Abstract
Prevailing guidelines advocate a low-salt diet to mitigate progression of kidney and cardiovascular disease in patients with diabetes mellitus. However, two recent cohort studies in patients with type 1 and type 2 diabetes mellitus associate lower salt intake with increased rates of end-stage renal disease, cardiovascular death and all-cause mortality.
The so-called “salt wars”1 pit those who view dietary salt as a toxin and favor its restriction as a matter of public policy against those who are skeptical that lowering salt intake would improve public health. The notion that dietary salt is harmful has garnered more backing to the point that authoritative guidelines now recommend a low-salt diet for the population in general and for patients with diabetes mellitus in particular.2 Nonetheless, a credible resistance to the anti-salt movement arose in the 1990s3 and has persisted to date. Recently, the skeptics’ position has been bolstered by two new sets of cohort data that associate a higher mortality and incidence of end-stage renal disease (ESRD) with lower salt intake in patients with diabetes mellitus.4,5
In the first study by Ekinci et al.,4 638 middle-aged Australians with established type 2 diabetes mellitus and a high baseline prevalence of obesity, retinopathy, hypertension, chronic kidney disease and cardiovascular disease were followed up for 10 years, during which time salt intake was monitored by measuring sodium excretion in 24-h urine samples.4 Sodium intake varied between individuals but was relatively stable within individuals over time. Urinary sodium excretion was distributed, with one-third excreting <150 mmol per day and one-third excreting >208 mmol daily. Survival tracked monotonically with urinary sodium; a daily 100 mmol increase in sodium intake predicted a 30% lower cardiovascular and all-cause mortality.4 The second study,5 conducted by the Finnish Diabetic Nephropathy (FinnDiane) study group, reported on 2,807 patients with longstanding type 1 diabetes mellitus and a high prevalence of retinopathy and hypertension. These individuals were also followed up for 10 years, with sodium intake determined by urine collection. The relationship of all-cause mortality to sodium intake was biphasic with a nadir at 100–200 mmol per day, a steep rise at lower salt intake and a gradual rise at higher intake. But the cumulative incidence of ESRD was monotonically associated with sodium intake such that reducing sodium intake from the 90th to the 10th percentile mapped to a 10-fold increase in the likelihood of developing ESRD (P<0.001).
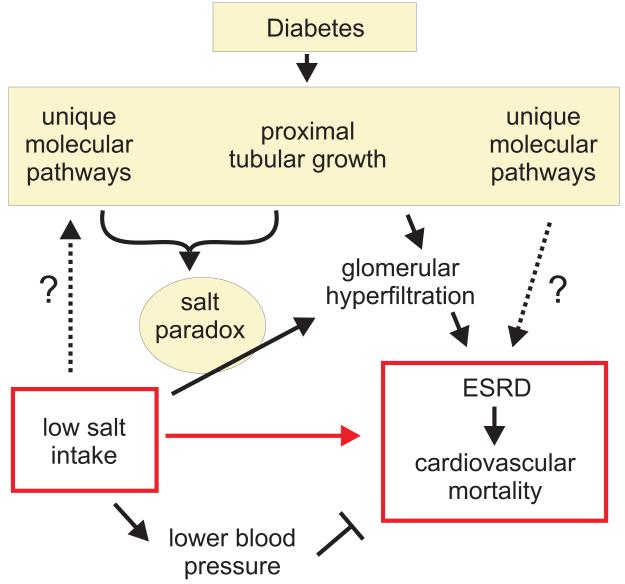
How might these findings arise from (patho)physiology (Figure 1)? First, some unmeasured trait that distributes randomly in diabetes mellitus might simultaneously suppress salt appetite while independently damaging the kidney and heart. Given how little is known about the regulation of salt appetite in humans, it is not currently possible to reject this hypothesis without an intervention trial. Second, another idea, which does not rely so heavily on ignorance, is that the diabetic kidney and cardiovascular system could be predisposed to damage by counter-regulatory nerves and hormones that are progressively activated by a decline in extracellular volume. For example, both the renin–angiotensin–aldosterone system (RAAS) and the sympathetic nervous system are naturally activated by salt restriction and are known for their ability to damage the heart and kidney. The argument that the salutary effect of salt restriction on blood pressure could be outweighed by the nefarious effects of provoking the RAAS and sympathetic nervous system has been made before and is noted by both Ekinici et al. and the FinnDiane study group. This effect is not exclusive to diabetes mellitus, but diabetic organs may be particularly susceptible to injury when the ratio of counter-regulators to dietary salt intake increases.
Figure 1.
A conceptual framework that links a low salt intake to end-stage renal disease (ESRD) and cardiovascular mortality via the salt paradox in the diabetic kidney—that is, an inverse relationship between salt intake and glomerular hyperfiltration.
