Abstract
Background
The interaction of megakaryocytes with matrix proteins of the osteoblastic and vascular niche is essential for megakaryocyte maturation and proplatelet formation. Fibrinogen is present in the vascular niche and the fibrinogen receptor αIIbβ3 is abundantly expressed on megakaryocytes, however the role of the interaction between fibrinogen and αIIbβ3 in proplatelet formation in humans is not yet fully understood. We have recently reported a novel congenital macrothrombocytopenia associated with a heterozygous mutation of the β3 subunit of αIIbβ3. The origin of thrombocytopenia in this condition remains unclear and this may represent an interesting natural model to get further insight into the role of the megakaryocyte fibrinogen receptor in megakaryopoiesis.
Methodology/Principal Findings
Patients' peripheral blood CD45+ cells in culture were differentiated into primary megakaryocytes and their maturation, spreading on different extracellular matrix proteins, and proplatelet formation were analyzed. Megakaryocyte maturation was normal but proplatelet formation was severely impaired, with tips decreased in number and larger in size than those of controls. Moreover, megakaryocyte spreading on fibrinogen was abnormal, with 50% of spread cells showing disordered actin distribution and more evident focal adhesion points than stress fibres. Integrin αIIbβ3 expression was reduced but the receptor was constitutively activated and a sustained, and substrate-independent, activation of proteins of the outside-in signalling was observed. In addition, platelet maturation from preplatelets was impaired.
Conclusions/Significance
Our data show that constitutive activation of αIIbβ3-mediated outside-in signalling in human megakaryocytes negatively influences proplatelet formation, leading to macrothombocytopenia.
Introduction
Mature megakaryocytes (Mks) migrate to the vascular niche of the bone marrow where they convert the bulk of their cytoplasm into multiple long processes, called proplatelets, that protrude through the vascular endothelium into the sinusoid lumen to release platelets [1], [2]. Recently a new intermediate stage in platelet maturation has been described: preplatelets, discoid particles circulating in blood, larger than platelets, that reversibly convert into barbell-shaped proplatelets that in turn generate each two mature platelets after a fission event [3].
Very little is known about the role of specific bone marrow proteins in megakaryocyte differentiation and function. Fibrinogen was shown to be localized in the bone marrow sinusoids of mice and to be essential for proplatelet formation by binding to megakaryocyte αIIbβ3 [4]. In fact, mouse megakaryocytes extend proplatelets when plated on fibrinogen, and treatment with αIIbβ3 antagonists strikingly reduces the percentage of megakaryocytes developing proplatelets [4]. However, the interaction between integrin αIIbβ3 and fibrinogen was shown to be essential for spreading but not for proplatelet formation by human megakaryocytes, and in fact while αIIbβ3 antagonists almost abolished adhesion and spreading they did not cause any significant reduction of human proplatelet formation [5]. Indeed, Glanzmann Thrombasthenia (GT), a rare hereditary autosomal recessive bleeding disorder affecting the megakaryocytic lineage and due to quantitative and/or qualitative abnormalities of αIIbβ3, is not associated with thrombocytopenia [6]. Therefore, the role of αIIbβ3 in proplatelet formation in humans is still controversial.
We have recently described two families with a novel autosomal dominant hereditary mucocutaneous bleeding disorder with macrothrombocytopenia and defective platelet function associated with a heterozygous mutation (2134+1 G>C) of the ITGB3 gene, coding for the β3 subunit of αIIbβ3 and producing a deletion (del647-686) of a large part of the β Tail Domain (βTD) [7], an extracellular domain of β3 involved in receptor activation [8]. This mutation, and in particular a mutation involving the β3 βTD domain, was never described before and it seemed of interest that it was associated with a reduced platelet number and altered platelet morphology.
Purpose of the present study was to analyse megakaryocyte maturation, spreading and proplatelet formation on fibrinogen, and other extracellular matrix proteins, in two patients with the Glanzmann variant macrothrombocytopenia associated with the β3 del647-686.
