Abstract
Traumatic brain injury (TBI) can trigger disturbances of cerebral pressure autoregulation that can translate into the generation of secondary insults and increased morbidity/mortality. Few therapies have been developed to attenuate the damaging consequences of disturbed autoregulatory control, although some suggest that hypothermia may exert such protection. Here we reexamine this issue of traumatically induced autoregulatory disturbances and their modulation by hypothermic intervention, examining these phenomena in two different models of TBI. Adult rats were subjected to either impact acceleration injury (IAI) or lateral fluid percussion injury (LFPI) followed by the insertion of cranial windows to assess the pial arteriolar cerebral autoregulatory vascular response to the post-traumatic induction of sequential reductions of arterial blood pressure. The potential for continued pial vasodilation in response to declining blood pressure was directly measured post-injury and compared with that in injured groups subjected to 33° C of hypothermia of 1–2 h duration initiated 1 h post-injury. We observed that the TBI resulted in either impaired or abolished cerebral vascular dilation in response to the sequential declines in blood pressure. Following IAI there was a 50% reduction in the vasculature's ability to dilate in response to the induced hypotension. In contrast, following LFPI, the vascular response to hypotension was abolished both ipsilateral and contralateral to the LFPI. In animals sustaining IAI, the use of 1 h post-traumatic hypothermia preserved vascular dilation in response to declines in blood pressure in contrast to the LFPI in which the use of the same strategy afforded no improvement. However, with LFPI, the use of 2 h of hypothermia provided partial vascular protection. These results clearly illustrate that TBI can alter the cerebral autoregulatory vascular response to sequentially induced hypotensive insult, whereas the use of post-traumatic hypothermia provides benefit. Collectively, these studies also demonstrate that different animal models of TBI can evoke different biological responses to injury.
Key words: animal models of injury, pial vessels, therapeutic intervention, vascular reactivity
Introduction
Cerebral pressure autoregulation is defined as a homeostatic mechanism subserved by the brain vasculature to maintain blood flow constant over a range of systemic blood pressures via arterial contraction and dilatation (Aaslid et al., 1989; Kontos et al., 1978; Harper, 1966; Lassen, 1959). The maintenance of cerebral pressure autoregulation represents one of the most important vascular functions to preclude the occurrence of secondary ischemic insults after traumatic brain injury (TBI) (Johnson et al., 2011). It is well known that cerebral pressure autoregulation can be impaired following severe TBI in patients and as such, can contribute to secondary insults to the injured brain (Bouma et al., 1992; Consonni et al., 2009; Czosnyka et al., 1996; Hlatky et al., 2002; Sviri et al., 2009). In animal experiments, cerebral hypoperfusion and impaired autoregulation have been described in cats subjected to fluid percussion injury (FPI) (DeWitt et al., 1992; Lewelt et al., 1980). Further, after impact acceleration injury (IAI) in rats, studies using laser Doppler flowmetry have suggested abnormalities in pressure autoregulation in response to increased blood pressure (Nawashiro et al., 1995) as well as hemorrhagic hypotension (Engelborghs et al., 2000). Lastly, in terms of the autoregulatory vascular response to hypotension following TBI, several investigators have reported impaired vascular reactivity following FPI in cats (Wei et al., 1980), in piglets (Armstead et al., 2010a,b), and in ex vivo rat cerebral vessels (Mathew et al., 1999).
Because of the importance of cerebral pressure autoregulation to maintain flow, coupled to its potential for alteration following TBI, investigations have explored various strategies to preserve cerebral autoregulation following TBI. To date, of the strategies employed, the use of hypothermia has received some attention. In animal experiments, post-traumatic hypothermia has been shown to exert multiple protective effects on various vascular abnormalities elicited by TBI (Jiang et al., 1992; Lotocki et al., 2009; Ueda et al., 2003, 2004; Wei et al., 2009). However, other than in an initial report by Bedell and colleagues (2004) the potential beneficial effects of hypothermia on cerebral autoregulation have not been fully evaluated.
Accordingly, to explore the potential beneficial effects of post-traumatic hypothermia on cerebral autoregulation, we examined using cranial windows, the autoregulatory vasoactive responses of pial arterioles in rats subjected to either moderate IAI or moderate lateral FPI (LFPI). Two models of injury were employed based on recent reports that different models of TBI can evoke different responses in both vascular reactivity (Fujita et al., 2011; Oda et al., 2011) and any potential hypothermic protection. Accordingly, via the use of two different TBI models, we felt well positioned to determine if any observed vascular responses and/or their hypothermic modification were operant across different models of moderate TBI with different biomechanical features.
