Abstract
Background:
Bevacizumab is a monoclonal antibody against vascular endothelial growth factor. Severe pulmonary haemorrhage (PH) is a rare but serious potential adverse event associated with bevacizumab therapy for advanced non-squamous non-small-cell lung cancer (NSCLC).
Methods:
A panel of expert oncologists, pulmonologists and radiologists reviewed the available data to identify predictive factors for PH in order to help guide physicians using bevacizumab in patients with NSCLC.
Results:
Patients with NSCLC are at an increased risk of PH owing to the underlying disease process. Patients with squamous histology and/or a history of grade ≥2 haemoptysis (≥2.5 ml per event) should not receive bevacizumab. No clinical or radiological features (including cavitation and central tumour location) reliably predict severe PH in bevacizumab-treated patients. Major blood vessel infiltration and bronchial vessel infiltration, encasement and abutting may predict PH; however, standardised radiological criteria for defining infiltration have not been established. Eligibility for bevacizumab is not affected by patient age, performance status or anticoagulation or antiplatelet therapy.
Conclusions:
An individualised risk–benefit assessment should be undertaken in all patients with NSCLC in whom bevacizumab is being considered. Further research is required to elucidate the mechanisms underlying PH and the clinical risk factors.
Keywords: bevacizumab, carcinoma, haemorrhage, haemoptysis, non-small-cell lung cancer, safety
introduction
Bevacizumab is a recombinant, humanised monoclonal antibody against vascular endothelial growth factor (VEGF) [1]. Bevacizumab is currently the only anti-angiogenesis agent approved for the treatment of lung cancer [1, 2]. In randomised phase II and III clinical trials, bevacizumab prolonged overall survival [3, 4] and progression-free survival [3–7] when added to standard platinum-based chemotherapy regimens for the first-line treatment of patients with recurrent or advanced (stage IIIB or IV) non-squamous NSCLC. European guidelines recommend that bevacizumab is included within the first-line treatment of selected patients with stage IV non-squamous NSCLC [8]. The USA National Cancer Institute also advises that certain patients with non-squamous NSCLC may benefit from bevacizumab [9]. Other anti-angiogenesis drugs are under evaluation for the treatment of NSCLC, but their value has yet to be established [10, 11].
The tolerability profile of bevacizumab in patients with NSCLC has been documented in phase III/IV trials and the Avastin Regimens: Investigation of treatment Effects and Safety (ARIES) observational cohort study [3, 5, 12–14; Dansin E, Cinieri S, Garrido P et al. (unpublished data)]. Concerns exist regarding the small increased risk of clinically significant (grade ≥3) or fatal (grade 5) pulmonary haemorrhage (PH) in patients treated with bevacizumab and other anti-VEGF agents. Two meta-analyses have found that the use of bevacizumab in combination with chemotherapy for the treatment of various tumour types conferred a significantly increased risk of severe and fatal bleeding events [15] and treatment-related mortality [16] versus chemotherapy alone. However, these meta-analyses pooled phase II and III studies of first- and second-line therapy and did not take account of the evolution in patient selection criteria that has been associated with a reduction in the risk of PH during first-line bevacizumab therapy [3, 5, 12–14; Dansin E, Cinieri S, Garrido P et al. (unpublished data)].
PH is distressing and potentially life threatening [17, 18]. Concerns and misunderstandings regarding the risk of severe PH during bevacizumab therapy for non-squamous NSCLC, and on the risk : benefit ratio of treatment, may cause clinicians to inappropriately avoid the use of the drug in patients who might benefit from it. Accordingly, a panel of expert oncologists, pulmonologists and radiologists reviewed the available data to identify predictive factors for PH in order to help guide physicians using bevacizumab in this setting.
PH in patients with NSCLC
Lung cancer accounts for ∼20% to 30% of cases of PH, as manifested by haemoptysis [19–21]. Haemoptysis is among the most frequent presenting symptoms of lung cancer, being reported in ∼30% to 60% of patients in case series [22, 23]. However, while patients with NSCLC at increased risk of PH, there are few robust data on its incidence in untreated patients. According to one retrospective case series, non-life-threatening PH occurred in 16.0% of 877 patients with lung cancer and was fatal in 3.3% [17]. Massive PH was significantly associated with squamous cell tumours, cavitation and with bronchial (versus peripheral) tumours [17]. Other estimates suggest that up to 90% of PH events occur from the bronchial artery [24].
The pathophysiology of PH in patients with NSCLC is poorly understood. Suggested mechanisms include neovascularisation, exposure of blood vessels by exfoliation of surface tumour cells, tumour necrosis, trauma from cough and invasive procedures (e.g. bronchoscopy) and the formation of airway-vascular fistulae [25].
PH in bevacizumab-treated patients
incidence and severity
Life-threatening PH occurred in 6 of 67 (9·0%) patients with NSCLC treated with bevacizumab during a phase II trial (AVF0757g); four events were fatal [26]. All six patients had ‘centrally located tumours close to major blood vessels’; five had cavitation or tumour necrosis and four had squamous cell carcinomas. There was only a small increased risk of grade ≥3 bleeding (4%) in patients with non-squamous tumours [26].
Phase III studies of bevacizumab in patients with NSCLC—the Eastern Cooperative Oncology Group (ECOG) 4599 and the Avastin in Lung study (AVAiL)—excluded patients with predominantly squamous cell tumours (i.e. >50% of squamous cells in the sample used for histological diagnosis by a pathologist) and those with significant PH (i.e. haemoptysis of ≥2.5 ml per event). Criteria concerning haemorrhagic diseases and anticoagulation therapy were also employed [3, 5]. AVAiL also excluded patients with tumours invading or abutting major blood vessels, based on a local radiological assessment [5]. In ECOG 4599, grade ≥3 PH was reported in eight (1.9%) bevacizumab recipients and was fatal in five (1.2%) (Table 1). One grade 3 event (0.2%) occurred in the chemotherapy group [3]. In AVAiL, grade ≥3 PH was reported in five patients (1.5%) treated with bevacizumab 7.5 mg/kg, three (0.9%) treated with 15.0 mg/kg and two (0.6%) who received chemotherapy alone. The incidences of fatal PH in these groups were 1.2%, 0.9% and 0.3%, respectively [5].
Table 1.
