Abstract
Fertilized mouse zygotes can reprogram somatic cells to a pluripotent state. Human zygotes might therefore be useful for producing patient-derived pluripotent stem cells. However, logistical, legal and social considerations have limited the availability of human eggs for research. Here we show that a significant number of normal fertilized eggs (zygotes) can be obtained for reprogramming studies. Using these zygotes, we found that when the zygotic genome was replaced with that of a somatic cell, development progressed normally throughout the cleavage stages, but then arrested before the morula stage. This arrest was associated with a failure to activate transcription in the transferred somatic genome. In contrast to human zygotes, mouse zygotes reprogrammed the somatic cell genome to a pluripotent state within hours after transfer. Our results suggest that there is a previously unappreciated barrier to successful human nuclear transfer, and that future studies should focus on the requirements for genome activation.
Keywords: zygote, reprogramming, mitosis, zygotic genome activation
Introduction
The generation of animals by nuclear transfer1,2 and the derivation of human embryonic stem cells3, suggested that these two approaches might be combined to generate patient-specific embryonic stem lines. Because they would carry the patient's genotype, such stem cells might be useful for the production of autologous transplants4,5 and for disease modeling6. Studies in mice have demonstrated the potential value of these combined approaches for treating severe combined immune deficiency and Parkinson's disease7,8.
Although it is now possible to produce pluripotent stem cell lines with patient genotypes using defined reprogramming factors9,10, the equivalency of these cells to embryonic stem cells has been questioned. Several groups have found differences in gene expression, DNA methylation and differentiation propensity between iPS cells and ES cells11-15. Further, it has been suggested that induced pluripotent stem cells are not reprogrammed to the same extent that is observed in embryonic stem cells following nuclear transfer16. Therefore, it remains important to pursue human nuclear transfer as an alternative approach for producing stem cell lines.
However, despite several attempts at human nuclear transfer17-24, these efforts have uniformly failed to produce stem cell lines. Instead, most studies reported developmental arrest during the cleavage stages. Consistent with this, the only stem cell line purportedly derived by human nuclear transfer25 has subsequently been shown to originate from parthenogenesis rather than reprogramming of a somatic nucleus26.
We have recently generated mouse embryonic stem cells by nuclear transfer into mitotic zygotes27, demonstrating that these cell-types might also be a useful supplement to oocytes for human studies. Here we report programs for the acquisition of human preimplantation embryos, resulting in the donation of a significant number of human zygotes. When we performed nuclear transfer, development proceeded through the cleavage stages but arrested around the time of compaction. Our investigation into the causes of this blockade revealed that this developmental arrest is the result of a failure to undergo transcriptional activation of the transferred somatic chromosomes. Thus, there is a barrier to reprogramming following human nuclear transfer that must be overcome before patient-specific embryonic stem cell lines can be derived.
Results
Donation of human zygotes
Because we have had difficulty in accessing sufficient numbers of unfertilized oocytes to proceed with nuclear transfer studies24, we considered the possibility that human zygotes could serve as a source of recipient cytoplasm for nuclear transfer. We therefore initiated a program for recruitment of couples willing to donate their frozen zygotes for research. These protocols were approved by the participating academic institutions’ committees on the use of human subjects in research, stem cell research oversight committees and by the Western Institutional Review Board, an Associate of the Accreditation of Human Research Protection Programs. Between January 2007 and May 2010, 4061 IVF embryos were donated by couples undergoing assisted reproduction. Though the majority of these were cryopreserved at either the cleavage- or blastocyst-stages, 461 (11.3%) were frozen at the 1 cell-stage and therefore useful for nuclear transfer (Fig.1a). These human zygotes were in excess of clinical need, but otherwise normal.
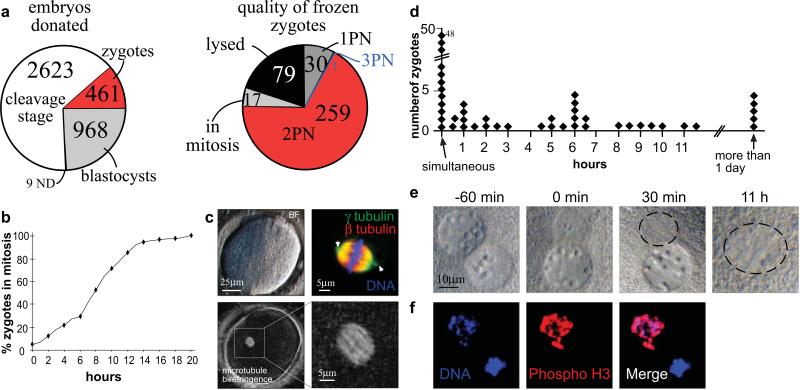
Figure 1. Donation of human zygotes for stem cell research.
a, embryos donated for stem cell research. PN= pronucleus, ND= not determined b, Mitotic entry time of human zygotes (n=107) in hours after thaw. Nuclear envelope breakdown of at least one of the two pronuclei was defined as the time point of mitotic entry. Some zygotes were mitotic at the time of thaw. c, mitotic spindle formation. Human zygote 30 minutes after nuclear envelope breakdown including brightfield image, microtubule immunohistochemistry (arrowheads point to the centrosome at both poles of the spindle) and spindle birefringence. d-f, asynchrony in nuclear envelope breakdown. d, Hours between the breakdown of the first pronuclear envelope and the second. e, Zygote with asynchronous pronuclear envelope breakdown. Numbers indicate the time from the breakdown of the first nuclear envelope. The location of mitotic chromatin is circled. f, 1PN zygote stained for phosphorylation of Ser 27 on Histone H3, a marker of mitosis.
Nuclear transfer into mitotic human zygotes
For successful reprogramming, we had previously cultured interphase mouse zygotes until they entered the first mitosis and only then carried out nuclear transfer27. To investigate the reprogramming potential of human zygotes, we thawed 386 frozen human zygotes of which 307 (80%) were viable. Of these, 259 (84.4%) contained one maternal and one paternal pronucleus, as is typical for the first interphase in a normal, diploid, zygote (Fig.1a). These zygotes entered mitosis within 0-12 hours after thaw (Fig.1b), assembled a bipolar spindle (Fig.1c), and of 28 control zygotes, 9 (32%) developed to the blastocyst stage (Supplementary Table S1). To better understand why not all zygotes developed to the blastocyst stage, and to exclude zygotes with low developmental potential from use in nuclear transfer experiments, we analyzed the first mitosis in more detail. Another group has previously reported that progression through the first mitosis can accurately predict developmental potential28. Closer inspection of zygotes entering into mitosis revealed asynchrony of nuclear envelope breakdown. 34/82 zygotes (41%) did not simultaneously break down their two pronuclei, but instead did so with a delay of 30min to up to 12 hours (Fig. 1d,e). When 1PN (pronuclear) zygotes were stained for phosphorylation of Histone H3, a mitosis-specific modification of chromatin imposed by AuroraB kinase, we observed extensive phosphorylation on chromosomes of the pronucleus that had broken down, but not on chromatin of the pronucleus that had retained a nuclear envelope (3 of 3 zygotes) (Fig.1f). In addition, we observed a variety of other idiosyncrasies in human zygotes entering into mitosis. These included failure to condense the chromosomes (Supplementary Figure S1a), a failure to completely dissolve nucleoli (Supplementary Figure S1b) and a failure to assemble a mitotic spindle (Supplementary Figure S1c). Zygotes exhibiting these abnormalities either arrested as single cells or eventually underwent fragmentation, and were excluded from use in nuclear transfer.
