Abstract
Background:
Our objective was to assess the global cost of the sentinel lymph node detection [axillary sentinel lymph node detection (ASLND)] compared with standard axillary lymphadenectomy [axillary lymph node dissection (ALND)] for early breast cancer patients.
Patients and methods:
We conducted a prospective, multi-institutional, observational, cost comparative analysis. Cost calculations were realized with the micro-costing method from the diagnosis until 1 month after the last surgery.
Results:
Eight hundred and thirty nine patients were included in the ASLND group and 146 in the ALND group. The cost generated for a patient with an ASLND, with one preoperative scintigraphy, a combined method for sentinel node detection, an intraoperative pathological analysis without lymphadenectomy, was lower than the cost generated for a patient with lymphadenectomy [€2947 (σ = 580) versus €3331 (σ = 902); P = 0.0001].
Conclusion:
ASLND, involving expensive techniques, was finally less expensive than ALND. The length of hospital stay was the cost driver of these procedures. The current observational study points the heterogeneous practices for this validated and largely diffused technique. Several technical choices have an impact on the cost of ASLND, as intraoperative analysis allowing to reduce rehospitalization rate for secondary lymphadenectomy or preoperative scintigraphy, suggesting possible savings on hospital resources.
Keywords: axillary lymphadenectomy, breast cancer, cost, sentinel lymph node
introduction
Breast cancer represents a major public health problem, with 50 000 new cases each year and an incidence estimated rate of 100/100 000 in France [1].
Surgical treatment in early cases consists of breast tumor removal and axillary lymph node dissection (ALND) or axillary sentinel lymph node detection (ASLND).
Axillary lymphadenectomy is a simple surgical procedure, needing conventional surgical supply.
ASLND needs radioisotopes and/or blue dyes. Using both techniques in combination, the sentinel node is surgically identified by either using a handheld gamma probe or visually identifying a blue-stained node. The removed ASLN are pathologically examined intraoperatively and through definitive analysis.
Pathological analysis of sentinel nodes, as defined by American Joint Committee on Cancer (AJCC) and International Union Against Cancer (UICC), allows a more precise evaluation of nodal disease and a more accurate staging of the axillary status than compared with ALND [2].
It has been demonstrated, through large randomized trials, that ASLND strategy brings less morbidity than ALND reducing hospital stay [3]. Currently, ASLND has become a worldwide validated technique, for patient with an early infiltrative breast carcinoma, instead of systematic ALND [4]. When an ASLND is carried out, ALND remains indicated in the case of sentinel node involvement or detection failure [5].
Introduction of a sophisticated technique instead of simple surgical procedure as standard treatment of a so frequent cancer would have economical consequences. Despite a huge publication rate of ASLN clinical series, the total cost of this innovative technique remains unknown, considering hospital stay, morbidity, need for reintervention and postoperative visits for complications. In nowadays international context of health resource restriction, physicians must evaluate the real cost of such innovative techniques, suggesting efficient allocation of hospital resources.
Our main objective was to assess the global cost of ASLND compared with standard ALND in the case of early breast cancer patients, including 1-month follow-up, in a prospective multi-institutional setting.
patients and methods
We conducted a cost consequence, observational, prospective, nonrandomized, multi-institutional study, with the financial support of the French national cancer institute. Our aim was to compare ASLND to ALND in terms of costs, quality of life, morbidity and pain. Quality of life has been assessed through QLQ-C30 version 3.0 from European Organization for Research and Treatment of Cancer completed by a specific questionnaire for breast cancer QLQ-BR23. Pain was assessed through the Visual Analogic Scale. Quality of life, pain and axillary control of local relapse were expected to be followed for 5 years. In this article, we only focused on the cost comparative analysis. Quality of life and pain will be treated when the 5 years follow-up will be reached.
patients and procedures
This study was validated by scientific and ethical boards.
We defined prospectively two groups according to axillary surgical procedure: ASLN group and ALND group. In ASLND group, patients underwent ASLND and a levels I–II axillary lymphadenectomy only in the case of a detection failure or involved sentinel node. In ALND group, patients underwent a systematic levels I–II axillary lymphadenectomy.
Patient eligibility criteria were the same for the two groups. Patients were not randomly assigned and the choice between groups was left to each institution depending on their practices.
eligibility
Inclusion criteria were patient ≤70 years, treated by conservative surgery for an infiltrative breast carcinoma, unifocal, clinically <2 cm (T1), without clinical axillary suspicious palpable lymph node (N0) and without distant metastasis.
Exclusion criteria were a multifocal tumor, a nonoperable inflammatory or metastatic breast cancer, neoadjuvant treatment, breast tumor previously removed, previous breast surgery as breast reduction, previous axillary surgery, pregnancy, clinically suspicious ipsilateral axillary lymph node, known allergy to blue dye and patient >70 years. Breast tumor must be neither a local relapse nor an intraductal carcinoma.
axillary procedure
lymphatic mapping.
