Abstract
Background
Carbamazepine, a sodium channel blocker and pro-autophagy agent used in the treatment of epilepsy and trigeminal neuralgia, is also an ionizing radiation mitigator and protector.
Materials and Methods
We measured the effect of carbamazepine, compared to other pro-autophagy drugs (i.e. lithium and valproic acid), on irradiation of autophagy incompetent (Atg5−/−) and competent (Atg5+/+) mouse embryonic fibroblasts, p53−/− and p53+/+ bone marrow stromal cells, and human IB3, KM101, HeLa, and umbilical cord blood cells, and in total body-irradiated or orthotopic tumor-bearing mice.
Results
Carbamazepine, but not other pro-autophagy drugs, was a radiation protector and mitigator for mouse cell lines, independent of apoptosis, autophagy, p53, antioxidant store depletion, and class I phosphatidylinositol 3-kinase, but was ineffective with human cells. Carbamazepine was effective when delivered 24 hours before or 12 hours after total body irradiation of C57BL/6HNsd mice and did not protect orthotopic Lewis lung tumors.
Conclusion
Carbamazepine is a murine radiation protector and mitigator.
Keywords: Radioprotection, radiation mitigation, autophagy, p53, carbamazepine
The recent discovery that drugs which promote autophagy, including carbamazepine, can clear misfolded protein from the liver (1) led to the investigation of other functions of carbamazepine, one of which was recently reported to be its effect as an ionizing irradiation protector and mitigator in vitro and in vivo (2). Carbamazepine is utilized clinically for the treatment of bipolar disorder, trigeminal neuralgia, and epilepsy (3, 5–6). The relatively safe history of administration of carbamazepine to patients with a variety of medical conditions, despite rare complications (7–8) led us to consider its use for radiation protection in humans. We therefore investigated its radiobiologic mechanism of action. We reasoned that identifying the specific molecular target of carbamazepine in radioprotection might facilitate its development for use in normal tissue protection during clinical radiotherapy, as well as for irradiation counter-terrorism.
The most frequently discussed mechanism of action of carbamazepine is in its amelioration of neurologic pathology by inactivation of voltage-gated sodium channels (3). How this action would affect cellular radiobiology is not known. Secondly, by up-regulating autophagy, carbamazepine promotes clearance of misfolded protein aggregates in α-anti-trypsin-deficient mice (1). Carbamazepine and other mood stabilizing drugs, including lithium and valproic acid (VPA), may therefore promote autophagy by depletion of intracellular inositol (4–7). Phosphoinositide 3-kinase (PI3K) is an enzyme involved in the inositol cycle and the production of inositol triphosphate (IP3), an important second messenger phospholipid that binds to IP3 receptors in the endoplasmic reticulum, releasing intracellular calcium stores, regulating both cell proliferation, and autophagy (9–11). Through a calcium surge regulated by IP3, apoptosis might be induced directly or indirectly (12) and therefore, by promoting autophagy, carbamazepine might reduce irradiation-induced apoptosis (13). Thirdly, since carbamazepine can deplete antioxidant levels (14), and may increase levels of radical oxygen species (ROS) (15), neither of which facilitate radioprotection (16), a rebound increase in antioxidants might be the explanation for its radiobiologic action.
We evaluated the effects of carbamazepine on radiation-induced cell death pathways that are associated with autophagy by utilizing autophagy incompetent Atg5−/− and control Atg5+/+ mouse embryonic fibroblast (MEF) cell lines (generously provided by Dr. Noboro Mizushima of Tokyo Medical and Dental University) (25). Other autophagy-promoting agents, including VPA and lithium chloride, were compared with carbamazepine. Since sodium channel inhibition by carbamazepine might alter intracellular p53, an important molecule in the DNA damage response to irradiation (17), we tested the effect of carbamazepine on the radiobiology of p53−/− compared to p53+/+ cell lines. Inhibitory complexes of p53 with B-cell lymphoma extra large (BclXL) and B-cell lymphoma 2 (Bcl2) may alter the mitochondria permeability, inducing cytochrome c release and apoptosis (18). Since p53 induces autophagy in response to DNA damage in a Damage-Regulated Autophagy Modulator (DRAM)-dependent manner (19), this action may be protective against radiation damage (20), and p53−/− cells would not exhibit the carbamazepine effects.
We also tested the effects of carbamazepine as a radiation protector in mice with orthotopic tumors to determine if therapeutic irradiation was also mitigated by the drug. Finally, to be assured of translation of the findings to human cells, we tested carbamazepine as a radioprotector or mitigator in human cell lines and fresh umbilical cord blood hematopoietic progenitors.
Materials and Methods
Cell culture
Murine hematopoietic progenitor cells (32Dcl3) (21, 22), murine p53+/+ and p53−/−bone marrow stromal cells (23), 3LL Lewis Lung Carcinoma cells (24), and Atg5+/+ Atg5−/− MEF cells (25) were cultured according to published methods. Briefly, 32Dcl3 cells were passaged in Iscove’s modified medium supplemented with 15% conditioned medium from Walter and Elizabeth Hall Institue-3 cells (WEHI-3) as a source of interleukin 3 (IL-3), 10% fetal bovine serum (FBS) (Hyclone Laboratories, Logan, UT, USA), 1% L-glutamine (GIBCO, Gaithersburg, MD, USA) and 1% penicillin-streptomycin (P/S) (GIBCO). Murine bone marrow stromal cell lines (p53+/+ and p53−/−), 3LL cells, and MEF cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Lonza, Walkersville, MD, USA) supplemented with 10% FBS, 1% L-glutamine and 1% P/S. Culture conditions for the human cell lines HeLa, IB3 (26) and KM101 (27) have been reported and were grown in DMEM supplemented with 10% FBS, 1% L-glutamine, and 1% P/S. Human umbilical cord blood cells were cultured and analyzed for CFU-GEMM multilineage colonies as published elsewhere (28).
In vitro irradiation experiments
Carbamazepine (Sigma Chemical Company, St. Louis, MO, USA) was prepared as a 10 mM stock solution in dimethyl sulfoxide (DMSO). Lithium chloride and VPA (Sigma Chemical Company) were prepared as 1 mM stock solutions in water. Cells were suspended at 1×106 cells/ml and irradiated with 0 to 8 Gy using a Shepherd Mark 1 irradiator with a cesium source (J.L. Shepherd, San Fernando, CA, USA). Carbamazepine was added at a final concentration of 10 μM (2) for one hour before or immediately after irradiation to murine Atg5-proficient and -deficient, murine p53-proficient and -deficient, and 3LL cells. With human IB3, HeLa, and KM101cells, 50 μM of carbamazepine was used. Lithium chloride or VPA was added to 32Dcl3 cell cultures at a final concentration of 0, 1 or 10 μM for one hour before or immediately after irradiation. 32Dcl3 cells were plated in triplicate in methylcellulose as previously described and incubated at 37°C with 5% CO2 for 7–14 days then colonies of >50 cells were counted (29). Adherent cells were plated in quadruplicate in 4-well Linbro plates (MP Biomedicals, LLC, Salon, OH, USA), incubated for 7 to 14 days at 37°C with 5% CO2, stained with crystal violet and colonies greater than 50 cells were counted with a colony counter (Oxford Optronix, Oxford, UK). Irradiated human umbilical cord blood mononuclear cells (MNC) were plated in triplicate in methylcellulose supplemented with recombinant human stem cell factor (rh SCF), granulocyte-macrophage colony-stimulating factor (GM-CSF), granulocyte colony-stimulating factor (G-CSF), IL 3 and erythropoietin (Stemcell Technologies, Vancouver, Canada). Colony-forming unit-granulocyte macrophage (CFU-GM), burst-forming unit erythroid (BFU-E), and colony-forming unit-granulocyte-erythroid-megakaryocyte-monocytes (CFU-GEMM) were scored on day 14. The radiosensitivity of human cord blood progenitor cells was measured according to published methods (30).
Immunoblot
Autophagy was assayed by immunoblot for microtubule-associated protein light chain 3 (LC3) as described previously (2). Briefly, Atg5+/+ and Atg5−/− MEF cells were harvested and lysed in NP-40 buffer [50 mM Tris, pH 7.8, 10 mM ethylenediaminetetaacetic acid (EDTA), 150 mM NaCl, 1 mM phenylmethylsulfonyl fluoride (PMSF), 1% NP-40 and a protease inhibitor cocktail tablet (Roche Diagnostics, Indianapolis, IN, USA)]. Protein samples were separated in 15% polyacrylamide gels by electrophoresis. Primary LC3 (Novus Biologicals, Littleton, CO, USA) or α-tubulin (Sigma Aldrich, St. Louis, MO, USA) antibody were used. Horseradish peroxidase anti-rabbit or anti-mouse secondary antibody (Promega, Pittsburgh, PA, USA) was applied and membranes developed with Super Signal West Dura ECL (Thermo Scientific, Rockford, IL, USA).
Immunofluorescent staining of autophagic vacuoles in Atg5 −/− and Atg5+/+ cell lines
Atg5+/+ and Atg5−/− cells were grown on glass coverslips in the presence or absence of 50 μM carbamazepine or 50 μM chloroquine (InvivoGen, San Diego, CA, USA) for 16 or 24 hours. Paraformaldehyde-fixed cells were stained with a rabbit polyclonal primary antibody against LC3II, AlexaFluor 488 secondary antibody and AlexaFluor 568 phalloidin (Invitrogen, Gaithersburg, MD, USA).
Lewis lung carcinoma (3LL) orthotopic tumor mode
A total of 1×106 3LL cells were injected subcutaneously into the left hind limbs of C57BL/6NTac 6 week old female mice (20 – 22 grams in weight) (Taconic Farms, Inc., Hudson, NY, USA). One week after injection, mice received an intra-peritoneal (i.p.) injection of 10 mg/kg carbamazepine in Cremphor-EL (29) prior to or immediately after 20 Gy irradiation to the tumor-containing leg using a LINAC (Varian Medical Systems, Palo Alto, CA, USA). Tumor diameter was monitored with caliper measurement.
