sequential pathway wherein FPI releases ET-1 to increase superoxide, which in turn releases ERK MAPK (Fig. 7)
FIG. 7.
Schematic depicting the effects of Phe on cerebral autoregulation during hypotension, and the roles of Endothelin-1(ET-1), extracellular signal-related kinase (ERK), mitogen-activated protein kinase (MAPK), and superoxide on such effects in male and female pigs. Arrow thickness reflects the relative degree to which this action occurs.
Conclusion
To summarize, more ET-1, activated oxygen, and ERK MAPK are released in males than in females, to sequentially impair K channel mediated cerebrovasodilation, contributing to impaired autoregulation during hypotension after TBI (Fig. 7). Systemic pressor support with Phe exacerbates dysregulation via aggravation of the sequential impairment of K channel mediated cerebrovasodilation in males but abrogates such impairment and is protective in females after TBI but not in males. The KATP channel agonist peptide adrenomedullin is released in response to TBI and serves as an endogenous vasoprotectant in preserving autoregulation (Fig. 7; Armstead et al., 2007). Exogenous administration of adrenomedullin to males after TBI is protective of autoregulation and thereby restores a naturally occurring vasoprotectant to the sex in which it is expressed at very low levels (Armstead et al., 2007). Taken together, these observations advocate for the consideration of development of sex-based therapies for treatment of hemodynamic sequelae of pediatric TBI.
Acknowledgment
This research was supported by grant HD57355 from the National Institutes of Health.
Author Disclosure Statement
No competing financial interests exist.
References
- Adelson P.D. Bratton S.L. Carney N.A. Chesnut R.M. du Coudray H.E. Goldstein B. Kochanek P.M. Miller H.C. Partington M.D. Selden N.R. Warden C.R. Wright D.W. American Association for Surgery of Trauma, Child Neurology Society, International Society for Pediatric Neurosurgery, International Trauma Anesthesia and Critical Care Society, Society of Critical Care Medicine, and World Federation of Pediatric Intensive and Critical Care Societies. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 4. Resuscitation of blood pressure and oxygenation and prehospitalbrain-specific therapies for the severe pediatric traumatic brain injury patient. Pediatr Crit. Care Med. 2003;4(Suppl. 3):S12–18. [PubMed] [Google Scholar]
- Armstead W.M. Age and cerebral circulation. Pathophysiology. 2005;12:5–15. doi: 10.1016/j.pathophys.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Armstead W.M. Age dependent cerebral hemodynamic effects of traumatic brain injury in newborn and juvenile pigs. Microcirculation. 2000;7:225–235. [PubMed] [Google Scholar]
- Armstead W.M. Brain injury impairs ATP-sensitive K channel function in piglet cerebral arteries. Stroke. 1997;28:2273–2280. doi: 10.1161/01.str.28.11.2273. [DOI] [PubMed] [Google Scholar]
- Armstead W. M. Hypotension dilates pial arteries by KATP and Kca channel activation. Brain Res. 1999a;816:158–164. doi: 10.1016/s0006-8993(98)01146-9. [DOI] [PubMed] [Google Scholar]
- Armstead W.M. Role of endothelin-1 in age dependent cerebrovascular hypotensive responses after brain injury. Am. J. Physiol. 1999b;277:H1884–1894. doi: 10.1152/ajpheart.1999.277.5.H1884. [DOI] [PubMed] [Google Scholar]
- Armstead W.M. Superoxide generation links protein kinase C activation to impaired ATP-sensitive K+ channel function after brain injury. Stroke. 1999c;30:153–159. doi: 10.1161/01.str.30.1.153. [DOI] [PubMed] [Google Scholar]
- Armstead W.M. Cines D.B. Bdeir K. Bdeir Y. Stein S.C. Higazi A.A.R. uPA modulates the age dependent effect of brain injury on cerebral hemodynamics through LRP and ERK MAPK. J. Cereb. Blood Flow Metab. 2009;29:524–533. doi: 10.1038/jcbfm.2008.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstead W.M. Kiessling J.W. Bdeir K. Kofke W.A. Vavilala M.S. Adrenomedullin prevents sex dependent impairment of cerebal autoregulation during hypotension after piglet brain injury through inhibition of ERK MAPK upregulation. J. Neurotrauma. 2010a;27:391–402. doi: 10.1089/neu.2009.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstead W.M. Kiessling J.W. Kofke W.A. Vavilala M.S. Impaired cerebral blood flow autoregulation during post traumatic arterial hypotension after fluid percussion brain injury is prevented by phenylephrine in female but exacerbated in male piglets by ERK MAPK upregulation. Crit. Care Med. 2010b;38:1868–1874. doi: 10.1097/CCM.0b013e3181e8ac1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstead W.M. Kiessling J.W. Riley J. Kofke W.A. Vavilala M.S. Phenylephrine infusion prevents impairment of ATP and Calcium sensitive K channel mediated cerebrovasodilation after brain injury in female but aggravates impairment in male piglets through modulation of ERK MAPK upregulation. J. Neurotrauma. 