ASLND was always carried out with the combined method with technetium and patent blue dye as previously described [6]. The use of preoperative scintigraphy was left to discretion of each team. For colorimetric detection, 2 ml of patent blue dye was injected under general anesthesia.
The intraoperative isotopic detection protocol for each patient included counts of each ASLN and ended with control of the lack of activity remaining in the axilla after ASLN resection. An ASLN may be radioactive and/or blue.
Each team was experienced with ASLND for years before the current study.
axillary lymphadenectomy.
A standard levels I–II axillary lymphadenectomy was carried out in ALND's patients or in ASLND's patients in case of a detection failure or a sentinel node involvement and in case of a multiple breast tumor found in definitive pathological analysis. A suction drain may be used or not into the axilla at the end of the procedure.
pathological analysis
Pathological examination of nodes from ASLND was carried out according to AJCC and UICC recommendations with thin serial sectioning of nodes at 2.0 mm intervals, embedding all sections and examining one section from the surface of the block.
One option was to carry out immunohistochemistry (IHC) with pancytokeratin in case of negative hematoxylin–eosin (H&E) examination.
Intraoperative examination of the sentinel nodes and its technique (touch imprint cytology, frozen section or both) was left to the discretion of participating teams.
Pathological node examination in the ALND was carried out in each center according to recommended guidelines with total embedding of each node and H&E examination of a single section by paraffin block without IHC and without intraoperative analysis.
studied parameters
clinical parameters.
Breast size (according to international definition of bra size: AB, CD, >DD), menopausal status (yes, no), clinical evaluation of breast tumor size (nonpalpable, T1, T2), detection rate (ratio: detection failure/injected patients), postoperative morbidity (abcess, seroma needing aspiration, bleeding) and the need for reintervention.
pathology.
Breast tumor histological subtype, pathological size (millimeters), number of resected nodes and number of involved nodes (macrometastasis—up to 2 mm—micrometastasis—from 0.2 to 2 mm, isolated cells <0.2 mm).
cost evaluation method
Health care resources from hospital perspectives have been included in the economic evaluation. Cost calculations have been realized with the micro-costing method from the cancer diagnosis until 1 month after the end of hospitalization for the last surgery. All consumed resources were integrated (not only the difference) in order to determine total direct medical costs for both procedures including the timing of each procedure and used devices. Moreover, specific surveys per center have allowed to determine further unit cost for nuclear medicine, surgery and pathology including mean salaries of concerned professionals, annual activity, type of equipment and cost of hospitalization in surgical units. Costs of adjuvant treatments (radiotherapy, chemotherapy, hormonotherapy) were not included.
Loss of productivity of active women was assessed through postoperative sick leave.
Actual French 2007 nomenclature of prices was used. Average cost per patient have been calculated and completed by a sensibility analysis.
Global cost will be presented by group of patients (ASLND and ALND) and subgroup analysis:
patients who have ASNLD in one step surgery,
patients who have ASNLD and ALND in one step and
patients who have ASNLD and ALND in two steps.
statistical considerations
Costs were described in terms of mean (standard deviation) or median (range). Tests for normality were carried out with the Kolmogorov–Smirnov tests. Comparisons of costs were based on a Student’s t-test, a Mann–Withney test or an analysis of variance. Sociodemographic characteristics, clinical information and all categorical variables were compared using a Chi-square test or a Fisher's exact test. Confounding factors were taken into account using multivariate regression linear models.
All tests are two sided with a significant level of 5%. Data were analyzed with SAS system software (version 9.1, SAS Institute Inc.).
findings
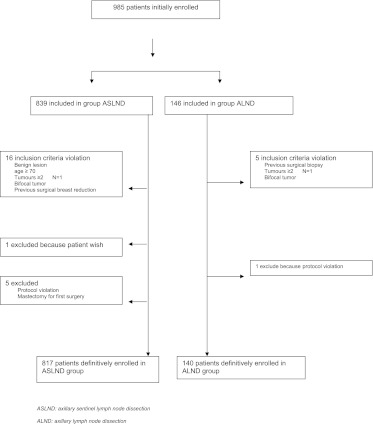
From September 2005 to December 2006, 839 patients were included in the ASLND group and 146 in the ALND group from 16 French cancer centers. The size of each group was coherent with the patient workload of each center involved in lymphadenectomy or in the sentinel node technique throughout the study. Patients with a violation of inclusion criteria were excluded (flowchart as Figure 1).
Figure 1.
Flowchart for inclusion of patients in cost analysis. ASLND, axillary sentinel lymph node dissection; ALND, axillary lymph node dissection.
patients’ characteristics
Patients’ characteristics did not significantly differ in the two groups, except for tumor size with respect to study eligibility criteria (Table 1).
