Abstract
Background
Socio-communicative impairments are salient features of autism spectrum disorder (ASD). Abnormal development of posterior superior temporal sulcus (pSTS), a key processing area for language, biological motion, and social context, may play a role in these deficits.
Methods
Functional connectivity MRI (fcMRI) was used to examine the synchronization of low frequency BOLD fluctuations during continuous performance on a visual search task. Twenty-one children and adolescents with ASD and 26 typically developing (TD) individuals, matched on age, sex, and IQ, participated in the study. Three subregions of pSTS were delineated with a data-driven approach, and differentiation of pSTS was examined by comparing the connectivity of each subregion.
Results
In TD individuals, differentiation of networks was positively associated with age and anatomical maturation (cortical thinning in pSTS, greater white matter volume). In the ASD group, differentiation of pSTS connectivity was significantly reduced and correlations with anatomical measures were weak or absent. Moreover, pSTS differentiation was inversely correlated with autism symptom severity.
Conclusions
Atypical maturation of pSTS suggests altered trajectories for functional segregation and integration of networks in ASD, potentially related to impaired cognitive and sensorimotor development. Furthermore, our findings provide a novel explanation for atypically increased connectivity in ASD observed in some fcMRI studies.
Keywords: intrinsic connectivity, low frequency BOLD fluctuations, cortical thickness, white matter volume, development, functional segregation
Introduction
In the past decade, the neurobiology of autism spectrum disorder (ASD) has come to be characterized with respect to abnormal neural connectivity and altered neurodevelopmental trajectories (1). For the detection of functional networks, many ASD studies have applied functional connectivity MRI (fcMRI) to assess temporal correlations of the blood oxygen level-dependent (BOLD) signal. FcMRI can either focus predominantly on task-evoked BOLD effects or on spontaneous BOLD fluctuations. In task-related fcMRI studies, functional connectivity describes the synchronization of task-driven activation effects between areas recruited for task performance. Spontaneous fluctuations, on the other hand, have most often been studied in a task-free, resting-state condition.
Synchronized spontaneous BOLD fluctuations during rest were first observed by Biswal and colleagues (2) in the motor system. Such “resting-state networks” may be more aptly called intrinsic connectivity networks (ICNs), as a large repertoire of connectivity patterns subserving domain-general and some domain-specific processes can be extracted from resting BOLD signals (3, 4). Slow spontaneous fluctuations of ICNs have been observed during sleep (5) and anesthesia (6), indicating that they exist separately from stimulus-dependent cognition. Notably, ICNs are simultaneously present during task performance and may equal task-evoked BOLD responses in amplitude (7). Low frequency BOLD (LF-BOLD) synchronizations are also strongly associated with anatomical connectivity (8, 9), although they are not constrained to monosynaptic relationships and connectivity may be modified with persistent use (10–13). Spontaneous fluctuations could therefore be a distinct neurobiological mechanism that reflects frequent and long-standing Hebbian co-activation, which supports the fundamental functional significance of LF-BOLD synchronizations (14). Task-driven fcMRI studies in ASD have generally found reduced coordination between areas of activation involving higher-order processing (15–19). Other fcMRI studies examining whole-brain effects have uncovered regions of partially enhanced functional connectivity in ASD (20–24).
One brain region reported as abnormal in ASD is the posterior superior temporal sulcus (pSTS). Situated at the temporo-parietal junction, pSTS receives polymodal input and subserves integrative functions that rely on convergent sensory processes (25). The STS in general participates in networks involved in socio-communicative processes such as language, biological motion, and intention understanding (26), which may be impaired in ASD (27–30). Abnormalities of the STS in ASD have been observed in anatomical studies (31–34) and have been indicated by reduced activation for socio-communicative tasks in PET (35) and fMRI studies (36). In addition, fMRI studies have detected abnormally enhanced activation in STS for tasks that do not activate STS in TD individuals (17, 28). Such atypical involvement across tasks may suggest abnormal recruitment of neurons in STS and reduced functional specificity. Task-evoked fcMRI studies have revealed reduced synchronization between pSTS and other regions, such as medial prefrontal cortex for theory of mind processing (17) and fusiform gyrus for face processing in ASD (18). One rs-fcMRI study examining LF-BOLD fluctuations in default mode network areas in ASD found greater intrinsic connectivity between posterior cingulate cortex and middle STS that positively correlated with autism symptom severity scores (37). However, no neuroimaging study in ASD has specifically investigated LF-BOLD fluctuations in pSTS.