Bleeding event rates in studies of bevacizumab in the treatment of advanced non-small-cell lung cancer
| Event type | Grade | Phase II | Phase III | Phase IV | ||||||||
| AVF0757g [26] | ECOG 4599 [3, 40] | AVAiL [5, 7] | SAiL [12, Dansin E, Cinieri S, Garrido P et al. (unpublished data)] | ARIES [14] | ||||||||
| B 7.5 mg/kg + CP (n = 32) | B 15 mg/kg + CP (n = 35) | CP (n = 32) | B 15 mg/kg + CP (n = 427) | CP (n = 440) | B 7.5 mg/kg + CG (n = 330) | B 15 mg/kg + CG (n = 329) | CG (n = 327) | B 7.5 or 15 mg/kg + chemoa (n = 2212) | B + chemoa (n = 1489) | |||
| All bleeding events, n (%) | ≥3 | 5 (15·6) | 1 (2·9) | 0 | 19 (4.4)* | 3 (0.7) | 14 (4.2) | 14 (4.3) | 6 (1.8) | 80 (3.6) | 50 (3.4) | |
| PH, n (%) | ≥3 | 5b (15·6) | 1 (2·9) | 0 | 8 (1.9) | 1 (0.2) | 5 (1.5) | 3 (0.9) | 2 (0.6) | 15 (0.7) | 13 (0.9) | |
| 5 | 4 (6·0) | 0 | 5 (1.2) | 0 | 4 (1.2) | 3 (0.9) | 1 (0.3) | 8 (0.4) | 4 (0.3) | |||
| Central tumour | ||||||||||||
| Definition | — | Not defined | Within 2 cm of bronchus and main and lobar bronchi | Not defined | Not defined | <2.0 cm between central-most tumour edge and trachea, main bronchi and lobular bronchi | ||||||
| Grade ≥3 PH incidence according to central location, n/N (%) | ||||||||||||
| Central | ≥3 | 5b/ND (ND)c | 1/ND (ND)c | 0 | 3/ND (ND)d | ND | 4/ND (ND)e | 4/578 (0.7) | 9/731 (1.2) | |||
| Non-central | ≥3 | 0 | 0 | 0 | 3/ND (ND)d | ND | 6/ND (ND)e | 11/1633 (0.7) | 4/758 (0.5) | |||
| Cavitation | ||||||||||||
| Grade ≥3 PH incidence according to cavitation, n/N (%) | ||||||||||||
| Cavitation | ≥3 | 5/ND (ND)f | 2/ND (ND)d | ND | ND | ND | ND | 0/56 (0) | 3/127 (1.4) | |||
| No cavitation | ≥3 | 0 | 0 | 4/ND (ND)d | ND | ND | ND | ND | 15/2155 (0.7) | 10/1272 (0.8) | ||
Chosen at discretion of investigator/physician.
Includes two cases described as haematemesis.
All patients with grade ≥3 PH events had central tumour location. Publication provides no data on the total number of patients with and without central tumours.
Data from retrospective analysis [40]. Publication provides no data on the total number of patients with/without central tumours and with/without cavitation.
Across all study arms, 4/10 (40%) of grade ≥3 events occurred in patients with central tumours. No data on the total number of patients with and without central tumours are given.
Five of six patients with grade ≥3 events had cavitation or necrosis of tumours, either at baseline or developing during bevacizumab therapy, but data according to dose group and overall rates of cavitation are not published.
*P < 0.001 versus chemotherapy group.
ARIES, Avastin Regimens: Investigation of treatment Effects and Safety; AVAiL. Avastin in Lung; B, bevacizumab; CG, cisplatin plus gemcitabine; CP, carboplatin plus paclitaxel; ECOG, Eastern Cooperative Oncology Group; ND, no data; PH, pulmonary haemorrhage; SAiL, Safety of Avastin in Lung.
According to the Safety of Avastin in Lung cancer (SAiL) study (MO19390) [12] and the observational ARIES registry [13, 14], rates of severe PH in clinical practice are low and similar to those in phase III trials. SAiL was a single-arm phase IV study performed in 400 centres in 40 countries [12]. Like AVAiL, SAiL excluded patients with a history of significant PH (haemoptysis of ≥2.5 ml per event) and radiological evidence of a tumour invading or abutting major blood vessels. However, a broader patient range was recruited, e.g. in terms of age, chemotherapy regimens, performance status and anticoagulation. At baseline in SAiL, 578 (26.1%) of 2212 patients treated had a centrally located tumour and 56 (2.5%) had cavitation. Overall, grade ≥3 bleeding events occurred in 80 patients (3.6%) and were fatal in 17 patients (0.8%). Bleeding caused the temporary interruption of bevacizumab treatment in 28 of 1347 events (2.0%) and its permanent cessation in 110 events (8.2%). Grade ≥3 PH occurred in 15 patients (0.7%), a rate considered by the authors to be in the normal background range. Eight patients (0.4%) died from PH events [12]. The ARIES cohort also represents a broader population than that treated in clinical trials. According to preliminary data from 1489 patients treated in ARIES, 131 (8.8%) had an ECOG performance status of two or more, 107 (7.2%) had a history of haemoptysis and 282 patients (18.9%) were aged ≥75 years. To date, severe PH has occurred in 13 patients (0.9%) and was fatal in four cases (0.3%) [14].
risk factors
The mechanisms by which anti-VEGF agents induce bleeding are not well understood. It may result from the inhibition of the physiological endothelial repair processes mediated by VEGF [27]. Pathological changes due to cancer, e.g. tumour erosion of vessels, may also be important [28]. Phase II data suggested that centrally located tumours close to major blood vessels might be linked to severe PH during bevacizumab therapy. However, this was based on only six cases and these tumour characteristics were not defined according to standardised radiological criteria [26]. Indeed, the validation of any relationship between PH and tumour centrality or vessel invasion, and the application of exclusion criteria for patient selection, is hampered by the lack of precise, standardised and well-accepted radiological definitions of tumour centrality or vessel invasion. Furthermore, any distinction between tumour centrality and vessel invasion is complicated by their interrelationship, one being a risk factor for the other.
Thoracic computed tomography (CT) is the principal tool in evaluating NSCLC and eligibility for bevacizumab [29–31]. However, CT has limited accuracy in differentiating mediastinal invasion from anatomic contiguity in lung cancer [32–36] (Figures 1 and 2). Similar problems have been reported during the development of CT-based grading systems for the prediction of haemorrhage risk in patients with pancreatic cancer [37, 38]. Multidetector CT may have advantages in this regard, but the clinical impact of this technology has not been assessed.