Apoptosis and mitochondria permeability
Cells from the IL3-dependent hematopoietic progenitor cell line 32Dcl3 (29) were incubated with 10 μM carbamazepine for one hour before or after irradiation with 5 or 10 Gy. Cells were harvested 48 hours after irradiation and apoptosis and mitochondrial membrane depolarization were quantified by commercial TUNEL stain (Promega, Madison, WI, USA) and JC1 (Immunochemistry Technologies, Bloomington, MN, USA) kits, respectively. Cell viability was calculated using an automated cell counter (Oxford Optronix, Milton Park, Oxford, UK). As a positive control, 32Dcl3 cells were grown in the absence of IL3, the deprivation of which induces apoptosis.
Antioxidant assay
Cells from the MEF cell lines Atg5+/+ and Atg5−/− were incubated with 10 μM carbamazepine for one hour prior to 6 Gy ionizing radiation. Cells were harvested after 10, 30, 60, 90 and 120 minutes and snap-frozen in liquid nitrogen. Cell pellets were then thawed and mechanically homogenized in cold phosphate buffer solution. Protein concentrations were standardized by Bradford assay and antioxidant levels measured using a commercial kit (Northwest Life Science Specialties, Vancouver, WA, USA).
Class I PI3K assay
The in vitro effect of carbamazepine on class I PI3K activity was measured by use of a commercial ELISA kit (Echelon Biosciences Inc., Salt Lake City, UT, USA). Each reaction mixture contained 0.025 ng/μl class I PI3K enzyme (Echelon Biosciences Inc.) and was incubated at 37°C for 1.5 hours in the presence of different concentrations (6.3 – 200 μM) of the control inhibitor LY-294,002 (Enzo Life Sciences Inc., Farmingdale, NY, USA) or carbamazepine. PI3K activity was quantified by phosphatidylinositol 3,4,5-trisphosphate (PIP3) production and the resultant absorbance change at 450 nm.
Statistics
The in vitro radiation survival curves were analyzed with the linear-quadratic model and the single-hit multi-target model, and were compared using the final slope representing multiple-event killing (D0) and the extrapolation number measuring the width of the shoulder on the radiation survival curve (ñ) (29, 31). Results for D0 and ñ are presented as the mean ± standard error of the mean (SEM) from multiple measurements. The two-sided two sample t-test was used to compare means of different groups.
The tumor volume data are summarized as the mean ± standard deviation for each of the four treatment groups (namely, 0 Gy, 20 Gy, carbamazepine before 20 Gy irradiation and carbamazepine after 20 Gy irradiation) at each day of measurement. Linear mixed models were built for the log-transformed tumor volume where group and day of measurement, as well as their interaction, were used as fixed explanatory variables, and day of measurement, the within subject variable, was used as a repeated measure. The F-test was used to examine the significance of interaction between group and day of measurement. A significant result indicates a significant difference in tumor growth rate between groups. For the in vivo mouse survival data, the two-sided log-rank test was used to compare each treatment group with the radiation only control group. For all these analyses, p-values less than 0.05 were interpreted as being significant.
Results
The autophagy-promoting drugs lithium chloride and VPA are not radiation dose modifiers in vitro
To test whether other autophagy-promoting drugs with similar clinical uses and effects on the inositol pathway were radiation protectors/mitigators, we tested lithium chloride and VPA in radiation clonogenic assays using 32Dcl3 cells. Lithium chloride or VPA added at 1 mM or 10 mM before or after irradiation did not change the ñ or D0 (Table I). Thus, unlike carbamazepine, neither lithium chloride nor VPA were radiation protectors or mitigators for 32Dcl3 cells (2). We next tested the autophagy dependence of CBZ radioprotection and mitigation.
Table I.
Effects of lithium chloride and valproic acid (VPA) on 32Dcl3 cell clonogenic radiation survival curves.
| Lithium (mM) | Pre-irradiation | Post-irradiation | ||
|---|---|---|---|---|
| Do (Gy) | ñ | Do (Gy) | ñ | |
| 0 | 1.3±0.1 | 1.3±0.3 | 1.3±0.1 | 1.3±0.3 |
| 1 | 1.3±0.1 (p=0.8340) | 1.0±0.1 (p=0.3465) | 1.5±0.1 (p=0.7415) | 1.0±0.1 (p=1.000) |
| 10 | 1.5±0.1 (p=0.1841) | 1.1±0.1 (p=0.2508) | 1.7±0.1 (p=0.1145) | 1.0±0.1 (p=0.7888) |
| VPA (mM) | ||||
| 0 | 1.5±0.1 | 1.0±0.1 | 1.5±0.1 | 1.0±0.1 |
| 1 | 1.6±0.1 (p=0.6037) | 1.5±0.5 (p=0.2529) | 1.2±0.1 (p=0.0602) | 1.5±0.5 (p=0.3122) |
| 10 | 1.3±0.1 (p=0.4431) | 1.1±0.1 (p=0.3801) | 1.4±0.1 (p=0.7159) | 1.3±0.3 (p=0.1527) |
Four-well plates with 500 or 1000 cells per plate were scored at day 7 for colonies of ≥50 cells as described in the Materials and Methods. The p-values compare cell survival with lithium and VPA to that of non-drug treated irradiated cells using the single-hit, multi-target model.
Radiation protection and mitigation by carbamazepine is autophagy independent
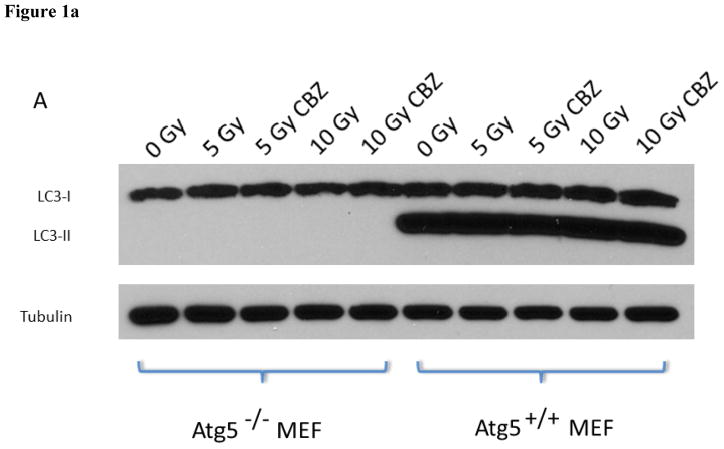
Western blot analysis of LC3 was first performed to confirm that Atg5−/− MEF cells were autophagy deficient. The absence of the LC3II band in control or carbamazepine-treated Atg5−/− cells and the absence of autophagosomes, the vacuoles necessary for autophagy (25), in control as well as in irradiation-, chloroquine-, or in carbamazepine-treated cells, confirmed that Atg5−/− cells were autophagy deficient (Figure 1 and 2).
Figure 1. Atg5−/− mouse embryonic fibroblast (MEF) cells exhibit markers of autophagy deficiency.
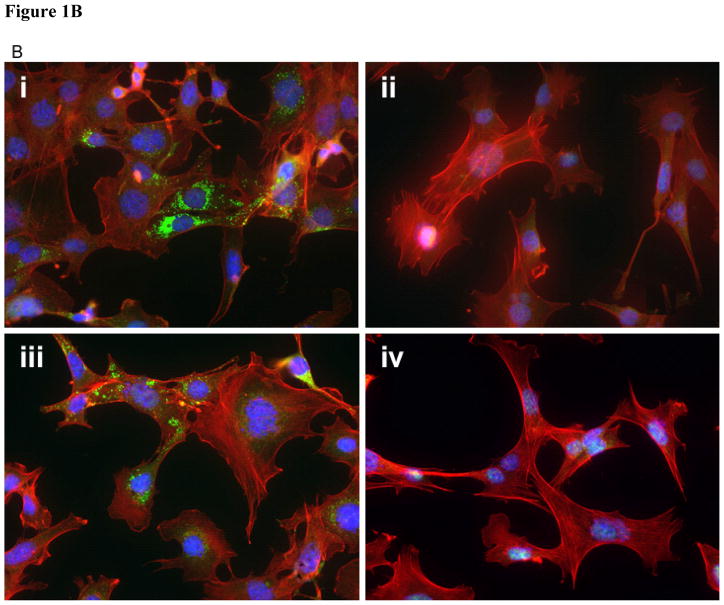
A: Immunoblot for microtubule associated light chain-3 type II (LC3II). Cells were treated with 5 or 10 μM carbamazepine (CBZ) and/or irradiated to 5 or 10 Gy to induce autophagy. There was a lack of LC3II in Atg5−/− cells B: LC3II staining of Atg5+/+ and Atg5−/−cells. Atg5+/+ and Atg5−/− cells were incubated with 50 mM CBZ 1 hour prior to or post 7 Gy irradiation, then stained for LC3II to identify the induction of autophagic vacuoles by irradiation. CBZ prior to 7 Gy induced autophagic vacuoles in Atg5+/+ cells (i) but not Atg5−/− cells (ii). Similar induction was evident in Atg5+/+ cells treated with 50 mM CBZ post 7 Gy (iii), but not with Atg5−/− cells (iv). Original magnification × 40.
Figure 2. Effect of chloroquine diphosphate and carbamazepine (CBZ) on induction of autophagic vacuoles in Atg5+/+ and Atg5−/− cell lines.
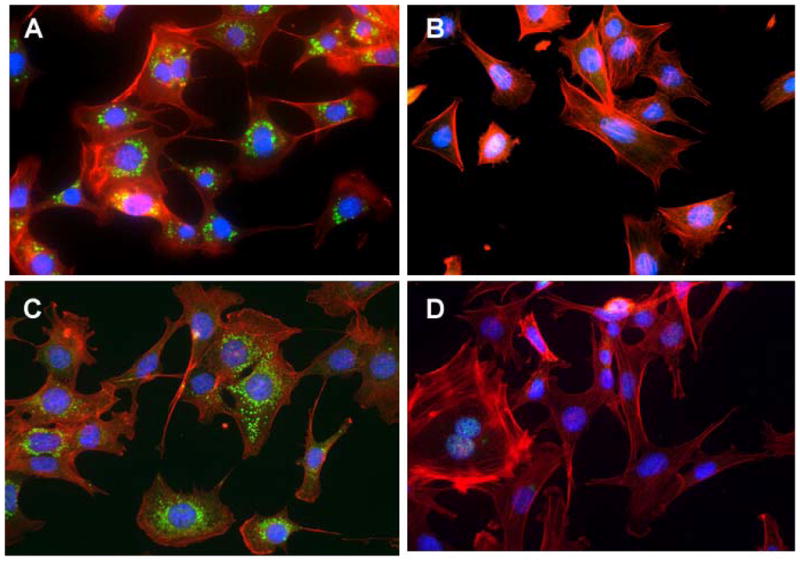
Atg5+/+ and Atg5−/− cells were incubated with 50 μM chloroquine for 16 hours or 50 μM CBZ for 24 hours, then stained for LC3II to identify the presence of autophagic vacuoles. Chloroquine induced autophagic vacuoles in Atg5+/+ cells (A) but not in Atg5−/− cells (B). Induction of autophagic vacuoles was evident in Atg5+/+ cells treated with 50 mM CBZ for 24 hours (C) but not in Atg5−/− cells (D). Original magnification × 40.