Results
Megakaryocyte differentiation and proplatelet formation
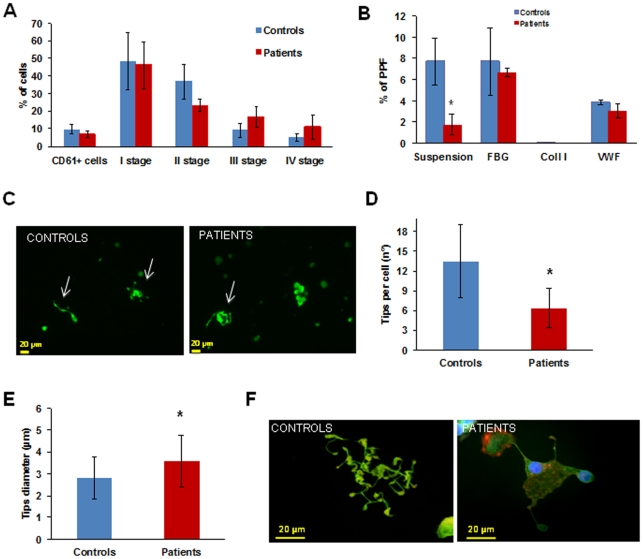
The percentage of CD45+ cells differentiated into megakaryocytes was comparable in patients and controls (7±1.8% vs 9.9±2.7%, respectively, p = ns). Megakaryocyte maturation profiles, classified according to standard criteria [9], were not significantly different between patients and controls, indicating that del647-686 of â3 integrin does not affect the differentiation or maturation of megakaryocytes ( Figure 1A ).
Figure 1. Megakaryocyte differentiation and proplatelet formation.
(A) Megakaryocyte maturation stages of patients did not differ from those of controls. (B) Proplatelet formation (PPF) from patient megakaryocytes in suspension was drastically reduced. When megakaryocytes were plated on type I collagen proplatelet formation was absent, similar to controls, while on fibrinogen and von Willebrand factor the number of megakaryocytes extending proplatelets was normal. *p<0.05 vs control. (C) Representative pictures of proplatelet formation in suspension, in a control subject and a patient (20× magnification). Arrows indicate pro-platelets, only one developing proplatelet is evident in the patient sample. (D, E) Patient megakaryocytes extended a reduced number of proplatelets with abnormal characteristics: a spread shape with shorter than normal proplatelet shafts and tips significantly decreased in number and larger in size than those of controls. *p<0.05 vs control. (F) Representative images of megakaryocytes from patients and controls releasing proplatelets upon adhesion to fibrinogen.
Proplatelets formed by megakaryocytes in suspension were instead reduced, with only 1.8±1.0% of patient megakaryocytes extending proplatelets vs 7.7±2.2% of controls after 16 hours (p<0.05) ( Figure 1B ), but with a normal morphology. Defective proplatelet formation was confirmed in experiments with a longer incubation time (24 hours) (data not shown).
On the contrary, when megakaryocytes were plated on fibrinogen proplatelets were numerically comparable to those of controls (7.7±3.2% vs 6.7±0.4% n = 3, p = ns), but presented important structural alterations, with megakaryocytes showing a spread shape, shorter proplatelet shafts and tips significantly decreased in number and larger in size than those of controls ( Figure 1C, 1D and 1E ). Interestingly, while pro-platelet formation usually starts from one pole of the megakaryocyte and rapidly leads to the conversion of the entire cytoplasm into proplatelets [2] , in our patients pro-platelet formation started at multiple poles of the megakaryocyte cell body ( Figure 1E , right). Finally, proplatelet formation from patient's megakaryocytes incubated with type I collagen or Von Willebrand factor was not different from that of control megakaryocytes [10] –[12] (on type I collagen: 0.1±0.2% vs 0.1±0.2%, p = ns; on VWF 3.0±0.6% vs 3.8±0.2%, p = ns) ( Figure 1B ).