Methods
General preparation
All experimental procedures were performed with a protocol approved by the Institutional Animal Care and Use Committee at Virginia Commonwealth University. Thirty adult male Sprague–Dawley rats, weighing 350–500 g, were used in the current study. Animals were housed in individual cages on a 12-h light/dark cycle with free access to water and food. Animals were initially anesthetized intraperitoneally with sodium pentobarbital at 60 mg/kg. The femoral artery was cannulated with a PE50 catheter (Becton Dickinson, Sparks, MD) for continuous monitoring of arterial blood pressure (PowerLab, AD Instruments, Colorado Springs, CO), rapid withdrawal of blood to induce hypotension, and periodic collection of blood samples for determination of arterial oxygen tension (PaO2), arterial carbon dioxide pressure (PaCO2), and pH values (Stat Profile® pHOx, Nova biomedical, Waltham, MA). Arterial blood gas samples (100 μL) were taken 10 min prior to injury and every hour post-injury. The femoral vein was cannulated with a PE50 catheter (Becton Dickinson) for administration of sodium pentobarbital. After completion of the tracheotomy, animals were mechanically ventilated (Harvard Apparatus, Holliston, MA) on room air. Postinjury pancuronium bromide (3 mg/kg) was administrated intravenously to produce skeletal muscle paralysis. The resting PaCO2 was maintained at a constant level, between 35 and 40 mm Hg, by adjusting the rate and/or volume of the respirator. Body temperature was maintained at 37°C with a heat lamp and/or a heating pad throughout the experiment except during the hypothermic period. As will be detailed below, two different models of TBI were employed based upon our previous findings that these distinct injury models can display differences in vascular responses to various therapeutic approaches, illustrating the complexity of TBI and the difficulties associated with a reliance on one animal model of injury.
Induction of hypothermia
Hypothermia was accomplished by whole body cooling achieved through use of ice packs. Body and brain temperatures were measured via a thermometer in the rectum (Yellow Spring Instruments, Yellow Spring, OH) and a thermistor in the temporalis muscle (Physitemp Instruments, Clifton, NJ), respectively. The temporalis muscle temperature was employed based upon the fact that the readings obtained therein closely parallel brain temperature (Jiang et al., 1991). Typically, 13–15 min of ice pack use was required to achieve the target rectal temperature of 33°C. Hypothermia was initiated 1 h post-injury and maintained at 33°C for 60 or 120 min, followed by re-warming over a span of 90 min. This re-warming was accomplished using a protocol detailed previously (Suehiro et al., 2003).
IAI
The IAI model of TBI employed the same model described previously (Fujita et al., 2011; Gao et al., 2010; Marmarou et al., 1994). Briefly, rats were placed in a stereotaxic frame; a middle sagittal incision was performed to expose the skull. A 10 mm circular stainless steel helmet was installed over the sagittal suture between bregma and lambda and secured with dental acrylic. The respiratory tube was disconnected from the tracheal tube, and the animal was rapidly placed prone on a foam pad. A weight of 450 g was dropped from a height of 2 m through a Plexiglas tube on the metal helmet, which was centered immediately under the lower end of the tube. The animal was returned to the respirator immediately after the injury with the continuation of blood gas and blood pressure monitoring. The helmet was then removed and the cranial window installed.
LFPI
The LFPI model of TBI used the same protocol previously described (Oda et al., 2011; Wei et al., 2009). Briefly, rats were placed in a stereotaxic frame, and a midline sagittal incision was performed to expose the skull bone. A 4.8 mm circular craniotomy was prepared on the left side of the skull between bregma and lambda. Two steel screws 3/16-inch long (Small Parts, Logansport, IN) for fixation were inserted into 1 mm holes drilled into the left frontal and occipital bones. The top portion of the Leur-Loc hub (Becton Dickinson, Franklin Lakes, NJ), which was cut away from the 20-gauge needle, was positioned over the exposed dura mater, and was rigidly affixed to the two screws using dental acrylic (Hygenic Corporation, Akron, OH). After the dental acrylic hardened, rats were disconnected from the respirator and then connected to a fluid percussion device (Custom Design and Fabrication, Virginia Commonwealth University, Richmond, VA) through the spacing tube filled with sterile saline. Rats were injured at a magnitude of 2.0 atm. The pressure pulse measured by the transducer was displayed on a storage oscilloscope (Tektronix, Beaverton, OR), and the peak pressure was recorded. Following injury, all animals were promptly ventilated with room air and reconnected to the blood pressure monitoring device. The assembly of the hub, screws, and dental acrylic was removed en bloc before installation of cranial window.
Visualization and assessment of the cerebral microcirculation
Visualization and assessment of the cerebral microcirculation was performed as described in our previous studies (Ellis et al., 1983; Fujita et al., 2011; Gao et al., 2010; Levasseur et al., 1975; Oda et al., 2011). Briefly, after midline skin incision on the skull of group 1, or after removal of the metal helmets for IAI in groups 2 and 3, a 2×4 mm rectangular craniotomy was made in the bone over the parietal cortex and then the underlying dura mater was incised. In groups 4, 5, and 6 subjected to LFPI, a 2×4 mm rectangular craniotomy was made over the parietal bone on the side contralateral to the injury pulse, and the underlying dura mater was carefully cut. The dura mater on the side ipsilateral to the LFPI was exposed post-injury through the 4.8 mm circular craniotomy in the parietal bone and cut. Next, a cranial window was installed over the exposed brain surface creating a singular window for the IAI preparation and a dual/bilateral window for the LFPI preparation. These were fixed in place by bone wax and dental acrylic. The cranial window consisted of a stainless steel ring with three outlets and a circular glass plate inside a ring. Two of the outlets served as inflow and outflow paths for the perfusion and clearance of selected vasoactive agents, whereas the free end of the other outlet was set at a predetermined height to achieve an intracranial pressure (ICP) of 5 mm Hg. The space under the cranial window and the three outlets were then filled with artificial cerebrospinal fluid (CSF), of which pH was adjusted to 7.35 by equilibration with a gas mixture containing 6% O2 and 6% CO2 balanced with N2. The underlying pial microcirculation was visualized with a microscope and pial arteriolar diameters were measured with a Vickers image-splitting device (Vickers Instruments Inc., Maiden, MA). Typically, in each window preparation a minimum of four arteriolar segments were evaluated. At 4 h post-TBI in groups 1, 2, 3, 4, and 5 or at 5 h post-TBI in group 6, vascular diameter was measured at resting blood pressure, followed by sequential reductions of mean arterial pressure (MAP) to 100, 80, 60, and 40 mm Hg achieved through rapid blood withdrawal via the femoral arterial line that resided in the abdominal aorta. After each reduced MAP had reached steady state for a minimum of 2–4 min, vessel diameter was measured. The vascular reactivity to the reductions of MAP was expressed as a percent change from the baseline diameter measured at the resting blood pressure.