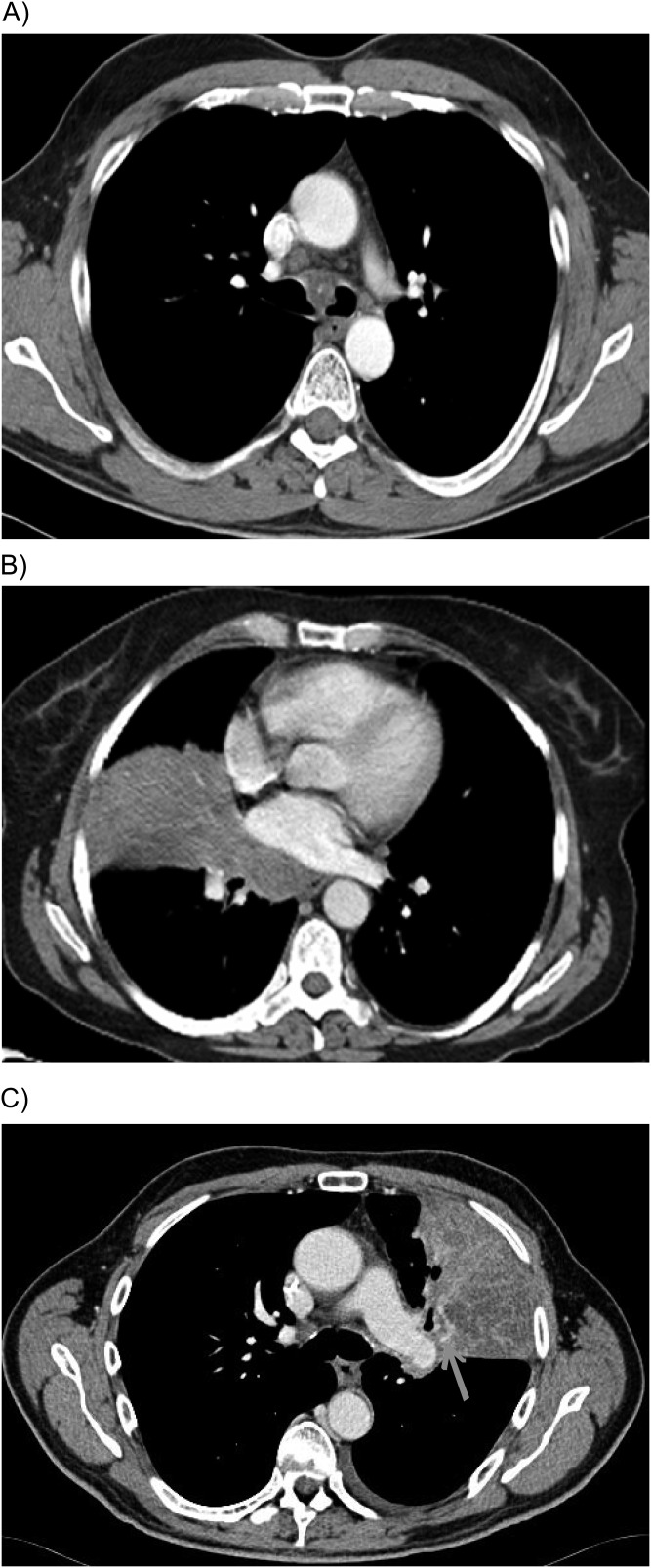
Figure 1.
Thoracic computed tomography images that show no clear vessel invasion by non-small-cell lung tumours. (A) Central tumour with infiltration of main carina and both main bronchi but no evident infiltration of large central blood vessels; (B) Central tumour with direct infiltration of the mediastinum but no clear infiltration of central blood vessels; (C) Central tumour touching without sign of pulmonary artery infiltration. The presence of an atelectasis makes the interpretation difficult and may necessitate multiplanar reconstructions to aid decision making.
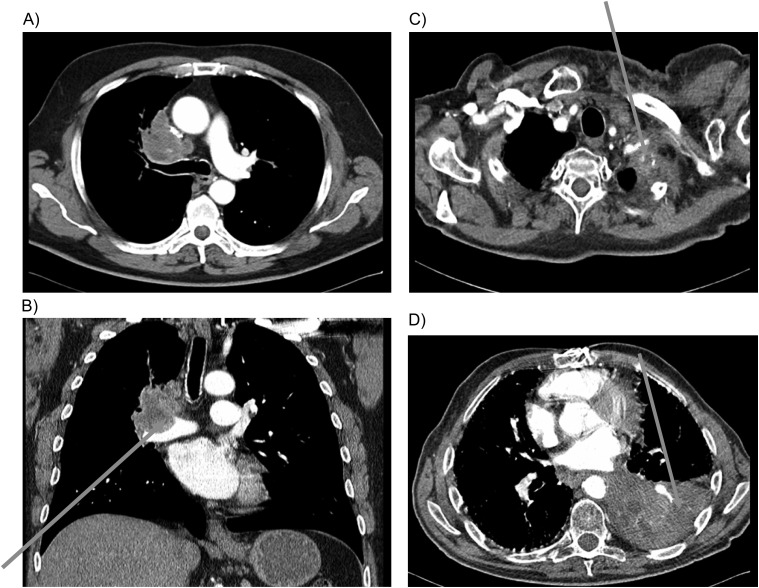
Figure 2.
Thoracic computed tomography images illustrating cases of clear vessel invasion by non-small-cell lung tumours. (A) Central tumour with clear circulation and infiltration of central blood vessels; (B) Concave impression of tumour tissue into the lumen of the pulmonary vessel as sign of vessel infiltration; (C) Diffuse infiltration of subclavian artery by tumour tissue; (D) Diffuse infiltration of left lower pulmonary artery by tumour tissue.
The development of standard radiological criteria for tumour centrality and vessel infiltration is crucial. It is also important to recognise that variations between assessments may occur even when criteria are standardised. Barlesi et al. [39] demonstrated discordance among radiologists and oncologists regarding decisions on the eligibility of patients with NSCLC for bevacizumab therapy based on proposed radiological criteria. In this retrospective multicentre study, radiologists and oncologists assessed proposed eligibility for bevacizumab based on 150 chest CT scans from patients with central NSCLC tumours. Discordance in eligibility decisions occurred in 55% of the scans, with significant differences among physicians independent of speciality (P < 0.05). Intraobserver variations between assessments were greater for oncologists than radiologists. On multivariate analysis, the assessment of the contact between tumour and the vessel was the only criterion significantly related to the risk of discrepancy between physicians [39].
The assessment of bronchial vessel infiltration by bronchoscopy is difficult because a direct infiltration of bronchial vessels located below the surface of the bronchial epithelium cannot be detected by conventional bronchoscopy. New techniques, such as autofluorescence bronchoscopy, might enable the detection of relevant infiltration of bronchial vessels in the future.
central tumour location and vessel invasion.
Although phase II data suggested that central tumour location may be a risk factor for PH in bevacizumab-treated patients [26], subsequent data do not support this conclusion. Sandler et al. [40] carried out a retrospective case–control analysis of grade ≥3 PH events in AVF757g [26] and ECOG 4599 [3]. Patients with PH potentially due to other confounding causes in ECOG 4599 were excluded. Patients with PH were compared with age- and sex-matched bevacizumab-treated controls. The incidence of PH was assessed according to potentially relevant baseline tumour characteristics, i.e. central tumour location (defined as within 2 cm of bronchus) versus peripheral epicentre (within 2 cm of a pleural surface or other location), presence and size of cavitation and longest diameter of largest nodal or tumour mass. Bronchial involvement was assessed according to six categories relating to contact of the tumour with the margin of the tracheobronchial tree, encasement narrowing, presence of a mass with or without consolidation and localised intralumenal soft tissue density. Cavitation was found to be significantly associated with severe PH, as discussed below. However, there was no significant association between PH and lesion location, size or vascular involvement and severe PH, although ‘suspicion of endobronchial involvement’ was a significant risk factor [odds ratio (OR) 12.8; P = 0.024] [40]. These authors concluded that patients with non-squamous NSCLC should not be excluded from receiving bevacizumab therapy based solely on central tumour location. In AVAiL, 4/10 (40.0%) patients with severe PH had tumours described as central, although tumour centrality was not defined prospectively in the protocol [5]. This proportion was similar to the proportion of all patients with central tumours (38%), further suggesting that central tumour location did not increase the risk of PH.