Megakaryocyte spreading
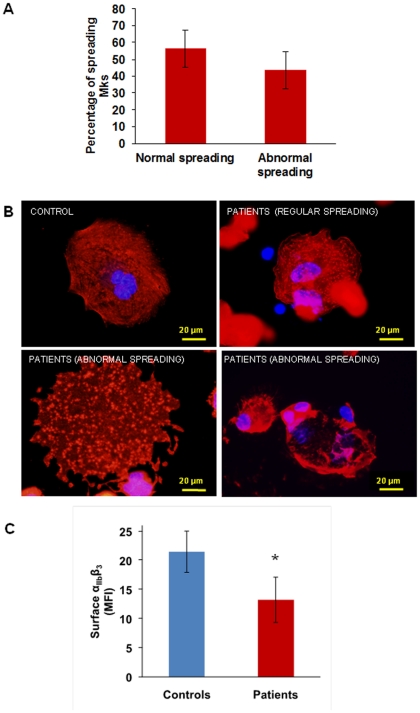
Megakaryocyte spreading on type I collagen was similar in patients and controls (15.4% vs 18.6±3.3% of the total adhering population, p = ns) as well as spreading on Von Willebrand factor (5.2% vs 5.9±1.3% of the total adhering population, p = ns). On the contrary, spreading on fibrinogen was increased: 31.7±5.6% of the total population of adhering megakaryocytes compared to 10±2% of controls (p<0.05). Two different populations were detectable among patient megakaryocytes: one spreading normally (56.4 ±11% of the total spread population), and the other abnormally (43.5±11% of the total spread population) ( Figure 2A ).
Figure 2. Megakaryocyte spreading on fibrinogen and αIIbβ3 expression.
(A) and (B) When plated on fibrinogen two populations of megakaryocytes are visible: half of the population spread regularly, while half showed abnormal spreading, with nuclei displayed towards cell periphery, a disordered distribution of actin and focal adhesion points more evident than stress fibres. (C) Flow cytometry showed decreased expression of αIIbβ3 on the surface of patient's megakaryocytes as compared with control megakaryocytes. *p<0.05 vs control.
Abnormally spread megakaryocytes showed nuclei displaced towards cell periphery, a disordered distribution of actin and focal adhesion points more evident than stress fibres ( Figure 2B lower panels). Normally spread megakaryocytes, instead, showed central nuclei and an ordered organization of actin in stress fibres and focal adhesion points, similar to controls ( Figure 2B upper panels).
αIIbβ3 expression and activation
Integrin αIIbβ3 was significantly less expressed on the surface of patients' megakaryocytes than on that of control megakaryocytes (mean fluorescence intensity: 13.2±2.1 vs 21.5±2.2%, respectively, p<0.05) ( Figure 2C ), in accordance with what we previously observed with the patients' platelets [7].
As β3 integrin is also a subunit of the αVβ3 receptor (CD51/61), we measured αVβ3 by flow cytometry and we found that its expression was comparable between patients and controls, both in platelets (patients 4.8±0.6% vs controls 4.8±0.4%, p = ns) and in megakaryocytes (patients 13±1.1% vs controls 10.7±1.6%, p = ns) (data not shown).
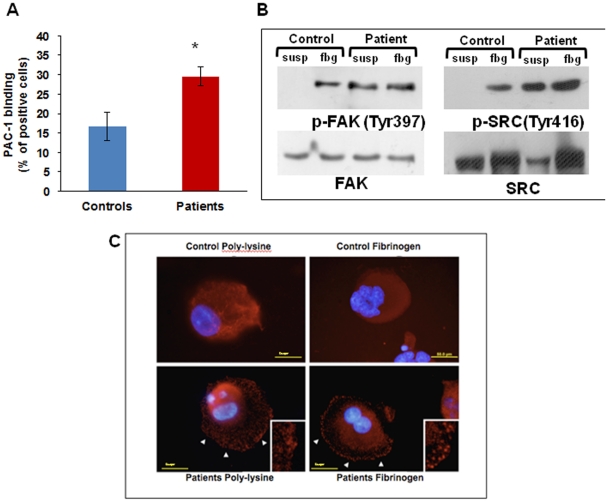
To study αIIbβ3 receptor activation we measured the binding of PAC-1, a monoclonal antibody that binds only to activated αIIbβ3, to un-stimulated megakaryocytes: 29.5±0.9% of patients' megakaryocytes bound PAC1 vs 16.7±3.6% of control megakaryocytes ( Figure 3A ), showing constitutive activation of αIIbβ3 integrin in patients' megakaryocytes.
Figure 3. Integrin αIIbβ3 activation and outside-in signalling.