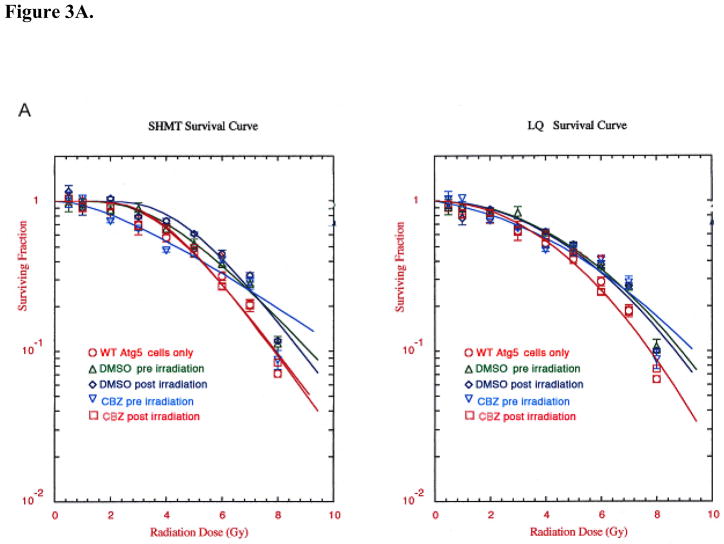
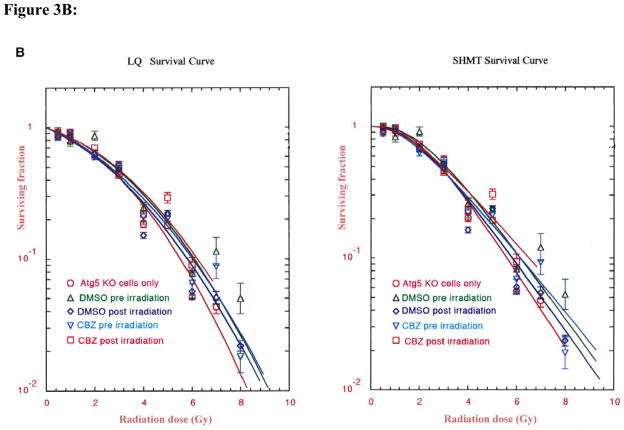
To determine whether carbamazepine protected and mitigated ionizing radiation damage in autophagy-deficient cells, Atg5−/− MEF cells were incubated with 50 μM CBZ for one hour before or immediately after irradiation. Autophagy-deficient MEF cells supplemented with 50 μM CBZ before irradiation had an increase in D0 from 1.53±0.05 to 1.99±0.05 (p=0.0028). Wild-type MEF cells had a similar increase in D0 from 1.67±0.09 to 2.90±0.35 (p=0.0260) (Table II, Figure 3A and B).
Table II.
Effect of carbamazepine (CBZ) on the radiosensitivity of Atg5+/+ and Atg5−/− mouse embryonic fibroblast cell lines.
| Experimental conditions | Pre or post irradiation | Cell type
|
|||
|---|---|---|---|---|---|
| ATG5+/+ | ATG5−/− | ||||
| D0 | ñ | D0 | ñ | ||
| Cells Only | 1.67±0.09 (n=3) | 9.76±0.84 (n=3) | 1.53±0.05 (n=3) | 7.43±2.70 (n=3) | |
| DMSO | Pre | 1.80±0.31 (n=3) | 7.0±0.30 (n=3) | 1.72±0.02 (n=3) | 7.39±3.48 (n=3) |
| p1=0.6965 | p1=0.0964 | p1=0.0622 | p1=0.9916 | ||
| Post | 2.01±0.25 (n=3) | 9.20±3.53 (n=3) | 1.69±0.01 (n=3) | 7.61±4.32 (n=3) | |
| p1=0.2794 | p1=0.8673 | p1=0.0932 | p1=0.9723 | ||
| CBZ | Pre | 2.90±0.35 (n=3) | 5.00±2.15 (n=3) | 1.99±0.05 (n=3) | 4.02±1.07 (n=3) |
| p1=0.0260 | p1=0.0687 | p1=0.0028 | p1=0.3089 | ||
| p2=0.0759 | p2=0.5245 | p2=0.0246 | p2=0.3342 | ||
| Post | 1.49±0.08 (n=3) | 15.15±1.71 (n=3) | 1.64±0.19 (n=3) | 13.23±7.74 (n=3) | |
| p1=0.2794 | p1=0.0299 | p1=0.6404 | p1=0.2622 | ||
| p3=0.1386 | p3=0.5245 | p3=0.8533 | p3=0.4125 | ||
Clonogenic survival curves were determined as described in the Materials and Methods. Data are summarized as the mean ± SEM. p-values were calculated with the two-sided two-sample t-test, where p1 is the p-value for comparison with the control cells only, p2 is for the comparison with the pre-irradiation dimethyl sulfoxide (DMSO) group, and p3 is for the comparison with the post-irradiation DMSO group. Significant p-values are written in bold.
Figure 3. Effect of (50 μM) carbamazepine (CBZ) treatment pre- and post-irradiation on Atg5+/+ and Atg5−/− cells.
Cells from cell lines Atg5+/+ (WT)(A) and Atg5−/− (KO)(B) were tested. MEF cells were irradiated to doses from 0 to 8 Gy then plated in clonogenic assay and colonies greater than 50 cells were scored at 7 days. CBZ added before irradiation or after irradiation protected and mitigated against radiation damage in both the autophagy-proficient and -deficient lines. CBZ added pre or post-irradiation to Atg5−/− clone 4 (KO) cells protected and mitigated against irradiation damage. Data are presented in single-hit multi-target and linear quadratic format.
We next evaluated the effects of carbamazepine on class I PI3K, an enzyme which inhibits autophagy and participates in the cellular inositol cycle (32, 33). We reasoned that if carbamazepine inhibited class I PI3K then autophagy would be up-regulated. Inhibition of PI3K might prevent production of IP3, calcium release, and cell death (34). To determine whether carbamazepine had an effect on IP3 levels, class I PI3K enzyme was incubated with carbamazepine and combined with PIP3 substrate. As a positive control LY-294,002 was added. With 32Dcl3 cells, there was nonspecific inhibition of the PI3K enzyme at 1000 μM carbamazepine but no inhibition at 250 μM or lower concentrations (Table III). The positive control PI3K inhibitor, LY-294,002, did inhibit PI3K activity at concentrations as low as 6.3 μM (Table III). Thus, the data indicated that carbamazepine did not directly inhibit class I PI3K in32Dcl3 cells. We cannot rule out possible carbamazepine interaction with a modulator of PI3K.
Table III.
Effects of carbamazepine (CBZ) on class I PI3K enzyme activity in 32Dcl3 cells in vitro.
| LY-294,002 | PIP3 | CBZ | PIP3 |
|---|---|---|---|
| 200 uM | 1.2 | 1000 uM | 9.5 |
| 100 uM | 1.8 | 250 uM | 25 |
| 50 uM | 1.5 | 62.5 uM | 25 |
| 25 uM | 2 | 15.6 uM | 28 |
| 12.5 uM | 4 | 3.9 uM | 28 |
| 6.3 uM | 6 | 1 uM | 25 |
| 0 uM | 22 | 0 uM | 25 |
Class I PI3K activity was measured in the presence of serial fold dilutions of control inhibitor LY-294,002 and CBZ. The amount of phosphatidylinositol 3,4,5-triphosphate (PIP3) produced is proportionate to the enzyme activity.
Carbamazepine induces the formation of autophagic vacuoles in Atg5+/+ cells but not in Atg5−/− cells
To test whether carbamazepine induced the formation of autophagic vacuoles in Atg5+/+ and autophagy-deficient Atg5−/− cells, we treated the cells with carbamazepine, pre and post 7 Gy irradiation, and used chloroquine diphosphate, which induces autophagosome production, as a positive control. Cells were stained for LC3II, which is a general marker for autophagic vacuoles. Atg5+/+ and Atg5−/− cells were incubated with 50 mM CBZ for one hour prior to or after 7 Gy irradiation. Cells were stained for LC3II to identify the presence of autophagic vacuoles induced by irradiation compared to chloroquine diphosphate or carbamazepine. Carbamazepine at 50 mM for one hour before 7 Gy irradiation induced the formation of autophagic vacuoles in Atg5+/+ cells but not Atg5−/− cells (Figure 1B). Similar induction was detected in Atg5+/+ cells treated with 50 mM carbamazepine after 7 Gy irradiation. Vacuoles were not detected in Atg5−/− cells (Figure 1B). When Atg5+/+ and Atg5−/− cells were incubated with 50 μM chloroquine for 16 hours, and were then stained for LC3II, autophagic vacuoles were detected in Atg5+/+ but not Atg5−/− cells (Figure 2A and B). When cells were treated with 50 μM carbamazepine for 24 hours prior to staining for LC3II, autophagic vacuoles were detected with Atg5+/+, but not Atg5−/− cells (Figure 2C and D). The data support the studies with PI3K and Atg5−/− cells, and indicate that carbamazepine acts as a radiation protector and mitigator independent of autophagy.
Carbamazepine does not alter mitochondrial permeability or prevent apoptosis
We next used Mito PT-JC1 and TUNEL staining to determine whether carbamazepine altered mitochondrial permeability and/or prevented apoptosis in irradiated cells. The percentage of cells with depolarized mitochondrial membrane after 5 Gy or 10 Gy irradiation did not change significantly when carbamazepine was added before or after irradiation (Table IV). Furthermore, the percentage of apoptotic cells after 5 or 10 Gy irradiation did not change significantly if carbamazepine was added before or after irradiation (Table IV). The viability of cells at 24 hours after irradiation was unchanged between the drug-treated and the irradiation-treated control group. Thus, carbamazepine did not alter irradiation-induced apoptosis in 32Dcl3 cells at 24 hours after irradiation and did not significantly alter mitochondrial membrane permeability. These results establish that the effect of carbamazepine on irradiated cells in vitro was mediated by events occurring after the first cell division not measurable by assays for apoptosis.