The underlying cause of abnormalities we observed in frozen zygotes was not entirely clear. There was substantial variation in the age of frozen zygotes donated to us, with the oldest having been cryopreserved for as many as 20 years (Supplementary Figure S2). We do not favor the idea that these problems are a direct side effect of the freeze-thaw process, as 3/3 human zygotes frozen in mitosis were able to assemble a spindle within 30min of thawing (Supplementary Figure S3) and because mouse zygotes frozen and thawed entered mitosis normally and could be used for nuclear transfer (Supplementary Table S2).
In the context of early nuclear transfer experiments, which controlled for micromanipulation of the human zygote, we found that the mitotic spindle could not properly direct cytokinesis if it's position or orientation was disturbed (Supplementary Figure S4-S6). We therefore devised a nuclear transfer strategy for human zygotes that was analogous to nuclear transfer into oocytes, thus overcoming this difficulty (Fig. 2a).
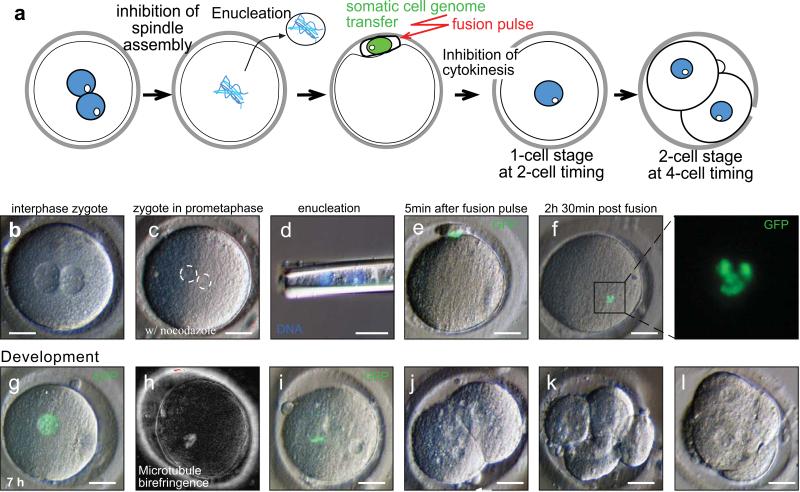
Figure 2. Somatic cell nuclear transfer into human zygotes.
a, Schematic of nuclear transfer into human zygotes. b, zygote at 2PN stage in the presence of nocodazole. Size bar applies to all panels except where otherwise indicated. c, zygote at mitosis in the presence of nocodazole. Maternal and paternal haploid genomes are circled. d, paternal and maternal haploid genomes removed from the zygote. e, Somatic cell nuclear transfer (green nucleus marked by H2B:GFP) by fusion, f, chromosome condensation, g, and after exit from mitosis. Time post exit from mitosis is indicated. h,spindle assembly i, chromosomes aligned at metaphase. j, cleavage, k, 6-cell stage, l, morula-like, on d4 post fertilization. Size bar: 25μm.
In this novel nuclear transfer strategy, 69 human interphase zygotes were placed into medium containing the microtubule-depolymerizing drug nocodazole (Fig.2b). In all cases, these human zygotes proceeded into the first mitosis (Fig.2c), but then as intended, arrested due to inhibition of spindle assembly. This arrest in mitosis induced by nocodazole was stable but reversible and nontoxic (Supplementary Figure S7), allowing us to perform micromanipulation. Once mitotic entry occurred, we removed the zygotic chromosomes (Fig.2d), and introduced interphase somatic donor nuclei (Fig.2e). Skin cells of an adult healthy subject, or an adult type 1 diabetic subject served as nuclear donors.
Because nuclear remodeling correlates with reprogramming in the rhesus monkey29, we monitored the transferred somatic chromatin hourly. We checked 46 of the 53 human zygotes (53/69, 77%) that survived these manipulations and found that 43 had undergone nuclear envelope breakdown and chromosome condensation within 6 hours after transfer (Fig.2f, Supplementary Figure S8). Therefore, we concluded that early nuclear remodeling was generally successful. As intended, the unreplicated donor chromosomes could not satisfy the spindle checkpoint and therefore these zygotes remained in mitosis (Supplementary Figure S9). To induce mitotic exit, while inhibiting abnormal cytokinesis, we incubated zygotes in the cyclin-dependent protein kinase inhibitor purvalanol A, and the kinase inhibitor, 6-DMAP. Importantly, nocodazole, purvalanol A, and 6-DMAP are compatible with preimplantation development after nuclear transfer in mammalian species including the rhesus monkey30-33, suggesting that little if any toxicity would be expected when they are used. Within 4 hours of drug treatment, 53/53 zygotes exited mitosis and entered the subsequent interphase (Fig.2g). Zygotes were then monitored for entry into the subsequent mitosis, and within 30 hours, 33/53 (62%) completed the second cell-cycle. Upon mitotic entry, 33/33 assembled a spindle (Fig. 2h,i), proceeded through chromosome segregation, subsequent rounds of cleavage division (Fig. 2j,k), and two initiated compaction (Fig.2l). However, we never observed further development to the blastocyst stage (n=0/33).
Genome activation fails after transfer into human zygotes
Because we observed that all cleaving nuclear transfer cells generated using normal human zygotes (33/33) arrested at a similar developmental stage (Fig. 3a), we wondered whether there was a fundamental blockade to their development. Due to two observations we considered the hypothesis that a failure to activate transcription was inhibiting development after nuclear transfer. Our first observation was that green fluorescence from the H2B-GFP protein transferred with the transgenic donor chromosomes was detectable at the first interphase. However, fluorescence intensity decreased with every cleavage and never returned (33/33 nuclear transfer specimen observed) (Fig. 3b). This observation suggested that even the H2B-GFP coding sequence under control of the strong CAAGS promoter was never again transcribed. In contrast, a similar transgene was routinely activated after mouse nuclear transfer31 (Fig.3c). Second, we found that development after nuclear transfer arrested at or shortly after the stage when major transcriptional activation in the embryo normally occurs34,35. The steep increase in transcriptional activity in the normal embryo on day3 after fertilization, or after the 4-cell stage, was also termed zygotic genome activation (ZGA).
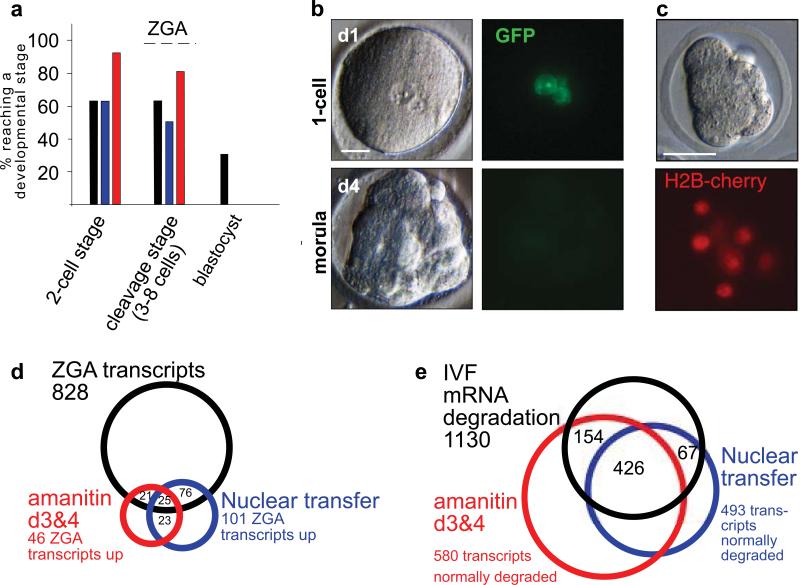
Figure 3. Developmental potential and ZGA after nuclear transfer into human zygotes.
a, Developmental potential of control IVF human zygotes (black columns, n=28), zygotes after nuclear transfer (blue columns, n=53), and zygotes cultured in the presence of alpha-amanitin (red columns, n=23) displayed as the percentage of cells developing to and beyond the indicated developmental stage. b, GFP expression after human nuclear transfer. c, Transgene reactivation after mouse nuclear transfer. Shown are nuclear transfer cells 2 days after transfer into mouse zygotes. d, Genome activation after human SCNT. Venn diagrams of genes upregulated >5-fold in the indicated groups, e, maternal mRNA degradation after human SCNT. Venn diagram of genes with transcript levels reduced by a factor of 5 or more in the indicated groups. NT=nuclear transfer, ZGA=zygotic genome activation. Size bar: 25μm.