(A) Flow cytometry analysis of PAC-1 binding to resting megakaryocytes is significantly increased in patients as compared with controls. *p<0.05 vs control. (B) Western blotting showed Src and FAK phosphorylation in patient megakaryocytes in suspension as well as after adhesion onto fibrinogen. (C) Differently from control cells (upper panels), patient megakaryocytes showed FAK clustering already after 1 hour of adhesion onto fibrinogen, and also in suspension (lower panels).
We therefore assessed αIIbβ3-triggered outside-in signalling by measuring the phosphorylation of FAK and Src after adhesion to fibrinogen by western blotting [13] .
Src and FAK were phosphorylated in patients' megakaryocytes also in suspension while in controls phosphorylation was observed only upon adhesion to fibrinogen ( Figure 3B ).
We also assessed FAK clustering at immunofluorescence: clustering was clearly evident in patients' megakaryocytes already one hour after plating on fibrinogen ( Figure 3C right panel ), while with control megakaryocytes it was evident only after 3 hours. Moreover, FAK clusters were observed in patients' megakaryocytes in suspension, differently from controls were they were evident only upon contact with fibrinogen ( Figure 3C left panel ), consistently with constitutive activation of αIIbβ3.
Conversion of preplatelets into platelets
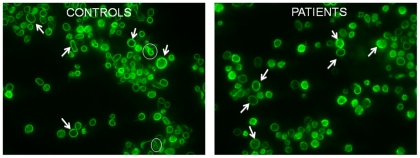
Very recently a new intermediate form between proplatelets and platelets, the preplatelet, was described [3]. A preplatelet can reversibly convert into a barbell-shaped proplatelet and generate two platelets passing through a “figure 8” structure. We therefore counted “figure 8” structures in platelet rich plasma (PRP) and we observed a significantly lower percentage of them in patients' PRP as compared to controls (0.5±0.7% vs 2.1±1.2% respectively, p<0.05) ( Figure 4 ).
Figure 4. Conversion of preplatelets into mature platelets.
In patient's peripheral blood less “figure 8” shapes are present. Two “figure 8” shapes are circled in white in control blood (left), while no “figure 8” shapes are visible in this picture of patient peripheral blood (right). Arrows show examples of preplatelets.
Discussion
Extracellular proteins play an important role in megakaryopoiesis and platelet formation by interacting with their receptors on megakaryocytes [5]. In particular, the vascular niche is enriched in fibrinogen and von Willebrand factor which drive the late phases of megakaryopoiesis and allow proplatelet formation and platelet release [4], [5].
Here we show that two patients with a variant form of Glanzmann Thrombasthenia (GT) associated with macrothrombocytopenia, which is not normally present in GT, due to a partial deletion of integrin β3 [7] have megakaryocytes that, despite normal differentiation, fail to extend proplatelets in suspension, form abnormal proplatelets on fibrinogen, and show reduced preplatelet maturation.
Patient megakaryocytes expressed significantly less αIIbβ3 on their surface, as already seen with platelets [7], but this was constitutively activated, as shown by PAC-1 binding under resting conditions, by faster spreading upon contact with fibrinogen and by FAK clustering and Src and FAK phosphorylation in suspension. A constitutively activated αIIbβ3 in our patient megakaryocytes is consistent with a deletion involving the βTD, a portion of αIIbβ3 with a key role in maintaining the receptor in its low affinity conformation, [8] and with our previous observation that CHO cells expressing the β3 del647-686 mutation bind fibrinogen without the need of activation [Bury L, Cecchetti L, Giannini S, Corazzi T, Appolloni V, et al. (2010) Impact of a novel integrin β3 mutation (del647-686), associated with a Glanzmann's variant hereditary platelet defect, on GPIIb/IIIa expression and signalling. Blood Transfus 8: OC067].
The expression of αVβ3, the receptor for vitronectin, was instead normal probably due to the structural differences between αIIb and αV in their calf2 domains [8], [14], the domain interacting with βTD.
Spreading on fibrinogen showed a peculiar pattern, with half of the population spreading normally, and half showing abnormal spreading, similar to patients’ platelets [7] suggesting that a preferential segregation of the mutant β3 subunits in clusters occurs in some cells but not in others upon ligand binding to αIIbβ3 [15].