Statistical analysis
Statistical analysis was performed using the statistical software PASW Statistics 17.0 (SPSS Inc., Chicago, IL). All data were presented as mean±standard error of the mean (SEM). The physiological parameters and laboratory data, which were normally distributed, were analyzed by one-way analysis of variance (ANOVA). When a significant difference was found, multiple comparisons among time points in the same group and groups at each time point were performed using the Bonferroni correction. As the difference in vascular reactivity between groups at each MAP level was not normally distributed, the data was evaluated by the Kruskal–Wallis test followed by multiple comparisons with Bonferroni correction. Additionally, as the difference in vascular reactivity between MAP levels in each group was not normally distributed, the data were analyzed by the Friedman test followed by multiple comparisons with Bonferroni correction. A value of p<0.05 was considered to be statistically significant.
Experimental design
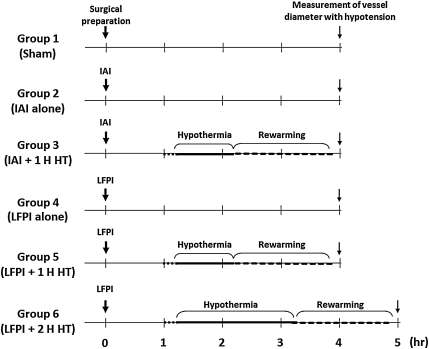
In this study, cerebral vascular responses to changing blood pressures were assessed after either IAI or LFPI with/without hypothermia. To this end, the animals were arbitrarily divided into six groups (Fig. 1). Each group contained five animals.
FIG. 1.
This chart shows the time course for each experimental group. IAI, impact acceleration injury; LFPI, lateral fluid percussion injury; HT, hypothermia.
Group 1 – sham surgery
These animals underwent the same surgical procedures used in all other animals, except for the induction of IAI or LFPI. Pial vascular reactivity to sequential reductions of the arterial blood pressure was assessed at 4 h following surgical preparation.
Group 2 – IAI with no treatment
These animals were subjected to IAI. The body temperature was maintained at normothermic levels for the duration of the experiment. Pial vascular reactivity to sequential reductions of the arterial blood pressure was assessed at 4 h following IAI.
Group 3 – IAI followed by I h hypothermic intervention
In this group, at 1 h following IAI, a 60 min period of hypothermia was induced, followed by a 90 min period of re-warming. Pial vascular reactivity to sequential reductions of the arterial blood pressure was assessed at 4 h following IAI.
Group 4 – LFPI with no treatment
These animals were subjected to LFPI. The body temperature was maintained at normothermic levels during the entire experimental period. Pial vascular reactivity to changes of blood pressure was assessed at 4 h following LFPI.
Group 5 – LFPI followed by 1 h hypothermic intervention
In this group, at 1 h following LFPI, a 60 min period of hypothermia was induced, followed by a 90 min period of re-warming. Pial vascular reactivity to sequential reductions of the arterial blood pressure was assessed at 4 h following LFPI.
Group 6 – LFPI followed by 2 h hypothermic intervention
In this group, at 1 h post-LFPI, cooling period was extended to 2 h, followed by a 90 min period of re-warming. Pial vascular reactivity to sequential reductions of the arterial blood pressure was assessed at 5 h following LFPI.
Results
General physiological observations
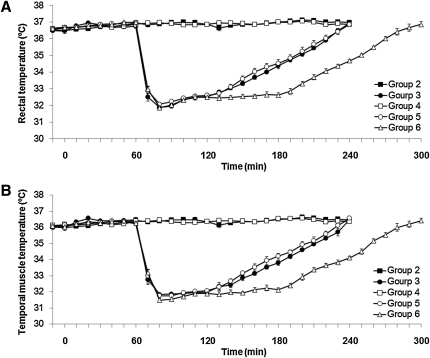
There were no significant differences in baseline body weight and hematocrit among all of the groups. The rectal temperature in the normothermic groups (groups 2 and 4) was maintained at 37°C over the entire experimental period (Fig. 2A). The temporalis muscle temperature was ∼ 0.5–1.0°C lower than the rectal temperature (Fig. 2B). In the hypothermic groups (groups 3, 5, and 6), the rats were cooled to the target rectal temperature of 33°C in 9.0±0.8, 10.0±0.6, and 10.4±0.4 min, respectively (Fig. 2A). The rats were maintained at this level for 60 min in groups 3 and 5, and for 120 min in group 6. They were then re-warmed to normothermia over a span of 90 min. There was no significant difference in either the induction or re-warming times among groups 3, 5, and 6. Table 1 shows the time course measurements of the mean arterial blood pressure and blood gas analyses. The mean values of PaO2 during hypothermic period (2 h post-injury) in groups 3, 5, and 6 tended to be higher than those in groups 2 and 4 (Table 1). The mean values of PaO2 in group 6 were significantly higher than those in group 2 at 2 h post-injury and in groups 2, 3, and 4 at 3 h post-injury (Table 1). Other than these physiological changes, all the other physiological variables reported in Table 1 were within normal physiological limits.