Table IV.
Effects of carbamazepine (CBZ) on 32Dcl3 cell line cell viability, mitochondrial membrane depolarization and apoptosis.
| Condition | Viability (%) | Mitochondrial membrane depolarization (%) | Apoptosis (%) |
|---|---|---|---|
| Control | 95.5±1.7 | 11.5±2.1 | 3.2±0.7 |
| Without IL3 | 35.0±7.2 | 55.2±6.5 | 76.1±6.6 |
| 5 Gy | 74.9±2.0 | 43.6±6.7 | 23.2±3.0 |
| CBZ + 5 Gy | 78.3±2.5 (p=0.3503#) | 38.7±5.4 (p=0.6093#) | 24.7±3.7 (p=0.8250#) |
| 5 Gy + CBZ | 76.7±2.7 (p=0.6362#) | 45.5±3.7 (p=0.8013#) | 25.4±3.1 (p=0.7330#) |
| 10 Gy | 64.1±2.6 | 58.6±5.7 | 46.0±4.6 |
| CBZ + 10 Gy | 61.2±3.2 (p=0.5233*) | 57.7±6.3 (p=0.9208*) | 41.9±3.9 (p=0.5274*) |
| 10 Gy + CBZ | 61.6±2.2 (p=0.4899*) | 51.9±6.9 (p=0.5333*) | 44.1±3.7 (p=0.7597*) |
p-value vs. 5 Gy;
p-value vs. 10 Gy.
Viability, mitochondrial depolarization and apoptosis were determined on 32Dcl3 cells treated with 10 μM CBZ either 1 hour before irradiation or added to the media after either 5 or 10 Gy irradiation. As a positive control, 32Dcl3 cells were grown in the absence of IL3, a condition which induces apoptosis. Cells were assayed 48 hours after either irradiation or removal of IL3. Viability was determined by trypan blue exclusion; mitochondrial membrane depolarization was determined using a MitoPT-JC1 Assay kit; and apoptosis using a TUNEL kit.
Protection by carbamazepine is P53 independent
We next evaluated whether carbamazepine-mediated ionizing irradiation protection and mitigation was dependent on p53. The effects of treatment with carbamazepine before or after irradiation of p53−/− murine bone marrow stromal cells were assessed by clonogenic survival curve assay and results were compared to those with a p53+/+ cell line. The p53−/− cells that were incubated with carbamazepine for one hour before or immediately after irradiation demonstrated both protection and mitigation, with an increase in ñ from 1.8±0.4 to 6.0±0.6 (p=0.0018) for protection (Table V, pFigure 4), and by an increase in ñ from 1.9±0.5 to 4.5±0.8 (=0.0318) for mitigation (data not shown). Thus, the mechanism by which carbamazepine modifies cellular irradiation damage was not dependent on p53.
Table V.
Effect of carbamazepine (CBZ) as a radiation protector in p53−/− compared to p53+/+ cell lines.
| Cell line | CBZ concentration (μM) | Do (Gy) | ñ |
|---|---|---|---|
| p53−/− | 0 | 3.9±0.8 | 1.8±0.4 |
| 1 | 2.0±0.1 | 6.0±0.6 (p=0.0018) | |
| 10 | 3.3±0.6 | 3.7±1.9 (p=0.3286) | |
| 100 | 3.1±0.6 | 3.6±1.5 (p=0.2433) | |
| p53+/+ | 0 | 1.9±0.5 | 3.0±0.7 |
| 1 | 4.0±0.2 (p=0.0179) | 1.5±0.1 | |
| 10 | 4.6±0.6 (p=0.0279) | 3.7±0.7 | |
| 100 | 4.3±2.5 | 1.9±0.9 |
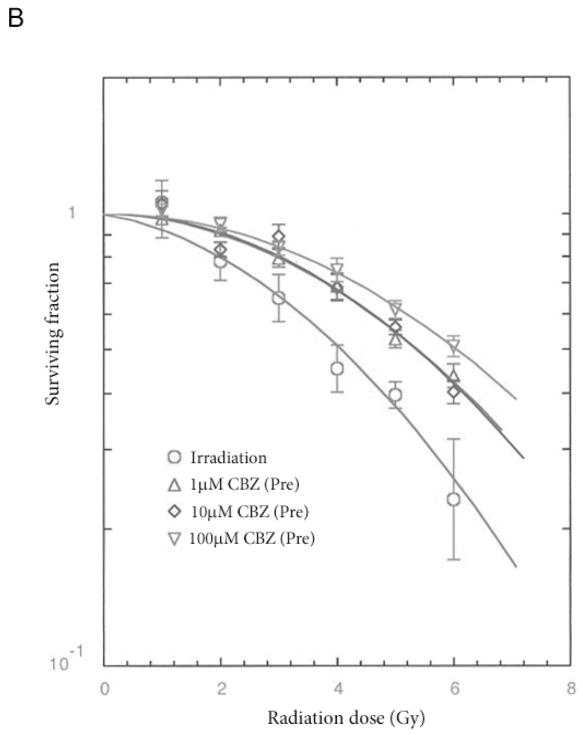
Cells from p53+/+ and p53−/− bone marrow stromal cell lines were incubated in the presence of 0, 1, 10 or 100 μM CBZ for one hour and then were irradiated to doses ranging from 0 to 8 Gy, plated in 4-well plates, incubated for 7 days at 37°C, stained with crystal violet and colonies of greater than 50 cells counted. The data was analysed using linear quadratic and single-hit, multi-target models. The p-values are in comparison to results using 0 μM. CBZ had different, but still protective effects on p53+/+ (increased D0) and p53−/− (increased ñ) cells.
Figure 4. Radiation protection/mitigation by carbamazepine (CBZ) is independent of p53.
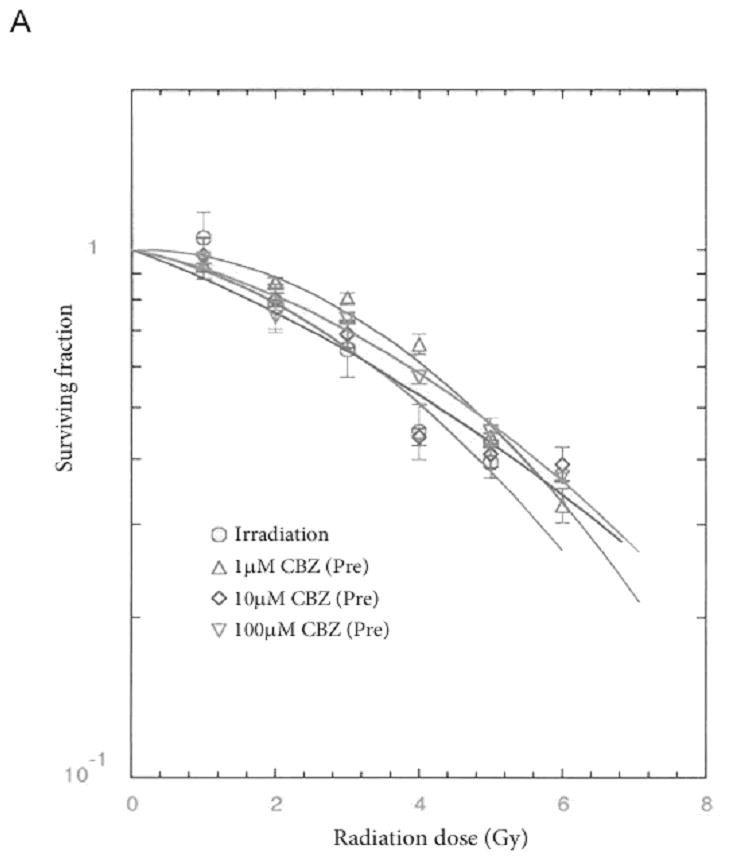
A: p53+/+ and B: p53−/− murine bone marrow stromal cells were irradiated to doses of 0 – 8 Gy then plated in clonogenic assay and colonies greater than 50 cells were scored at 14 days. CBZ protected (pre) and mitigated (post) radiation damage in both the p53-containing and -deficient lines.
Carbamazepine increases antioxidant levels in Atg5+/+ MEF cells
The cell lines Atg5+/+ and Atg5−/− were incubated with carbamazepine for one hour prior to 6 Gy irradiation and were then harvested at various time points. Atg5+/+ cells that were supplemented with 10 μM carbamazepine demonstrated an increase in antioxidant levels 10 minutes after irradiation, compared to control irradiated cells (Figure 5). This result may reflect a rebound from the response of cells to carbamazepine-induced oxidative stress which was caused by adding carbamazepine one hour prior to irradiation. However, carbamazepine was also effective as a radiation mitigator with continual exposure to drug in the medium in the mitigation experiment. Continuous exposure would have been expected to deplete antioxidant levels through any such rebound. Increased antioxidant levels were not detected in autophagy-deficient Atg5−/− cells, which were also protected and mitigated from irradiation by carbamazepine. Atg5−/− cells demonstrated consistently low antioxidant (Figure 5) and glutathione (GSH) levels (Table VI). The data support the conclusion that radiation protection and mitigation of cells by CBZ was not mediated by alterations in cellular antioxidant levels.
Figure 5. Carbamazepine (CBZ) increases antioxidant levels in irradiated Atg5+/+ but not Atg5−/− cells.
