Abstract
Background
Accurate quantification of soft tissue properties, specifically the stress-relaxation behavior of viscoelastic tissues such as plantar tissue, requires precise testing under physiologically relevant loading. However, limitations of testing equipment often result in target strain errors that can contribute to large stress errors and confound comparative results to an unknown extent. Previous investigations have modeled this artifact but they have been unable to obtain empirical data to validate their models. Moreover, there are no studies that address this issue for plantar tissue. The purpose of this research was to directly measure the difference in peak force for a series of small target strain errors within the range of our typical stress-relaxation experiments for subcutaneous plantar soft tissue.
Method of Approach
Five plantar tissue specimens were tested to seven incremental target strain error levels of −0.9%, −0.6%, −0.3%, 0.0%, 0.3%, 0.6%, and 0.9%, so as to undershoot and overshoot the target displacement in 0.3% increments. The imposed strain errors were accurately attained using a special compensation feature of our materials testing software that can drive the actuator to within 0% (1 to 2μm) of the target level for cyclic tests. Since stress-relaxation tests are not cyclic, we emulated the ramp portion of our stress relaxation tests with 5Hz triangle waves.
Results
The average total stress variation for all specimens was 25±5%, with the highest and lowest stresses corresponding to the largest and smallest strain errors of 0.9% and −0.9% respectively. A strain overshoot of 0.3%, the target strain error observed in our typical stress relaxation experiments, corresponded to an average stress overshoot of 3±1%.
Conclusions
Plantar tissue in compression is sensitive to small target strain errors that can result in stress errors that are several fold larger. The extent to which overshoot may affect the peak stress will likely differ in magnitude for other soft tissues and loading modes.
Keywords: overshoot, diabetes, subcutaneous, soft tissue, viscoelastic
Introduction
It is essential to accurately quantify the compressive properties of plantar tissue and changes that occur with disease that compromise these tissues in order to develop solutions that can compensate for these changes. For example, many people with diabetes suffer aberrant plantar pressures and are hence prone to plantar ulceration and ultimately amputation of the affected foot [1, 2]. Knowledge of diabetic tissue properties could be used to develop impedance matched orthoses that could potentially alleviate plantar pressures under critical regions. Stress-relaxation tests are routinely used for quantifying the viscoelastic properties inherent in most soft tissues including plantar subcutaneous tissue [3, 4]. Further, quasi-linear viscoelastic (QLV) theory [5] is extensively used to quantify the relaxation response since it provides meaningful coefficients that can be applied to a wide range of tissues. Our research group is currently in the process of quantifying the differences between healthy and diabetic soft tissue using stress relaxation tests and QLV theory.
A limitation of QLV theory is that its accuracy is dependent on the assumption that the applied strain includes an instantaneous ramp to a prescribed strain level. Since instantaneous ramps are experimentally unachievable, this assumption leads to errors [6]; hence, amendments to QLV theory have been made to incorporate fast finite ramps [7]. However, these changes do not take into account the experimental target strain error (i.e., overshoot and undershoot) resulting from fast ramps. There have been few studies that address target strain error [8–10], but none that address this issue for plantar soft tissue in compression. Previous solutions to target strain errors in other tissues have included using a very slow ramp time to minimize overshoot experimentally [10]. It is unclear whether this approach is suited for plantar tissue since it requires extremely slow ramp rates that preclude the characteristic initial relaxation observed at high, physiologic ramp rates. Funk et al. replaced the erroneous part of the relaxation curve (i.e., the experimentally obtained force peak) by back extrapolating from an accurate section of the force curve [8]. This method is limited as it relies on a model (i.e., back extrapolation) to predict how the tissue would behave had the target error not been present. Gimbel et al. used a direct fit approach to account for overshoot in the strain history [9], but this method still had a nearly 20% mean square error for a 7.5% overshoot. They also explored the effect of varying overshoot, but this was conducted as a simulated experiment, which assumed the tissue behaved as an ideal QLV system.
Our current approach [3] is to aggressively tune our testing control system to a fast ramp and hold test. Using this approach, we can successfully minimize our target strain error (i.e., overshoot) to approximately 0.3%. Although this error is considerably smaller than 7.5% reported for supraspinatus tendons [9], even this small strain error might contribute to significant load errors for plantar tissue. In fact, we previously observed that a strain difference of 0.8% can lead to considerable artifact in the observed stress (on the order of at least 10%) [11]. It would be extremely useful to know to what extent small strain errors can affect stress values to minimize any unnecessary confounding of future results.