FIG. 2.
This graph shows the changes of the mean rectal temperature and mean temporalis muscle temperature throughout the duration of this study (A and B, respectively). The data points represent 10 min intervals. Values are expressed as the mean±SEM. TBI, traumatic brain injury.
Table 1.
Physiological Parameters
| |
|
Measurement period |
|||||
|---|---|---|---|---|---|---|---|
| Variable | Group | Pre-injury | 1 h | 2 h | 3 h | 4 h | 5 h |
| MAP (mmHg) | 1 | 122±4 | |||||
| 2 | 117±3 | 117±5 | 109±6 | 109±1 | 111±2 | ||
| 3 | 118±1 | 121±2 | 120±1 | 118±1 | 115±2 | ||
| 4 | 121±2 | 109±4 | 112±6 | 110±5 | 110±4 | ||
| 5 | 115±3 | 110±3 | 115±1 | 109±5 | 111±4 | ||
| 6 | 116±2 | 111±3 | 114±2 | 112±2 | 108±5 | 111±6 | |
| pH | 1 | 7.41±0.02 | |||||
| 2 | 7.42±0.01 | 7.41±0.01 | 7.40±0.01 | 7.40±0.01 | 7.40±0.01 | ||
| 3 | 7.41±0.01 | 7.41±0.01 | 7.41±0.01 | 7.41±0.01 | 7.42±0.01 | ||
| 4 | 7.41±0.01 | 7.41±0.01 | 7.41±0.01 | 7.42±0.01 | 7.41±0.01 | ||
| 5 | 7.41±0.01 | 7.39±0.01 | 7.38±0.02 | 7.42±0.02 | 7.40±0.01 | ||
| 6 | 7.41±0.01 | 7.41±0.01 | 7.37±0.01 | 7.39±0.02 | 7.42±0.01 | 7.41±0.01 | |
| PaO2 (mmHg) | 1 | 93±6 | |||||
| 2 | 90±4 | 73±4 | 79±3a | 79±4* | 86±5 | ||
| 3 | 93±7 | 78±5 | 100±7 | 81±4* | 80±1 | ||
| 4 | 88±3 | 79±3 | 86±4 | 88±2* | 88±3 | ||
| 5 | 84±5 | 76±4 | 100±8 | 97±7 | 92±6 | ||
| 6 | 97±6 | 88±8 | 105±5 | 113±7 | 98±8 | 90±9 | |
| PaCO2 (mmHg) | 1 | 38±1 | |||||
| 2 | 37±1 | 36±1 | 37±1 | 37±1 | 36±1 | ||
| 3 | 37±1 | 38±1 | 37±1 | 37±1 | 36±1 | ||
| 4 | 37±1 | 37±1 | 37±1 | 38±1 | 37±1 | ||
| 5 | 38±1 | 39±1 | 37±1 | 37±1 | 37±1 | ||
| 6 | 37±1 | 38±1 | 37±1 | 37±1 | 35±1 | 35±1 | |
Significant difference compared with the values of group 6 at same measurement point (p<0.05).
MAP, mean arterial blood pressure; PaO2, arterial oxygen tension; PaCO2, arterial carbon dioxide pressure.
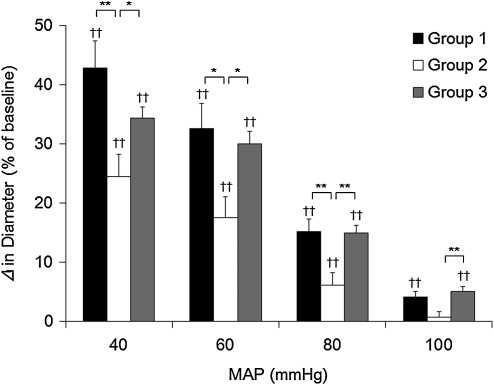
Brain arteriolar reactivity to arterial hypotension after IAI
The vascular reactivity to arterial hypotension after IAI is shown in Figure 3. Pial arteriolar diameter in sham-injured animals (group 1) dilated significantly with progressive reductions of MAP (42.8±4.5, 32.5±4.3, 15.1±2.2, and 4.2±0.9% at MAP of 40, 60, 80, and 100 mm Hg, respectively). In group 2, animals subjected to IAI without treatment, vascular dilation to hypotension was significantly reduced by ∼50% compared with those in group 1 (24.5±3.7, 17.5±3.6, 6.1±2.1, and 0.7±1.0% at MAP of 40, 60, 80, and 100 mm Hg, respectively (p<0.01 at 40 and 80 mm Hg of MAP, p<0.05 at 60 mm Hg of MAP, Fig. 3). However, in group 3, which received 1 h hypothermia following IAI, vascular reactivity to hypotension was virtually preserved (34.3±1.9, 29.9±2.2, 14.9±1.2, and 5.1±0.8% at MAP of 40, 60, 80, and 100 mm Hg, respectively) improving significantly in comparison with those in group 2 (p<0.01 at 80 and 100 mm Hg of MAP, p<0.05 at 40 and 60 mm Hg of MAP).
FIG. 3.
This bar graph shows the vascular reactivity to arterial hypotension in rats subjected to impact acceleration injury (IAI). Values are expressed as the mean±SEM. *Significant differences (p<0.05). **Significant differences (p<0.01). ††Significant differences compared with corresponding value at resting mean arterial pressure (MAP) in each group (p<0.01).