Table 1.
Patients' characteristics
| ASLND (SD) (n = 817) | ALND (SD) (n = 140) | P | |
| Average age (years) | 55.8 (8.3) | 56.1 (9.5) | 0.7 |
| Average pathological tumor size (mm) | 13.5 (7) | 16.3 (12) | 0.0001 |
| Carcinoma (%) | |||
| Infiltrative ductal | 87 | 84 | 0.23 |
| Infiltrative lobular | 2 | 1 | |
| Others | 10 | 15 | |
| Tumor–node–metastasis (%) | |||
| Nonpalpable | 34 | 21 | 0.003 |
| T1 | 65 | 79 | |
| Tis | 1 | 0 | |
| Bra cup sizea (%) | |||
| AB | 52.4 | 42.1 | 0.09 |
| CD | 43.2 | 54.4 | |
| EF | 4.4 | 3.5 | |
| Menopausal status (%) | 62.8 | 65 | 0.32 |
Mean (SD).
According to international definition AB, CD, >DD.
ASLND, axillary sentinel lymph node dissection; ALND, axillary lymph node dissection; SD, standard deviation; Tis, tumor in situ.
Among the 817 patients of the ASLND group, the detection rate was 97.5% (797/817), 589 patients had sentinel detection as a unique surgical procedure, while 228 patients had a complementary ALND in one- (n = 95) or two-step (n = 133) surgical procedure according to intraoperative analysis. The pathological results for the 627 patients with intraoperative analysis are listed in Table 2. Surgical management of the axilla in the ASLND group are listed in Table 3. Macrometastases were found in 130 patients (130/817; 16%); 93 in the group with intraoperative analysis, 20 in the group without intraoperative analysis and 17 among the 20 patients who underwent an ALND because of a detection failure. Intraoperative imprints were positives in 59 of the 93 cases of macrometastasis (59/93; 63.4%), allowing to carry out the complementary lymphadenectomy in one step. The false-positive rate of intraoperative imprints was 0.3% (2/627; 0.3%).
Table 2.
Sentinel nodes: intraoperative and definitive pathological results for the 627 cases with intraoperative analysis
| Pathological definitive node status | Total | Intraoperative ASLND diagnosis ‘negative’ | Intraoperative ASLND diagnosis ‘positive’ |
| pN1 | 93 | 34 (37%) | 59 (63%) |
| pN1 mi | 49 | 46 (94%) | 3 (6%) |
| pN0i+ | 18 | 17 (94%) | 1 (6%) |
| Subtotal | 160 | 97 (61%) | 63 (39%) |
| pN0 | 467 | 465 | 2a |
| Total | 627 (100%) | 562 (90%) | 65 (10%) |
Two false-positive cases of intraoperative ASLN analysis (0.3%)
PN, pathological results of node status; ASLND, axillary sentinel lymph node dissection.
Table 3.
Surgical management of the axilla in the ASLND group
| Intraoperative examination |
No intraoperative examination | Total | |||
| Diagnosis negative | Diagnosis positive | ||||
| Patients with ASLND alone | 461 | 0 | 128 | 589 | |
| Patients with axillary lymphadenectomy | In one-step surgery | 5a | 65 | 25b | 95 |
| In two-step surgery | 96 | 0 | 37 | 133 | |
| Total | 101 | 65 | 62 | 228 | |
| Total | 562 (69%) | 65 (8%) | 190 (23%) | 817 (100%) | |
Suspicious nonsentinel nodes discovered intraoperatively after sentinel node examination.
Twenty sentinel node detection failure and five multifocal carcinoma diagnosed intraoperatively.
ASLND, axillary sentinel lymph node dissection.
Among the 140 patients of ALND group, definitive pathological analysis found a macrometastasis in 38 patients (38/140; 27%).
resource consumption
The global resource consumptions per group, including 1 month follow-up, are listed in Table 4.
Table 4.