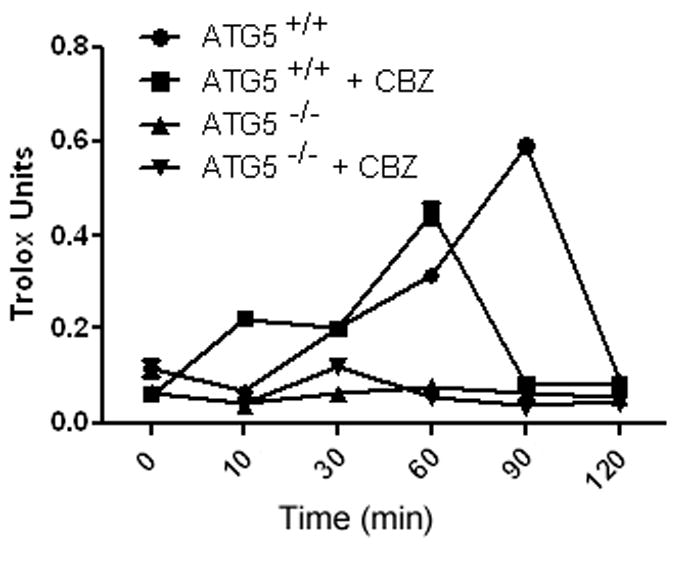
Cells from Atg5+/+ and Atg5−/− cell lines were treated with 10 μM CBZ for 1 hour prior to 6 Gy irradiation, harvested at different time points and antioxidant levels measured. Baseline antioxidants remained higher in autophagy-proficient Atg5+/+ cells. CBZ-treated Atg5+/+ cells had an increase in antioxidants at 10 minutes, peaking at 60 minutes after irradiation.
Table VI.
Effect of carbamazepine (CBZ) on antioxidant stores in irradiated Atg5+/+ compared to Atg5−/− (WT) cell lines.
| Cell Line | Total antioxidants (Trolox equivalents) | GSH (μM) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 Gy | 24 h after 4 Gy | 0 Gy | 6 h after 6 Gy | |||||
| 0 μM CBZ | 10 μM CBZ | 0 μM CBZ | 10 μM CBZ | 0 μM CBZ | 10 μM CBZ | 0 μM CBZ | 10 μM CBZ | |
| WT 1 | 0.026 | 0.039 | 0.217 | 2.111 | <0.010 | |||
| WT 3 | 0.064 | 0.050 | 0.067 | <0.010 | ||||
| KO 2 | 0.49 | 0.67 | 0.039 | 0.043 | <0.010 | <0.010 | <0.010 | <0.010 |
| KO 4 | 0.042 | 0.133 | <0.010 | <0.010 | ||||
Clonal cell lines of Atg5+/+ (WT1 and 3) and Atg5−/− (KO2 and 4) were irradiated to doses from 0, 4 or 6 Gy as described in the methods. Total antioxidants (Trolox units) and levels of glutathione (GSH) were measured using a commercial kit at 24 hours following 4 Gy or 6 hours after irradiation of 6 Gy, respectively.
Carbamazepine does not protect tumor cells in vitro
An effective radioprotector for use in clinical radiation therapy should protect normal tissues but not tumor cells. To determine if carbamazepine protected tumor cells from ionizing irradiation, Lewis lung carcinoma (3LL) cells were incubated in 10 μM carbamazepine before or after irradiation and plated for clonogenic survival assay. Cells that received carbamazepine before or after irradiation did not exhibit a statistically different survival curve from control irradiated cells (Table VII). The results establish that carbamazepine did not protect 3LL tumor cells in vitro.
Table VII.
Effect of carbamazepine (CBZ) on radiosensitivity of Lewis lung carcinoma in 3LL cells assayed by clonogenic radiation survival curve in vitro.
| CBZ Treatment | D0 (Gy) | ñ |
|---|---|---|
| 0 μM | 2.0±0.4 | 5.7±2.4 |
| 10 μMPre-Irradiation | 2.0±0.1 (p=0.9441) | 3.3±1.0 (p=0.3520) |
| 10 μM Post-Irradiation | 2.0±0.2 (p=0.09522) | 3.2±1.3 (p=0.3795) |
We evaluated 4-well plates with 500 or 1000 cells per plate irradiated to doses of 0 – 8 Gy. The plates were screened 14 days after irradiation for colonies of >50 cells as described in the Materials and Methods. The p-values compare cell survival of 3LL cell lines treated with CBZ before or after irradiation to those treated with irradiation alone, using linear quadratic or single-hit, multi-target models.
Carbamazepine does not modulate the radiation response of 3LL orthotopic tumors in vivo
We next tested the effect of carbamazepine on irradiated 3LL tumors in vivo. 3LL tumor cells were injected into the leg of mice and allowed to grow to a measurable 5 mm diameter mass prior to irradiation with 20 Gy to the hind limb, a dose known to reduce tumor growth. The irradiated tumors in mice that received intraperitoneal injection of CBZ before or after irradiation did not show faster regrowth compared to tumors in mice that received irradiation only (p=0.2431 and 0.5439, respectively) (Figure 6). Thus carbamazepine did not reduce the irradiation response of 3LL tumors in vivo. These data confirm and extend prior studies, showing that CBZ was an effective mitigator against total body irradiation when delivered at 12 but not at 24 hours after irradiation (2).
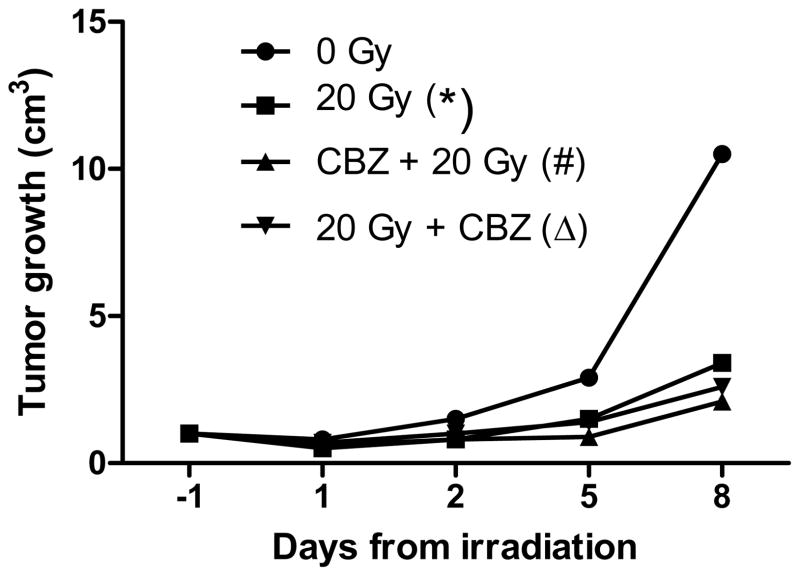
Figure 6. Effect of carbamazepine (CBZ) on irradiation-mediated size reduction of 3LL carcinoma cell line-derived orthotopic tumors in vivo.
We injected 106 3LL cells into the hind leg of C57BL/6 HNsd mice and tumors grew to a solid mass for 7 days. Mice then received intraperitoneal injection of CBZ at 20 mg/kg in 0.1/ml and tumors were then irradiated to 20 Gy and monitored for tumor growth. Tumors in mice receiving 20 Gy only to the tumor (*) grew significantly slower than tumors in 0 Gy mice (p=0.0019). Tumors in mice treated with CBZ before 20 Gy (#) grew slower than the tumors in 0 Gy mice but not tumors receiving 20 Gy only (p=0.0001 or 0.2431, respectively). Tumors in mice administered 20 Gy and followed by CBZ (Δ) had a decreased growth rate compared to tumors in 0 Gy mice but not in 20 Gy irradiated mice (p=0.0005 or 0.5439, respectively). CBZ treatment did not protect tumors from size reduction due to irradiation.
Carbamazepine is not a radioprotector or mitigator for human cell lines or fresh human umbilical cord blood hematopoietic progenitor cells in vitro
We tested the effect of carbamazepine pre and post-irradiation on three human cell lines: i) IB3, bronchoalveolar cells, ii) KM101 human bone marrow stromal cells, and iii) cervical cancer derived HeLA cells (Table VIII). We evaluated the effect of carbamazepine on human umbilical cord blood MNCs that form multilineage hematopoietic colonies in vitro (Table IX). We also tested the effects on the sorted and purified cord blood CD34+ progenitor cells (Table X). The results showed no detectable radioprotection or mitigation by carbamazepine of any of the human cell sources (Tables VIII - X).
Table VIII.
Lack of radioprotection or mitigation of radiation-induced effects in human cell lines by carbamazepine (CBZ).
| CBZ concentration | Pre or post irradiation | HeLA | KM101 | IB3 | |||
|---|---|---|---|---|---|---|---|
| D0 (Gy) | ñ | D0 (Gy) | ñ | D0 (Gy) | ñ | ||
| 0 μM | Pre | 1.7 ± 0.1 | 7.6 ± 2.5 | NA | NA | NA | NA |
| Post | 1.79 ± 0.2 | 5.1 ± 1.3 | 1.3 ± 0.1 | 3.0 ± 0.3 | 1.6 ± 0.1 | 7.1 ± 4.6 | |
| 1 μM | Pre | 1.9 ± 0.3 | 7.6 ± 4.0 | NA | NA | NA | NA |
| Post | 1.6 ± 0.2 | 7.3 ± 2.7 | 1.3 ± 0.1 | 3.2 ± 0.3 | 1.6 ± 0.1 | 5.5 ± 2.7 | |
| 10 μM | Pre | 1.8 ± 0.2 | 9.0 ± 4.1 | NA | NA | NA | NA |
| Post | 1.8 ± 0.2 | 4.7 ± 1.0 | 1.2 ± 0.1 | 3.5 ± 0.5 | 1.8 ± 0.1 | 4.6 ± 2.2 | |
| 100 μM | Pre | 1.8 ± 0.1 | 6.6 ± 1.6 | NA | NA | NA | NA |
| Post | 1.4 ± 0.2 | 6.1 ± 2.2 | 1.2 ± 0.1 | 2.2 ± 0.1 | 1.7 ± 0.1 | 6.6 ± 4.6 | |
Cell lines were irradiated to doses of 0 – 8 Gy in the presence of CBZ one hour pre-irradiation (protection) or plated in CBZ (mitigation). Colonies were scored on day 7 as described in the Materials and Methods. Data are summarized as mean ± SEM. NA, not assayed pre-irradiation.
Table IX.