In SAiL, 578 patients (26.1%) had a centrally located lung tumour, although no standardised definition was used. The incidence of PH of any grade was 8.1% in patients with central tumours and 8.6% in those with non-central tumours. The groups with central and non-central tumours were also similar with regard to the rate of grade ≥3 PH (0.7% and 0.7%, respectively) [Dansin E, Cinieri S, Garrido P et al. (unpublished data)] and the proportion of all PH events that were fatal (5.6% and 3.2%, respectively) [41].
In ARIES, central tumours were defined as those showing a distance of <2.0 cm between central-most tumour edge and the trachea, main bronchi and lobular bronchi. As of February 2010, ∼49% of patients (n = 1489) had at least one central tumour at baseline [14]. The rate of grade ≥3 PH in patients with centrally located tumours was 1.2% compared with 0.5% in patients without centrally located tumours (Table 1). Grade ≥3 PH was not significantly associated with central tumour location, the presence or size of cavitations or the size or number of measurable tumours. Although the number of cases is small, this cohort is larger, and hence potentially more reliable, than the original phase II study that raised concerns regarding central tumours and PH risk [26].
cavitation.
Approximately 15% of patients may have cavitations before the initiation of bevacizumab in clinical practice [14]. Approximately 15%–24% of patients with NSCLC develop pulmonary cavitation after treatment with angiogenesis inhibitors (Figure 3) [42, 43]. This is thought to be due to the central necrosis of lesions after inhibition of angiogenesis and appears more likely to occur in squamous cell tumours [43].
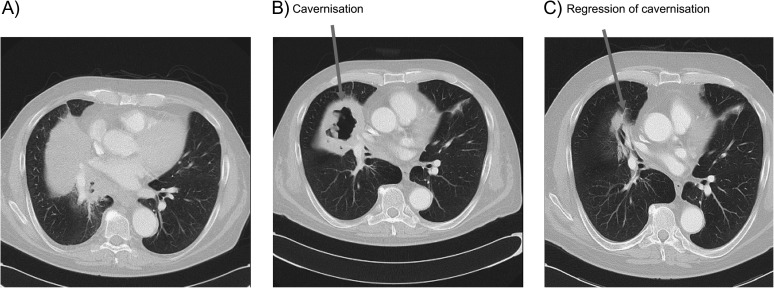
Figure 3.
Thoracic computed tomography illustrating cavitation occurring during bevacizumab therapy. The patient was diagnosed with stage IV cT4N2M1 pulmo-adenocarcinoma and was treated with bevacizumab (7.5 mg/kg) in combination with carboplatin and gemcitabine. (A) Image before therapy; (B) Image after two cycles of bevacizumab plus chemotherapy; (C) After 10 cycles of bevacizumab maintenance therapy.
A retrospective analysis showed that cavitation was the only significant predictive factor for severe PH during bevacizumab therapy [40]. In ECOG 4599, cavitation was present in 2 of 6 patients with severe PH compared with 1 of 29 matched controls (P = 0.034). There was a nonsignificant association between baseline cavitation and severe PH when phase II/III data were pooled. Post-baseline cavitation was associated with PH (P = 0.02).
In contrast, other data do not show a relationship between cavitation and PH during anti-angiogenesis therapy. According to a retrospective single-centre study, pre-existing cavitations were present in 13% of 124 patients treated with anti-angiogenesis agents in clinical trials and in 9% of those with non-squamous tumours [43]. Overall, cavity formation occurred in 16% of patients with no pre-existing cavitations and was more frequent in patients with squamous cell tumours (P = 0.04) and current smokers (P = 0.02). Haemoptysis was associated with tumour size (P = 0.04) but not with pre- or on-treatment cavitation, or with cavity size or cavity : mass ratio, histology or central tumour location. Haemoptysis occurred more commonly with tumours involving a segmental airway or artery rather than a main bronchus or artery [43].
In SAiL, the rates of grade ≥3 PH were similar in patients with (0.0%) and without cavitation (0.7%) [Dansin E, Cinieri S, Garrido P et al. (unpublished data)] (Table 1). Preliminary data from the ARIES cohort also suggest that cavitation does not predict PH during bevacizumab therapy. The rate of grade ≥3 PH in patients with cavitation in measurable tumours was 1.4% compared with 0.8% in patients without cavitation (Table 1). Analysis revealed no significant associations between the presence and size of cavitations [14]. A substudy of 198 patients showed that 25 (12.6%) had cavitation at baseline and 59 (29.8%) developed new or enlarged cavitations during bevacizumab therapy. Presence of pre-existing cavitations, multiple measurable tumours and large tumours were associated with the development of on-study cavitations. Two patients developed severe PH during bevacizumab therapy; neither event was fatal and neither patient had new or enlarged cavitations post-baseline. No statistically significant association was found between the development of on-treatment cavitation and severe PH incidence [44]. These findings, although limited by the small number of events, suggest that neither baseline cavitation nor the development of cavitations alone is a risk factor for severe PH.
other factors.
Phase II evidence suggesting that severe PH during bevacizumab therapy was linked with squamous histology [26] led to the exclusion of patients with predominantly squamous tumours from subsequent trials. However, as squamous cell tumours are more likely to be centrally located and to be cavitated compared with adenocarcinoma, it is not clear whether this histology is an independent risk factor or simply a surrogate marker [26]. The Avastin in Squamous NSCLC (AVASQ) trial (BO19734) evaluated the safety of bevacizumab added to cisplatin–gemcitabine from cycle 2 onwards, as compared with cisplatin–gemcitabine alone, in patients with advanced squamous NSCLC [45]. Eligible patients were at risk of PH owing to the presence of central tumours or peripheral tumours with longest diameter ≥2 cm, which had not been irradiated previously. Patients who had experienced grade ≥2 haemoptysis up to 3 months before study entry were not eligible. All patients received a short course of radiation therapy 3 weeks before starting chemotherapy. The study was terminated early after 2 of the first 20 bevacizumab-treated patients (10.0%) experienced grade ≥3 PH [45]. The implications of these data for current patient selection are unclear owing to the multiple potential PH risk factors present in this population, including squamous histology.
The open-label, single-arm phase II BRIDGE study investigated whether the risk of PH in patients with squamous NSCLC could be reduced by delaying the initiation of bevacizumab and selecting patients without baseline risk factors for PH [45]. Exclusion criteria for BRIDGE included a history of gross haemoptysis and cavitation at baseline. Patients received carboplatin/paclitaxel for two cycles, followed by carboplatin/paclitaxel and bevacizumab in cycles 3–6 and then bevacizumab monotherapy. Grade ≥3 PH occurred in 1 of 31 patients, i.e. 3.2% [45]. Although this incidence was lower than that in the AVF0757g study [26], the use of bevacizumab in squamous NSCLC remains experimental.