Megakaryocyte spreading and proplatelet formation on Von Willebrand Factor (VWF) were instead normal, which may be unexpected because VWF is a ligand for αIIbβ3. Given that in platelets [16] and in αIIbβ3 expressing CHO cells [17] the αIIbβ3-VWF interaction occurs only after integrin activation and that signalling through GPIb-IX-V activates αIIbβ3 [18], it is conceivable that in megakaryocytes contact with VWF GPIb-IX-V activates αIIbβ3 that, in turn, promotes spreading. If this were the case, in our patients a constitutively activated αIIbβ3 would not perturb VWF-mediated megakaryocyte spreading while it would affect fibrinogen-mediated spreading, where this activation is not required.
Also proplatelet formation on fibrinogen was abnormal in our patients, with a reduced number of proplatelets with enlarged tips, in agreement with two recent reports describing patients with gain-of-function mutations of αIIbβ3 associated with thrombocytopenia, one at the cytoplasmic tail of β3 [19] and the second at the cytoplasmic domain of αIIb [20]. Differently from these reports, that demonstrated constitutive αIIbβ3 activation only in cells transfected with the mutant integrin [19], [20], our study shows for the first time a constitutively activated αIIbβ3 in patients' megakaryocytes. Altogether these observations show that an absent αIIbβ3 is less disruptive to thrombopoiesis than a hyper-active receptor, suggesting that outside-in signalling must be “switched off” during platelet production.
Our data also suggest that actin remodelling is critical in the late phases of fibrinogen-induced proplatelet formation. In fact, ligand-binding to αIIbβ3 induces the activation of c-Src, normally associated with the cytoplasmic tail of β3 in resting megakaryocytes, and then FAK activation that in turn stimulates actin remodelling leading to cell spreading [21]. A constitutively activated FAK-Src signalling, as observed in our patients' megakaryocytes, leads to permanent actin polymerization and this may cause abnormal proplatelet formation, as shown by treatment of megakaryocytes with an inhibitor of actin assembly, cytochalasin [22], or by macrothrombocytopenia in mice genetically deficient of ADF or cofilin, two proteins involved in actin depolymerization [23].
Recently it has been shown that in the late maturation steps leading to the formation of platelets, a malleable cytoplasm is essential for the passage from preplatelets, large, oval-shaped circulating platelet precursors, into barbell-shapes, by the twisting of their microtubule cytoskeleton around their centre to yield “figure 8” structures, and finally into two individual platelets [3]. It is therefore conceivable that a rigid, constitutively activated actin network may hinder proplatelet formation and lead to the formation of platelets of an abnormal size, compatible with the reduced maturation of preplatelets into platelets observed in our patients' blood.
In conclusion, impaired proplatelet formation from megakaryocytes, together with a normal number of reticulated platelets excluding enhanced platelet destruction, lead us to conclude that macrothrombocytopenia in our patients is due to defective platelet formation. Our results show that constitutive activation of αIIbβ3-mediated outside-in signalling in human megakaryocytes negatively influences proplatelet formation and open new perspectives in the study of the role of the αIIbβ3–fibrinogen axis in platelet formation and related diseases.
Materials and Methods
Cell culture and immunofluorescence
CD45+ cells were separated from peripheral blood of the patients and healthy controls and cultured as previously described [5], [11], [24].
All subjects gave written informed consent to the study, which was approved by the Committee on Bioethics of the University of Perugia.
Megakaryocyte differentiation was evaluated at day 14 of culture on cells (1×105) cytospun on poly-L-lysine-coated glass coverslips (Sigma-Aldrich, Milan, Italy) and stained with an anti CD41 antibody or with May Grunwald Giemsa, as previously described [10], [25]. CD41 positive cells were classified according to dimensions and nuclear configuration.
To evaluate proplatelet formation and megakaryocyte spreading onto adhesive substrates megakaryocytes at day 14 of culture were separated on a BSA gradient (3–4%), plated onto glass coverslips coated with 100 µg/ml human fibrinogen (Sigma-Aldrich, St. Louis, MO, USA), 10 µg/ml VWF-rich concentrate (Haemate P; Aventis-Behring, Milan, Italy) or 25 µg/ml type I collagen from bovine tendon (kind gift of prof M.E. Tira, University of Pavia) in 24-well plates (1×105 cells per well), and allowed to adhere for 16 h at 37°C and 5% CO2. To evaluate proplatelet formation in suspension, megakaryocytes were seeded in 24 well plates and incubated for 16 h at 37°C and 5% CO2.