Although low frequency fcMRI studies typically examine ICNs at rest, individual and systematic differences in intrinsic connectivity have been attributed to differences in cognitive states and spontaneous thoughts (10, 38–40), which may present serious confounds in participants with ASD. Several studies have indeed observed differences in mentation between ASD and TD individuals (41–44). As an alternative approach, fMRI data acquired during task engagement are suitable for the study of intrinsic connectivity, since network-specific, LF-BOLD signals can be computationally separated from modeled task-evoked BOLD responses (7, 45, 46). However, residual effects of task may still remain in the time series; therefore, the approach of examining LF-BOLD fluctuations from task-related data may be more comparable to low frequency steady-state fcMRI (ss-fcMRI) (47). In contrast to resting-state, ss-fcMRI attempts to control for inter- and intra-individual variations in spontaneous thought processes through continuous task performance, thus offering an alternative to examining ICNs when systematic differences in mentation between groups cannot be ruled out.
In typical development, the shaping of synaptic connectivity patterns through experience-based selective pruning and stabilization results in the emergence of distinctly organized functional networks, associated with domain-specific learning and functional efficiency (48). On the local level, this maturational process is accompanied by increasing functional differentiation (49). Although the sculpting of connectivity patterns and the emergence of local functional specialization are often examined separately, they are more adequately understood as two aspects of the same developmental process of network constitution. Any local cortical site thus achieves functional specialization through its unique afferent and efferent connectivity patterns (50, 51). While this view of typical brain development is generally accepted, surprisingly little experimental evidence is available addressing potential disturbances of these basic developmental mechanisms in ASD.
Using ss-fcMRI to examine LF-BOLD fluctuations, we identified three functional subdivisions in pSTS with a data-driven approach and investigated their individual patterns of connectivity. Based on fMRI findings of atypical activation in pSTS, we hypothesized diminished functional differentiation within the pSTS region in ASD, accompanied by reduced segregation of networks and increased connectivity with regions outside of typical networks. Further, we expected that in the TD brain, anatomical maturation would be associated with increases in functional differentiation within pSTS, whereas the links between the developmental trajectories of structure and function would be disturbed in ASD.
Methods and Materials
Participants
Twenty-one high-functioning children and adolescents with ASD and 26 TD participants, matched on age, verbal IQ, and nonverbal IQ, were included (Table 1). IQ was assessed using the Wechsler Abbreviated Scale of Intelligence (WASI; 52) and hand preference using the Edinburgh Handedness Inventory (53). Informed consent was collected from all participants in accordance with the Institutional Review Boards of University of California, San Diego, and San Diego State University. Diagnosis of ASD was established using Autism Diagnostic Interview-Revised (ADI-R; 54), Autism Diagnostic Observation Schedule (ADOS; 55), and expert clinical opinion. Children with autism-related medical conditions (e.g. Fragile-X, tuberous sclerosis, epilepsy) were excluded. Participants in the TD group had no reported personal or family history of autism, autism-related symptoms, or any other neurological or psychiatric conditions.
Table 1.
Demographic and Diagnostic Information
| TD | ASD | p | |
|---|---|---|---|
| N (male) | 26 | 21 | n/a |
| Handedness | 24 Right; 2 Left |
19 Right; 2 Left |
n/a |
| Age in years* | 14.3 (2.9) 8–19 |
14.3 (2.8) 8–18 |
.92 |
| Verbal IQ* | 110.5 (13.0) 74–133 |
108.2 (16.8) 79–147 |
.87 |
| Nonverbal IQ* | 112.1 (12.1) 85–129 |
111.8 (13.7) 70–131 |
.80 |
| Full Scale IQ* | 112.6 (13.2) 77–140 |
111.4 (15.5) 73–141 |
.93 |
| ADOS Algorithm Score* | |||
| Communication | n/a | 3.5 (1.9) 0–6 |
n/a |
| Social reciprocity | n/a | 7.7 (2.6) 3–13 |
n/a |
| Repetitive | n/a | 1.7 (1.5) 0–5 |
n/a |
Values for age, IQ, and ADOS scores are presented as Mean (SD) and Range. Significance value, p, from independent t-tests for differences between groups. IQ scores were missing for two individuals in the TD and ASD group. ADOS scores were not available for one individual.
MRI Acquisition
Imaging data were acquired on a GE 3T HD Signa Excite scanner with an 8-channel head coil. High-resolution structural images were acquired with a standard FSPGR T1-weighted sequence (TR: 11.08-ms; TE: 4.3-ms; flip angle: 45°; field of view [FOV]: 256-mm; 256×256 matrix; 172 slices; 1-mm3 resolution). Functional T2*-weighted images were obtained using a single-shot gradient-recalled, echo-planar pulse sequence. Four 5:20 minute runs were acquired, each including 128 volumes of 39 or 40 interleaved 3.2-mm axial slices (TR: 2.5-s; TE: 30-ms; flip angle: 90°; FOV: 220-mm; 64×64 matrix; 3.4 mm2 in-plane resolution).