Thus, the objective of this study was to determine empirically how peak stress is affected by small target strain errors for subcutaneous plantar soft tissue. While we experimentally cannot reduce this error any further for a ramp and hold test, we are capable of achieving 0% error (1 to 2μm) using a compensation feature of our testing software for cyclic tests. In our current relaxation testing protocol, we keep the ramp time constant for all tests at 0.1s so as to prevent relaxation from occurring during the ramp, i.e., < τ1, the short-term QLV time constant determined from previous plantar tissue tests. This ramp time of 0.1s is equivalent to the ramp portion of a 5Hz triangle wave. Hence, by using 5Hz triangle waves in conjunction with compensation, we can accurately achieve the desired target strain error.
Methods
Experimental Protocol
Five specimens were obtained from four fresh frozen cadaveric feet (Table 1), which were purchased from the National Disease Research Interchange. Institutional Review Board approval was obtained for this study from the Human Subjects Division at the University of Washington.
Table 1.
Specimen and donor information
| Specimen | Thickness (mm) | BMI (kg/m2) | Age (yrs) | Disease | Gender |
|---|---|---|---|---|---|
| 1 | 9.37 | 24.4 | 55 | Diabetic | M |
| 2 | 5.39 | 21.5 | 90 | None | F |
| 3 | 5.25 | 20.8 | 81 | None | F |
| 4 | 4.03 | 21.5 | 90 | None | F |
| 5 | 6.32 | 22.0 | 78 | None | M |
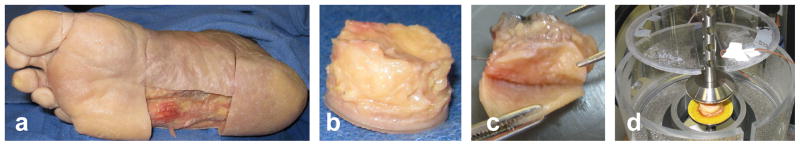
The plantar soft tissue was dissected free from the lateral midfoot, cut into cylindrical specimens using a 1.905cm diameter punch, and further dissected from the skin using a scalpel (Fig. 1). Each specimen was then placed in an environmental chamber between two platens covered with 220 grit sandpaper (Fig. 1d). The chamber was designed to heat a water bath below the platen and create a moist environment near 100% humidity and at 35°C to approximate conditions in vivo. This setup was attached to an ElectroForce 3200 materials testing machine (Bose Corporation, Minnetonka, MN). The bottom platen was raised to apply a 0.1N compressive load and specimen initial thickness was measured (Table 1).
Fig. 1.
Dissection of specimen showing (a) removal of plantar tissue flap at lateral midfoot location from which a (b) cylindrical specimen was punched and the (c) skin was removed. The specimen was then placed (d) between two platens covered with sandpaper in a humidity chamber at 35°C and sealed with plastic wrap (not shown).
The target load, based on specimen cross-sectional area, donor weight, and normative ground reaction force and contact area [12], was used to determine the target displacement. In load control, the specimen underwent ten 1Hz sine waves from 10N to the target load; the maximal absolute displacement was noted as the target displacement. Target strain was calculated as target displacement divided by the initial thickness. Six additional “erroneous” target strains were calculated to undershoot and overshoot the target displacement in 0.3% increments leading to seven test groups of −0.9%, −0.6%, −0.3%, 0.0%, 0.3%, 0.6%, and 0.9% strain error. A special compensation function of the testing software (Wintest v4.0, Bose Corporation, Minnetonka, MN) enabled us to achieve 0% error (1 to 2μm) for cyclic tests; we elected to use 5Hz triangle waves to emulate the ramp portion of our typical relaxation test. The sample was allowed to recover in an unloaded state for 25 minutes after the load control test followed by a brief tuning and recovery period and then seven triangle wave tests corresponding to each strain level. Each triangle wave test consisted of 30 cycles to the prescribed strain, to ensure sufficient cycles for compensation and preconditioning, followed by 10 minutes of recovery before the next triangle wave test. The force and displacement data were acquired at a rate of 5000Hz. Stress (force divided by original specimen area) between the maximum undershoot and maximum overshoot (i.e., −0.9% and 0.9% strain error) was used to calculate the total stress variation for each specimen.
Statistical Analysis
Linear mixed effects regression was used to determine the relationship between % target strain error (the independent variable) and peak stress (the dependent variable). Specimen number was modeled as a random effect, with variability assessed for both the intercept and slope of % target strain error across specimens. Analyses were carried out using R 2.9.0 (R Foundation for Statistical Computing, Vienna, Austria).