Resources consumed for each phase of ASLND or ALND including 1 month follow-up
| ASLND (n = 817) | ALND (n = 140) | P | |
| Preoperative phase | |||
| Preoperative diagnostic | |||
| Realized and obtained (%) | 97 | 89 | 0.0001 |
| Average isotopic dose injected (MBq) | 41.5 | – | – |
| Preoperative scintigraphy frequency (%) | 80 | ||
| Average scintigraphy per patienta (mean, standard deviation) | 1.2 (σ = 0.4) | – | – |
| Detection of sentinel lymph node (%) | 97 | – | – |
| Subtotal cost (€, 2007) | 582 (σ = 213) | 176 (σ = 141) | 0.001 |
| Operative and hospitalization phase | |||
| Frequency of intraoperative analysis (%) | 77 | – | – |
| Mean duration of pathological analysis (min) | 18 | – | – |
| Mean time laboratory technician (min) | 21 | – | – |
| Mean occupation time operating room (min) | 98 (σ = 34) | 106 (σ = 31) | 0.01 |
| Complications during hospitalization | 17 (2.1%) | 7 (5%) | 0.07 |
| Due to breast surgery | 16 | 3 | |
| Due to axillary surgery | 1 | 4 | |
| Mean length of hospital stay (days) | 3.8 (σ = 1.4) | 6.4 (σ = 3.2) | 0.000 |
| Without breast complications | 3.79 (σ = 1.3) | 6.4 (σ = 3.2) | 1 |
| Total costs excluding follow-up (€, 2007) | 3007 (σ = 687) | 3340 (σ = 1174) | 0.002 |
| Hospital follow-up (1 month) | |||
| Total number of surgical reintervention | 253 (31%) | 24 (17%) | 0.001 |
| Number of second surgery | 242 (30%) | 23 (16%) | 0.001 |
| For breast | 110 (13%) | 23 (16%) | – |
| For axillary nodes | 132 (16%) | 0 | – |
| Mean length of stay (days) for second surgery | 4.5 (σ = 2) | 5.6 (σ = 5) | 0.3 |
| For breast | 4 (σ = 3) | 5.6 (σ = 5) | |
| For axillary nodes | 5.2 (σ = 2.2) | – | |
| Number of third surgery | 11 (+1%) | 1 (<1%) | – |
| For breast margins | 10 | 1 | – |
| For axillary nodes | 1 | 0 | – |
| Mean length of stay (days) for third surgery | 4.4 (σ = 2.5) | 8 | |
| For breast | 4.5 (σ = 2) | 8 | |
| For axillary nodes | 4 | – | |
| Number of patients who have had complications at 1 month after the first surgery | 130 (16%) | 41 (29%) | 0.0001 |
| Localization of complication at the first surgery | |||
| Breast | 82 (10%) | 18 (12%) | 0.049 |
| Axilla | 49 (6%) | 23 (16%) | 0.0001 |
| Type of complicationsb | |||
| Abcess and hematoma | 40 (5%) | 5 (4%) | – |
| Seroma aspiration | 49 (6%) | 26 (19%) | – |
| Lymphedema | 0 | 2 (2%) | – |
| Othersc | 40 (5%) | 5 (4%) | – |
| Number of complications leading to another hospital stayd | 9 | 2 | – |
| Breast margin (and others) complications | 8 | 2 | |
| For axillary node complications | 1 | 0 | |
| Mean length of stay (days) for complication | 5.3 (σ = 4) | 2 (σ = 1) | – |
| For breast margins | 5 (σ = 3.8) | 2 (σ = 1) | |
| For axillary nodes | 6 | – | |
| Number of seroma aspiration at hospital | 0.5 (σ = 1.3) | 1.2 (σ = 12.8) | 0.0003 |
| Number of physiotherapist visits at hospital | 2 (σ = 5.9) | 6.3 (σ = 8.7) | 0.001 |
| Number of surgeon visits at hospital | 1.3 (σ = 1.3) | 1.3 (σ = 1.2) | 0.75 |
| Total costs including follow-up (€, 2007) | 3.7 (σ = 1.4) | 3.8 (σ = 20) | 0.62 |
For patients who had a scintigraphy.
Axillary and breast complications.
Discharge, erythema, brides, bruise etc.
Complications following the first, second or third surgery.
ASLND, axillary sentinel lymph node dissection; ALND, axillary lymph node dissection.
Preoperative step parameters were initial biopsy, preoperative visits, radiological guiding for nonpalpable tumor, injection of technetium solution and scintigraphy. The majority of patients had a preoperative diagnostic with core biopsy (97% in ASLND versus 89% in ALND; P = 0.001). At least one scintigraphy was carried out for 80% of patients from ASLND group (n = 657/817 patients) in 14 of 16 cancer centers of the study.
Operative step parameters included blue stain injection, ASLND with a gamma probe, intraoperative analysis, surgery under general anesthesia and definitive pathological analysis. The mean surgical room occupation was greater for ALND group than for ASLND group (106 versus 98 min; P = 0.01).
The mean length of conventional hospitalization was significantly different between ASLND and ALND groups [3.8 days (σ = 1.4) versus 6.4 days (σ = 3.2), respectively; P = 0.0001]. In one center, ASLND was an outpatient procedure for 15 patients accounting for a lower cost (€2215, σ = 1475). This small group did not allow any statistical consideration.
The rate of surgical reoperation was significantly higher in the ASLND groups than ALND groups [30% (253/817) versus 17% (24/140); P = 0.001]. ALND patients have on average more significant axillary complications than ASLND patients: 16% (23/140) versus 6% (49/817); P = 0.0001. The ALND most frequent complication was seroma aspiration inducing need for additional medical or nurse visits.