Data Preprocessing
Functional images were processed using the Analysis of Functional NeuroImages software (AFNI; 56). The first four time points of each run were discarded and slice-time correction was performed. Due to the potential sensitivity of our analysis to effects of head motion, several precautions were taken. 1) To reduce interpolation-related blurring, motion correction of functional images and co-registration to the anatomical image were performed in one combined transformation matrix. 2) Data were spatially smoothed to a global full-width-at-half-maximum (FWHM) of 5-mm using AFNI’s 3dBlurtoFWHM, which incrementally smooth and estimates the FWHM value at each step. 3) Six rigid-body motion parameters were modeled as nuisance variables and removed with regression. 4) Time points with excessive motion, estimated as the magnitude of displacement from one time point to the next (Equation S1), were censored. 5) Each participant’s total motion over the entire session was quantified as the root mean square of displacement (Equation S2). Total motion was not significantly different between groups (p > .05). Nonetheless, comprehensive post hoc analyses of head motion were performed to confirm the effects observed in the present study (see Supplement).
We examined LF-BOLD fluctuations from fMRI data acquired during continuous performance on a visual search task, presented in an event-related design. There were generally no significant group differences in performance (see Supplement). To isolate the LF-BOLD signal, we applied a band-pass filter (.008<f<.08-Hz) (57) and modeled orthogonal task regressors (46, 58–60). Specifically, to model stimulus-evoked BOLD effects as accurately as possible for removal, we estimated variable-shape (rather than fixed-shape) response functions for each trial-type by interpolating seven cubic spline basis functions over 15 seconds.
Linear effects attributable to scanner drift, the mean BOLD signal time series in white matter, and six motion parameters were included in the general linear model. The mean white matter signal was extracted with a binary mask, derived from Freesurfer’s automated segmentation output (excluding the cerebellum) and shrunk by 3 voxels in all directions (61). All participants had at least 180 remaining time points (corresponding to 7.5 minutes), which is sufficient to produce reliable ICNs (62). There were no differences between groups (p > .05). For group analysis, images were standardized to the N27 Talairach template (63). Intensity normalization was performed on each run.
Identification of Subdivisions in PSTS
A flow chart of the entire process for investigating pSTS subregions is available as Figure S1 in the Supplement. Anatomical images were processed and labeled by Freesurfer’s automated segmentation and cortical parcellation algorithm version 4.5.0 (61). The superior and inferior banks of the STS, parcellated by Freesurfer according to the Desikan-Killiany cortical atlas (64), were selected in each hemisphere in native anatomical space as our pSTS region-of-interest (ROI). We further performed a connectivity-based parcellation of pSTS, which identified three functional subdivisions (Figure S2 in the Supplement). A data-driven procedure was applied, as functional parcellation of pSTS in the developing brain is not well understood (for details on methods, see Supplement).
Functional Connectivity of PSTS Subregions
Conjunction Analysis of Whole-Brain FcMRI Effects
At the individual-subject level, we obtained the intrinsic connectivity maps of each pSTS subregion by correlating its average time series with every voxel in the brain. Fisher’s r-to-z' transformations were applied and one-sample t-tests were performed to construct group-wise connectivity maps for each seed. For qualitatively visualizing within-group ICNs, the maps were uniformly thresholded across all six seeds and included a minimum cluster size of 10 neighboring voxels. Different thresholds for the TD (p < 1×10−8) and ASD (p < 6.5×10−5) groups were chosen so that the average number of voxels showing connectivity with rostral- and caudal-pSTS were approximately equal, as those seeds were derived from the same principle component (Figure S2 in the Supplement). Between-group comparisons of whole-brain effects were performed with two-sample independent t-tests for each pSTS seed separately. Group differences in connectivity were protected against false positives with Monte-Carlo simulations (65). All six pSTS connectivity maps were binarized and combined to examine conjunction effects.
Connectional Fingerprints
We constructed fingerprints (51) by depicting a subregion’s strength of connectivity with all regions that showed significant connectivity with any of the three pSTS subregions. A 600-µl sphere was placed at coordinates centered on peak connectivity (t > 10) across all fcMRI effects in the TD group (Table 2 and Tables S5-S10 in the Supplement).
Table 2.