We next asked whether there was a more global deficit in transcription following human nuclear transfer. To this end, we isolated RNAs from nuclear transfer and control embryos at equivalent developmental stages (Supplementary Figure S10a) then analyzed their transcriptional profiles using microarrays. To understand the normal changes in transcription that occur during early stages of human cleavage development, we compared the profiles of unmanipulated 1-cell zygotes collected on day 1 post IVF, to blastomeres of 6 to 8-cell embryos collected on day 3 (approx. 72-84h post IVF) and day 4 of development (approx. 90-100h post IVF). Relative to the maternal RNAs present in fertilized zygotes (n=2 embryos), we found that 828 transcripts were significantly elevated (> 5-fold, p<0.01, see Methods for statistical analysis) in all IVF samples (n=19 embryos, 4 biological replicates). This result suggests there is substantial transcriptional activity already occurring at the 6 to 8-cell stage, as has previously been reported 35. In stark contrast, when the profiles of developmentally advanced nuclear transfer samples were examined (n=2, 2 independent biological replicates, 6-8 cell stage), only 101 (12%) of these 828 transcripts were increased in their abundance relative to the 1-cell zygote (Fig. 3d) (>5-fold, p<0.01 for both samples).
As a control to ensure that we could accurately monitor transcriptional changes in nuclear transfer blastomeres, we also monitored the decay of transcripts. At the cleavage stage, we observed that 1130 maternal transcripts were subject to decay in all four control samples collected on day 3 and day 4 after IVF. The levels of each of these transcripts declined by a factor of 5 or more during normal development from the 1-cell stage to the 6 to 8-cell stage (<0.2-fold, p<0.01). Consistent with normal decay of a substantial fraction, but not all maternal RNAs, we found that 493 (44%) of these transcripts were also reduced at the cleavage stage following nuclear transfer (<0.2-fold, p<0.01) (Fig.3e).
To examine whether a somatic rather than an embryonic gene expression program was active after nuclear transfer, we defined a set of 2153 transcripts that are transcribed at higher levels in the somatic donor cells than in human zygotes (>10-fold, p<0.001 for two somatic cell lines). Of the 2153 transcripts, 85 (4%) were elevated in nuclear transfer samples (>5-fold higher levels than in zygotes, p<0.01 for 2 samples), while 350 (16%) were elevated in control IVF embryos (>5-fold higher levels than in zygotes, p<0.01 for 4 samples). Expression of these 350 transcripts after nuclear transfer would not require reprogramming, as they are already active in the somatic donor cell. Nevertheless, only 50 of them (14%) were elevated after nuclear transfer. This indicates that neither an embryonic nor a somatic transcriptional program was being expressed, and even genes that do not require reprogramming failed to be normally expressed after nuclear transfer.
Inhibition of transcription induces cleavage stage arrest
Our transcriptional analyses demonstrated that there was an unexpected failure to activate transcription at the appropriate time following human nuclear transfer. These results raise the possibility that development might arrest when the RNAs provided by the maternal stores are no longer sufficient to promote development. If this hypothesis is correct, then culture of normal fertilized zygotes in the transcriptional inhibitor alpha-amanitin should be sufficient to induce a developmental arrest identical to that observed following nuclear transfer. To test this idea, we cultured 23 zygotes for 5 days in the presence of the transcriptional inhibitor alpha-amanitin (Fig.3a). We observed that 22/23 of these zygotes cleaved and 10/23 developed to the 6 to 8-cell stage (Supplementary Table S1). However no further development to the blastocyst stage occurred. In these arrested blastomeres, the number of transcripts with elevated levels was very similar to nuclear transfer samples: only 46 of the 828 transcripts (5.5%, p<0.01) normally rising in abundance over the first three days of development were increased >5 fold over the same time frame in alpha-amanitin treated samples (n=2 specimen, 2 biological replicates)(Fig.3d). This result also suggests that as many of the 101 ZGA transcripts elevated in nuclear transfer blastomeres may be attributed to differential mRNA stability rather than active transcription. As observed after nuclear transfer, treatment with alpha-amanitin did not completely interfere with the degradation of maternal RNAs. We found that 590 of 1130 (47%, <0.2-fold, p<0.01) transcripts that normally declined during cleavage also fell after alpha-amanitin treatment (samples collected on days 3 and 4 after IVF) (Fig.3e). Therefore, pharmacological inhibition of transcription was sufficient to induce changes in mRNA levels and a developmental arrest identical to that observed after human nuclear transfer.
Reprogramming within hours after transfer into mouse zygotes
Although our experiments thus far suggest that there is a blockade to reprogramming using human zygotes that is not present when mouse zygotes are used, our human nuclear transfer protocol was technically distinct from the approach we had previously used for nuclear transfer using mouse zygotes27. To rule out the possibility that technical aspects of our human nuclear transfer procedure were preventing reprogramming, we used methods that we developed here for human nuclear transfer and asked whether it could support efficient development and reprogramming following nuclear transfer in mouse zygotes. To monitor the state of reprogramming, we used tail tip fibroblasts carrying an Oct4-GFP transgene 36. In striking contrast to our human experiments, we observed efficient development to the morula and the blastocyst stages (significance of difference mouse-human: χ2=0.002, Chi-square test) (Fig. 4a, Supplementary Table S3). We also found that within less than 36h, GFP became activated from the somatic chromosomes, suggesting that reprogramming of the Oct4 promoter had occurred and transcription initiated (Fig. 4a). This reprogramming following nuclear transfer was far more rapid than observed following induction of pluripotency in mouse fibroblasts using defined transcription factors37,38 (Fig. 4b). We also controlled for the effects of cryopreservation by performing nuclear transfer into frozen-thawed mouse zygotes. These zygotes gave rise to blastocysts after nuclear transfer (Supplementary Table S2). Thus, we can conclude that even when precisely the same nuclear transfer methods are used, mouse zygotes supported reprogramming, while human zygotes could not.
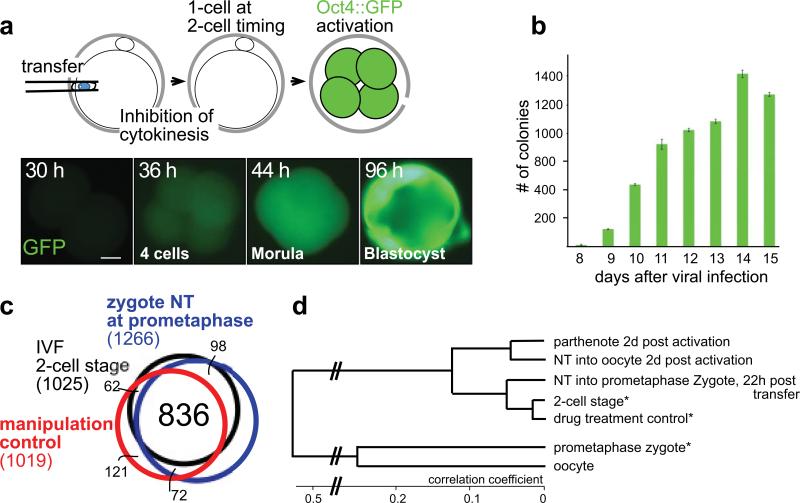
Figure 4. Transcriptional reprogramming within hours after mouse nuclear transfer.
a, Oct4::GFP reprogramming after somatic cell nuclear transfer into mouse zygotes. b, Number of Oct4-GFP+ colonies during mouse iPS generation. Kinetics of Oct4::GFP reactivation in somatic cells after transduction with retroviruses carrying the reprogramming factors Oct4, Sox2, Klf4 and c-Myc. Day 8 is the 8th day after the first exposure of somatic cells to viral vectors. c,d, ZGA and reprogramming 22h after nuclear transfer into mouse zygotes. c, Venn diagram of transcripts elevated at the 2-cell stage. d, cluster diagram of the global gene expression profile after nuclear transfer into oocytes or zygotes. * from ref. 31. Size bar 10μm.