Effect of carbamazepine (CBZ) pre or post irradiation on colony formation by human cord blood mononuclear cells.
| Experimental conditions | Pre or post irradiation | CFU-GM | BFU-E | CFU-GEMM | |||
|---|---|---|---|---|---|---|---|
| D0 | ñ | D0 | ñ | D0 | ñ | ||
| Cells only | 1.60±0.28 | 1.71±0.41 | 1.40±0.19 | 3.31±0.73 | 2.08±0.05 | 1.22±0.12 | |
| DMSO | Pre | 1.57±0.20 | 2.73±1.73 | 1.61±0.18 | 1.81±0.30 | 2.13±0.10 | 1.22±0.13 |
| p1=0.9340 | p1=0.5971 | p1=0.4598 | p1=0.1307 | p1=0.6221 | p1=0.9858 | ||
| Post | 1.68±0.20 | 1.43±0.25 | 1.30±0.17 | 11.98±9.55 | 2.11±0.14 | 1.24±0.04 | |
| p1=0.8202 | p1=0.5923 | p1=0.7331 | p1=0.4600 | p1=0.8004 | p1=0.9445 | ||
| CBZ 1 μM | Pre | 1.69±0.07 | 1.38±0.35 | 1.46±0.01 | 3.31±1.30 | 2.02±0.23 | 1.35±0.16 |
| p1=0.7558 | p1=0.5738 | p1=0.7872 | p1=0.9983 | p1=0.8293 | p1=0.5526 | ||
| p2=0.5831 | p2=0.4868 | p2=0.4760 | p2=0.3213 | p2=0.6781 | p2=0.5572 | ||
| Post | 1.84±0.06 | 1.16±0.16 | 1.66±0.07 | 1.63±0.23 | 2.11±NA | 1.00±NA | |
| p1=0.4346 | p1=0.2797 | p1=0.2762 | p1=0.0941 | p1=NA | p1=NA | ||
| p3=0.4833 | p3=0.4015 | p3=0.1191 | p3=0.3917 | p3=NA | p3=NA | ||
| CBZ 10 μM | Pre | 1.50±0.21 | 2.46±1.46 | 1.46±0.16 | 2.69±1.14 | 2.06±NA | 1.34±NA |
| p1=0.7864 | p1=0.6463 | p1=0.8141 | p1=0.6693 | p1=NA | p1=NA | ||
| p2=0.8179 | p2=0.9120 | p2=0.5594 | p2=0.4960 | p2=NA | p2=NA | ||
| Post | 1.91±0.07 | 1.00±0.00 | 1.67±0.03 | 1.61±0.22 | 2.37±NA | 1.00±NA | |
| p1=0.3354 | p1=0.2287 | p1=0.2436 | p1=0.0908 | p1=NA | p1=NA | ||
| p3=0.3436 | p3=0.2224 | p3=0.0973 | p3=0.3910 | p3=NA | p3=NA | ||
| CBZ 50 μM | Pre | 1.63±0.06 | 1.83±0.19 | 1.47±0.04 | 3.01±0.23 | - | - |
| p1=0.9123 | p1=0.8009 | p1=0.7313 | p1=0.7161 | ||||
| p2=0.7747 | p2=0.6567 | p2=0.4812 | p2=0.0322 | ||||
| Post | 1.72±0.27 | 1.27±0.14 | 1.70±0.32 | 1.71±0.40 | - | - | |
| p1=0.7715 | p1=0.3724 | p1=0.4553 | p1=0.1296 | ||||
| p3=0.9184 | p3=0.6041 | p3=0.3249 | p3=0.3949 | ||||
| CBZ 100μM | Pre | 1.74±0.04 | 1.26±0.18 | 1.50±0.24 | 3.09±2.01 | 2.05±NA | 1.00±NA |
| p1=0.7258 | p1=0.4742 | p1=0.7693 | p1=0.9091 | p1=NA | p1=NA | ||
| p2=0.5576 | p2=0.5581 | p2=0.7116 | p2=0.4676 | p2=NA | p2=NA | ||
| Post | 1.55±0.19 | 1.00±0.00 | 1.32±0.18 | 2.38±0.66 | - | - | |
| p1=0.9014 | p1=0.2287 | p1=0.8053 | p1=0.4480 | ||||
| p3=0.6786 | p3=0.2224 | p3=0.9514 | p3=0.4933 | ||||
We evaluated nucleated cells from 3 separate human umbilical cord blood samples as described in the Materials and Methods. Colonies were analyzed at day 14 as described in the methods. Data are summarized as mean ± SEM. p-values were calculated with the two-sided two-sample t-test, where p1 is the p-value for comparison with the cells only control; p2 is for the comparison with the pre-irradiation dimethyl sulfoxide (DMSO) group; and p3 is for the comparison with the post-irradiation DMSO group. Significant p-values are shown in bold.
Table X.
Effect of carbamazepine (CBZ) pre or post irradiation on colony formation by human cord blood CD34+ cells.
| Experimental conditions | Pre or post irradiation | CFU-GM | BFU-E | CFU-GEMM | |||
|---|---|---|---|---|---|---|---|
| D0 | ñ | D0 | ñ | D0 | ñ | ||
| Cells only | 1.73 ±0.10 | 1.07 ±0.07 | 1.68 ±0.06 | 1.70 ±0.28 | 2.07 ±0.21 | 1.00 ±0.0 | |
| DMSO | Pre | 2.59 ± 0.29 | 1.04 ± 0.04 | 2.33 ± 0.07 | 1.44 ± 0.44 | 2.03 ±0.32 | 1.00 ±0.0 |
| p1=0.0248 | p1=0.7214 | p1=0.0008 | p1=0.6225 | p1=0.9151 | p1=1.0000 | ||
| Post | 2.15 ±0.16 | 1.08 ±0.80 | 2.25 ±0.28 | 1.00 ±0.0 | 2.30 ±0.56 | 1.00 ±0.0 | |
| p1=0.0776 | p1=0.9354 | p1=0.0398 | p1=0.0845 | p1=0.6494 | p1=1.0000 | ||
| CBZ 1 μM | Pre | 1.50 ±0.05 | 1.36 ±0.36 | 2.75 ±0.09 | 1.07 ±0.07 | 2.14 ±0.30 | 1.00 ±0.0 |
| p1=0.1971 | p1=0.3007 | p1=0.0005 | p1=0.2004 | p1=0.8522 | p1=1.0000 | ||
| p2=0.0640 | p2=0.5333 | p2=0.0327 | p2=0.5571 | p2=0.8241 | p2=1.0000 | ||
| Post | 1.66 ±0.08 | 2.07 ±1.07 | 2.22 ±0.39 | 1.04 ±0.04 | no colonies | no colonies | |
| p1=0.6647 | p1=0.5212 | p1=0.3902 | p1=0.1857 | p1=NA* | p1=NA | ||
| p3=0.1114 | p3=0.4537 | p3=0.9552 | p3=0.5000 | p3=NA | p3=NA | ||
| CBZ 10 μM | Pre | 2.13 ±0.38 | 1.00 ±0.0 | 2.26 ±0.31 | 1.21 ±0.15 | 2.03 ±0.35 | 1.00 ±0.0 |
| p1=0.2236 | p1=0.3910 | p1=0.0489 | p1=0.3056 | p1=0.9162 | p1=1.0000 | ||
| p2=0.3932 | p2=0.4226 | p2=0.8058 | p2=0.7099 | p2=0.9975 | p2=1.0000 | ||
| Post | 1.64 ±0.26 | 3.71 ±2.71 | 1.95 ±0.29 | 1.29 ±0.29 | 1.69 ± NA | 1.00 ± NA | |
| p1=0.6732 | p1=0.5083 | p1=0.2389 | p1=0.4120 | p1=NA | p1=NA | ||
| p3=0.2293 | p3=0.5095 | p3=0.5278 | p3=0.5000 | p3=NA | p3=NA | ||
| CBZ 50 μM | Pre | 1.80 ±0.02 | 1.63 ±0.06 | 2.16 ±0.22 | 1.30 ±0.30 | 2.43 ±0.08 | 1.00 ± 0.0 |
| p1=0.6715 | p1=0.0073 | p1=0.0392 | p1=0.4183 | p1=0.3248 | p1=1.0000 | ||
| p2=0.1275 | p2=0.0028 | p2=0.4397 | p2=0.8244 | p2=0.4074 | p2=1.0000 | ||
| Post | 1.22 ±0.18 | 35.75 ±33.30 | 2.57 ± NA | 1.00 ± NA | no colonies | no colonies | |
| p1=0.0468 | p1=0.4871 | p1= NA | p1= NA | p1= NA | p1= NA | ||
| p3=0.0587 | p3=0.4872 | p2= NA | p2= NA | p2= NA | p2= NA | ||
| CBZ 100 μM | Pre | 1.94 ±0.44 | 1.41 ±0.41 | 2.20 ±0.45 | 1.00 ±0.0 | 2.22 ±0.12 | 1.00 ±0.0 |
| p1=0.5423 | p1=0.2797 | p1=0.4488 | p1=0.0845 | p1=0.6622 | p1=1.0000 | ||
| p3=0.2785 | p3=0.5291 | p3=0.7397 | p3=0.4226 | p3=0.6768 | p2=1.0000 | ||
| Post | 1.13 ± NA | 15.13 ± NA | no colonies | no colonies | no colonies | no colonies | |
| p1=NA | p1=NA | p1=NA | p1=NA | p1=NA | p1=NA | ||
| p3=NA | p3=NA | p3=NA | p3=NA | p3=NA | p3=NA | ||
NA = Not Available
Data are summarized as mean ± SEM. p-values were calculated with the two-sided two-sample t-test, where p1 is the p-value for comparison with the cells only control; p2 is for the comparison with the pre-irradiation DMSO group; and p3 is for the comparison with the post-irradiation dimethyl sulfoxide (DMSO) group. Significant p-values are shown in bold.
Discussion
An aggressive search for small-molecule radiation protectors and mitigators has been necessitated by both the need for such agents in clinical radiotherapy (35) and in radiation counter-terrorism (36). In clinical radiotherapy, the availability of novel modalities of intensity modulated radiotherapy (37), stereotactic radiosurgery (38), and high-dose rate brachytherapy (39) still does not prevent the normal tissue toxicity of ionizing irradiation and often prevents radiation dose-escalation protocols. We were encouraged by the discovery that carbamazepine was a radioprotector and mitigator (2). Carbamazepine is a Food and Drug Administration approved drug for clinical use for epilepsy, trigeminal neuralgia, and bipolar disorder, and is a commonly prescribed drug with a well-known safety and side-effect profile. Serious but rare hematologic complications after chronic use have been identified (7–8). Therefore, while attractive for potential clinical use (2), its safety in irradiated humans must be carefully evaluated.