All active patients were on sick leave after surgery during radiotherapy.
total direct medical costs.
The global unit costs are summarized in Table 5.
Table 5.
Comparison of unit costs for each phase of procedure
| Techniques |
||
| ASLND (in €) | ALND (in €) | |
| Preoperative phase | ||
| Consultation with surgeon | 44 | 44 |
| Consultation with anesthesiologist | 44 | 44 |
| Preoperative diagnosis | ||
| With punction or cytopunction ultrasound guided | 19 | 19 |
| Microbiopsy ultrasound guided | 77 | 77 |
| Macrobiopsy with Mammotome® (or Vacora®) ultrasound guided | 531 | 531 |
| Macrobiopsy with Mammotome® (or Vacora®) radiology guided | 512 | 512 |
| Preoperative reperage | ||
| Ultrasound guided wire | 38 | – |
| Stereotactic guided wire | 82 | – |
| Isotope injection in nuclear medicine | 48 | – |
| Scintigraphy | 230 | – |
| Operative phase and hospitalization | ||
| Patent blue dye injection | 8.4 | – |
| Intraoperative examination | 71 | – |
| Operative room occupation (according to average times observed in two groups) | 853 | 922 |
| Histopathological analysis (HES immunohistochemistry examination) | 138 | 103 |
| Conventional hospitalization (daily cost in breast surgery department) | 340 | 340 |
| Ambulatory hospitalization (daily cost for outpatient surgery) | 200 | 200 |
| Follow-up phase | ||
| Complications (daily cost) | 505 | 505 |
| Rehospitalization for complications (according to length of stay observed in our study) | ||
| For breast margins and others | 2651 | 1010 |
| For axillary | 3030 | – |
ASLND, axillary sentinel lymph node dissection; ALND, axillary lymph node dissection; HSE, hematoxylin eosin saffron.
Considering ASLND group, it appears that the cost generated for a patient with an ASLND, with one preoperative scintigraphy, a combined method for ASLND, an intraoperative pathological analysis and no standard lymphadenectomy, was lower than the cost of a patient with a standard lymphadenectomy [€2947 (σ = 580) versus €3331 (σ = 902); P = 0.0001]. The costs of the main subgroups are summarized in Table 6. Patients with a complementary lymphadenectomy in one single surgical procedure, thanks to intraoperative analysis, involved a lower cost than the cost observed for patients undergoing ASLND and ALND in two different surgical procedures [€4032 (σ = 852) versus €5325 (σ = 1232); P = 0.0001]. IHC has resulted in a relative additional cost of definitive pathological analysis [€138 (σ = 61) versus €103 (σ = 66); P = 0.01].
Table 6.
Total hospital cost at 1 month
| Type of subgroups | Mean (standard deviation) | P global | |
| Strategy ASLND (n = 817) | ASLND alone (n = 496) | €2947 (580) | P = 0.0001 |
| ASLND and ALND in the second time (n = 131) | €5325 (1232) | ||
| ASLND and ALND in the same time (n = 56) | €4032 (852) | ||
| Strategy ALND (n = 140) | ALND (n = 116) | €3331 (902) |
Patients with reintervention for breast complications were excluded.
ASLND, axillary sentinel lymph node dissection; ALND, axillary lymph node dissection.
Sick leaves were linked with systematic postoperative radiotherapy and not with postoperative morbidity. Sick leaves were ineffective for loss of productivity assessment.
sensibility analysis
All parameters were fixed, 30% daily hospital cost variation as well as operating room cost variation; the incremental cost remains significantly different between two strategies.
discussion
In the current study, we showed that ASLND, with the following recommendations of the combined detection method, one preoperative scintigraphy and intraoperative pathological examination of sentinel nodes, without sentinel node involvement, was less expensive than ALND.
sentinel lymph node concept is characterized by a heterogeneous use of the recommended methods
The current study has revealed the heterogeneity of clinical practices with direct and indirect cost impacts: isotopic methods, choice of injected colloids, number of preoperative lymphoscintigraphy, pathological method with IHC, intraoperative analysis and type of hospital stay and its duration.
Isotopic detection method induces costs (colloids preparation, scintigraphy and handed gamma probe) but allows a high detection rate, ∼94% to 97%, rarely reached with blue dye alone [7]. Scintigraphy allows to find extra axillary nodes, which is not part of recommendations [8]. In the context of an exclusive axillary search for sentinel nodes, the use of a systematic scintigraphy is controversial. Even in the case of negative scintigraphy, Dupont et al. [9] found 84.5% isotopic detection rate. In the series of McMasters et al. [10], the detection rate was 89% in the group of 248 patients with a preoperative scintigraphy and 92% in the group of 240 patients without preoperative scintigraphy. Systematic scintigraphy may be used for learning curve period and for overweighed patient [11]. For experienced surgeons, seeking only axillary sentinel nodes, avoiding a systematic preoperative scintigraphy may allow to reduce the cost of sentinel node technique, about €230 in the current study.