To more broadly determine whether transcriptional initiation was occurring normally after mouse nuclear transfer into zygotes, we performed transcriptional profiling. In contrast to the situation in human development, where ZGA occurs at the 4-8 cell stage35, in mouse, ZGA occurs at the 2-cell stage39. Surprisingly, we found that transcriptional reprogramming was essentially complete by the end of first the cell cycle, or 22-24 hours after nuclear transfer. 934/1025 (91%) of transcripts that were upregulated between control mouse zygotes and the 2-cell stage, were also upregulated after nuclear transfer (>5x, P<0.01) (Fig. 4c). Chemically mock-treated control zygotes upregulated a similar number of transcripts (898/1025, 88%). Remarkably, of 179 transcripts that were upregulated at the 2-cell stage relative to the zygote (>5-fold, P<0.01) and that were not expressed in tail tip fibroblasts, 151 were also upregulated after nuclear transfer, and 154 in mock-treated controls. This level of reprogramming was identical to that observed after nuclear transfer into mouse oocytes; the transcriptome of nuclear transfer embryos generated with zygotes clustered closely with unmanipulated 2-cell embryos, and nuclear transfer embryos generated with oocytes clustered closely with parthenotes (Fig. 4d).
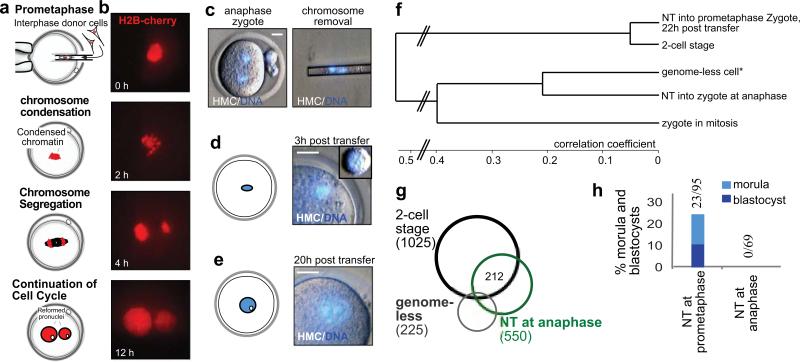
To better understand the mechanism of reprogramming in mouse zygotes, we transferred somatic cells at various time points of mitosis. When somatic nuclei were transferred at prometaphase, chromosome condensation occurred within 2 hours post transfer (Fig. 5a,b). In contrast, when nuclei were transferred at anaphase of mitosis, chromosome condensation did not occur and nuclear remodeling required 20 or more hours (Fig. 5c-e). Reprogramming and development after nuclear transfer into mouse zygotes was strictly dependent on nuclear remodeling by chromosome condensation. The transcriptome of zygotes transferred at anaphase clustered most closely with genome-less embryos, (Fig. 5f). Only 212/1025 (20.7%) ZGA genes were normally expressed after nuclear transfer at anaphase (Fig. 5g), and of 179 ZGA genes silent in the somatic donor cell, only 23 (12.8%) were normally upregulated (Supplementary Figure S11a). Furthermore, all embryos arrested at the 2-cell stage when interphase nuclei were transferred (Fig. 5h, Supplementary Table S3). This observation raised the question whether a failure to condense somatic chromatin could be responsible for the transcriptional and developmental phenotype after nuclear transfer into human zygotes. However, this was not the case, as we found that 40/46 human zygotes underwent nuclear envelope breakdown and chromosome condensation within 3 hours after transfer (Supplementary Figure S8).
Figure 5. Chromosome condensation is required for development and reprogramming after nuclear transfer into mouse zygotes.
a, schematic for nuclear transfer into prometaphase and b, corresponding images. Nuclei of fibroblasts at interphase are transferred into a zygote at prometaphase of mitosis. Chromosomes are marked with the red fluorescent fusion protein H2B-cherry. Shown is the mitotic progression after nuclear transfer. Time indicates the hours after nuclear transfer. Note the condensation of chromosomes and their separation into two groups, followed by formation of 2 pronuclei after inhibition of cytokinesis and entry into interphase. c, removal of the genome from a zygote at anaphase. d, Nuclear morphology after transfer of interphase nuclei. Small inset: a somatic donor cell before transfer. Note that somatic donor chromatin is not condensed. e, nuclear remodeling is slow and requires about 1 day to restructure the nucleus into a large blastomere-like morphology. f, cluster analysis of gene expression after mouse SCNT. g, Venn diagram of transcripts elevated 22-24h after nuclear transfer at anaphase. h, development to morula and blastocyst stage (as % of transferred). Size bar 10μm.
Abnormal karyotypes do not cause transcriptional failure
It has been suggested that mitotic abnormalities after primate nuclear transfer 40 cause karyotypic aberrations that contribute to developmental arrest. We therefore used fluorescence in situ hybridization to investigate whether similar abnormalities occurred after human nuclear transfer and whether they might induce the transcriptional failures we observed. Although some chromosome abnormalities were observed, (Supplementary Figure S12), abnormalities were also found in IVF blastomeres (Supplementary Table S4), many of which continue development to the morula and blastocyst stage.
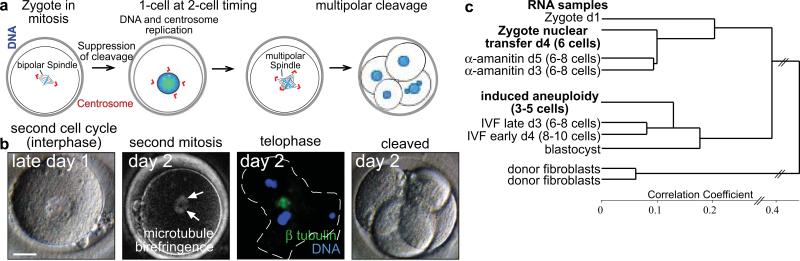
To directly test whether or not karyotypic abnormalities could be causing transcriptional failures, we intentionally induced aneuploidy in otherwise normal fertilized controls, then monitored their transcriptional activity. To induce karyotypic abnormalities, we suppressed the first cleavage division, thus generating tetraploid cells with supernumerary centrosomes (Fig. 6a). These cells formed multipolar spindles at the next mitosis and directly cleaved into either 3 or 4 cells instead of 2 (Fig.6b, Supplementary Table S5). As a consequence of the asymmetric segregation of chromosomes, the resulting blastomeres would be expected to have abnormal karyotypes. Despite their presumably abnormal karyotypes, we found that these blastomeres (n=2, 1 replicate) initiated transcription (489/828 ZGA genes or 59% were upregulated at least 5 fold, p<0.001) (Supplementary Figure S13), and therefore their transcriptional profiles clustered closely together with normal IVF samples, and not with the nuclear transfer samples (Fig.6c). Therefore, karyotypic abnormalities are not sufficient to explain the transcriptional failures we have observed following human nuclear transfer. Furthermore, as these zygotes were treated with nocodazole and the kinase inhibitors 6-DMAP and purvalanol A to inhibit cytokinesis, the same drugs as in our nuclear transfer protocols, they control for the effect of these drugs on transcriptional activation of the genome. We found that the transcriptional profile of these drug treated control zygotes clustered with untreated control embryos and clustered separately from nuclear transfer embryos (Fig. 6c). Therefore, the drugs used are not directly responsible for the transcriptional failures observed after somatic cell nuclear transfer.