The phase III trials of bevacizumab excluded patients with a history of clinically significant PH. More recently, the observational ARIES study did not exclude patients with a history of PH. According to preliminary data, grade ≥3 PH events were reported in 4 of 140 patients with a history of haemoptysis at baseline [2.9%; 95% confidence interval (CI) 0.8% to 7.2%], compared with 11 of 1814 patients (0.6%; 95% CI 0.3% to 1.1%) without a history of haemoptysis [13].
A cohort analysis of phase II and ECOG 4599 data showed no statistically significant association between severe PH and baseline sex, age, ECOG performance score, prior treatment with radiation therapy or prior thoracic surgery [40]. The safety of NSCLC treatments in the elderly is an important consideration, as the mean age lung cancer diagnosis is 70 years [46]. According to a retrospective analysis of ECOG 4599, 1 of 111 (0.9%) bevacizumab recipients aged >70 years experienced grade 3 PH and 2 (1.8%) died from PH [47]. However, in AVAiL, no elderly patients (aged ≥65 years) experienced PH, as compared with frequencies of 1.2% and 1.3% among younger patients treated with bevacizumab 7.5 mg/kg and 15.0 mg/kg, respectively [48]. In SAiL, the incidence of all bleeding was similar in patients aged >65 years (43.0% of 623 patients) and ≤65 years (42.1% of 1589), as was the rate of grade ≥3 PH (0.6% vs 0.7%, respectively) [Dansin E, Cinieri S, Garrido P et al. (unpublished data)]. Also, in ARIES there was no increase in severe PH in elderly patients as compared with the overall population [14]. Taken together, these data suggest that the risk of PH during bevacizumab therapy is not increased in the elderly as compared with younger patients.
The eligibility for bevacizumab is not affected by anticoagulation [1]. Overall, 9% of patients in the AVAiL study received therapeutic anticoagulation post-baseline and no PH events were observed in these patients [5]. Pooled data from three randomised phase III studies showed similar rates of bleeding events with bevacizumab and placebo in patients with NSCLC or metastatic colorectal cancer treated concurrently with full-dose therapeutic anticoagulation [49]. In the SAiL study, 83 patients (3.8%) were receiving anticoagulant therapy at baseline, while 328 patients (14.8%) received anticoagulants during the study. The incidence of bleeding events, including grade ≥3 PH, was similar in patients receiving anticoagulants versus those not receiving anticoagulation therapy [Dansin E, Cinieri S, Garrido P et al. (unpublished data)]. The use of bevacizumab is not affected by antiplatelet therapy and 23.3% of patients in the ARIES cohort were using antiplatelet therapy at the initiation of bevacizumab therapy [14].
practical management of PH in bevacizumab-treated patients
There are few robust data on the management of PH in patients with advanced lung cancer. The incidence of severe PH in patients treated with anti-VEGF agents is low and to our knowledge, no studies have specifically investigated its optimal treatment. As there are no specific recommendations available for the treatment of PH associated with bevacizumab therapy, guidelines for the general management of PH are also used for this purpose.
Severe PH is a medical emergency requiring specialised management and patients with massive bleeding are typically cared for in the intensive care unit [50]. Bevacizumab should be discontinued permanently in patients who experience grade 3 or 4 bleeding during therapy [1]. Supportive measures include airway maintenance with endotracheal intubation, together with cardiorespiratory monitoring, correction of hypoxia, stabilisation of blood pressure and blood transfusion as necessary [25, 30]. Bronchoscopic investigation should be performed, although this has limited accuracy in identifying the source of bleeding. Rigid bronchoscopy is considered most suitable for massive PH, but a flexible instrument is often preferred because it is more convenient and can be used in therapeutic irrigation [30, 50]. A chest CT scan is needed to identify the bleeding site [30]. Conventional endobronchial therapy (irrigation with cold saline or epinephrine solution, according to local guidelines and drug approval status) may have a role, but is thought to have limited effectiveness [25, 50]. Various other bronchoscopic therapies are considered useful, including laser coagulation, electrocautery and argon plasma coagulation [25]. For example, endobronchial argon plasma coagulation was rapidly effective in a study of 56 patients with PH due to lung neoplasms, although the stage of cancer was not stated [51]. Patients with lung cancer were included in another case series in which bronchoscopy-guided haemostatic tamponade was effective in controlling life-threatening PH, but the stage of cancer and the efficacy of therapy in cancer patients was not reported [52].
Bronchial arterial embolisation (BAE) has an important role as first-line therapy for PH, for persistent PH and in preoperative stabilisation [30]. However, data in lung cancer patients are limited [25] and in some cases suggestive of a greater likelihood of recurrence compared with non-cancer patients [53–55]. In one small recent series, BAE provided symptom palliation with an immediate decrease or resolution of bleeding in 24 out of 27 patients (89%). However, survival was significantly lower in patients with tumour-related PH than in those with non-tumour-related bleeding (P = 0.004) [55].
The use of external beam radiation therapy has been recommended for use in the management of non-massive PH caused by unresectable lung cancer [25]. Radiotherapy has been reported to resolve PH in ≥70% of treated patients [56, 57]. The optimal radiotherapy regimen has not been established, although evidence suggests that lower doses or shorter courses (two to five fractions) may be as effective as higher doses or longer courses [56, 57]. Results were recently reported from a large cohort of 1250 patients with NSCLC treated with short-course split-dose palliative radiotherapy, of whom 250 had moderate or severe PH (definition not provided). Treatment was reported to improve or alleviate PH in 68% of patients [58]. Surgery may be considered when BAE is unavailable or ineffective/likely to be ineffective [59] and appears most effective when preceded by non-surgical methods of bleeding control [60]. However, patients with massive PH due to advanced lung cancer are generally not candidates for surgery [25].
discussion and conclusions
Bevacizumab is an important treatment option that extends survival in patients with advanced non-squamous NSCLC. Severe PH is a rare but serious potential adverse effect that may occur during treatment with bevacizumab and other anti-VEGF agents, and which can also be a sequela of NSCLC itself. The criteria by which patients are selected for suitability for bevacizumab have evolved since PH was first observed during its use. In particular, patients with predominantly squamous histology and those with a history of clinically significant PH have been excluded from therapy. These changes have been associated with a reduction in the incidence of severe PH to <1% in the most recent studies of bevacizumab in patients with NSCLC [12, 14].
An individualised risk–benefit assessment should be undertaken in all patients with NSCLC in whom bevacizumab is being considered. However, this assessment is hampered by the lack of robust predictive factors for severe PH. Historical assumptions regarding risk factors for severe PH based on limited phase II data (e.g. central tumour location) have not been confirmed by more recent research involving larger numbers of patients. An important obstacle to further refinements in patient selection criteria is the lack of precise, standardised and well-accepted radiological definitions of tumour centrality the contact between tumour and mediastinal structures. It remains to be seen whether advances in CT scanning can help better define tumour location and invasion.