Regions of Greatest Connectivity in pSTS Networks of the Typically Developing Group
| Region of interest (Brodmann area) | Peak t | Talairach coordinates |
||
|---|---|---|---|---|
| x | y | z | ||
| Right hemisphere regions | ||||
| Caudate Nucleus | 19.6 | 11 | 5 | 15 |
| Middle Temporal Gyrus, V5/MT+ (37) | 17.6 | 44 | −62 | 9 |
| Medial Superior Frontal Gyrus (6) | 16.9 | 8 | 48 | 36 |
| Middle Cingulate Cortex (31) | 15.7 | 2 | −23 | 45 |
| Precuneus (7) | 15.1 | 2 | −50 | 42 |
| Superior Frontal Gyrus (8) | 13.5 | 26 | 23 | 51 |
| Middle Frontal Gyrus (8) | 13.1 | 47 | 11 | 42 |
| Fusiform Gyrus (37) | 13.1 | 29 | −38 | −7 |
| Left hemisphere regions | ||||
| Precuneus (7) | 19.6 | −7 | −53 | 34 |
| Medial Superior Frontal Gyrus (8) | 15.1 | −2 | 29 | 46 |
| Pallidum | 14.4 | −17 | 2 | 9 |
| Precentral Gyrus (6) | 14.4 | −47 | 2 | 39 |
| Middle Cingulate Cortex (24) | 13.9 | −5 | −14 | 39 |
| Inferior Frontal Gyrus (47) | 13.9 | −44 | 23 | −1 |
| Pulvinar | 13.8 | −8 | −26 | 3 |
| Fusiform Gyrus (37) | 13.5 | −44 | −56 | −13 |
| Caudate Nucleus | 13.4 | −11 | 5 | 18 |
Peak t-value obtained from within-TD group functional connectivity maps. See Supplementary Tables S1–6 for a full listing of significant fcMRI effects.
Functional Brain Development
Differentiation of Intrinsic Network Connectivity
In functional brain development, connectional specificity increases as neurons become more specialized to respond to a smaller range of sensory stimuli or cognitive tasks (49). High consistency among the BOLD responses across a large cortical expanse would suggest an immature pattern of connectivity. To examine pSTS within each individual, a differentiation index (DI) was devised as a coarse measure to describe the segregation of intrinsic networks subserved by a region. DI values can range from 0, similarly connected, to 1, distinctly segregated, and is calculated by
| (1) |
which incorporates a commonly used measure of internal consistency, Cronbach’s alpha (66). Alpha is given by
| (2) |
where i,j = {1, …, k}, k is the number of ROIs in a given anatomically defined region (i.e., pSTS), and r̅ij is the average correlation between all ROI pairs. Composite DIs averaged across both hemispheres were computed separately for temporal correlations between time courses and spatial correlations between connectivity maps, DIT and DIS, respectively. The distribution of DI was found to be approximately normal; therefore, two-sample independent t-tests were appropriate.
Anatomical Maturation
To compare functional and anatomical maturation, measures of cortical thickness in pSTS and total cerebral white matter volume were obtained for each participant using Freesurfer’s segmentation and parcellation software (61). To avoid potentially nonlinear effects, the two youngest participants in each group were excluded to restrict the age range to 11–19 years.