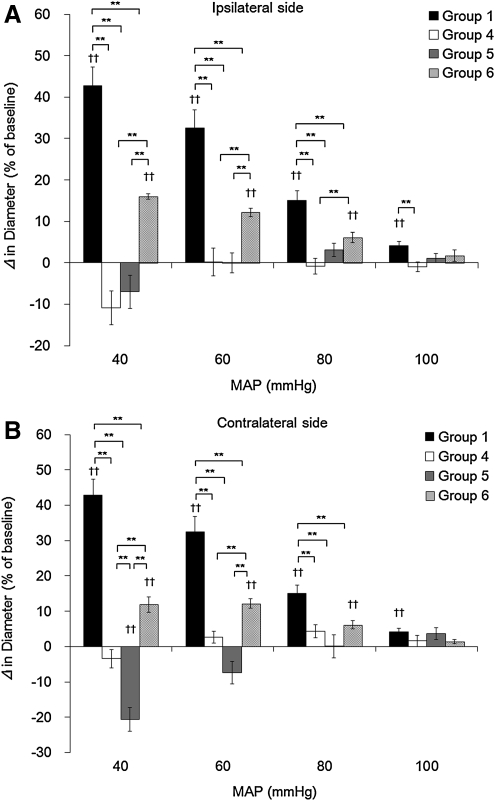
Brain arteriolar reactivity to arterial hypotension after LFPI
The vascular reactivity to arterial hypotension after LFPI on the side ipsilateral as well as contralateral to injury is shown in Figure 4A and B, respectively. In group 4, animals subjected to LFPI without treatment, the pial vascular response to hypotension was completely abolished on both the side ipsilateral (-10.8±4.1, 0.2±3.4, −0.9±1.9, and −0.9±1.2% at MAP of 40, 60, 80, and 100 mm Hg, respectively) and contralateral to the injury (-3.4±2.6, 2.7±1.7, 4.3±1.8, and 1.6±1.6% at MAP of 40, 60, 80, and 100 mm Hg, respectively) compared with group 1 (p<0.01, at MAP of 40, 60, 80, and 100 mm Hg on both the ipsilateral and contralateral sides, with the exception of the 100 mm Hg pressure on the contralateral side). The vascular response in group 5, which received 1 h hypothermia following LFPI, was not improved on the ipsilateral side (-7.0±4.0, 0.1±2.4, 3.1±1.7, and 1.1±1.1% at MAP of 40, 60, 80, and 100 mm Hg, respectively), and was actually exacerbated on the contralateral side (-20.7±3.4, −7.3±3.2, 0.1±3.2, and 3.7±1.7% at MAP of 40, 60, 80, and 100 mm Hg, respectively). However, the vascular response in group 6, which received 2 h of hypothermia following LFPI, was significantly improved compared with groups 4 and 5 (on the ipsilateral side: 16.0±0.7, 12.2±1.0, 6.0±1.2, and 1.7±1.4% at MAP of 40, 60, 80, and 100 mm Hg, respectively, and on the contralateral side: 11.9±2.2, 12.2±1.4, 6.2±1.1, and 1.3±0.6% at MAP of 40, 60, 80, and 100 mm Hg, respectively (p<0.01, vs. group 4 at 40 and 60 mm Hg of MAP on both sides, at 80 mm Hg of MAP on the ipsilateral side; p<0.01, vs. group 5 at 40 and 60 mm Hg of MAP on both sides).
FIG. 4.
This bar graph shows the vascular reactivity to arterial hypotension on the side Ipsilateral (A) and contralateral (B) to the site of lateral fluid percussion injury (LFPI). Values are expressed as the mean±SEM. *Significant differences (p<0.05). **Significant differences (p<0.01). ††Significant differences compared with corresponding value at resting mean arterial pressure (MAP) in each group (p<0.01).
Discussion
The findings of this study demonstrate clearly that TBI, evoked by either IAI or LFPI, disturbs the ability of cerebral arterioles to appropriately dilate in the face of decreasing blood pressure, with the caveat that LFPI causes more disruption in this cerebral autoregulatory vascular response than that seen in IAI. Further, in this regard, the impaired vasodilatory/autoregulatory response following IAI could be fully restored by use of delayed treatment with hypothermia of 1 h. In contrast, longer cooling periods were required in order to attain partial recovery in rodents subjected to LFPI. Collectively, these findings further confirm the vulnerability of the brain vasculature following experimentally induced brain injury in response to changes in arterial blood pressure, while emphasizing that animal modeling can elicit different vascular responses to injury and hypothermic intervention.
Our findings regarding the impaired cerebral arteriolar responsiveness to induced hypotension post-TBI are in agreement with our previous reports in cats subjected to central FPI (Wei et al., 1980). Further, these findings of impaired cerebral pressure autoregulation following either IAI or FPI are also consistent with the observations of other laboratories (Engelborghs et al., 2000; Lewelt et al., 1980) employing different TBI models. Previously, we have reported the beneficial effects of post-traumatic hypothermia on the cerebral vascular responsiveness to ACh and arterial hypercapnia (Suehiro et al., 2003; Ueda et al., 2003). We now provide evidence that the protective effects of hypothermia also extend to autoregulatory adjustments in vessel size in response to changing blood pressures in the brain-injured rats.