Further retrospective analyses of PH cases could be useful to explore whether PH is related to a composite of clinical and radiological risk factors. A prospective international observational registry of severe PH cases may also be useful, although there are considerable challenges in establishing and maintaining such a resource. Challenges in establishing the risk factors for severe PH in NSCLC include the small number of cases and the difficulty in collecting accurate and complete information (e.g. due to rapid fatality, a lack of autopsy data and the lack of a standardised data collection procedure). As well as the need for clinical research, preclinical research (e.g. using tumour xenograft models) is also required to elucidate the pathophysiology of PH and its relationship with anti-VEGF agents. Key aspects for such research include the importance of squamous histology and the contributions of vessel permeability, hypertension and vessel wall erosion.
In conclusion, while acknowledging the limitations in the data, this group offers the following consensus conclusions regarding bevacizumab therapy and PH:
Patients with NSCLC are at an increased risk of PH owing to the underlying disease process, although the pathophysiological mechanisms are poorly understood and robust data on the background incidence of clinically significant and severe PH are lacking.
PH is a rare but serious potential adverse event during treatment of non-squamous NSCLC with bevacizumab and other anti-VEGF agents. The mechanisms by which anti-VEGF agents induce PH are unclear and other factors/co-morbidities are insufficient to guide treatment selection.
Patients with predominantly squamous histology and/or history of grade ≥2 PH (defined as bright red blood of ≥2.5 ml within 3 months before randomisation) should not receive bevacizumab, as these groups were excluded from pivotal trials.
No clinical or radiological features (including cavitation and central tumour location) have been shown to be reliable predictive factors for severe PH in bevacizumab-treated patients. Major blood vessel infiltration and bronchial vessel infiltration, encasement and abutting, may predict PH. However, standardised radiological criteria for defining infiltration have not been established. Hypothetically, dilation of the bronchial artery could be a risk factor for PH, but no data exist to support this.
Eligibility for bevacizumab is not affected by patient age, performance status, anticoagulant or antiplatelet therapy.
Oncologists, pulmonologists and radiologists should collaborate closely in the evaluation and management of patients with NSCLC.
The risk : benefit ratio for bevacizumab therapy should be discussed with the patient.
A high-resolution CT scan should be used to assess PH occurring in non-squamous NSCLC patients treated with bevacizumab.
The optimal management of clinically significant PH in patients with advanced non-squamous NSCLC has not been established. Bronchoscopic therapies (e.g. laser coagulation, electrocautery and argon plasma coagulation) and BAE may be useful. Radiotherapy and surgery may have a role in salvage therapy on an individualised basis.
Key areas for future research include the pathophysiology of PH and its relationship with anti-VEGF agents and the clinical risk factors for PH in patients with NSCLC.
It is hoped that this guidance—based on available evidence—helps practising clinicians to undertake an individualised risk–benefit assessment with regard to PH in patients with advanced non-squamous NSCLC in whom bevacizumab therapy is being considered.
funding
This work was supported by F. Hoffman-La Roche, both for third-party writing assistance for this manuscript as well as the Expert Panel meetings that resulted in the development of this paper.
disclosure
All authors (except LC), or their institutions, received an honorarium (from F. Hoffman-La Roche) and reimbursement of travel costs for attending the first Expert Panel meeting that resulted in the development of this paper. All authors received reimbursement for travel costs for attendance at the manuscript review meeting. MR has acted as a consultant (compensated) for Hoffmann-La Roche, Lilly, Pfizer, AstraZeneca and BMS and has accepted honoraria for academic presentations from Hoffmann-La Roche, Lilly and AstraZeneca. FB has acted as a consultant (compensated) for Hoffmann-La Roche. CH is a named inventor on a number of patents and patent applications relating to the evaluation of pulmonary nodules on CT scans of the chest, which are owned by Cornell Research Foundation (CRF). As of April 2009, CH signed away any financial benefit including royalties and any other proceeds related to the patents and patent applications. F. Hoffman-La Roche Ltd provided funding support to the Early Diagnosis and Treatment Research Foundation for the Advisory Board Meeting summarising information presented in this manuscript. CH is an Officer of the Early Diagnosis and Treatment Research Foundation and is not compensated for these services. MR, LC, LI, SS and DS have declared no other conflicts of interest.
References
- 1.Hoffmann-La Roche Ltd. AVASTIN® (Bevacizumab). Summary of Product Characteristics. Welwyn Garden City, UK: Roche Registration Ltd; 2010. [Google Scholar]
- 2.Genentech, Inc. AVASTIN® (Bevacizumab). Solution for Intravenous Infusion, US label. San Francisco, CA: Genentech, Inc; 2010. [Google Scholar]
- 3.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 4.Soria JC. Meta-analysis of randomized phase II/III trials adding bevacizumab to platin-based chemotherapy as first-line treatment in patients with advanced non-small cell lung cancer. Ann Oncol. 2010;21(Suppl 8):viii147. doi: 10.1093/annonc/mds590. (Abstr 437P) [DOI] [PubMed] [Google Scholar]
- 5.Reck M, von PJ, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAiL. J Clin Oncol. 2009;27:1227–1234. doi: 10.1200/JCO.2007.14.5466. [DOI] [PubMed] [Google Scholar]
- 6.Murakami H, Yamamoto N, Kunitoh H, et al. Randomized phase II study of bevacizumab combined with CBDCA-PTX in Japanese patients with advanced non-sq NSCLC. Ann Oncol. 2010;21(Suppl 9):ix11–ix12. (Abstr PL-O3) [Google Scholar]