2011;28:105–111. doi: 10.1089/neu.2010.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstead W.M. Raghupathi R. Endothelin and the neurovascular unit in pediatric traumatic brain injury. Neurol. Res. 2011;33:127–132. doi: 10.1179/016164111X12881719352138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstead W.M. Vavilala M.S. Adrenomedullin reduces gender dependent loss of hypotensive cerebrovasodilation after newborn brain injury through activation of ATP-dependent K channels. J. Cereb. Blood Flow Metab. 2007;27:1702–1709. doi: 10.1038/sj.jcbfm.9600473. [DOI] [PubMed] [Google Scholar]
- Bratton S.L. Chestnut R.M. Ghajar J. McConnell Hammond F.F. Harris O.A. Hartl R. Manley G.T. Nemecek A. Newell D.W. Rosenthal G. Schouten J. Shutter L. Timmons S.D. Ullman J.S. Videtta W. Wilberger J.E. Wright D.W. Brain Trauma Foundation, American Association of Neurological Surgeons, Congress of Neurological Surgeons, Joint Section on Neurotrauma and Critical Care, and AANS/CNS. Guidelines for the management of severe traumatic brain injury. J. Neurotrauma. 2007;24(Suppl. 1):S7–13. doi: 10.1089/neu.2007.9995. S59–64. [DOI] [PubMed] [Google Scholar]
- Català–Temprano A. Claret Teruel G. Cambra Lasaosa F.J. Pons O.M. Noguera J.A. Palomeque R.A. Intracranial pressure and cerebral perfusion pressure as risk factors in children with traumatic brain injury. J. Neurosurg. 2007;106(Suppl. 6):463–466. doi: 10.3171/ped.2007.106.6.463. [DOI] [PubMed] [Google Scholar]
- Coles J.P. Steiner L.A. Johnston A.J. Fryer T.D. Coleman M.R. Smieleweski P. Chatfield D.A. Aigbirhio F. Williams G.B. Boniface S. Rice K. Clark J.C. Pickard J.D. Menon D.K. Does induced hypertension reduce cerebral ischemia within traumatized human brain? Brain. 2004;127:2479–2490. doi: 10.1093/brain/awh268. [DOI] [PubMed] [Google Scholar]
- Cremer O.L. van Dijk G.W. van Wensen E. Brekelmans G.J. Moons K.G. Leenen L.P. Kalkman C.J. Effect of intracranial pressure monitoring and targeted intensive care on functional outcome after severe head injury. Crit. Care Med. 2005;33:2207–2213. doi: 10.1097/01.ccm.0000181300.99078.b5. [DOI] [PubMed] [Google Scholar]
- DiGennaro J.L. Mack C.D. Malakouti A. Zimmerman J.J. Chesnut R. Armstead W. Vavilala M.S. Use and effect of vasopressors after pediatric traumatic brain injury. Dev. Neurosci. 2011;32:420–430. doi: 10.1159/000322083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvinsson L. Ekman R. Jansen I. McCulloch J. Uddman R. Calcitonin gene related peptide and cerebral blood vessels: distribution and vasomotor effects. J. Cereb. Blood Flow Metab. 1987;7:720–728. doi: 10.1038/jcbfm.1987.126. [DOI] [PubMed] [Google Scholar]
- Faraci F.M. Heistad D.D. Regulation of the cerebral circulation: role of endothelium and potassium channels. Physiol. Rev. 1998;78:53–97. doi: 10.1152/physrev.1998.78.1.53. [DOI] [PubMed] [Google Scholar]
- Freeman S.S. Udomphorn Y. Armstead W.M. Fisk D.M. Vavilala M.S. Young age as a risk factor for impaired cerebral autoregulation after moderate-severe pediatric brain injury. Anesthesiology. 2008;108:588–595. doi: 10.1097/ALN.0b013e31816725d7. [DOI] [PubMed] [Google Scholar]
- Ishikawa S. Ito H. Yokoyama K. Makita K. Phenylephrine ameliorates cerebral cyotoxic edema and reduces cerebral infarction volume in a rat model of complete unilateral carotid occlusion with severe hypotension. Anesth. Analg. 2009;108:1631–1637. doi: 10.1213/ane.0b013e31819d94e3. [DOI] [PubMed] [Google Scholar]
- Keenan H.T. Nocera M. Bratton S.L. Frequency of intracranial pressure monitoring in infants and young toddlers with traumatic brain injury. Pediatr. Crit. Care Med. 2005;6:537–541. doi: 10.1097/01.PCC.0000164638.44600.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laher I. Zhang J.H. Protein kinase C and cerebral vasospasm. J. Cereb. Blood Flow Metab. 2001;21:887–906. doi: 10.1097/00004647-200108000-00001. [DOI] [PubMed] [Google Scholar]
- Langlois J.A. Rutland–Brown W. Thomas K.E. The incidence of traumatic brain injury among children in the United States: differences by race. J. Head Trauma Rehabil. 2005;20:229–238. doi: 10.1097/00001199-200505000-00006. [DOI] [PubMed] [Google Scholar]