Intraoperative pathological analysis induces a moderate cost for an important benefit. It allows to carry out the complementary lymphadenectomy in case of lymph node involvement at the same time avoiding rehospitalization for secondary lymphadenectomy. Recently, Kaminski et al. [12] found a surcharge of $10 000 per patient in the group of patients who did not undergo any intraoperative pathological analysis. Intraoperative false-negative rate is ∼9% to 50% for frozen section and 5%–70% for cytological apposition, higher for micrometastasis than for macrometastasis [13]. Introducing intraoperative IHC staining of the sentinel node biopsy allows some diagnosis of micrometastasis intraoperatively, with an overall cost saving by reducing rehospitalization rate [14]. Intraoperative analysis may be avoided in case of very low risk of sentinel node involvement [15].
Intraoperative molecular analysis dramatically increases the diagnosis of sentinel node involvement, reducing the risk of complementary lymphadenectomy [16]. The relative overcost of this technique should be evaluated.
The question of clinical interest of detecting isolated cells remains controversial and a complementary lymphadenectomy in this case is not yet a warranted standard procedure [17].
hospital stay is the cost driver
In a recent meta-analysis of axillary lymphadenectomy, the length of hospital stay was ranged from 2.9 to 6.0 days [18].
Reducing the length of hospital stay to 2 days or less, thanks to ASLND strategy, results in a reduction of total cost when compared with ALND strategy [€3164 (σ = 1404) for 2 days in ASLND strategy versus €3394 (σ = 1397) for 5 days in ALND strategy; P < 0.05].
The length of stay after ALND is essentially due to the suction drain, systematically placed into the axilla by the surgeon, which is more infrequent after ASLND [19]. Axillary surgery without any drain makes easier outpatient surgery [20]. On the contrary, the simple option of early delivery with the axillary drain in situ brings hidden costs from hospital such as nurses and general practitioners’ visits [21].
Hospital stay is the cost driver of cost comparison between ASLND and ALND when other costs are stabilized. Thus, reducing hospital stay, directly with a policy of short hospital stay after axillary lymphadenectomy or indirectly by reducing the need for secondary axillary lymphadenectomy, reduces the cost of ASLND. In the current study, the ASLND strategy remains less expensive than systematic lymphadenectomy even though the hospital stay for lymphadenectomy is reduced to 3 days. The development of screening campaigns, inducing the diagnosis of smaller tumors with low risk of lymph node involvement leading a low risk of secondary lymphadenectomy, highlights the economical benefit of sentinel node detection [22].
comparison and comparability of our results with other studies
Our study is the first prospective multi-institutional study that compares the two studied strategies, ALND and ASLND, with the micro-costing technique with 1 month follow-up in the two groups, including nearly a thousand patients.
Perrier et al. [23] carried out a retrospective cost comparison of 48 patients with an ASLND and 43 patients with an ALND based on total medical cost assessment. They observed a significant lower cost for ASLND as compared with ALND. As in our study, the total cost for ASLND decreased even further for patients who underwent ASLND alone. In comparison with our study, the same proportion of production process is observed. Hospital stay represented 57% of the cost of ASLND and 37% of ALND cost.
Chirikos et al. [24] have used the method of modelization to evaluate the cumulative cost for ASLND procedure compared with ALND without follow-up after the day of axillary surgery. In conclusion, these authors found that ASLN did not reduce the cost of treatment, probably because they did not include postoperative follow-up in their study. In 2004, Ronka et al. [25] carried out a prospective analysis of the costs of a series of 237 patients with ASLND without follow-up after surgery. As a comparison, they used the theoretical cost of ALND based on the hypothesis that patients with ALND have exactly the same length of hospital stay as patients with ASLND. They stated that ASLND brings 24% overcost as compared with ALND [25]. This study shows that global cost evaluation of an innovative surgical technique with possible impact on patient follow-up, pain and morbidity rate must include the postoperative period. Unfortunately, in our study, active patients were on sick leave after surgery during radiotherapy; thus, it was ineffective for indirect cost evaluation.
conclusion
ASLND strategy enables to determine the axillary status of a patient treated for an early breast cancer with a lower cost than ALND. Our observational economic evaluations have pointed heterogeneous practices. Therefore, this study helps in a more efficient allocation of hospital resources.
Intraoperative pathological analysis, allowing to reduce the rate of rehospitalization for secondary lymphadenectomy, must be recommended. The interest of a systematic preoperative scintigraphy must be questioned. Any initiative leading to reduce the length of hospital stay should be encouraged, as avoiding the systematic use of suction drain in the axilla or the development of 1-day surgery.
funding
National Cancer Institute grant dedicated to economic studies of innovative techniques.
disclosure
The authors have declared no conflicts of interest with this study.