The present study indicates that carbamazepine is a radiation protector and mitigator for normal murine tissues, but not of tumor cells, both in vitro and in vivo. The data establish that radiobiologic effects of carbamazepine are not associated with changes in mitochondrial membrane permeability or radiation-induced apoptosis. Since carbamazepine increases radioresistance of the mouse hematopoietic progenitor cell line 32Dcl3 in clonogenic survival curve assays (2), the mechanism may be subtle and not detectable at the level of initial apoptosis of single cells. Consistent with this data was the observation that carbamazepine protected both p53−/− and p53+/+ cell lines from ionizing irradiation. The combined evidence indicates that carbamazepine acts by an apoptosis-independent mechanism.
Since carbamazepine is a pro-autophagy agent (1), we evaluated two other autophagy-promoting drugs, lithium chloride and VPA, as radiation-dose modifiers. These drugs have similar clinical indications and effects on the inositol cycle, and also are known to up-regulate autophagy (40). Neither lithium chloride nor VPA were effective radiation protectors or mitigators in vitro. Both Atg5−/− cells, which demonstrated absence of autophagy in western blot assay for LC3, and Atg5+/+ MEF cells were significantly radioprotected and mitigated by carbamazepine in clonogenic survival curve assays. The data indicate that the mechanism of radiation protection and mitigation by carbamazepine is independent of autophagy.
We demonstrated that Atg5−/− cells had a lower baseline level of antioxidants compared to the autophagy proficient Atg5+/+ wild-type cells. This result was consistent with other data showing that ROS oxidize Atg4, a process that induces and is essential for autophagy (41). Atg5−/− MEF cells accumulate ROS since ROS oxidation of Atg4 (ROS consumption) is upstream of Atg5 (41). Since carbamazepine causes oxidative stress after acute administration (42), this oxidative stress might result in higher levels of ROS and thus more Atg4 oxidation, and increased autophagy (41). It is possible that carbamazepine might up-regulate autophagy by this mechanism, but this process was independent of radioprotection or mitigation.
Intracellular glutamate transport is known to be altered by gamma irradiation in astrocytes and neurons (43). Since PI3K is involved in the regulation of glutamate transport, carbamazepine may enhance the affinity of transporters for their substrates. PI3K inhibitors LY-294,002 and wortmannin inhibit carbamazepine enhancement of glutamate transport activity (42). Since class III PI3K is upstream of Atg4 oxidation and the completion of autophagy (41), it is possible that effects of irradiation were propagated through a class III PI3K pathway and that carbamazepine ameliorates radiation damage in a class III PI3K-dependent manner (44). Our data suggest that it is unlikely that carbamazepine is inhibiting class III PI3K. Beclin 1 is a physiologic activator of class III PI3K (45). When the inhibitory complex of Beclin 1 with Bcl2 and BclX is disrupted, Beclin 1 can up-regulate class III PI3K activity (46). One potential mechanism by which carbamazepine may activate class III PI3K might be by preventing Bc12 or BclX interaction with Beclin 1. Further studies will be required to evaluate this possible mechanism of radiation mitigation and protection by carbamazepine.
The potential clinical value of carbamazepine as a radiation protector and mitigator was further supported in the present studies by our observation that 3LL tumor cells were not protected or mitigated in vitro by carbamazepine, and that in vivo orthotopic mouse tumors derived from 3LL cells were not protected by carbamazepine from single fraction irradiation. The data suggested that normal tissue protection and mitigation by carbamazepine should not extend to tumor cells. If carbamazepine acts by a novel mechanism of radioprotection, it might be additive or synergistic with other known small-molecule radiation protectors and mitigators that function through known anti-apoptotic pathways (29).
The present data indicated that there was no detectably significant carbamazepine radiation protection or mitigation of human cells in vitro. Furthermore, in other retrospective analysis studies of the use of carbamazepine during intracranial radiotherapy of patients with trigeminal neuralgia (47) or in other patients receiving radiotherapy for head and neck or lung cancer (48), there was no detectable decrease in radiation side-effects. Further studies will be required to determine how carbamazepine functions as a radiation protector and mitigator in mouse cells and in mice. Investigating the role of class III PI3K in carbamazepine-mediated protection and mitigation may reveal targets for future drug design, modification, and development which may increase its spectrum of activity to include human cells. Such data might lead to potential use of a modified agent in clinical radiotherapy and in radiation counter-measures.
Acknowledgments
This study was supported by NIH/NIAID grant U19A1068021 and NIH T32AG21885. The authors gratefully acknowledge Dr. Noboru Mizushima for providing the Atg5−/− and Atg5+/+ cell lines, and thank Dr. David Perlmutter for helpful discussions.
References
- 1.Hidvegi T, Ewing M, Hale P, Dippold C, Beckett C, Kemp C, Maurice N, Mukherjee A, Goldbach C, Watkins S, Michalopoulos G, Perlmutter DH. An autophagy-enhancing drug promotes degradation of mutant α1-Antitrypsin Z and reduces hepatic fibrosis. Science. 2010;329:229–235. doi: 10.1126/science.1190354. [DOI] [PubMed] [Google Scholar]
- 2.Kim H, Bernard ME, Flickinger J, Jr, Epperly MW, Wang H, Dixon TM, Shields D, Houghton F, Zhang X, Greenberger JS. The autophagy-inducing drug carbamazepine is a radiation protector and mitigator. Int J Radiat Biol. 2011;87(10):1052–1060. doi: 10.3109/09553002.2011.587860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacDonald RL, Kelly KM. Antiepileptic drug mechanisms of action. Epilepsia. 1995;36(Supplemental 2):S2–12. doi: 10.1111/j.1528-1157.1995.tb05996.x. [DOI] [PubMed] [Google Scholar]
- 4.Allison JH, Stewart MA. Reduced brain inositol in lithium-treated rats. Nat New Biol. 1971;233(43):267–268. doi: 10.1038/newbio233267a0. [DOI] [PubMed] [Google Scholar]
- 5.Vadnal RE, Bazan NG. Carbamazepine inhibits electroconvulsive shock-induced inositol triphosphate (IP3) accumulation in rat cerebral cortex and hippocampus. Biochem Biophys Res Commun. 1988;153(1):129–134. doi: 10.1016/s0006-291x(88)81198-7. [DOI] [PubMed] [Google Scholar]
- 6.Williams RSB, Cheng L, Mudge AW, Harwood AJ. A common mechanism of action for three mood-stabilizing drugs. Nature. 2002;417(6886):292–295. doi: 10.1038/417292a. [DOI] [PubMed] [Google Scholar]
- 7.McCormack M, Alfirevic A, Bourgeois S, Farrell JJ, Kasperaviciute D, Carrington M, Sills GJ, Marson T, Jia X, Eng M, deBakker PIW, Chinthapalli K, Molokhia M, Johnson MR, Phil D, O’Connor GD, Chaila E, Alhusaini S, Shianna KV, Radtke RA, Heinzen EL, Walley N, Pandolfo M, Pichler W, Park BK, Depondt C, Sisodiya SM, Goldstein DB, eloukas P, Delanty N, Cavalleri GL, Pirmohamed M. HLA-A*3101 and carbamazepine-induced hypersensitivity reactions in Europeans. N Engl J Med. 2011;364:1134–1143. doi: 10.1056/NEJMoa1013297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen P, Lin J-J, Lu C-S, Ong C-T, Hsieh PF, Yang C-C, Tai C-T, Wu S-L, Lu C-H, Hsu Y-C, Yu H-Y, Ro L-S, Lu C-T, Chu C-C, Tsai J-J, Su Y-H, Lan S-H, Sung S-F, Lin S-Y, Chuang H-P, Huang L-C, Chen Y-J, Tsai P-J, Liao H-T, Lin Y-H, Chen C-H, Chung W-H, Hung S-I, Wu J-Y, Chang C-F, Chen L, Chen Y-T, Shen C-Y. Carbamazepine-induced toxic effects and HLA-B*1502 screening in Taiwan. N Engl J Med. 2011;364:1126–1133. doi: 10.1056/NEJMoa1009717. [DOI] [PubMed] [Google Scholar]
- 9.Seglen PO, Gordon PB. 3-Methyladenin: specific inhibitor of autophagic/lysosomal protein degradation in isolated rat hepatocytes. Proc Natl Sci Acad USA. 1982;79(6):1889–1892. doi: 10.1073/pnas.79.6.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lindmo K, Stenmark H. Regulation of membrane traffic by phosphoinositide 3-kinases. J Cell Sci. 2006;119(Pt 4):605–614. doi: 10.1242/jcs.02855. [DOI] [PubMed] [Google Scholar]
- 11.Nakatogawa H, Suzuki K, Kamada Y, Ohsumi Y. Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nat Rev Mol Cell Biol. 2009;10(7):458–467. doi: 10.1038/nrm2708. [DOI] [PubMed] [Google Scholar]
- 12.Szalai G, Krishnamurthy R, Hacnóczky G. Apoptosis driven by IP3-linked mitochondrial calcium signals. EMBO J. 1999;18(22):6349–6361. doi: 10.1093/emboj/18.22.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moretti I, Cha YI, Niermann, Lu B. Switch between apoptosis and autophagy: radiation-induced endoplasm reticulum stress? Cell Cycle. 2007;6(7):793–798. doi: 10.4161/cc.6.7.4036. [DOI] [PubMed] [Google Scholar]