Samples were then analyzed by immunofluorescence as previously described [5] . Analysis was performed on 20 different fields for each sample. For tips diameter and number measurements, at least one hundred tips per sample were measured.
Proplatelet formation and megakaryocyte spreading onto different substrates were calculated as the percentage of proplatelets or spread megakaryocytes over the total megakaryocyte population adhering to the substrate. Normal and abnormal spreading on fibrinogen are expressed as the percentage of normal or abnormal shapes out of the total population of spreading megakaryocytes.
FAK phosphorylation by immunofluorescence was assessed on megakaryocytes plated for 1 hour onto fibrinogen or poly-L-lysine using an anti phospho-FAK (Tyr 397) antibody (Cell Signalling, Danvers, MA, USA).
Flow cytometry
CD41 expression was analyzed by flow cytometry after incubation of Mks for 30 minutes with the FITC-conjugated mAb anti-CD41 clone P2 (Beckman Coulter, Miami, FL, USA). CD51 expression was analyzed incubating Mks and platelets for 30 minutes with a FITC-conjugated anti-CD51 mAb (Immunotech, Marseille, France). To measure αIIbβ3 activation Mks were incubated for 30 minutes with the PAC-1 FITC mAb (BD Biosciences, Milan, Italy), that recognizes activated αIIbβ3. PAC-1 binding is expressed as the percentage of megakaryocytes that bind PAC-1 out of the total number of CD41-expressing cells.
For every mAb an isotypic antibody was used as a negative control. Samples were analysed in an EPICS XL-MCL flow cytometer (Beckman Coulter, Miami, FL, USA), equipped with an argon laser operating at 488 nm [26].
SDS page and Western Blotting
Patient and control megakaryocytes were plated for 3 h at 37°C on 12-well plates pre-coated with 100 µg/ml of purified human fibrinogen or 1% BSA. Cells were then washed twice with PBS and lysed with lysis buffer (40 mM Tris- HCl, 0.3 M NaCl, 1 mM EDTA, 1 mM NaF, 1 mM Na3VO4, 10 μl NP-40, 10 µg/ml leupetin/pepstatin).
An equal amount of proteins were resolved by 8% SDS-polyacrylamide gel electrophoresis (PAGE) and transferred onto nitrocellulose. Membranes were probed with a rabbit anti-phospho–FAK (Tyr 397) or an anti-FAK MoAb, with a rabbit anti-phospho-Src (Tyr416) or an anti-Src (Cell Signalling Technology, Danvers, MA) MoAb and immunoreactive bands were detected using peroxidase-conjugated secondary antibodies and chemiluminescence detection.
Preplatelet “figure 8” counting
Patient and control blood was centrifuged at 100 g for 20 minutes to obtain PRP; 106 platelets were then cytospun on poly-L-lysine-coated glass coverslips (Sigma-Aldrich, St. Louis, MO, USA), fixed with 4% PFA for 20 minutes, permeabilized with 0.1% Triton-X for 5 minutes, blocked with 3% BSA for 2 hours, stained with an anti-β1 tubulin antibody (a kind gift of professor Joseph Italiano, Boston, USA) and then with a secondary antibody conjugated with Alexa Fluor 488 (Invitrogen, Life Technologies, Grand Island, NY, USA). Specimens were mounted in Mowiol (Calbiochem, Merck, Darmstadt, Germany) and analyzed through a Carl Zeiss Axio Observer. A1 fluorescence microscope, using a 100X/1,4 Plan-Apochromat oil-immersion objective.
“figure 8” preplatelets were counted as the percentage of “figure 8” shapes over the total number of β1 tubulin-positive elements plated on the slide; the analysis was performed on 20 different fields for each sample.
Statistic analysis
Data are presented as means ± SD. T test for unpaired data or two way ANOVA were used to analyze data, with a significant difference set at p<0.05.