Acknowledgments
Sandra Pelissier, Institut Curie, Paris; Cecile Simondi, Institut Curie, Paris; Catherine Buron, Hospices Civils de Lyon, Lyon; Marianne Doz, CEMKA, Paris; Myriam Benamor, Institut Curie, Paris; Christine Sagan, University Hospital Laennec, Nantes; Delphine Loussouarn, University Hospital Laennec, Nantes; Gaetan Mac Grogan, Institut Bergonié, Bordeaux; Françoise Bonichon, Institut Bergonié, Bordeaux; Florent Cachin, Center Jean Perrin, Clermont-Ferrand; Frédérique Penault LLorca, Center Jean Perrin, Clermont-Ferrand; Laurent Arnould, Center GF Leclerc, Dijon; Alina Riedinger, Center GF Leclerc, Dijon; Véronique Cabaret, Center Oscar Lambret, Lille; Philippe Carpentier, Center Oscar Lambret, Lille; Isabelle Treilleux, Center Leon Berard, Lyon; Francesco Giammarile, Center Leon Berard, Lyon; Jocelyne Jacquemier, Center Paoli Calmettes, Marseille; Isabelle Brenot-Rossi, Center Paoli Calmettes, Marseille; Marie-Christine Château, Center Val d'Aurelle, Montpellier; Marie-Claude Eberle, Center Val d'Aurelle, Montpellier; Agnes Leroux, Center Alexis Vautrin, Vandoeuvre les Nancy; Jean-Claude Mayer, Center Alexis Vautrin, Vandoeuvre les Nancy; Francette Ettore, Center A Lacassagne, Nice; Jacques Darcourt, Center A Lacassagne, Nice; Jean-Michel Picquenot, Center H Becquerel, Rouen; Pierre Vera, Center H Becquerel, Rouen; Jean-Pierre Ghnassia, Center P Strauss, Strasbourg; Olivier Schneegans, Center P Strauss, Strasbourg; Eliane Mery, Center C Regaud, Toulouse; Frederic Courbon, Center C Regaud, Toulouse; Françoise Bertrand, Center R Huguenin, Saint Cloud; Jean-Louis Alberini, Center R Huguenin, Saint Cloud; Claude Rigaud, Center J Godinot, Reims; Roland Amir, Center J Godinot, Reims.
A list of where and when the study has been presented in part elsewhere, if applicable—San Antonio breast cancer symposium, poster session no. 1021, 2009.
References
- 1.Hill C, Doyon F. The frequency of cancer in France in year 2002, and trends since 1968] Bull Cancer. 2006;93:7–11. [PubMed] [Google Scholar]
- 2.Turner RR, Ollila DW, Krasne DL, et al. Histopathologic validation of the sentinel lymph node hypothesis for breast carcinoma. Ann Surg. 1997;226:271–276. doi: 10.1097/00000658-199709000-00006. discussion 276–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mansel RE, Fallowfield L, Kissin M, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst. 2006;98:599–609. doi: 10.1093/jnci/djj158. [DOI] [PubMed] [Google Scholar]
- 4.Benson JR, della Rovere GQ. Management of the axilla in women with breast cancer. Lancet Oncol. 2007;8:331–348. doi: 10.1016/S1470-2045(07)70103-1. [DOI] [PubMed] [Google Scholar]
- 5.Lyman GH, Giuliano AE, Somerfield MR, et al. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol. 2005;23:7703–7720. doi: 10.1200/JCO.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Rodier JF, Velten M, Wilt M, et al. Prospective multicentric randomized study comparing periareolar and peritumoral injection of radiotracer and blue dye for the detection of sentinel lymph node in breast sparing procedures: FRANSENODE trial. J Clin Oncol. 2007;25:3664–3669. doi: 10.1200/JCO.2006.08.4228. [DOI] [PubMed] [Google Scholar]
- 7.Krag DN, Weaver DL, Alex JC, et al. Surgical resection and radiolocalization of the sentinel lymph node in breast cancer using a gamma probe. Surg Oncol. 1993;2:335–339. doi: 10.1016/0960-7404(93)90064-6. discussion 340. [DOI] [PubMed] [Google Scholar]
- 8.Victorzon M, Hamalainen E, Svartback M, et al. Extra-axillary sentinel node biopsy in breast cancer staging—is it necessary? Eur J Surg Oncol. 2003;29:604–606. doi: 10.1016/s0748-7983(03)00101-x. [DOI] [PubMed] [Google Scholar]
- 9.Dupont EL, Kamath VJ, Ramnath EM, et al. The role of lymphoscintigraphy in the management of the patient with breast cancer. Ann Surg Oncol. 2001;8:354–360. doi: 10.1007/s10434-001-0354-4. [DOI] [PubMed] [Google Scholar]