Results
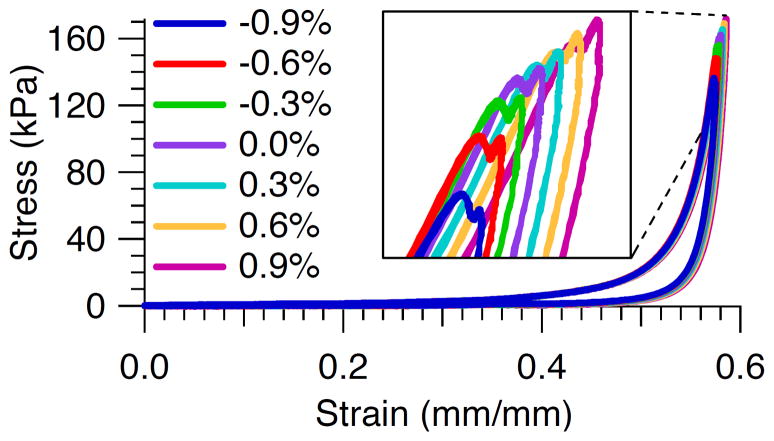
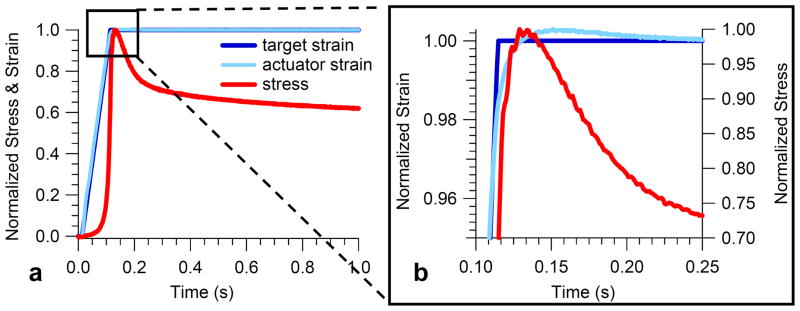
The peak stress for all specimens was highest for the largest strain error of 0.9% and lowest for the smallest strain error of −0.9% (Table 2). This result is also illustrated in the stress versus strain response for a typical specimen for all seven target strain errors (Fig. 2). The average total stress variation for all specimens was 25% (Table 2). A strain overshoot of 0.3%, the target strain error observed in our typical stress relaxation experiments, corresponded to a stress overshoot of 2.2%, 5.1%, 1.2%, 2.7%, and 2.7% for specimens 1–5 respectively, i.e., an average stress overshoot of 3±1%. Further examination of the tuning results of a representative specimen (Fig. 3) demonstrated that the peak stress is reached just prior to the maximal target strain error of 0.3% and just after the time at which the actuator first reaches target.
Table 2.
Stress variation with target strain error (standard deviation)
| Strain error (%) | Peak stress for each specimen (kPa)
|
Ave σ error (%) | Stdev σ error (%) | ||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||
| −0.9 | 135 (0) | 61 (0) | 89 (0) | 58 (0) | 78 (0) | −16 | 2 |
| −0.6 | 147 (0) | 67 (0) | 100 (1) | 62 (0) | 83 (0) | −9 | 1 |
| −0.3 | 155 (1) | 72 (0) | 106 (1) | 66 (0) | 87 (0) | −4 | 1 |
| 0 | 161 (1) | 75 (0) | 109 (0) | 69 (0) | 89 (0) | 0 | 0 |
| 0.3 | 164 (1) | 79 (0) | 110 (1) | 71 (0) | 92 (1) | 3 | 1 |
| 0.6 | 168 (1) | 82 (0) | 111 (1) | 73 (0) | 94 (0) | 5 | 3 |
| 0.9 | 170 (1) | 86 (0) | 112 (1) | 75 (0) | 97 (0) | 8 | 4 |
| Total stress variation (%) | 22 | 33 | 22 | 25 | 22 | 25 | 5 |
Fig. 2.
Stress versus strain response for a typical specimen for all seven target strain errors.
Fig. 3.
Results of tuning the material testing machine to a ramp and hold wave for a representative specimen showing (a) normalized stress and strain during the first second of the ramp and hold and (b) close-up of overshoot showing the peak stress is reached just prior to the target strain error of 0.3%.
There was a significant linear relationship between target strain error and stress (p=.0002). However, upon plotting the data in Table 1 (not shown), a non-linear component in the association between target strain error and stress was evident, whereby the difference in peak stress tended to decrease with increasing total displacement (−16% stress error at −0.9% strain error vs. 8% stress error at 0.9% strain error). We carried out another regression with target strain error modeled as an orthogonal polynomial and found a significant cubic relationship (p<.0001) that was stronger than the linear relationship.