Results
Intrinsic Connectivity Networks of PSTS
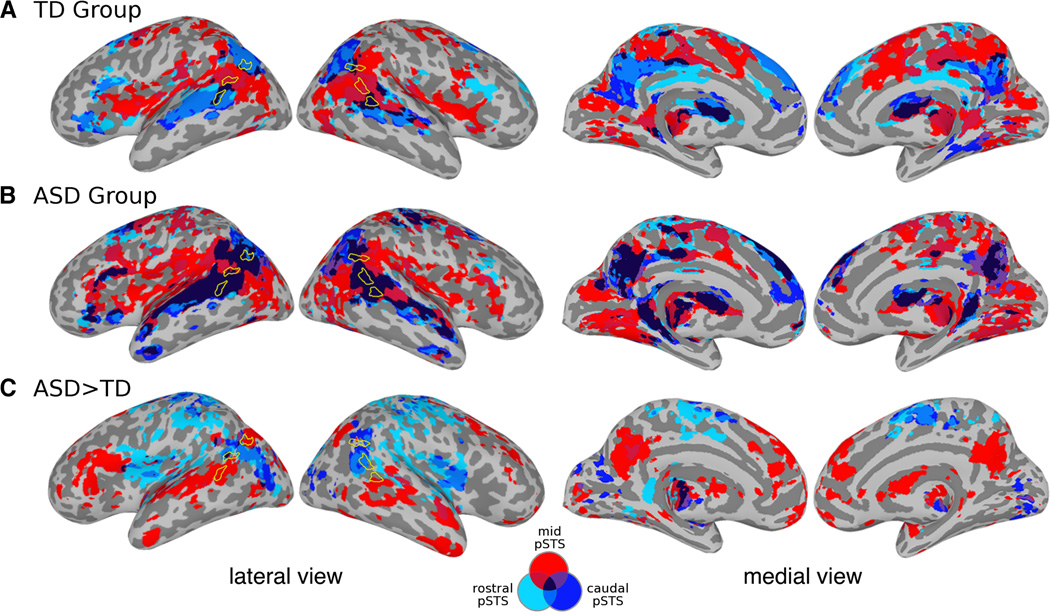
In the TD group, the conjunction analysis of fcMRI effects indicated that rostral-pSTS and caudal-pSTS had similar patterns of connectivity, while mid-pSTS participated in a distinctly different network (Figure 1A). The connectional fingerprints were similar between rostral- and caudal-pSTS and between homotopic seeds in contralateral regions (Figure 2). In the ASD group, extensive three-way conjunction effects indicated greater degrees of overlap among the networks subserved by pSTS (darkest clusters in Figure 1B). On direct-group comparisons of fcMRI effects, significantly greater connectivity in the ASD group compared to the TD group with regions belonging to adjacent pSTS networks suggests that each subregion was functionally less distinct from its neighbor (Figure 1C). No inverse effects (TD > ASD) were detected after correcting for multiple comparisons. Effects for each seed are presented in Tables S5-S10 in the Supplement. Since one study (60) suggested an effect of global signal regression (GSR) on fcMRI group differences, we performed an identical between-group analysis including GSR, which produced similar results (Figure S3 in the Supplement).
Figure 1.
Seed-based connectivity maps show distinct, mid-pSTS connectivity and overlapping rostral–caudal pSTS networks. Connectivity maps for all subregion were combined: light blue = rostral-pSTS, red = mid-pSTS, and dark blue = caudal-pSTS. Color code also indicated in a Venn diagram. Conjunction of whole-brain effects within the TD group (A), within the ASD group (B), and from between-group comparisons of each seed (ASD > TD only) (C). No significant TD > ASD effects were observed after correcting for multiple comparisons (p < 0.05). See Tables S5-S10 in the Supplement for a full listing of significant clusters.
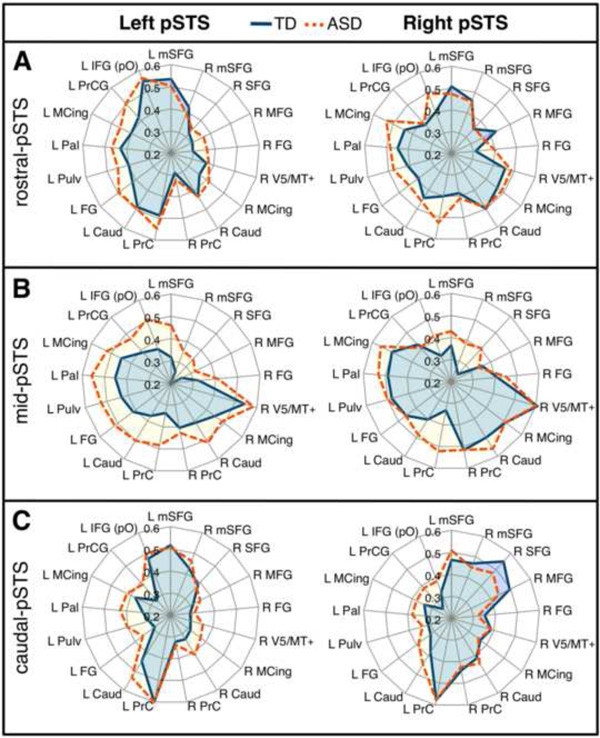
Figure 2.
Connectional fingerprints. PSTS subregions show distinct patterns of connectivity that is relatively consistent between TD (blue) and ASD (orange) groups: rostral-pSTS (A), mid-pSTS (B) and caudal-pSTS (C) of the left and right hemispheres. Radial distances indicate the strength of connectivity (between specified pSTS seed and labeled region from Table 2). There is greater similarity between rostral- and caudal-pSTS and between homotopic seeds. Connectivity in ASD is overall more diffuse, interacting with regions belonging to networks of other distinct subregions. L = left; R = right. Clockwise from mSFG = medial superior frontal gyrus; MFG = middle frontal gyrus; FG = fusiform gyrus; MCing = middle cingulate gyrus; Caud = caudate; PrC = precuneus; Pulv = pulvinar; Pal = pallidum; PrCG = precentral gyrus; IFG (pO) = inferior frontal gyrus pars orbitalis.