In contrast to the above noted responses to injury and hypothermia, Bedell and colleagues (2004), using laser Doppler flowmetry, reported no change in cerebral autoregulation following central FPI. Whereas this finding cannot be fully reconciled with ours, there are significant differences between their study and ours, other than the obvious differences in the approaches used in assessing the integrity of cerebral autoregulation (laser Doppler flowmetry vs. cranial window technique). In the study of Bedell and colleagues (2004), the intensity of FPI was reached at 1.8 atm, in contrast to ours which was set at 2.0 atm. We have reported previously in cats that autoregulatory adjustment of cerebral vessels at 1.6 atm was still operant, although not fully preserved (Wei et al., 1980) at 1 h post-FPI. It is of note that Bedell and colleagues (2004) also observed a significantly lowered CBF following FPI. Whereas it was conceivable that the autoregulatory ability of vessel size was somewhat diminished as we have previously reported (Wei et al., 1980), this diminished vasoaction could be sufficient to maintain resting CBF relatively constant at varied blood pressure levels. Further complicating comparisons between our work and that of Bedell and colleagues (2004) is the fact that this study measured regional CBF at 30 or 60 min post-injury which was significantly earlier than other reports (Engelborghs et al., 2000; Lewelt et al., 1980) as well as ours. We have previously reported that the therapeutic window in rats subjected to moderate IAI approximates 1 h (Suehiro et al., 2003; Ueda et al., 2003), implying that cerebral vascular reactivity can remain functional immediately post-injury or shortly thereafter. In fact, as evidenced in the present study, the pial arterioles remained partially responsive to changes of arterial blood pressure up to 4 h following IAI.
The overall mechanisms by which hypothermia preserves cerebrovascular function described in the present study remain unclear. Originally, hypothermia was thought to attenuate changes in signaling events, including adenosine-5'-triphosphate (ATP) depletion, glutamate release, Ca2+ mobilization, anoxic depolarization, the generation of free radicals, inflammation, the blood–brain barrier permeability, and necrotic and apoptotic pathways (Zhao et al., 2007). Hypothermia has also been shown to reduce contusion volume and lower elevated ICP, while attenuating diffuse axonal injury (Dietrich et al., 1994; Kawai et al., 2000; Koizumi et al., 1998). Our finding that hypothermia may restore the impaired microvascular response to autoregulatory challenges post-TBI perhaps constitutes another mechanism associated with the beneficial effects of therapeutic hypothermia.
In the present study, 1 h of hypothermia did not show any protective effects on the reduced microvascular reactivity to hypotension following LFPI, although the reduced reactivity to hypotension following IAI was fully restored by this same approach. Importantly, however, with LFPI, partial recovery was observed when the cooling period was extended to 2 h. These findings are comparable to the results of our previous reports demonstrating that delayed hypothermia can restore impaired microvascular responsiveness to ACh and arterial hypocapnia following IAI, with partial recovery seen at 1 h, and full recovery following 2 h of hypothermic treatment (Ueda et al., 2003). These findings again demonstrate that extended periods of hypothermic intervention can protect the cerebrovascular response to physiological challenges post-TBI, even when a shorter period of hypothermic intervention exerts no protective effect. This may imply that more severe TBI requires longer durations of hypothermia to achieve cerebrovascular protection.
In the current investigation, impairment of microvascular responsiveness to hypotension was more dramatic in LFPI than that in IAI. Further, as noted LFPI required longer duration of post-injury hypothermia (2 h) to achieve partial restoration of microvascular responsiveness to hypotension. Our previous reports using combination therapy of delayed hypothermia and FK506 in IAI and LFPI models also indicated that LFPI induced more severe vascular damage than did IAI (Fujita et al., 2011; Oda et al., 2011). Whereas the potential exists that these injuries induce insults of differing severity, we do not believe that this is the case. However, we cannot discount biomechanical differences in that one model involves direct impact of the pial arterioles (LFPI), whereas the other (IAI) does not, a finding that could explain the varied vascular responses observed (Baranova et al., 2008; Fujita et al., 2011; Oda et al., 2011). Irrespective of these issues however, the fact remains that animal modeling itself remains an extremely important variable in any assessment of vascular injury and/or its therapeutic modulation. Accordingly, this observation warrants more careful consideration in future studies focusing on TBI and its related sequelae.
Acknowledgments
We thank Susan Walker and Lynn Davis for their excellent technical assistance. This study is supported by NIH grants HD055813 and NS047463.
Author Disclosure Statement
No competing financial interests exist.