- 7.Reck M, von PJ, Zatloukal P, et al. Overall survival with cisplatin-gemcitabine and bevacizumab or placebo as first-line therapy for nonsquamous non-small-cell lung cancer: results from a randomised phase III trial (AVAiL) Ann Oncol. 2010;21:1804–1809. doi: 10.1093/annonc/mdq020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D'Addario G, Fruh M, Reck M, et al. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v116–v119. doi: 10.1093/annonc/mdq189. [DOI] [PubMed] [Google Scholar]
- 9.National Cancer Institute. Stage IV Non-Small Cell Lung Cancer. http://www.cancer.gov/cancertopics/pdq/treatment/non-small-cell-lung/healthprofessional/page11. 2011 (16 February 2011, date last accessed) [Google Scholar]
- 10.Horn L, Sandler AB. Angiogenesis in the treatment of non-small cell lung cancer. Proc Am Thorac Soc. 2009;6:206–217. doi: 10.1513/pats.200807-066LC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan R, Kay A, Berg WJ, Lebwohl D. Targeting tumorigenesis: development and use of mTOR inhibitors in cancer therapy. J Hematol Oncol. 2009;2:45. doi: 10.1186/1756-8722-2-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crinò L, Dansin E, Garrido P, et al. Safety and efficacy of first-line bevacizumab-based therapy in advanced non-squamous non-small-cell lung cancer (SAiL, MO19390): a phase 4 study. Lancet Oncol. 2010;11:733–740. doi: 10.1016/S1470-2045(10)70151-0. [DOI] [PubMed] [Google Scholar]
- 13.Wozniak AJ, Garst J, Jahanzeb M, et al. Clinical outcomes (CO) for special populations of patients (pts) with advanced non-small cell lung cancer (NSCLC): results from ARIES, a bevacizumab (BV) observational cohort study (OCS) J Clin Oncol. 2010;28(Suppl):15s. (Abstr 7618) [Google Scholar]
- 14.Kumar P, Fischbach NA, Brahmer JR, et al. Baseline (BL) radiographic characteristics and severe pulmonary hemorrhage (SPH) in bevacizumab (BV)-treated non-small cell lung cancer (NSCLC) patients (pt): results from ARIES, an observational cohort study (OCS) J Clin Oncol. 2010;28(Suppl):15s. (Abstr 7619) [Google Scholar]
- 15.Hapani S, Sher A, Chu D, Wu S. Increased risk of serious hemorrhage with bevacizumab in cancer patients: a meta-analysis. Oncology. 2010;79:27–38. doi: 10.1159/000314980. [DOI] [PubMed] [Google Scholar]
- 16.Ranpura V, Hapani S, Wu S. Treatment-related mortality with bevacizumab in cancer patients: a meta-analysis. JAMA. 2011;305:487–494. doi: 10.1001/jama.2011.51. [DOI] [PubMed] [Google Scholar]
- 17.Miller RR, McGregor DH. Hemorrhage from carcinoma of the lung. Cancer. 1980;46:200–205. doi: 10.1002/1097-0142(19800701)46:1<200::aid-cncr2820460133>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 18.Corey R, Hla KM. Major and massive hemoptysis: reassessment of conservative management. Am J Med Sci. 1987;294:301–309. doi: 10.1097/00000441-198711000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Hirshberg B, Biran I, Glazer M, Kramer MR. Hemoptysis: etiology, evaluation, and outcome in a tertiary referral hospital. Chest. 1997;112:440–444. doi: 10.1378/chest.112.2.440. [DOI] [PubMed] [Google Scholar]
- 20.Santiago S, Tobias J, Williams AJ. A reappraisal of the causes of hemoptysis. Arch Intern Med. 1991;151:2449–2451. [PubMed] [Google Scholar]
- 21.Uzun O, Atasoy Y, Findik S, et al. A prospective evaluation of hemoptysis cases in a tertiary referral hospital. Clin Respir J. 2010;4:131–138. doi: 10.1111/j.1752-699X.2009.00158.x. [DOI] [PubMed] [Google Scholar]
- 22.Hyde L, Hyde CI. Clinical manifestations of lung cancer. Chest. 1974;65:299–306. doi: 10.1378/chest.65.3.299. [DOI] [PubMed] [Google Scholar]
- 23.Chute CG, Greenberg ER, Baron J, et al. Presenting conditions of 1539 population-based lung cancer patients by cell type and stage in New Hampshire and Vermont. Cancer. 1985;56:2107–2111. doi: 10.1002/1097-0142(19851015)56:8<2107::aid-cncr2820560837>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 24.Remy J, Remy-Jardin M, Voisin C. Endovascular management of bronchial bleeding. In: Butler J, editor. The Bronchial Circulation. New York: Dekker; 1992. pp. 667–723. [Google Scholar]
- 25.Kvale PA, Selecky PA, Prakash UB. Palliative care in lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132:368S–403S. doi: 10.1378/chest.07-1391. [DOI] [PubMed] [Google Scholar]
- 26.Johnson DH, Fehrenbacher L, Novotny WF, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2004;22:2184–2191. doi: 10.1200/JCO.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 27.Schmidinger M, Bellmunt J. Plethora of agents, plethora of targets, plethora of side effects in metastatic renal cell carcinoma. Cancer Treat Rev. 2010;36:416–424. doi: 10.1016/j.ctrv.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Kamba T, McDonald DM. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer. 2007;96:1788–1795. doi: 10.1038/sj.bjc.6603813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bidwell JL, Pachner RW. Hemoptysis: diagnosis and management. Am Fam Physician. 2005;72:1253–1260. [PubMed] [Google Scholar]
- 30.Chun JY, Morgan R, Belli AM. Radiological management of hemoptysis: a comprehensive review of diagnostic imaging and bronchial arterial embolization. Cardiovasc Intervent Radiol. 2010;33:240–250. doi: 10.1007/s00270-009-9788-z. [DOI] [PubMed] [Google Scholar]
- 31.Mori H, Ohno Y, Tsuge Y, et al. Use of multidetector row CT to evaluate the need for bronchial arterial embolization in hemoptysis patients. Respiration. 2010;80:24–31. doi: 10.1159/000253882. [DOI] [PubMed] [Google Scholar]
- 32.Herman SJ, Winton TL, Weisbrod GL, et al. Mediastinal invasion by bronchogenic carcinoma: CT signs. Radiology. 1994;190:841–846. doi: 10.1148/radiology.190.3.8115637. [DOI] [PubMed] [Google Scholar]
- 33.Glazer HS, Kaiser LR, Anderson DJ, et al. Indeterminate mediastinal invasion in bronchogenic carcinoma: CT evaluation. Radiology. 1989;173:37–42. doi: 10.1148/radiology.173.1.2781028. [DOI] [PubMed] [Google Scholar]
- 34.Izbicki JR, Thetter O, Karg O, et al. Accuracy of computed tomographic scan and surgical assessment for staging of bronchial carcinoma. A prospective study. J Thorac Cardiovasc Surg. 1992;104:413–420. [PubMed] [Google Scholar]
- 35.Verschakelen JA, Bogaert J, De WW. Computed tomography in staging for lung cancer. Eur Respir J Suppl. 2002;35:40s–48s. doi: 10.1183/09031936.02.00270802. [DOI] [PubMed] [Google Scholar]
- 36.Munden RF, Swisher SS, Stevens CW, Stewart DJ. Imaging of the patient with non-small cell lung cancer. Radiology. 2005;237:803–818. doi: 10.1148/radiol.2373040966. [DOI] [PubMed] [Google Scholar]