Discussion
Stress-relaxation tests are a useful method for quantifying the viscoelastic properties of soft tissues, especially with regard to changes that occur with diseases such as diabetes. However, accurate modeling of these results depends on accurate strain inputs [9]. Experimental limitations of testing equipment often create target strain errors that can result in significant stress response errors [3, 8, 9]. It is unclear to what extent these errors could confound comparative results and complicate modeling of the stress response. To our knowledge, nobody has empirically determined a relationship between strain and stress error for any soft tissues. The purpose of this study was to measure the effect of varying amounts of target strain error on peak stress in plantar tissue in compression. Peak stress is an important parameter because high plantar pressures can cause ulceration [2]. We emulated the ramp portion of our typical stress relaxation tests using triangle waves at a frequency consistent with our stress relaxation test ramp time. We used a special compensation function of the testing software to drive the machine to varying target strain error levels including the typical amount of overshoot present in our stress relaxation tests.
As anticipated, increased displacements resulted in increased peak stress wherein the largest strain error of 0.9% resulted in the largest peak stress (Table 2). Previous investigations of overshoot in other soft tissues have demonstrated that strain overshoots contribute to notably higher peak stresses, e.g., for supraspinatus tendon, a 7.5% strain error resulted in a 30% increase in the elastic QLV parameter A which was indicative of increased peak force [9]. For our own small range of 1.8% target strain error, the average total stress variation for all specimens was considerably high at 25%, indicating that plantar soft tissue in compression is sensitive to small target strain errors. In our case a strain overshoot of 0.3%, as would be obtained by tuning in our typical stress relaxation experiments, corresponded to a stress error of 3%. While this stress error is ten-fold larger than the strain error, it is the smallest average strain error for any of the target strain errors tested. Thus, although we cannot experimentally reduce this error any further, we can at least account for it. Additionally, by maintaining our target strain error at a consistent level, we can ensure fair comparisons between plantar tissue specimen groups and eliminate those results that have variable strain errors. In this manner, we can limit the extent to which target strain errors might confound our results.
We purposely varied the donor population to see how different tissues respond. Although we were limited by our small sample size, the observed trends should apply to the wide range of plantar tissue tested including healthy, diabetic, and older plantar tissue. As expected the diabetic specimen had higher stresses overall. A small anomaly was the greater thickness of the diabetic specimen; diabetic tissue is generally thinner than healthy tissue [13]. However, this discrepancy can be explained since the diabetic specimen was harvested from an aspect of the lateral midfoot in closer proximity to the heel or it could be due to interspecimen variability.
Of note, the strain versus stress error had a significant non-linear relationship whereby the stress error increased with overall displacement but at a decreasing rate for all specimens. For example, the stress error for a 0.9% strain undershoot was twice as large as an overshoot of equivalent magnitude (Table 2). Although this might be indicative that it is better to overshoot than undershoot the target strain in a stress relaxation test, this result might be an artifact of our testing order. We deliberately did not randomize the order of testing but went from the smallest displacement to highest displacement (i.e., −0.9% through to +0.9% strain error). Otherwise, if we had randomized the testing order, greater strains could have affected subsequent lesser strains [14]. These effects could potentially be mitigated by avoiding plastic deformation and/or allowing for long recovery periods between tests. Because we did not randomize the order of testing, the fact that the first tested strain level (−0.9%) is most stiff and the last strain level (0.9%) is least stiff may be a cumulative effect.
The fact that the stress during tuning peaked just prior to the maximal target strain error of 0.3% and just after the actuator first reached target (Fig. 3) was anticipated given the viscous stress component dominates the tissue response during a fast ramp. However, it appears that the duration of the overshoot affects the shape of the relaxation curve as it prevents the stress from relaxing immediately, and hence could lead to errors in quantifying this region of the relaxation curve. Although we did not explore the duration of overshoot in this study, we are typically able to minimize this time to <0.1s, i.e. < τ1 (the short-term QLV time constant determined from previous tests of plantar tissue). One of the limitations of this study is that since we were unable to perform repeatable stress relaxation tests with varying target strain errors but were instead limited to triangle tests, we could not directly study the effects of overshoot on the QLV parameters themselves. It seems likely that the parameters would be affected given the model’s sensitivity to small changes [9].