Differentiation of PSTS Connectivity with Age
Temporal and spatial differentiation indices (DI) comparing the connectivity of pSTS subregions were computed for each individual. Differentiation was overall decreased in ASD relative to TD participants [DIT: t(45) = 2.43, p = .019; DIS: t(45) = 2.45, p = .018]. As expected given increasing functional differentiation in neurotypical development, we observed positive correlations of age with both DIT [r(24) = 0.60, p = .001] and DIS[r(24) = 0.60, p = .001] in the TD group (Figure S4 in the Supplement). In the ASD group, correlation with age was marginally significant for DIT [r(19) = 0.45, p = .040], but not for DIS [r(19) = 0.39, p = .085]. The difference in slopes for the TD and ASD groups was nonsignificant [z(45) = 0.86, p = .19]. DI was not associated with IQ in either group. In ASD, abnormal responses in pSTS have been associated with impaired socio-communicative functions; therefore, Spearman’s rank correlations between DI and symptom severity were performed. Greater ADOS combined communication and social scores were significantly associated with reduced DIT [ρ(18) = −0.59, p = .006]; a negative relationship with DIS trended toward significance [ρ(18) = −0.43, p = .057].
Relationship between Anatomy and Intrinsic Connectivity
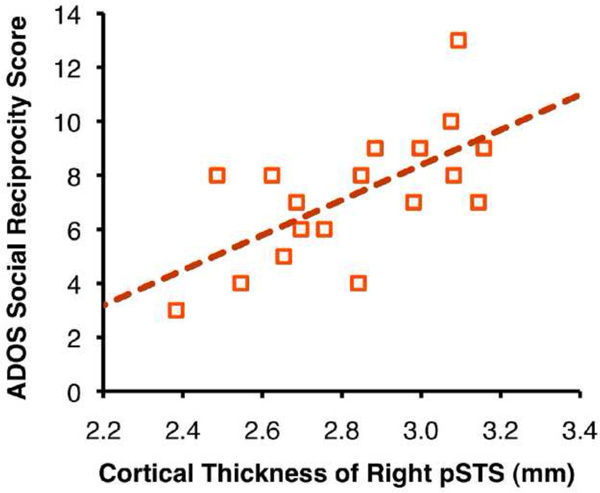
We investigated the relationship of our differentiation indices with anatomical measures of cortical thickness and total white matter volume (Table 3). We observed significant negative correlations between cortical thicknesses in bilateral pSTS and age in the TD group. Increasing differentiation was associated with cortical thinning in pSTS and greater cerebral white matter volume (Figure S4 in the Supplement). The relationship was strongest for spatial differentiation, which supports the notion that functional brain development involves myelination of large-scale networks throughout childhood and adolescence. In the ASD group, we did not observe any significant correlations between our measures for anatomical and functional maturation. We observed, however, a positive relationship between the cortical thickness of right pSTS and ADOS social reciprocity scores [r(16) = 0.64, p = .004] (Figure 3). No significant differences between groups were detected in cortical thickness and white matter volume.
Table 3.
Correlations Between pSTS Differentiation and Anatomical Measures
| TD |
ASD |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cortical thickness |
Total WM volume |
Cortical thickness |
Total WM volume |
|||||||||
| Left pSTS |
Right pSTS |
Left pSTS |
Right pSTS |
|||||||||
| r(22) | p | r(22) | p | r(22) | p | r(17) | p | r(17) | p | r(17) | p | |
| Age | −0.59 | .002 | −0.48 | .018 | 0.47 | .020 | 0.01 | .968 | −0.26 | .282 | 0.07 | .776 |
| DIT | −0.41 | .047 | −0.30 | .154 | 0.44 | .031 | −0.05 | .839 | −0.20 | .412 | 0.02 | .935 |
| DIS | −0.50 | .013 | −0.32 | .127 | 0.66 | .0004 | 0.13 | .596 | −0.10 | .684 | 0.11 | .654 |
Temporal and spatial differentiation indices, DIT and DIS respectively, were computed as coarse measures of functional maturation of pSTS intrinsic connectivity networks. See Supplementary Figure S4 for scatter plots. WM = white matter.
Figure 3.
Scatter plot depicts a relationship between cortical thickness of right pSTS and symptom severity in the ASD group [r(16) = 0.64, p = 0.004].