Figure 6. Failure to initiate transcription is not caused by karyotypic abnormalities.
a, Induction of aneuploidy in human zygotes. Schematic showing development after suppression of cleavage at the first mitosis. b, Development after suppression of cleavage at the first mitosis. Arrows point to a multipolar spindle as detected by microtubule birefringence. Immunohistochemistry at anaphase of mitosis shows the asymmetric segregation of chromatin to three poles (outer surface of the cleaving cell is outlined). Final panel: cleavage directly to 4 cells. NT=nuclear transfer. Days indicated the time post IVF. c, Clustering of the transcriptome of the indicated samples. Size bar 25μm.
Nuclear transfer into zygotes with supernumerary pronuclei
Human zygotes fertilized by more than one sperm41, or which do not complete meiosis normally, are routinely discarded because of their abnormal chromosome content. We have shown that mouse polyspermic zygotes can serve as competent recipient cells27 and therefore attempted to use 29 analogous human zygotes for nuclear transfer.
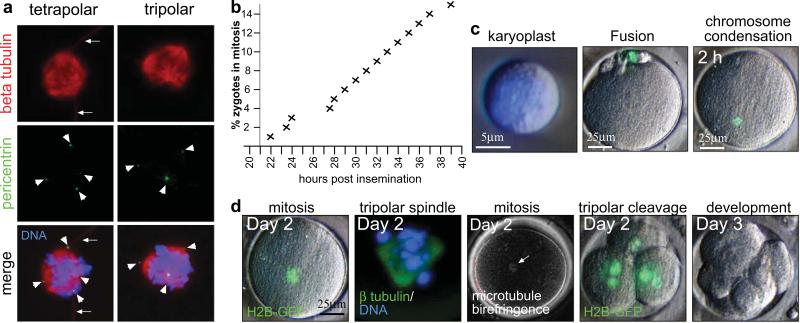
As has been observed previously42,43, when the development of 7 of these zygotes was monitored, they underwent abnormal cytokinesis (Supplementary Table S5). We found that the unusual cleavage pattern that resulted was caused by the nucleation of tripolar or tetrapolar mitotic spindles with supernumerary centrosomes at the spindle poles (Fig. 7a).
Figure 7. Nuclear transfer into human polyspermic zygotes.
a, Immunocytochemistry of dispermic human zygotes at mitosis. Arrowheads point to pericentrin positive centrosomes, arrows to tubulin positive sperm tails. b, Entry of polyspermic zygotes into mitosis. c, Nuclear transfer into human polyspermic zygotes. Genome removed at mitosis, fusion with a somatic donor cell and chromosome condensation of the somatic cell genome 2h post transfer are shown. d, Cleavage and development after nuclear transfer into polyspermic zygotes. Time indicates the days post fertilization. The arrow points to a birefringent spindle.
We hoped that by arresting these zygotes in mitosis (Fig. 7b) and then removing their abnormal spindles, we might replace them with donor nuclei that would support normal cytokinesis. After we transferred nuclei into these mitotic zygotes, we again observed nuclear envelope breakdown and chromosome condensation (Fig. 7c). Following mitotic exit and inhibition of cytokinesis, entry into the second interphase occurred normally. However, in all instances, the next cleavage division was multipolar (Supplementary Table S5), resulting in more than two, presumably aneuploid daughter cells and developmental arrest on day3 after fertilization (Fig.7d). Our results suggest that we were unable to remove supernumerary centrosomes during enucleation. Multipolar cleavage in human tetraploid zygotes was in striking contrast to both mouse and rabbit zygotes. In those species, tetraploid or polyspermic zygotes made a normal cleavage division, and efficiently developed to the blastocyst stage (Supplementary Table S6). These results suggest that the centrosome has a more dominant role in directing spindle assembly and cytokinesis in human development than it does in other species. This observation has substantial ramifications for human nuclear transfer and suggests that if more than one centrosome is present in the zygote after manipulations are completed, then abnormal patterns of spindle assembly and cleavage will occur.
Discussion
Here, we report our attempts to overcome the logistical and scientific obstacles impeding the production of patient-specific stem cell lines by nuclear transfer. While it has been difficult to recruit “altruistic” oocyte donors24 we did succeed in sourcing more than 400 normally fertilized eggs (zygotes) for our nuclear transfer studies.
Development after nuclear transfer was normal through the cleavage stages, but, unlike IVF controls, all cleaving nuclear transfer cells arrested before or at the time of compaction with severe transcriptional abnormalities. An identical phenotype could be induced by inhibition of transcription in IVF controls, but not by the deliberate induction of karyotypic abnormalities, suggesting that transcriptional defects are more proximally responsible for the developmental arrest.
Our findings are not the trivial result of a small sample size. Instead our results of more than 160 nuclear transfer experiments and more than 200 control manipulations indicate that there is a robust species-specific blockade to reprogramming that must be overcome before human stem cell lines can be derived. Our work and those of others suggests that the developmental arrest we observed is not simply the result of using zygotes for nuclear transfer as a temporally similar arrest is commonly observed after nuclear transfer into to human unfertilized oocytes18,20,25. Although a single group has reported efficient transcriptional reprogramming after human nuclear transfer20, they compared somatic cells to nuclear transfer samples and their results are therefore confounded by the presence of maternal mRNAs, which were not appropriately accounted for by their analyses. Another group has generated a single blastocyst after transfer of embryonic stem cell nuclei into human oocytes17, but development arrested when fibroblasts were transferred using identical methods18. This suggests that development and activation of the transferred genome depend on the epigenetic state of the injected nucleus.
In contrast to human zygotes, when we performed nuclear transfer into mouse zygotes, we found that reprogramming was essentially complete within hours and indeed within a single cell cycle. This result also points to a fundamental difference between reprogramming after nuclear transfer and iPS reprogramming: at least in animals, nuclear transfer mediates an immediate transition from a somatic to a pluripotent gene expression pattern, while reprogramming by defined factors seems to be a gradual process, requiring days or weeks37,38. It is interesting to consider that this could explain why stem cells generated by nuclear transfer are indistinguishable44 from stem cells derived from fertilized blastocysts, while in mouse16,45 and human12,15,46-48 iPS cell reprogramming may at times be less complete.
Incomplete reprogramming and developmental defects after somatic cell nuclear transfer have been described in other vertebrate species36,49-54. For example, in bovine nuclear transfer embryos, 3.8% of genes were found to be incompletely reprogrammed53. This is comparable to the results reported here with mouse zygotes, where the majority of transcripts (>91%) are normally expressed within 24 hours after nuclear transfer. In contrast, the transcriptional defects after nuclear transfer into human zygotes, extend far beyond incomplete reprogramming of a few genes: the majority of transcripts, or more than 88%, were not normally expressed. Surprisingly, even genes that were active in the somatic donor cell were not normally expressed, suggesting a failure to properly activate the transferred somatic cell genome.
The species-specific differences in transcription after nuclear transfer might be due to a property of the human egg, or of the human somatic cells. Our results suggest that investigating the requirements for transcriptional activation of the donor cell genome may help to overcome the developmental arrest commonly observed after human nuclear transfer.
Methods
Human zygotes were obtained using protocols reviewed and approved by the Committee on the Use of Human Subjects (IRB) and the Stem Cell Research Oversight Committee (ESCRO) at Harvard University.