References
- Aaslid R. Lindegaard K.F. Sorteberg W. Nornes H. Cerebral autoregulation dynamics in humans. Stroke. 1989;20:45–52. doi: 10.1161/01.str.20.1.45. [DOI] [PubMed] [Google Scholar]
- Armstead W.M. Kiessling J.W. Bdeir K. Kofke W.A. Vavilala M.S. Adrenomedullin prevents sex-dependent impairment of autoregulation during hypotension after piglet brain injury through inhibition of ERK MAPK upregulation. J. Neurotrauma. 2010a;27:391–402. doi: 10.1089/neu.2009.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstead W.M. Kiessling J.W. Kofke W.A. Vavilala M.S. SNP improves cerebral hemodynamics during normotension but fails to prevent sex dependent impaired cerebral autoregulation during hypotension after brain injury. Brain Res. 2010b;1330:142–150. doi: 10.1016/j.brainres.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranova A.I. Wei E.P. Ueda Y. Sholley M.M. Kontos H.A. Povlishock J.T. Cerebral vascular responsiveness after experimental traumatic brain injury: The beneficial effects of delayed hypothermia combined with superoxide dismutase administration. J. Neurosurg. 2008;109:502–509. doi: 10.3171/JNS/2008/109/9/0502. [DOI] [PubMed] [Google Scholar]
- Bedell E.A. DeWitt D.S. Uchida T. Prough D.S. Cerebral pressure autoregulation is intact and is not influenced by hypothermia after traumatic brain injury in rats. J. Neurotrauma. 2004;21:1212–1222. doi: 10.1089/neu.2004.21.1212. [DOI] [PubMed] [Google Scholar]
- Bouma G.J. Muizelaar J.P. Bandoh K. Marmarou A. Blood pressure and intracranial pressure-volume dynamics in severe head injury: relationship with cerebral blood flow. J. Neurosurg. 1992;77:15–19. doi: 10.3171/jns.1992.77.1.0015. [DOI] [PubMed] [Google Scholar]
- Consonni F. Abate M.G. Galli D. Citerio G. Feasibility of a continuous computerized monitoring of cerebral autoregulation in neurointensive care. Neurocrit. Care. 2009;10:232–240. doi: 10.1007/s12028-008-9151-2. [DOI] [PubMed] [Google Scholar]
- Czosnyka M. Smielewski P. Kirkpatrick P. Menon D.K. Pickard J.D. Monitoring of cerebral autoregulation in head-injured patients. Stroke. 1996;27:1829–1834. doi: 10.1161/01.str.27.10.1829. [DOI] [PubMed] [Google Scholar]
- DeWitt D.S. Prough D.S. Taylor C.L. Whitley J.M. Deal D.D. Vines S.M. Regional cerebrovascular responses to progressive hypotension after traumatic brain injury in cats. Am. J. Physiol. 1992;263:H1276–H1284. doi: 10.1152/ajpheart.1992.263.4.H1276. [DOI] [PubMed] [Google Scholar]
- Dietrich W.D. Alonso O. Busto R. Globus M.Y. Ginsberg M.D. Post-traumatic brain hypothermia reduces histopathological damage following concussive brain injury in the rat. Acta Neuropathol. 1994;87:250–258. doi: 10.1007/BF00296740. [DOI] [PubMed] [Google Scholar]
- Ellis E.F. Wei E.P. Cockrell C.S. Choi S. Kontos H.A. The effect of PGF2 alpha on in vivo cerebral arteriolar diameter in cats and rats. Prostaglandins. 1983;26:917–923. doi: 10.1016/0090-6980(83)90154-5. [DOI] [PubMed] [Google Scholar]
- Engelborghs K. Haseldonckx M. Van Reempts J. Van Rossem K. Wouters L. Borgers M. Verlooy J. Impaired autoregulation of cerebral blood flow in an experimental model of traumatic brain injury. J. Neurotrauma. 2000;17:667–677. doi: 10.1089/089771500415418. [DOI] [PubMed] [Google Scholar]
- Fujita M. Oda Y. Wei E.P. Povlishock J.T. The combination of either tempol or FK506 with delayed hypothermia: implications for traumatically induced microvascular and axonal protection. J. Neurotrauma. 2011;28:1209–1218. doi: 10.1089/neu.2011.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G. Oda Y. Wei E.P. Povlishock J.T. The adverse pial arteriolar and axonal consequences of traumatic brain injury complicated by hypoxia and their therapeutic modulation with hypothermia in rat. J. Cereb. Blood Flow Metab. 2010;30:628–637. doi: 10.1038/jcbfm.2009.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper A.M. Autoregulation of cerebral blood flow: influence of the arterial blood pressure on the blood flow through the cerebral cortex. J. Neurol. Neurosurg. Psychiatry. 1966;29:398–403. doi: 10.1136/jnnp.29.5.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hlatky R. Furuya Y. Valadka A.B. Gonzalez J. Chacko A. Mizutani Y. Contant C.F. Robertson C.S. Dynamic autoregulatory response after severe head injury. J. Neurosurg. 2002;97:1054–1061. doi: 10.3171/jns.2002.97.5.1054. [DOI] [PubMed] [Google Scholar]
- Jiang J.Y. Lyeth B.G. Clifton G.L. Jenkins L.W. Hamm R.J. Hayes R.L. Relationship between body and brain temperature in traumatically, brain-injured rodents. J. Neurosurg. 1991;74:492–496. doi: 10.3171/jns.1991.74.3.0492. [DOI] [PubMed] [Google Scholar]
- Jiang J.Y. Lyeth B.G. Kapasi M.Z. Jenkins L.W. Povlishock J.T. Moderate hypothermia reduces blood–brain barrier disruption following traumatic brain injury in the rat. Acta Neuropathol. 1992;84:495–500. doi: 10.1007/BF00304468. [DOI] [PubMed] [Google Scholar]