- 14.Li Z-H, Zlabek V, Velisek J, Grabic R, Machova J, Kolarova J, Li P, Randak T. Acute toxicity of carbamazepine to juvenile rainbow trout (Oncorhynchus mykiss): Effects on antioxidant responses, hematological parameters and hepatic EROD. Exotoxicol Environ Saf. 2011;74(3):319–327. doi: 10.1016/j.ecoenv.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Underwood BR, Imarisio S, Fleming A, Rose C, Krishna G, Heard P, Quick M, Korolchuk VI, Renna M, Sarkar S, Garcia-Arencibia M, O’Kane CJ, Murphy MP, Rubinsztein DC. Antioxidants can inhibit basal autophagy and enhance neurodegeneration in models of polyglutamine disease. Hum Mol Genet. 2010;19(17):3413–3429. doi: 10.1093/hmg/ddq253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Epperly MW, Wang H, Jones JA, Dixon T, Montesinos CA, Greenberger JS. Antioxidant-chemoprevention diet ameliorates late effects of total-body irradiation and supplements radioprotection by MnSOD-plasmid liposome administration. Rad Res. 2011;175(6):759–765. doi: 10.1667/RR2398.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brahma MK, Dohare P, Varma S, Rath SK, Garg P, Biswal PK, Chowdhury PD, Madhur R. The neuronal apoptotic death in global cerebral ischemia in gerbil: important role for sodium channel modulator. J Neurosci Res. 2009;87(6):1400–1411. doi: 10.1002/jnr.21960. [DOI] [PubMed] [Google Scholar]
- 18.Mihara M, Erster S, Zaika A, Petrenko O, Chittenden T, Pancoska P, Moll UM. p53 has a direct apoptogenic role at the mitochondria. Mol Cell. 2003;11(3):577–590. doi: 10.1016/s1097-2765(03)00050-9. [DOI] [PubMed] [Google Scholar]
- 19.Crighton D, Wilkinson S, O’Prey J, Syed N, Smith P, Harrison PR, Gasco M, Garrone O, Crook T, Ryan KM. DRAM, a p53-induced modulator of autophagy, is critical for apoptosis. Cell. 2006;126(1):121–134. doi: 10.1016/j.cell.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 20.Paglin S, Hollister T, Delohery T, Hackett N, McMahill M, Sphicas E, Domingo D, Yahalom J. A novel response of cancer cells to radiation involves autophagy and formation of acidic vesicles. Cancer Res. 2001;61(2):439–444. [PubMed] [Google Scholar]
- 21.Ralph P, Moore MAS, Nilsson K. Lysozyme synthesis by established human and murine histiocytic lymphoma cell lines. J Exp Med. 1976;143(6):1528–1533. doi: 10.1084/jem.143.6.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenberger JS, Eckner RJ, Sakakeeny M, Reid D, Nabel G, Hapel A, Ihle JN, Humphries KG. Interleukin 3-dependent hematopoietic progenitor cell lines. Fed Proc. 1983;42(10):2762–2771. [PubMed] [Google Scholar]
- 23.Epperly MW, Bray JA, Carlos TM, Prochownik E, Greenberger JS. Biology of marrow stromal cell lines derived from long-term bone marrow cultures of Trp53-deficient mice. Radiat Res. 1999;152(1):29–40. [PubMed] [Google Scholar]
- 24.Greenberger JS, Epperly MW, Zeevi A, Brunson KW, Goltry KL, Pogue-Geile KL, Bray J, Berry LA. Stromal cell involvement in leukemogenesis and carcinogenesis. In Vivo. 1996;10(1):1–17. [PubMed] [Google Scholar]
- 25.Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, Ohsumi Y, Tokuhisa T, Mizushima N. The role of autophagy during the early neonatal starvation period. Nature. 2004;432(7020):1032–1036. doi: 10.1038/nature03029. [DOI] [PubMed] [Google Scholar]
- 26.Rosenstein M, Epperly MW, Hughey R, Prezioso J, Greenberger JS. Overexpression of the gamma glutamyltranspeptidase transgene does not alter the gamma irradiation sensitivity of the IB3-1 normal bronchoepithelial or A549 human lung carcinoma cell line. Rad Oncol Invest: Clin Basic Res. 1995;3(1):9–16. [Google Scholar]
- 27.Santucci MA, FitzGerald TJ, Harigaya K, Woda B, Sakakeeny MA, Anklesaria P, Kase K, Holland CA, Greenberger JS. Gamma-irradiation response of co-cultivated bone marrow stromal cell lines of differing intrinsic radiosensitivity. Int J Radiat Oncol Biol Phys. 1990;18:1083–1092. doi: 10.1016/0360-3016(90)90444-o. [DOI] [PubMed] [Google Scholar]
- 28.Goff JP, Shields DS, Greenberger JS. The influence of cytokines on the growth kinetics and immunophenotype of daughter cells resulting from the first division of single CD34+thy-1+lin− cells. Blood. 1998;92(11):4098–4107. [PubMed] [Google Scholar]
- 29.Rwigema J-CM, Beck B, Wang W, Doemling A, Epperly MW, Shields D, Franicola D, Dixon T, Frantz M-C, Wipf P, Tyurina Y, Kagan VE, Wang H, Greenberger JS. Two strategies for the development of mitochondrial-targeted small molecule radiation damage mitigators. Int J Radiat Oncol Biol Phys. 2011;80(3):860–868. doi: 10.1016/j.ijrobp.2011.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.FitzGerald TJ, Rothstein L, Kase K, Greenberger JS. Radiosensitivity of human bone marrow granulocyte-macrophage progenitor cells: effect of dose rate on purified target cell populations. Radiat Res. 1986;107:205–215. [PubMed] [Google Scholar]
- 31.Hall EJ, Giaccia AJ. Radiobiology for the Radiologist. 6. Lippincott Williams & Wilkins; New York: 2006. [Google Scholar]
- 32.Liu B, Cheng Y, Liu Q, Bao J-K, Yang J-M. Autophagic pathways as new targets for cancer drug development. Acta Pharmacol Sin. 2010;31(9):1154–1164. doi: 10.1038/aps.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toker A, Cantley LC. Signaling through the lipid products of phosphoinositide-3-OH kinase. Nature. 1997;387(6634):673–676. doi: 10.1038/42648. [DOI] [PubMed] [Google Scholar]
- 34.Norberg E, Gogvadze V, Ott M, Horn M, Uhlen P, Orrenius S, Zhivolovsky B. An increase in intracellular Ca2+ is required for the activation of mitochondrial calpain to release AIF during cell death. Cell Death Differ. 2008;15(12):1857–1864. doi: 10.1038/cdd.2008.123. [DOI] [PubMed] [Google Scholar]
- 35.Brizel DM. Pharmacologic approaches to radiation protection. J Clin Oncol. 2007;25(26):4084–4089. doi: 10.1200/JCO.2007.11.5816. [DOI] [PubMed] [Google Scholar]
- 36.Stone HB, Moulder JE, Coleman CN, Ang KK, Anscher MS, Barcellos-Hoff MH, Dynan WS, Fike JR, Grdina DJ, Greenberger JS, Hauer-Jensen M, Hill RP, Kolesnick RN, MacVittie TJ, Marks C, McBride WH, Metting N, Pellmar T, Purucker M, Robbins ME, Schiestl RH, Seed TM, Tomaszewski JE, Travis EL, Wallner PE, Wolpert M, Zaharevitz D. Models for evaluating agents intended for the prophylaxis, mitigation, and treatment of radiation injuries. Report of an NCI workshop December 3–4, 2003. Radiat Res. 2004;162(6):711–728. doi: 10.1667/rr3276. [DOI] [PubMed] [Google Scholar]
- 37.Veldeman L, Madani I, Hulstaert F, DeMeerleer G, Mareel M, DeNeve W. Evidence behind use of intensity-modulate radiotherapy: a system review of comparative clinical studies. Lancet Oncol. 2008;9(4):367–375. doi: 10.1016/S1470-2045(08)70098-6. [DOI] [PubMed] [Google Scholar]
- 38.Minniti G, Clarke E, Lanzetta G, Osti MF, Trasimeni G, Bozzao A, Romano A, Enrici RM. Stereotactic radiosurgery for brain metastases: analysis of outcome and risk of brain radionecrosis. Radiat Oncol. 2011;15(6):48. doi: 10.1186/1748-717X-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beriwal S, Kim H, Heron DE, Selvaraj R. Comparison of 2D vs. 3D dosimetry for Rotte Y applicator high-dose rate brachytherapy for medically inoperable endometrial cancer. Technol Cancer Res Treat. 2006;5(5):521–527. doi: 10.1177/153303460600500509. [DOI] [PubMed] [Google Scholar]
- 40.Harwood AJ. Lithium and bipolar mood disorder: the inositol-depletion hypothesis revisited. Mol Psychiatry. 2005;10(1):117–126. doi: 10.1038/sj.mp.4001618. [DOI] [PubMed] [Google Scholar]
- 41.Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007;26(7):1749–1760. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee G, Huang Y, Washington JM, Briggs NW. Carbamazepine enhances the activity of glutamate transporter type 3 via phosphatidylinositol 3-kinase. Epilepsy Res. 2005;66(1–3):145–153. doi: 10.1016/j.eplepsyres.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 43.Sanchez MC, Nelson GA, Green LM. Effects of protons and HZE particles on glutamate transport in astrocytes, neurons, and mixed cultures. Radiat Res. 2010;174(6):669–678. doi: 10.1667/RR2106.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller S, Tavshanjian B, Oleksy A, Perisic O, Houseman BT, Shokat KM, Williams RL. Shaping development of autophagy inhibitors with the structure of the lipid kinase Vps34. Science. 2010;327(5973):1638–1642. doi: 10.1126/science.1184429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maiuri MC, Toumelin GL, Criollo A, Rain J-C, Gautier F, Juin P, Tasdemir E, Pierron G, Troulinaki K, Tavernarakis N, Hickman JA, Geneste O, Kroemer G. Functional and physical interation between Bcl-XL and a BH3-like domain in Beclin-1. EMBO J. 2007;26(10):2527–2539. doi: 10.1038/sj.emboj.7601689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malik SA, Orhon I, Morselli E, Criollo A, Shen S, Marino G, Younes AB, Benit P, Rustin P, Maiuri MC, Kroemer G. BH3 mimetics activate multiple pro-autophagic pathways. Oncogene. 2011;30:3918–3929. doi: 10.1038/onc.2011.104. [DOI] [PubMed] [Google Scholar]
- 47.Flickinger JC, Jr, Kim H, Kano H, Greenberger JS, Lunsford LD, Kondziolka D, Flickinger JC., Sr Do carbamazepine, gabapentin, or other anticonvulsants exert sufficient radioprotective effects to alter responses to trigeminal neuralgia radiosurgery? Int J Radiat Oncol Biol Phys. doi: 10.1016/j.ijrobp.2012.01.016. (In Press) [DOI] [PubMed] [Google Scholar]
- 48.Greenberger JS, Clump D, Kagan V, Bayir H, Lazo J, Wipf P, Li S, Gao X, Epperly MW. Strategies for discovery of small molecule radiation protectors and radiation mitigators. Front Oncol. 2011;1(59):1–12. doi: 10.3389/fonc.2011.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]