Nuclear transfer into human zygotes
Human zygotes were thawed with Quinn's Advantage Thaw Kit (Cooper Surgical). Zygotes were thawed in small groups, from 6-12 for a single experiment. Upon thaw, zygotes were washed and placed in microdrops of Global media under mineral oil, equilibrated over night at 37 deg., 5% CO2. The 307 zygotes surviving the thaw were used as follows: 151 for nuclear transfer experiments, 77 for other experimental manipulations, 12 for immunohistochemistry and RNA analysis, 28 as developmental controls, and the remainder for developmental controls and the characterization of zygotic mitosis.
The following nuclear transfer protocol emerged as the most practical: nocodazole was added 1-2 hours post thaw at a concentration of 50μg/ml. Lower nocodazole (Sigma) concentrations (0.1 μg/ml) were ineffective for human zygotes (Supplementary Figure S13, Supplementary Table S7, Supplementary Figure S14). Without addition of nocodazole prior to spindle removal, most spindle material was removed with the zygotic genome (Supplementary Fig.S15), resulting in developmental arrest and the formation of abnormal spindles at the next mitosis (Supplementary Table S1). Upon entry into mitosis, zygotes were stained with Hoechst 33342 (2μg/ml) (Sigma) for 5-10 minutes in GMOPS-plus containing nocodazole. Zygotes were then placed on the heated stage in microdrops containing 10μg/ml CytoB and 50μg/ml nocodazole to generate a fluid cytoplasm. A hole was made in the zona pellucida with a XYClone Laser (Hamilton Thorne) using two to four 500-600μs pulses. The DNA was removed using a fire-polished pipette to prevent lysis (73/116 zygotes, 63%, survived the enucleation step). Chromosomes were visualized by low intensity UV illumination and/or Hoffman Modulation Contrast Optics. Removal of genomic DNA was verified by the presence of Hoechst/DNA complex in the removed material and the absence in the zygote. A second hole was made and a somatic cell inserted below the zona pellucida. Somatic cells were allowed to reach confluency to induce cell cycle arrest (for both human or mouse somatic cells, this usually occurred within a week after passage), and maintained as confluent cultures until use for nuclear transfer (between one week to two months after reaching confluency). In some instances, cells were exposed to medium containing 0.5% serum for 1 day before use for nuclear transfer. Fibroblasts obtained from a male adult T1D subject (ID#1011) or an adult healthy male (ID#1003) with a normal karyotype (Supplementary Figure S16) were used as nuclear donors. . Fusion of the somatic cell was done using two DC pulses of 1.6kV/cm of 20ms width using LF101 (NEPA GENE) in cell fusion medium 0.26M mannitol, 0.1mM MgSO4, 0.05% BSA, 0.5mM HEPES. 94 of 95 zygotes (99%) fused under these conditions, and none of them lysed. Using Piezo-mediated injection only 7/24, or 29% recovered from the lesion. Upon fusion, zygotes were returned to the incubator in Global culture medium. Chromosome condensation was monitored at least every hour using GFP fluorescence. After 2-3 hours chromosome condensation had occurred and zygotes were stimulated to exit mitosis without cleavage using 2.5mM 6-DMAP and 25μM purvalanolA (Sigma P4484), in Global medium. After 4-5 hours, when interphase nuclei were apparent, zygotes were thoroughly washed and then cultured in Global medium supplemented with 15% plasmanate.
Incubation of zygotes in α-amanitin (Sigma) was done beginning 3-4 hours after thaw at a concentration of 50μg/ml. Our total survival rate for the total of 160 nuclear transfer manipulations was 108/160 or 67%.
Microarray analysis
For microarray analysis, total RNA was isolated using PicoPure RNA isolation kit (Molecular Devices), and RNA was amplified by two to three rounds of T7 transcription using the Illumina TotalPrep RNA Amplification Kit. Amplified RNA was hybridized to the to Illumina® Sentrix Human Gene Expression BeadChip® RefSeq 8 v3.0 and read by the Illumina Bead Array Reader. Analysis was done using the Illumina Genome Studio Program and Microsoft Excel. Data was normalized to average and a differential gene expression score calculated in comparison to 1-cell zygotes as reference. Fold change to 1-cell zygotes were calculated based on the average signal for a particular transcript using Microsoft Excel. The average fold change for two samples was calculated (e.g. two samples on d3 post IVF, 2 samples on d4 post IVF, 2 samples treated with amanitin, 2 NT samples, etc). If the average fold change was >5 or <0.2, and the differential gene expression score was >22 or <-22, corresponding to a p-value of <0.01 for both samples, a transcript was considered differentially expressed. Differential gene expression score (DIffscore) is calculated by the Illumina Genome Studio program using an Illumina custom algorithm. A diffscore of >33 or <-33 corresponds to a P-value of <0.001. All p-values indicated in the manuscript were calculated in this manner. The 828 ZGA transcripts and the 1130 maternal mRNA decay transcripts were at least 5x differentially expressed in the set of both d3 and d4 IVF data points, each consisting of two samples.
Nuclear transfer into mouse zygotes and oocytes
Nuclear transfer protocols developed on human zygotes were used for nuclear transfer into mouse zygotes. In brief, tail tip fibroblasts used for nuclear transfer and iPS generation were obtained from adult B6jcBA-Tg (Pou5fI-EGFP)2Mnn/J mice. Mouse somatic cells were prepared for nuclear transfer as described above for human somatic cells. Zygotes and oocytes were obtained from BDF1 mice (Charles River). Zygotes were arrested in mitosis by nocodazole and had their genome removed shortly (5-10min.) after release from nocodazole with or without the use of Hoechst/low intensity UV illumination. Nuclear transfer was done by two DC pulses of 1.6 kV/cm in fusion buffer (71/98 or 72% of tail tip cells fused and integrated into the recipient zygote). Cytokinesis was inhibited by incubation inhibited by the same combination of kinase inhibitors as used for nuclear transfer in human zygotes: 10μM of the cyclin-dependent kinase inhibitor purvalanol A (Sigma P4484), 2mM 6-DMAP (Sigma D2629), 0.5μg/ml nocodazole, and optionally with the addition of 20μM of the aurora B kinase inhibitor ZM 447439 (Tocris Bioscience) for 90-120 minutes. For nuclear transfer at anaphase, zygotes had their genome removed at anaphase (40-50min post nocodazole release) or, alternatively, the genome was removed at prometaphase and zygotes were stimulated to enter anaphase as above (cytokinesis inhibitors purvalanol A, 6-DMAP and nocodazole) for 45-60 minutes prior to transfer. Control zygotes that had cytokinesis inhibited with these same cytokinesis kinase inhibitors efficiently developed to the morula and blastocyst stage (9 blastocysts and 1 morula of 10 zygotes), suggesting that the combination of these drugs is not toxic to mouse preimplantation development. Nuclear transfer into mouse oocytes was done as follows: oocytes were obtained 14 h post hCG injection, their spindle-chromosome complex was removed in the presence of 5μg/ml cytochalasinB. Tail tip fibroblasts were transferred into enucleated oocytes by direct injection, and activated by 5 hours incubation in 1mM SrCl2 in ca-free MZCB and 5μg/ml cytochalasinB to inhibit polar body extrusion, as described elsewhere 55,56. Array data on nuclear transfer into interphase zygotes and on genome less zygotes were obtained from Ref. 31. Drug treatment only controls were arrested as zygotes with nocodazole, and cytokinesis was inhibited with the use of purvalanol A and 6-DMAP.
For gene expression analysis, RNA was isolated from groups of approx. 20 cells, amplified by 2 rounds of T7 polymerase transcription and hybridized to Illumina arrays. Differentially expressed genes were defined as having a Diffscore of > 22 or < than -22 in two samples of the same kind, and an at least 5-fold difference in the average signal (average of both samples). Interphase zygotes were harvested 25-28h post hCG pulse, mitotic zygotes were obtained by nocodazole mediated arrest until 33h post hCG.
iPS generation
Plat-E packaging cells were transfected (Fugene, Roche) with retroviral plasmids for Oct4, Sox2, Klf4 or c-Myc and viral supernatent was collected, filtered, combined to contain virus of all four transcription factors, and placed on tail tip cells obtained from adult B6jcBA-Tg (Pou5fI-EGFP)2Mnn/J mice. In brief,cells on 3 consecutive days, as previously described (Ichida et al. 2009). The first application of viral supernatant was counted as day 0.