- Johnson U. Nilsson P. Ronne–Engström E. Howells T. Enblad P. Favorable outcome in traumatic brain injury patients with impaired cerebral pressure autoregulation when treated at low cerebral perfusion pressure levels. Neurosurgery. 2011;68:714–721. doi: 10.1227/NEU.0b013e3182077313. [DOI] [PubMed] [Google Scholar]
- Kawai N. Nakamura T. Okauchi M. Nagao S. Effects of hypothermia on intracranial pressure and brain edema formation: studies in a rat acute subdural hematoma model. J. Neurotrauma. 2000;17:193–202. doi: 10.1089/neu.2000.17.193. [DOI] [PubMed] [Google Scholar]
- Koizumi H. Povlishock J.T. Posttraumatic hypothermia in the treatment of axonal damage in an animal model of traumatic axonal injury. J. Neurosurg. 1998;89:303, 309. doi: 10.3171/jns.1998.89.2.0303. [DOI] [PubMed] [Google Scholar]
- Kontos H.A. Wei E.P. Navari R.M. Levasseur J.E. Rosenblum W.I. Patterson J.L., Jr Responses of cerebral arteries and arterioles to acute hypotension and hypertension. Am. J. Physiol. 1978;234:H371–383. doi: 10.1152/ajpheart.1978.234.4.H371. [DOI] [PubMed] [Google Scholar]
- Lassen N.A. Cerebral blood flow and oxygen consumption in man. Physiol. Rev. 1959;39:183–238. doi: 10.1152/physrev.1959.39.2.183. [DOI] [PubMed] [Google Scholar]
- Levasseur J.E. Wei E.P. Raper A.J. Kontos A.A. Patterson J.L. Detailed description of a cranial window technique for acute and chronic experiments. Stroke. 1975;6:308–317. doi: 10.1161/01.str.6.3.308. [DOI] [PubMed] [Google Scholar]
- Lewelt W. Jenkins L.W. Miller J.D. Autoregulation of cerebral blood flow after experimental fluid percussion injury of the brain. J. Neurosurg. 1980;53:500–511. doi: 10.3171/jns.1980.53.4.0500. [DOI] [PubMed] [Google Scholar]
- Lotocki G. Vaccari J.P. Perez E.R. Sanchez–Molano J. Furones–Alonso O. Bramlett H.M. Dietrich W.D. Alterations in blood–brain barrier permeability to large and small molecules and leukocyte accumulation after traumatic brain injury: effects of post-traumatic hypothermia. J. Neurotrauma. 2009;26:1123–1134. doi: 10.1089/neu.2008.0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmarou A. Foda M.A. van den Brink W. Campbell J. Kita H. Demetriadou K. A new model of diffuse brain injury in rats. Part I: Pathophysiology and biomechanics. J. Neurosurg. 1994;80:291–300. doi: 10.3171/jns.1994.80.2.0291. [DOI] [PubMed] [Google Scholar]
- Mathew B.P. DeWitt D.S. Bryan R.M., Jr Bukoski R.D. Prough D.S. Traumatic brain injury reduces myogenic responses in pressurized rodent middle cerebral arteries. J. Neurotrauma. 1999;16:1177–1186. doi: 10.1089/neu.1999.16.1177. [DOI] [PubMed] [Google Scholar]
- Nawashiro H. Shima K. Chigasaki H. Immediate cerebrovascular responses to closed head injury in the rat. J. Neurotrauma. 1995;12:189–197. doi: 10.1089/neu.1995.12.189. [DOI] [PubMed] [Google Scholar]
- Oda Y. Gao G. Wei E.P. Povlishock J.T. Combinational therapy using hypothermia and the immunophilin ligand FK506 to target altered pial arteriolar reactivity, axonal damage, and blood–brain barrier dysfunction after traumatic brain injury in rat. J. Cereb. Blood Flow Metab. 2011;31:1143–1154. doi: 10.1038/jcbfm.2010.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suehiro E. Ueda Y. Wei E.P. Kontos H.A. Povlishock J.T. Posttraumatic hypothermia followed by slow rewarming protects the cerebral microcirculation. J. Neurotrauma. 2003;20:381–390. doi: 10.1089/089771503765172336. [DOI] [PubMed] [Google Scholar]
- Sviri G.E. Aaslid R. Douville C.M. Moore A. Newell D.W. Time course for autoregulation recovery following severe traumatic brain injury. J. Neurosurg. 2009;111:695–700. doi: 10.3171/2008.10.17686. [DOI] [PubMed] [Google Scholar]
- Ueda Y. Suehiro E. Wei E.P. Kontos H.A. Povlishock J.T. Uncomplicated rapid posthypothermic rewarming alters cerebrovascular responsiveness. Stroke. 2004;35:601–606. doi: 10.1161/01.STR.0000113693.56783.73. [DOI] [PubMed] [Google Scholar]
- Ueda Y. Wei E.P. Kontos H.A. Suehiro E. Povlishock JT. Effects of delayed, prolonged hypothermia on the pial vascular response after traumatic brain injury in rats. J. Neurosurg. 2003;99:899–906. doi: 10.3171/jns.2003.99.5.0899. [DOI] [PubMed] [Google Scholar]
- Wei E.P. Dietrich W.D. Povlishock J.T. Navari R.M. Kontos H.A. Functional, morphological, and metabolic abnormalities of the cerebral microcirculation after concussive brain injury in cats. Circ. Res. 1980;46:37–47. doi: 10.1161/01.res.46.1.37. [DOI] [PubMed] [Google Scholar]
- Wei E.P. Hamm R.J. Baranova A.I. Povlishock J.T. The long-term microvascular and behavioral consequences of experimental traumatic brain injury after hypothermic intervention. J. Neurotrauma. 2009;26:527–537. doi: 10.1089/neu.2008.0797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H. Steinberg G.K. Sapolsky R.M. General versus specific actions of mild-moderate hypothermia in attenuating cerebral ischemic damage. J. Cereb. Blood Flow Metab. 2007;27:1879–1894. doi: 10.1038/sj.jcbfm.9600540. [DOI] [PubMed] [Google Scholar]