In conclusion, the results of this study demonstrate that plantar tissue in compression is sensitive to small target strain errors that can result in stress errors that are several fold larger. Since these errors are difficult to completely eliminate experimentally while maintaining fast physiologic ramp rates, we recommend understanding the extent to which overshoot may affect the peak stress for a particular tissue and loading regime. In the case of plantar tissue in compression, we are able to maintain a target strain error of 0.3% and hence a small peak stress error of 3%. We expect that these findings will differ in magnitude for other soft tissues. Future studies should examine strain vs. stress errors for plantar tissue for other physiologic loading modes, e.g., shear and combined loading, where the tissue properties and associated overshoot error are likely to differ.
Acknowledgments
This study was supported by NIH grant 1R01 DK75633-03 and the Department of Veterans Affairs, RR&D Service.
References
- 1.CDC. Technical Report No. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta, GA: 2005. Center for Disease Control and Prevention. National Diabetes Surveillance System. [Google Scholar]
- 2.Van Schie CH. A Review of the Biomechanics of the Diabetic Foot. Int J Low Extrem Wounds. 2005;4(3):160–70. doi: 10.1177/1534734605280587. [DOI] [PubMed] [Google Scholar]
- 3.Ledoux WR, Blevins JJ. The Compressive Material Properties of the Plantar Soft Tissue. J Biomech. 2007;40(13):2975–81. doi: 10.1016/j.jbiomech.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Miller-Young JE, Duncan NA, Baroud G. Material Properties of the Human Calcaneal Fat Pad in Compression: Experiment and Theory. J Biomech. 2002;35(12):1523–31. doi: 10.1016/s0021-9290(02)00090-8. [DOI] [PubMed] [Google Scholar]
- 5.Fung YC. Biomechanics: Mechanical Properties of Living Tissues. Springer-Verlag; New York: 1993. Bioviscoelastic Solids. [Google Scholar]
- 6.Dortmans LJ, Sauren AA, Rousseau EP. Parameter Estimation Using the Quasi-Linear Viscoelastic Model Proposed by Fung. Journal of Biomechanical Engineering. 1984;106(3):198–203. doi: 10.1115/1.3138483. [DOI] [PubMed] [Google Scholar]
- 7.Kwan MK, Lin TH, Woo SL. On the Viscoelastic Properties of the Anteromedial Bundle of the Anterior Cruciate Ligament. Journal of Biomechanics. 1993;26(4–5):447–52. doi: 10.1016/0021-9290(93)90008-3. [DOI] [PubMed] [Google Scholar]
- 8.Funk JR, Hall GW, Crandall JR, Pilkey WD. Linear and Quasi-Linear Viscoelastic Characterization of Ankle Ligaments. J Biomech Eng. 2000;122(1):15–22. doi: 10.1115/1.429623. [DOI] [PubMed] [Google Scholar]
- 9.Gimbel JA, Sarver JJ, Soslowsky LJ. The Effect of Overshooting the Target Strain on Estimating Viscoelastic Properties from Stress Relaxation Experiments. J Biomech Eng. 2004;126(6):844–8. doi: 10.1115/1.1824132. [DOI] [PubMed] [Google Scholar]
- 10.Abramowitch SD, Woo SL. An Improved Method to Analyze the Stress Relaxation of Ligaments Following a Finite Ramp Time Based on the Quasi-Linear Viscoelastic Theory. J Biomech Eng. 2004;126(1):92–7. doi: 10.1115/1.1645528. [DOI] [PubMed] [Google Scholar]
- 11.Pai S, Ledoux WR. Structural Properties of Diabetic and Normal Plantar Soft Tissue. State College, PA: 2009. [Google Scholar]
- 12.Ledoux WR, Hillstrom HJ. The Distributed Plantar Vertical Force of Neutrally Aligned and Pes Planus Feet. Gait Posture. 2002;15(1):1–9. doi: 10.1016/s0966-6362(01)00165-5. [DOI] [PubMed] [Google Scholar]
- 13.Gooding GA, Stess RM, Graf PM, Moss KM, Louie KS, Grunfeld C. Sonography of the Sole of the Foot. Evidence for Loss of Foot Pad Thickness in Diabetes and Its Relationship to Ulceration of the Foot. Investigative Radiology. 1986;21(1):45–8. [PubMed] [Google Scholar]
- 14.Cheng S, Clarke EC, Bilston LE. The Effects of Preconditioning Strain on Measured Tissue Properties. J Biomech. 2009;42(9):1360–2. doi: 10.1016/j.jbiomech.2009.03.023. [DOI] [PubMed] [Google Scholar]