Immunostaining
Zygotes, blastomeres and blastocysts were fixed in 4% paraformaldehyde over night at 4°C, permeabilized in PBS with 0.5% Triton-X100 (PBS/T) for 20min., blocked in blocking solution consisting of 0.1% PBS/T with 10% FBS over night at 4°C, incubated in primary antibody at 4°C in blocking solution, then washed for 1h at room temperature in 0.1% PBS/T, incubated with secondary conjugated antibody in 0.1% PBS/T at room temperature for 1h, washed as above, stained with Hoechst 33342 for 5 minutes and used for confocal imaging. Oct4 antibody (Santa Cruz, sc5279) and Cdx-2 antibody (Biogenex) was used at a concentration of 1:200, and Brg-1 antibody (Santa Cruz sc-10768) was used at a concentration of 1:50. Conditions were maintained between different samples. BrdU labeling was done using the Amersham cell proliferation kit (RPN20). Cells were incubated into the labeling solution diluted 1:1000 in KSOM for 18h post transfer.
Supplementary Material
Acknowledgments
We thank George Kenty, Rachel Orler, Anne Danforth and Christine Baugh for thawing human specimen. We thank the donor couples for providing zygotes to our research. We thank Doctors and Staff of the Reproductive Science Center (Lexington, MA) for 3PN zygotes and Miguel Verbitsky for microarray hybridization. This work was supported by the Stowers Medical Institute, a seed grant to D.E. from the Harvard Stem Cell Institute through the support of the Singer Family Foundation, and by the New York Stem Cell Foundation. Work on mouse SCNT was supported by NIH grant R01 HD045732-03 to K.E. K.E. is a fellow of the John D. and Catherine T. MacArthur Foundation, DE is a NYSCF-Druckenmiller fellow.
Footnotes
Author Contributions K.E., D.M. and G.S. established IRB and consent documents. D.E., A.E.C., K.E. and D.M. conceived and planned experiments, D.E. and A.E.C. performed work with human zygotes, D.E. performed mouse experiments, R.G. and R.L. recruited and consented skin cell donors, W.G.K. did blastomere karyotyping, M.B. provided cryopreserved mouse zygotes, K.G., N.A., J.P. provided 3PN zygotes, C.F. provided general assistance, D.E. and K.E. wrote the paper with input from R.L., D.M., and A.E.C.
Additional information
Supplementary Information accompanies this paper at http://www.nature.com/naturecommunications
Competing financial interests: The authors declare no competing financial interests.
Illumina array data have been deposited at GEO under accession number GSE28811 and GSE24631. Reprints and permission information is available online at http://npg.nature.com/reprintsandpermissions/
References
- 1.Gurdon JB. Adult frogs derived from the nuclei of single somatic cells. Dev Biol. 1962;4:256–273. doi: 10.1016/0012-1606(62)90043-x. [DOI] [PubMed] [Google Scholar]
- 2.Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KH. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997;385(6619):810–813. doi: 10.1038/385810a0. [DOI] [PubMed] [Google Scholar]
- 3.Thomson JA, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 4.Lanza RP, Cibelli JB, West MD. Prospects for the use of nuclear transfer in human transplantation. Nat Biotechnol. 1999;17(12):1171–1174. doi: 10.1038/70709. [DOI] [PubMed] [Google Scholar]
- 5.Kind A, Colman A. Therapeutic cloning: needs and prospects. Semin Cell Dev Biol. 1999;10(3):279–286. doi: 10.1006/scdb.1999.0277. [DOI] [PubMed] [Google Scholar]
- 6.Kiskinis E, Eggan K. Progress toward the clinical application of patient-specific pluripotent stem cells. J Clin Invest. 2010;120(1):51–59. doi: 10.1172/JCI40553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tabar V, et al. Therapeutic cloning in individual parkinsonian mice. Nat Med. 2008;14(4):379–381. doi: 10.1038/nm1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rideout WM, 3rd, Hochedlinger K, Kyba M, Daley GQ, Jaenisch R. Correction of a genetic defect by nuclear transplantation and combined cell and gene therapy. Cell. 2002;109(1):17–27. doi: 10.1016/s0092-8674(02)00681-5. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi K, et al. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 10.Dimos JT, et al. Induced Pluripotent Stem Cells Generated from Patients with ALS Can Be Differentiated into Motor Neurons. Science. 2008;321(5893):1218–1221. doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- 11.Chin MH, et al. Induced pluripotent stem cells and embryonic stem cells are distinguished by gene expression signatures. Cell Stem Cell. 2009;5(1):111–123. doi: 10.1016/j.stem.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lister R, et al. Hotspots of aberrant epigenomic reprogramming in human induced pluripotent stem cells. Nature. 2011;471:68–73. doi: 10.1038/nature09798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng J, et al. Targeted bisulfite sequencing reveals changes in DNA methylation associated with nuclear reprogramming. Nat Biotechnol. 2009;27(4):353–360. doi: 10.1038/nbt.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stadtfeld M, et al. Aberrant silencing of imprinted genes on chromosome 12qF1 in mouse induced pluripotent stem cells. Nature. 2010;465(7295):175–181. doi: 10.1038/nature09017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu BY, et al. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc Natl Acad Sci U S A. 2010;107(9):4335–4340. doi: 10.1073/pnas.0910012107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim K, et al. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467(7313):285–290. doi: 10.1038/nature09342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stojkovic M, et al. Derivation of a human blastocyst after heterologous nuclear transfer to donated oocytes. Reprod Biomed Online. 2005;11(2):226–231. doi: 10.1016/s1472-6483(10)60962-5. [DOI] [PubMed] [Google Scholar]
- 18.Hall VJ, et al. Developmental competence of human in vitro aged oocytes as host cells for nuclear transfer. Hum Reprod. 2007;22(1):52–62. doi: 10.1093/humrep/del345. [DOI] [PubMed] [Google Scholar]
- 19.French AJ, et al. Development of human cloned blastocysts following somatic cell nuclear transfer with adult fibroblasts. Stem Cells. 2008;26(2):485–493. doi: 10.1634/stemcells.2007-0252. [DOI] [PubMed] [Google Scholar]
- 20.Chung Y, et al. Reprogramming of human somatic cells using human and animal oocytes. Cloning Stem Cells. 2009;11(2):213–223. doi: 10.1089/clo.2009.0004. [DOI] [PubMed] [Google Scholar]
- 21.Cibelli J, et al. Somatic Cell Nuclear Transfer in Humans: Pronuclear and Early Embryonic Development. The Journal of Regenerative Medicine. 2001;2:25–31. [Google Scholar]
- 22.Lavoir MC, Weier J, Conaghan J, Pedersen RA. Poor development of human nuclear transfer embryos using failed fertilized oocytes. Reprod Biomed Online. 2005;11(6):740–744. doi: 10.1016/s1472-6483(10)61693-8. [DOI] [PubMed] [Google Scholar]
- 23.McElroy SL, et al. Developmental competence of immature and failed/abnormally fertilized human oocytes in nuclear transfer. Reprod Biomed Online. 2008;16(5):684–693. doi: 10.1016/s1472-6483(10)60483-x. [DOI] [PubMed] [Google Scholar]
- 24.Egli D, Chen AE, Melton D, Eggan K. Impracticality of Egg Donor Recruitment in the Absence of Compensation. Cell Stem Cell. 2011 doi: 10.1016/j.stem.2011.08.002. (in the press) [DOI] [PubMed] [Google Scholar]