Acknowledgments
The authors thank the patients for their kind collaboration and professor Joseph Italiano for helpful suggestions and for the protocols for the study of preplatelets. Editorial handling from Dr. Sara Orsini is gratefully acknowledged.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by a Telethon grant (GGP10155) to PG and by a Cariplo Foundation grant (2006.0596/10.8485) to AB. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Avecilla ST, Hattori K, Heissig B, Tejada R, Liao F, et al. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat Med. 2004;10:64–71. doi: 10.1038/nm973. [DOI] [PubMed] [Google Scholar]
- 2.Junt T, Schulze H, Chen Z, Massberg S, Goerge T, et al. Dynamic visualization of thrombopoiesis within bone marrow. Science. 2007;317:1767–70. doi: 10.1126/science.1146304. [DOI] [PubMed] [Google Scholar]
- 3.Thon JN, Montalvo A, Patel-Hett S, Devine MT, Richardson JL, et al. Cytoskeletal mechanics of proplatelet maturation and platelet release. J Cell Biol. 2010;191:861–74. doi: 10.1083/jcb.201006102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larson MK, Watson SP. Regulation of proplatelet formation and platelet release by integrin αIIbβ3. Blood. 2006;108:1509–14. doi: 10.1182/blood-2005-11-011957. [DOI] [PubMed] [Google Scholar]
- 5.Balduini A, Pallotta I, Malara A, Lova P, Pecci A, et al. Adhesive receptors, extracellular proteins and myosin IIA orchestrate proplatelet formation by human megakaryocytes. J Thromb Haemost. 2008;6:1900–7. doi: 10.1111/j.1538-7836.2008.03132.x. [DOI] [PubMed] [Google Scholar]
- 6.Nurden AT. Glanzmann Thrombasthenia. Orphanet J Rare Dis. 2006;6:1–10. doi: 10.1186/1750-1172-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gresele P, Falcinelli E, Giannini S, D'Adamo P, D'Eustacchio A, et al. Dominant inheritance of a novel integrin β3 mutation associated with a hereditary macrothrombocytopenia and platelet dysfunction in two Italian families. Haematologica. 2009;94:663–9. doi: 10.3324/haematol.2008.002246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu J, Luo BH, Xiao T, Zhang C, Nishida N, et al. Structure of a complete integrin ectodomain in a physiologic resting state and activation and deactivation by applied forces. Mol Cell. 2008;32:849–61. doi: 10.1016/j.molcel.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams N, Levine RF. The origin, development and regulation of megakaryocytes. Br J Haematol. 1982;52:173–80. doi: 10.1111/j.1365-2141.1982.tb03878.x. [DOI] [PubMed] [Google Scholar]
- 10.Balduini A, Malara A, Pecci A, Badalucco S, Bozzi V, et al. Proplatelet formation in heterozygous Bernard-Soulier syndrome type Bolzano. J Thromb Haemost. 2009;7:478–84. doi: 10.1111/j.1538-7836.2008.03255.x. [DOI] [PubMed] [Google Scholar]
- 11.Pecci A, Malara A, Badalucco S, Bozzi V, Torti M, et al. Megakaryocytes of patients with MYH9-related thrombocytopenia present an altered proplatelet formation. Thromb Haemost. 2009;102:90–6. doi: 10.1160/TH09-01-0068. [DOI] [PubMed] [Google Scholar]
- 12.Chen Z, Naveiras O, Balduini A, Mammoto A, Conti MA, et al. The May-Hegglin anomaly gene MYH9 is a negative regulator of platelet biogenesis modulated by the Rho-ROCK pathway. Blood. 2007;100:171–9. doi: 10.1182/blood-2007-02-071589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parsons JT, Martin KH, Slack JK, Taylor JM, Weed SA. Focal Adhesion Kinase: a regulator of focal adhesion dynamics and cell movement. Oncogene. 2000;19:5606–13. doi: 10.1038/sj.onc.1203877. [DOI] [PubMed] [Google Scholar]