- 10.McMasters KM, Wong SL, Tuttle TM, et al. Preoperative lymphoscintigraphy for breast cancer does not improve the ability to identify axillary sentinel lymph nodes. Ann Surg. 2000;231:724–731. doi: 10.1097/00000658-200005000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goyal A, Newcombe RG, Mansel RE, et al. Role of routine preoperative lymphoscintigraphy in sentinel node biopsy for breast cancer. Eur J Cancer. 2005;41:238–243. doi: 10.1016/j.ejca.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Kaminski JP, Case D, Howard-McNatt M, et al. Sentinel lymph node intraoperative imprint cytology in patients with breast cancer—costly or cost effective? Ann Surg Oncol. 2010;17(11):2920–2925. doi: 10.1245/s10434-010-1130-0. [DOI] [PubMed] [Google Scholar]
- 13.Cserni G, Amendoeira I, Apostolikas N, et al. Pathological work-up of sentinel lymph nodes in breast cancer. Review of current data to be considered for the formulation of guidelines. Eur J Cancer. 2003;39:1654–1667. doi: 10.1016/s0959-8049(03)00203-x. [DOI] [PubMed] [Google Scholar]
- 14.Holm M, Paaschburg B, Balslev E, et al. Intraoperative immunohistochemistry staining of sentinel nodes in breast cancer: clinical and economical implications. Breast. 2008;17:372–375. doi: 10.1016/j.breast.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Canavese G, Bruzzi P, Catturich A, et al. Intra-operative evaluation of the sentinel lymph node for T1-N0 breast-cancer patients: always or never? A risk/benefit and cost/benefit analysis. Eur J Surg Oncol. 2010;36:737–744. doi: 10.1016/j.ejso.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 16.Cutress RI, McDowell A, Gabriel FG, et al. Observational and cost analysis of the implementation of breast cancer sentinel node intraoperative molecular diagnosis. J Clin Pathol. 2010;63:522–529. doi: 10.1136/jcp.2009.072942. [DOI] [PubMed] [Google Scholar]
- 17.Reed J, Rosman M, Verbanac KM, et al. Prognostic implications of isolated tumor cells and micrometastases in sentinel nodes of patients with invasive breast cancer: 10-year analysis of patients enrolled in the prospective East Carolina University/Anne Arundel Medical Center Sentinel Node Multicenter Study. J Am Coll Surg. 2009;208:333–340. doi: 10.1016/j.jamcollsurg.2008.10.036. [DOI] [PubMed] [Google Scholar]
- 18.Droeser RA, Frey DM, Oertli D, et al. Volume-controlled vs no/short-term drainage after axillary lymph node dissection in breast cancer surgery: a meta-analysis. Breast. 2009;18:109–114. doi: 10.1016/j.breast.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Roses DF, Brooks AD, Harris MN, et al. Complications of level I and II axillary dissection in the treatment of carcinoma of the breast. Ann Surg. 1999;230:194–201. doi: 10.1097/00000658-199908000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Classe JM, Dupre PF, Francois T, et al. Axillary padding as an alternative to closed suction drain for ambulatory axillary lymphadenectomy: a prospective cohort of 207 patients with early breast cancer. Arch Surg. 2002;137:169–172. doi: 10.1001/archsurg.137.2.169. discussion 173. [DOI] [PubMed] [Google Scholar]
- 21.Bundred N, Maguire P, Reynolds J, et al. Randomised controlled trial of effects of early discharge after surgery for breast cancer. BMJ. 1998;317:1275–1279. doi: 10.1136/bmj.317.7168.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cady B, Stone MD, Schuler JG, et al. The new era in breast cancer. Invasion, size, and nodal involvement dramatically decreasing as a result of mammographic screening. Arch Surg. 1996;131:301–308. doi: 10.1001/archsurg.1996.01430150079015. [DOI] [PubMed] [Google Scholar]
- 23.Perrier L, Nessah K, Morelle M, et al. Cost comparison of two surgical strategies in the treatment of breast cancer: sentinel lymph node biopsy versus axillary lymph node dissection. Int J Technol Assess Health Care. 2004;20:449–454. doi: 10.1017/s0266462304001345. [DOI] [PubMed] [Google Scholar]
- 24.Chirikos TN, Berman CG, Luther SL, et al. Cost consequences of sentinel lymph node biopsy in the treatment of breast cancer. A preliminary analysis. Int J Technol Assess Health Care. 2001;17:626–631. doi: 10.1017/s026646230110718x. [DOI] [PubMed] [Google Scholar]
- 25.Ronka R, Smitten K, Sintonen H, et al. The impact of sentinel node biopsy and axillary staging strategy on hospital costs. Ann Oncol. 2004;15:88–94. doi: 10.1093/annonc/mdh019. [DOI] [PubMed] [Google Scholar]