- 37.Loyer EM, David CL, Dubrow RA, et al. Vascular involvement in pancreatic adenocarcinoma: reassessment by thin-section CT. Abdom Imaging. 1996;21:202–206. doi: 10.1007/s002619900046. [DOI] [PubMed] [Google Scholar]
- 38.Lu DS, Reber HA, Krasny RM, et al. Local staging of pancreatic cancer: criteria for unresectability of major vessels as revealed by pancreatic-phase, thin-section helical CT. AJR Am J Roentgenol. 1997;168:1439–1443. doi: 10.2214/ajr.168.6.9168704. [DOI] [PubMed] [Google Scholar]
- 39.Barlesi F, Balleyguier C, Besse B, et al. Inter- and intraobserver consistency in assessing eligibility for bevacizumab (BVZ) in non-small-cell lung cancer (NSCLC) patients with centrally located tumors. Ann Oncol. 2010;21:1682–1686. doi: 10.1093/annonc/mdp590. [DOI] [PubMed] [Google Scholar]
- 40.Sandler AB, Schiller JH, Gray R, et al. Retrospective evaluation of the clinical and radiographic risk factors associated with severe pulmonary hemorrhage in first-line advanced, unresectable non-small-cell lung cancer treated with carboplatin and paclitaxel plus bevacizumab. J Clin Oncol. 2009;27:1405–1412. doi: 10.1200/JCO.2008.16.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Griesinger F, Bearz A, Eberhardt W, et al. Safety of first-line bevacizumab (BV)-based therapy in the sail (MO19390) trial: central tumour location (CTL) and hypertension (HTN) in patients (PTS) with advanced non-small cell lung cancer (NSCLC) Ann Oncol. 2010;21(Suppl 8):viii144. [Google Scholar]
- 42.Crabb SJ, Patsios D, Sauerbrei E, et al. Tumor cavitation: impact on objective response evaluation in trials of angiogenesis inhibitors in non-small-cell lung cancer. J Clin Oncol. 2009;27:404–410. doi: 10.1200/JCO.2008.16.2545. [DOI] [PubMed] [Google Scholar]
- 43.Marom EM, Martinez CH, Truong MT, et al. Tumor cavitation during therapy with antiangiogenesis agents in patients with lung cancer. J Thorac Oncol. 2008;3:351–357. doi: 10.1097/JTO.0b013e318168c7e9. [DOI] [PubMed] [Google Scholar]
- 44.Spigel DR, Brahmer JR, Fischbach NA, et al. Risk of severe pulmonary hemorrhage (SPH) associated with development of cavitation while on bevacizumab (BV) therapy in patients (PTS) with non small cell lung cancer (NSCLC): results from ARIES, a BV treatment observational cohort study (OCS) J Thorac Oncol. 2010;5:S84. [Google Scholar]
- 45.Hainsworth JD, Fang L, Huang JE, et al. BRIDGE: an open-label phase II trial evaluating the safety of bevacizumab + carboplatin/paclitaxel as first-line treatment for patients with advanced, previously untreated, squamous non-small cell lung cancer. J Thorac Oncol. 2011;6:109–114. doi: 10.1097/JTO.0b013e3181f94ad4. [DOI] [PubMed] [Google Scholar]
- 46.Coleman MP, Forman D, Bryant H, et al. Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995–2007 (the International Cancer Benchmarking Partnership): an analysis of population-based cancer registry data. Lancet. 2011;377:127–138. doi: 10.1016/S0140-6736(10)62231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramalingam SS, Dahlberg SE, Langer CJ, et al. Outcomes for elderly, advanced-stage non small-cell lung cancer patients treated with bevacizumab in combination with carboplatin and paclitaxel: analysis of Eastern Cooperative Oncology Group Trial 4599. J Clin Oncol. 2008;26:60–65. doi: 10.1200/JCO.2007.13.1144. [DOI] [PubMed] [Google Scholar]
- 48.Leighl NB, Zatloukal P, Mezger J, et al. Efficacy and safety of bevacizumab-based therapy in elderly patients with advanced or recurrent nonsquamous non-small cell lung cancer in the phase III BO17704 study (AVAiL) J Thorac Oncol. 2010;5:1970–1976. doi: 10.1097/JTO.0b013e3181f49c22. [DOI] [PubMed] [Google Scholar]
- 49.Leighl NB, Bennouna J, Yi J, et al. Bleeding events in bevacizumab-treated cancer patients who received full-dose anticoagulation and remained on study. Br J Cancer. 2011;104:413–418. doi: 10.1038/sj.bjc.6606074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haponik EF, Fein A, Chin R. Managing life-threatening hemoptysis: has anything really changed? Chest. 2000;118:1431–1435. doi: 10.1378/chest.118.5.1431. [DOI] [PubMed] [Google Scholar]
- 51.Morice RC, Ece T, Ece F, Keus L. Endobronchial argon plasma coagulation for treatment of hemoptysis and neoplastic airway obstruction. Chest. 2001;119:781–787. doi: 10.1378/chest.119.3.781. [DOI] [PubMed] [Google Scholar]
- 52.Valipour A, Kreuzer A, Koller H, et al. Bronchoscopy-guided topical hemostatic tamponade therapy for the management of life-threatening hemoptysis. Chest. 2005;127:2113–2118. doi: 10.1378/chest.127.6.2113. [DOI] [PubMed] [Google Scholar]
- 53.Hayakawa K, Tanaka F, Torizuka T, et al. Bronchial artery embolization for hemoptysis: immediate and long-term results. Cardiovasc Intervent Radiol. 1992;15:154–158. doi: 10.1007/BF02735578. [DOI] [PubMed] [Google Scholar]
- 54.Swanson KL, Johnson CM, Prakash UB, et al. Bronchial artery embolization: experience with 54 patients. Chest. 2002;121:789–795. doi: 10.1378/chest.121.3.789. [DOI] [PubMed] [Google Scholar]
- 55.Wang GR, Ensor JE, Gupta S, et al. Bronchial artery embolization for the management of hemoptysis in oncology patients: utility and prognostic factors. J Vasc Interv Radiol. 2009;20:722–729. doi: 10.1016/j.jvir.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 56.Fairchild A, Harris K, Barnes E, et al. Palliative thoracic radiotherapy for lung cancer: a systematic review. J Clin Oncol. 2008;26:4001–4011. doi: 10.1200/JCO.2007.15.3312. [DOI] [PubMed] [Google Scholar]
- 57.Beli I, Koukourakis G, Platoni K, et al. Hypofractionated radiotherapy in non small cell lung cancer: a review of the current literature. Rev Recent Clin Trials. 2010;5:103–111. doi: 10.2174/157488710791233608. [DOI] [PubMed] [Google Scholar]
- 58.Reinfuss M, Mucha-Malecka A, Walasek T, et al. Palliative thoracic radiotherapy in non-small cell lung cancer. An analysis of 1250 patients. Palliation of symptoms, tolerance and toxicity. Lung Cancer. 2011;71:344–349. doi: 10.1016/j.lungcan.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 59.Shigemura N, Wan IY, Yu SC, et al. Multidisciplinary management of life-threatening massive hemoptysis: a 10-year experience. Ann Thorac Surg. 2009;87:849–853. doi: 10.1016/j.athoracsur.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 60.Andrejak C, Parrot A, Bazelly B, et al. Surgical lung resection for severe hemoptysis. Ann Thorac Surg. 2009;88:1556–1565. doi: 10.1016/j.athoracsur.2009.06.011. [DOI] [PubMed] [Google Scholar]