- 25.Kennedy D. Editorial retraction. Science. 2006;311(5759):335. doi: 10.1126/science.1124926. [DOI] [PubMed] [Google Scholar]
- 26.Kim K, et al. Recombination signatures distinguish embryonic stem cells derived by parthenogenesis and somatic cell nuclear transfer. Cell Stem Cell. 2007;1:346–352. doi: 10.1016/j.stem.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Egli D, Rosains J, Birkhoff G, Eggan K. Developmental reprogramming after chromosome transfer into mitotic mouse zygotes. Nature. 2007;447(7145):679–685. doi: 10.1038/nature05879. [DOI] [PubMed] [Google Scholar]
- 28.Wong CC, et al. Non-invasive imaging of human embryos before embryonic genome activation predicts development to the blastocyst stage. Nat Biotechnol. 2010;28(10):1115–1121. doi: 10.1038/nbt.1686. [DOI] [PubMed] [Google Scholar]
- 29.Mitalipov SM, et al. Reprogramming following somatic cell nuclear transfer in primates is dependent upon nuclear remodeling. Hum Reprod. 2007;22(8):2232–2242. doi: 10.1093/humrep/dem136. [DOI] [PubMed] [Google Scholar]
- 30.Kawakami M, et al. Effect of demecolcine and nocodazole on the efficiency of chemically assisted removal of chromosomes and the developmental potential of nuclear transferred porcine oocytes. Cloning Stem Cells. 2003;5(4):379–387. doi: 10.1089/153623003772032871. [DOI] [PubMed] [Google Scholar]
- 31.Egli D, Eggan K. Recipient cell nuclear factors are required for reprogramming by nuclear transfer. Development. 2010;137(12):1953–1963. doi: 10.1242/dev.046151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wakayama T, Yanagimachi R. Effect of cytokinesis inhibitors, DMSO and the timing of oocyte activation on mouse cloning using cumulus cell nuclei. Reproduction. 2001;122(1):49–60. doi: 10.1530/rep.0.1220049. [DOI] [PubMed] [Google Scholar]
- 33.Byrne JA, et al. Producing primate embryonic stem cells by somatic cell nuclear transfer. Nature. 2007;450(7169):497–502. doi: 10.1038/nature06357. [DOI] [PubMed] [Google Scholar]
- 34.Vassena R, et al. Waves of early transcriptional activation and pluripotency program initiation during human preimplantation development. Development. 2011;138(17):3699–3709. doi: 10.1242/dev.064741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Braude P, Bolton V, Moore S. Human gene expression first occurs between the four- and eight-cell stages of preimplantation development. Nature. 1988;332(6163):459–461. doi: 10.1038/332459a0. [DOI] [PubMed] [Google Scholar]
- 36.Boiani M, Eckardt S, Scholer HR, McLaughlin KJ. Oct4 distribution and level in mouse clones: consequences for pluripotency. Genes Dev. 2002;16(10):1209–1219. doi: 10.1101/gad.966002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brambrink T, et al. Sequential expression of pluripotency markers during direct reprogramming of mouse somatic cells. Cell Stem Cell. 2008;2(2):151–159. doi: 10.1016/j.stem.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stadtfeld M, Maherali N, Breault DT, Hochedlinger K. Defining molecular cornerstones during fibroblast to iPS cell reprogramming in mouse. Cell Stem Cell. 2008;2(3):230–240. doi: 10.1016/j.stem.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flach G, Johnson MH, Braude PR, Taylor RA, Bolton VN. The transition from maternal to embryonic control in the 2-cell mouse embryo. Embo J. 1982;1(6):681–686. doi: 10.1002/j.1460-2075.1982.tb01230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simerly C, et al. Molecular correlates of primate nuclear transfer failures. Science. 2003;300(5617):297. doi: 10.1126/science.1082091. [DOI] [PubMed] [Google Scholar]
- 41.Van der Ven HH, et al. Polyspermy in in vitro fertilization of human oocytes: frequency and possible causes. Ann N Y Acad Sci. 1985;442:88–95. doi: 10.1111/j.1749-6632.1985.tb37508.x. [DOI] [PubMed] [Google Scholar]
- 42.Craven L, et al. Pronuclear transfer in human embryos to prevent transmission of mitochondrial DNA disease. Nature. 2010;465(7294):82–85. doi: 10.1038/nature08958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kola I, Trounson A, Dawson G, Rogers P. Tripronuclear human oocytes: altered cleavage patterns and subsequent karyotypic analysis of embryos. Biol Reprod. 1987;37(2):395–401. doi: 10.1095/biolreprod37.2.395. [DOI] [PubMed] [Google Scholar]
- 44.Brambrink T, Hochedlinger K, Bell G, Jaenisch R. ES cells derived from cloned and fertilized blastocysts are transcriptionally and functionally indistinguishable. Proc Natl Acad Sci U S A. 2006;103(4):933–938. doi: 10.1073/pnas.0510485103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Polo JM, et al. Cell type of origin influences the molecular and functional properties of mouse induced pluripotent stem cells. Nat Biotechnol. 2010;28(8):848–855. doi: 10.1038/nbt.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ghosh Z, et al. Persistent donor cell gene expression among human induced pluripotent stem cells contributes to differences with human embryonic stem cells. PLoS One. 2010;5(2):e8975. doi: 10.1371/journal.pone.0008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feng Q, et al. Hemangioblastic derivatives from human induced pluripotent stem cells exhibit limited expansion and early senescence. Stem Cells. 28(4):704–712. doi: 10.1002/stem.321. [DOI] [PubMed] [Google Scholar]
- 48.Doi A, et al. Differential methylation of tissue- and cancer-specific CpG island shores distinguishes human induced pluripotent stem cells, embryonic stem cells and fibroblasts. Nat Genet. 2009;41(12):1350–1353. doi: 10.1038/ng.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eggan K, et al. Hybrid vigor, fetal overgrowth, and viability of mice derived by nuclear cloning and tetraploid embryo complementation. Proc Natl Acad Sci U S A. 2001;98(11):6209–6214. doi: 10.1073/pnas.101118898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Humpherys D, et al. Abnormal gene expression in cloned mice derived from embryonic stem cell and cumulus cell nuclei. Proc Natl Acad Sci U S A. 2002;99(20):12889–12894. doi: 10.1073/pnas.192433399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gao S, et al. Somatic cell-like features of cloned mouse embryos prepared with cultured myoblast nuclei. Biol Reprod. 2003;69(1):48–56. doi: 10.1095/biolreprod.102.014522. [DOI] [PubMed] [Google Scholar]
- 52.Ng RK, Gurdon JB. Epigenetic memory of active gene transcription is inherited through somatic cell nuclear transfer. Proc Natl Acad Sci U S A. 2005;102(6):1957–1962. doi: 10.1073/pnas.0409813102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rodriguez-Osorio N, et al. Transcriptional reprogramming of gene expression in bovine somatic cell chromatin transfer embryos. BMC Genomics. 2009;10:190. doi: 10.1186/1471-2164-10-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bortvin A, et al. Incomplete reactivation of Oct4-related genes in mouse embryos cloned from somatic nuclei. Development. 2003;130(8):1673–1680. doi: 10.1242/dev.00366. [DOI] [PubMed] [Google Scholar]
- 55.Egli D, Eggan K. Nuclear transfer into mouse oocytes. J Vis Exp. 2006;(1):116. doi: 10.3791/116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wakayama T, Perry AC, Zuccotti M, Johnson KR, Yanagimachi R. Full-term development of mice from enucleated oocytes injected with cumulus cell nuclei. Nature. 1998;394(6691):369–374. doi: 10.1038/28615. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.