Discussion
Using ss-fcMRI, we assessed functional differentiation in pSTS by examining low frequency BOLD (LF-BOLD) fluctuations in TD children and adolescents and those with ASD. Three subdivisions (caudal-, mid-, and rostral-pSTS) in each hemisphere were delineated with a data-driven approach, producing six connectional fingerprints with unique topographical characteristics. Comparable fingerprints were seen in the ASD group, although the patterns of connectivity were more diffuse, involving atypical LF-BOLD synchronizations with areas outside of the networks identified for TD participants. In a conjunction analysis combining the fcMRI effect maps of all pSTS seeds, we observed extensively overlapping patterns in the ASD group. Direct group comparisons of functional connectivity maps indicated many areas of increased connectivity in ASD, whereas no inverse effects (TD > ASD) were found. This finding may appear unexpected given numerous previous studies reporting underconnectivity (15–19); however methodological differences, especially the focus on intrinsic BOLD fluctuations in the present study may explain inconsistencies (as discussed in; 67). In general, significantly greater correlations in the ASD group appeared in areas where atypical, between-seed overlap of connectivity was observed in the within-group maps (dark clusters in Figure 1B).
Such overlapping patterns of connectivity suggest impaired functional network differentiation in ASD. We therefore investigated age-dependent correlates of pSTS differentiation and anatomical maturation. The emergence of distinct ICNs may directly relate to functional maturation of connections. We observed significantly lower differentiation of pSTS in participants with ASD. Further, positive associations between differentiation and age were significant in the TD group for both spatial and temporal differentiation, whereas they were marginal to nonsignificant in the ASD group. However, the relationship between age and DI was not significantly different between groups, which may be related to the relatively high functional level of our ASD participants compared to the overall ASD population. Interestingly, inverse relationships between differentiation of pSTS connectivity and autism symptom severity were observed. Correlations were also found between DI and anatomical measures in TD children and adolescents, which were absent in the ASD group. However, cortical thickness of right pSTS positively correlated with ADOS social reciprocity scores, which is consistent with results from Hadjikhani et al. (68) and may reflect impaired regressive mechanisms of cortical development in a region considered crucial for socio-emotional processing (27).
The current evidence suggests that the synchronization of spontaneous BOLD fluctuations may have widespread implications in functional brain development. Consistent with this hypothesis, rs-fcMRI studies have provided support for a model of brain development involving functional segregation of local connections and strengthened integration between distant regions of maturing large-scale networks (69–72). Thus, the differentiation of networks subserved by pSTS may be a developmental processes occurring through late childhood and adolescence that is intimately related to functional maturation and specialization. One mechanism for functional specialization involves tuning of neurons to activate selectively for a specific set of stimuli (73). Analogously, since LF-BOLD synchronization may reflect mechanisms of learning and network development possibly related to Hebbian plasticity (74), increasing specialization via sharpening of neural responses would be associated with more specific and distinct connections between functionally interacting regions.
Reduced connectional specificity in pSTS indicates that the region may be less functionally specialized in ASD. Impaired differentiation of pSTS networks could be reflected in reports of atypically enhanced BOLD responses in STS/pSTS in ASD for nonsocial tasks (17, 28, 43, 44), in addition to findings of reduced STS activation for socio-emotional tasks (18, 36). In the neurotypical brain, pSTS is involved in the processing of language, theory-of-mind, biological motion, faces, gaze perception, intention inferences, and other socio-communicative functions (26, 75). Some of these functions may arise from activity within distinct subregions, while others may require the interaction among subregions. Therefore, it is of note that attributing distinctly specialized functions to the networks observed in the present study (Figure 1) would appear overly ambitious and simplified, particularly since the relationship between LF-BOLD networks and task-specific activation has yet to be fully elucidated. One study employing independent component analysis to compare rs-fcMRI-derived ICNs and BrainMap meta-data from 7,342 fMRI activation maps found overall close correspondence between the two (3). However, some functions involved more than one network and some networks subserved more than one function, which is expected given the limits on localization imposed by spatial resolution in fMRI.
Although the lack of association between functional and anatomical maturation in the ASD group in part reflect greater individual variability often observed in ASD (76) or the existence of ASD subtypes (77), it is probably indicative of aberrant neurobiological mechanisms underlying functional brain development (78). Reductions in pSTS gray matter with age, particularly during late childhood through early adulthood, have been documented by several anatomical studies in typically developing brains (79, 80). However, findings of abnormal gray matter volume in ASD in areas such as the temporal cortices (31) suggests that abnormal regressive and constructive mechanisms may be involved at specific points in development.
Furthermore, deviant maturation of LF-BOLD synchronization in pSTS comes in the context of consistent findings of early white matter overgrowth (33, 81, 82) and abnormal trajectories in cortical development (83, 84). The theory of interactive specialization posits that basic neural scaffoldings are set in place, but further development depends on the ongoing interaction between neurobiological and experience-dependent mechanisms (49, 85, 86). Thus, insufficient social interaction may further hamper the emergence of networks for socio-communicative processes in ASD. Conversely, networks supporting restricted interests may be overdeveloped, occupying the resources of other processes that are recruited less often. Improper input during specific periods of development may lead to disorganized or altered cortical connectivity (87, 88). Altered developmental time courses for functional segregation and integration of networks in ASD may thus result in subtly altered topographical characteristics, especially in functional domains and associated regions that are subject to sensitive or critical periods.