Another mechanism could make the diabetic kidney vulnerable to chronic damage on a low-sodium diet. This phenomenon is unique to diabetes mellitus and involves an anomalous tendency for the glomerular filtration rate (GFR) in the diabetic kidney to vary inversely with salt intake, as observed in rodents and humans with diabetes mellitus. An inverse relationship between dietary salt and GFR is counterintuitive with regard to salt homeostasis and, therefore, we refer to it as the salt paradox.6,7 Much of the evidence for the salt paradox has been gained in animal models and patients with type 1 diabetes mellitus, as little information is available on type 2 diabetes mellitus. The salt paradox occurs because diabetes mellitus sensitizes proximal tubular reabsorption to changes in dietary salt such that eating less salt causes proximal reabsorption to increase. This increase in proximal reabsorption lessens the amount of fluid and NaCl reaching the macula densa. This leads, in turn, to an increase in GFR through the normal process of tubuloglomerular feedback, a mechanism that serves to stabilize salt delivery to the further distal nephron. In fact, salt delivery to the distal nephron is reduced among the subset of diabetics with glomerular hyperfiltration. Hyperfiltration with decreased distal salt delivery cannot occur together without a primary increase in proximal reabsorption, which suggests that excessive proximal reabsorption promotes diabetic hyperfiltration in animal models and humans.6,7,8 A low-salt diet can also augment diabetic kidney growth, and patients with diabetes mellitus and large hyperfiltering kidneys are predisposed to renal failure later in life.7,9 This principle was recently confirmed in a cross-section of older patients with longstanding diabetes mellitus and an estimated GFR <60 ml/min, among whom those with larger kidneys were more likely to develop ESRD after 5 years than those who entered the study with smaller kidneys.10 Hence, kidney size and relative hyperfiltration remain identifiable risk factors for progression to ESRD in the types of patients studied by the FinnDiane and Australian groups, and a low-sodium diet could act through the salt paradox to augment kidney growth and hyperfiltration in these individuals.
The enhanced sensitivity of proximal tubular reabsorption to dietary salt is related to diabetic tubular growth.7 This growth includes aspects of cell proliferation, hypertrophy, and cellular senescence, a phenomenon that is observed in old cells or to prevent replication of damaged DNA. These tubular cells may have lost the programming of a differentiated proximal tubule cell, which includes not responding to moderate changes in dietary salt. The molecular signature of this growth phenotype has been linked to inflammation, which may contribute to the development of renal damage.7 Further studies are needed to establish the influence of dietary salt on these molecular pathways in the diabetic kidney.
In summary, two new cohort studies suggest that salt restriction could do more harm than good in patients with diabetes mellitus. Effects of sodium intake from obligatory intervention trials in diabetes mellitus are obviously called for. Meanwhile, established features of the diabetic kidney make these novel findings seem less surprising. It will be interesting to see how much weight the FinnDiane and Australian reports receive in future dietary guidelines.
Acknowledgments
Work by the authors was supported by the NIH (R01DK56248, R01HL094728, P30DK079337), the American Heart Association (GRNT3440038) and the Department of Veterans Affairs.
References
- 1.Tierney J. Salt wars. The New York Times TierneyLab Blog [online] 2010 http://Tierneylab.blogs.nytimes.com/2010/02/22/salt-wars/ [Google Scholar]
- 2.Bantle JP, et al. Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2008;31(Suppl. 1):S61–S78. doi: 10.2337/dc08-S061. [DOI] [PubMed] [Google Scholar]
- 3.Taubes G. The (political) science of salt. Science. 1998;281:898–901. 903–907. doi: 10.1126/science.281.5379.898. [DOI] [PubMed] [Google Scholar]
- 4.Ekinci EI, et al. Dietary salt intake and mortality in patients with type 2 diabetes. Diabetes Care. 2011;34:703–709. doi: 10.2337/dc10-1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas MC, et al. The association between dietary sodium intake, ESRD, and all-cause mortality in patients with type 1 diabetes. Diabetes Care. 2011;34:861–866. doi: 10.2337/dc10-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomson SC, Vallon V, Blantz RC. Kidney function in early diabetes: the tubular hypothesis of glomerular filtration. Am. J. Physiol. Renal. Physiol. 2004;286:F8–F15. doi: 10.1152/ajprenal.00208.2003. [DOI] [PubMed] [Google Scholar]
- 7.Vallon V. The proximal tubule in the pathophysiology of the diabetic kidney. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011;300:R1009–R1022. doi: 10.1152/ajpregu.00809.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pruijm M, et al. Glomerular hyperfiltration and increased proximal sodium reabsorption in subjects with type 2 diabetes or impaired fasting glucose in a population of the African region. Nephrol. Dial. Transplant. 2010;25:2225–2231. doi: 10.1093/ndt/gfq008. [DOI] [PubMed] [Google Scholar]
- 9.Mogensen CE. Early glomerular hyperfiltration in insulin-dependent diabetics and late nephropathy. Scand. J. Clin. Lab. Invest. 1986;46:201–206. doi: 10.3109/00365518609083660. [DOI] [PubMed] [Google Scholar]
- 10.Rigalleau V, et al. Large kidneys predict poor renal outcome in subjects with diabetes and chronic kidney disease. BMC Nephrol. 2010;11:3. doi: 10.1186/1471-2369-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]