- 14.Xiong JP, Mahalingham B, Alonso JL, Borrelli LA, Rui X, et al. Crystal structure of the complete integrin αVβ3 ectodomain plus an α/β transmembrane fragment. J Cell Biol. 24; 2009;186:589–600. doi: 10.1083/jcb.200905085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coller BS, Shattil SJ. The GPIIb/IIIa (integrin áIIbâ3) odyssey: a technology-driven saga of a receptor with twists, turns, and even a bend. Blood. 2008;112:3011–25. doi: 10.1182/blood-2008-06-077891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kieffer N, Fitzgerald LA, Wolf D, Cheresh DA, Phillips DR. Adhesive Properties of the β3 Integrins: comparison of GP IIb-IIIa and the Vitronectin Receptor Individually Expressed in Human Melanoma Cells. The Journal of Cell Biology. 1991;113:451–461. doi: 10.1083/jcb.113.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mekrache M, Legendre P, Kieffer N, Baruch D. Activation of integrin áIIbâ3 expressed in Chinese hamster ovary cells is required for interaction with solid-phase von Willebrand factor. British Journal of Haematology. 2009;119:1024–1032. doi: 10.1046/j.1365-2141.2002.03960.x. [DOI] [PubMed] [Google Scholar]
- 18.Kasirer-Friede A, Cozzi MR, Mazzucato M, De Marco L, Ruggeri ZM, j etal. Signalling through GP Ib-IX-V activates αIIbβ3 independently of other receptors. Blood. 2004;103:3403–3411. doi: 10.1182/blood-2003-10-3664. [DOI] [PubMed] [Google Scholar]
- 19.Ghevaert C, Salsmann A, Watkins NA, Schaffner-Reckinger E, Rankin A, et al. A non-synonymous SNP in the ITGB3 gene disrupts the conserved membrane-proximal cytoplasmic salt bridge in the αIIbβ3 integrin and co-segregates dominantly with abnormal proplatelet formation and macrothrombocytopenia. Blood. 2008;111:3407–14. doi: 10.1182/blood-2007-09-112615. [DOI] [PubMed] [Google Scholar]
- 20.Kunishima S, Kashiwagi H, Otsu M, Takayama N, Eto K, et al. Heterozygous ITGA2B R995W mutation inducing constitutive activation of the αIIbβ3 receptor affects proplatelet formation and causes congenital macrothrombocytopenia. Blood. 2011;117:5479–84. doi: 10.1182/blood-2010-12-323691. [DOI] [PubMed] [Google Scholar]
- 21.Obergfell A, Eto K, Mocsai A, Buensuceso C, Moores SL, et al. Coordinate interactions of Csk, Src, and Syk kinases with αIIbβ3 initiate integrin signalling to the cytoskeleton. J Cell Biol. 2002;157:265–75. doi: 10.1083/jcb.200112113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Italiano JE, Lecine P, Shivdasani RA, Hartwig JH. Blood platelets are assembled principally at the ends of proplatelet processes produced by differentiated megakaryocytes. J Cell Biol. 1999;147:1299–312. doi: 10.1083/jcb.147.6.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bender M, Eckly A, Hartwig JH, Elvers M, Pleines I, et al. ADF/n-cofilin-dependent actin turnover determines platelet formation and sizing. Blood. 2010;116:1767–75. doi: 10.1182/blood-2010-03-274340. [DOI] [PubMed] [Google Scholar]
- 24.Nurden P, Gobbi G, Nurden A, Enouf J, Youlyouz-Marfak I, et al. Abnormal VWF modifies megakaryopoiesis: studies of platelets and megakaryocyte cultures from patients with von Willebrand disease type 2B. Blood. 2010;115:2649–56. doi: 10.1182/blood-2009-07-231886. [DOI] [PubMed] [Google Scholar]
- 25.Balduini A, D'Apolito M, Arcelli D, Conti V, Pecci A, et al. Cord blood in vitro expanded CD41 cells: identification of novel components of megakaryocytopoiesis. J Thromb Haemost. 2006;4:848–60. doi: 10.1111/j.1538-7836.2006.01802.x. [DOI] [PubMed] [Google Scholar]
- 26.Giannini S, Mezzasoma AM, Guglielmini G, Rossi R, Falcinelli E, et al. A New case of acquired Glanzmann's thrombasthenia: diagnostic value of flow cytometry. Cytometry B Clin Cytom. 2008;74:194–9. doi: 10.1002/cyto.b.20396. [DOI] [PubMed] [Google Scholar]