Our approach of using ss-fcMRI allowed us to examine intrinsic spontaneous BOLD fluctuations, while minimizing confounds related to differences in mentation between TD and ASD groups. The statistical removal of task-evoked effects has distinct advantages, providing greater control in isolating intrinsic LF-BOLD fluctuations; however, these fluctuations may subtly differ between resting and task states and subtle effects of task could remain even after task-regression and low-pass filtering. Indeed, the relationship between spontaneous BOLD fluctuations and task-driven BOLD synchronization is likely intertwined, as one can influence the other (11, 89). Arguably the more critical issue, head motion can be a potentially serious confound in fcMRI studies, since it may result in inflated and diminished signal correlations. Although our two groups did not significantly differ in total head motion, we nonetheless implemented several precautionary processing steps and conducted additional analyses to examine any residual effects on our main findings, which were found to be minimal (see Supplement).
The current study is, to our knowledge, the first to present fcMRI results directly addressing the issue of impaired cortical functional differentiation in ASD. Our findings of reduced differentiation in pSTS accompanied by slowed developmental increase in differentiation contribute novel clues to understanding the neurofunctional bases of cognitive and behavioral impairment in ASD. Furthermore, they generate a potential explanation for findings of increased connectivity in ASD relative to age-matched TD groups, as observed in some fcMRI studies (20–24, 37). Reduced local functional differentiation thus appears to be accompanied by more diffuse (less distinct) connectivity patterns, which is consistent with the concept of specialized local function being a reflection of distinctive connectivity (50). Increasing differentiation of network connectivity throughout childhood and adolescence in typical development, as observed in our study, is a reflection of constructive and regressive principles of functional and anatomical maturation (48, 90, 91). In ASD, early maturational schedules appear disturbed, as documented by aberrations in early brain growth, possibly resulting in disruptions of local functional differentiation and associated sculpting of distributed specialized networks. These disruptions result in the appearance of “immature” differentiation of connectivity patterns, as observed for pSTS in this study; however, our use of the term immaturity does not imply any claim that the brain is expected to ultimately reach maturity and full functional differentiation at a later age. Longitudinal studies with a wider age range including older adults will be needed for further elucidation. The lack of typical relationships between measures of functional and anatomical maturation in ASD suggests that functional brain development may be partially deviant, rather than simply delayed, with pervasive alterations in neurodevelopmental trajectories.
Supplementary Material
Acknowledgements
This work was supported by the National Institutes of Health (R01-DC006155, R01-MH081023); and the National Institute on Deafness and Other Communicative Disorders (1T32-DC007361-03 to B.K.). We are very grateful for all the children and families who participated. We would also like to thank Pamela Moses for the insightful discussions on brain and cognitive development and Carlos Bazan for comments on the mathematical appendix.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data from this study were presented at the 2010 International Meeting for Autism Research in Philadelphia, PA and the 2010 annual meeting for the Society for Neuroscience in San Diego, CA.
All authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends Neurosci. 2008;31:137–145. doi: 10.1016/j.tins.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Biswal BB, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 3.Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, et al. Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Damoiseaux JS, Rombouts SARB, Barkhof F, Scheltens P, Stam CJ, Smith SM, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Larson-Prior LJ, Zempel JM, Nolan TS, Prior FW, Snyder AZ, Raichle ME. Cortical network functional connectivity in the descent to sleep. Proc Natl Acad Sci U S A. 2009;106:4489–4494. doi: 10.1073/pnas.0900924106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moeller S, Nallasamy N, Tsao DY, Freiwald WA. Functional connectivity of the macaque brain across stimulus and arousal states. J Neurosci. 2009;29:5897–5909. doi: 10.1523/JNEUROSCI.0220-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fox MD, Snyder AZ, Zacks JM, Raichle ME. Coherent spontaneous activity accounts for trial-to-trial variability in human evoked brain responses. Nat Neurosci. 2006;9:23–25. doi: 10.1038/nn1616. [DOI] [PubMed] [Google Scholar]
- 8.Skudlarski P, Jagannathan K, Calhoun VD, Hampson M, Skudlarska BA, Pearlson GD. Measuring brain connectivity: Diffusion tensor imaging validates resting state temporal correlations. Neuroimage. 2008;43:554–561. doi: 10.1016/j.neuroimage.2008.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R, et al. Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci U S A. 2009;106:2035–2040. doi: 10.1073/pnas.0811168106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stevens WD, Buckner RL, Schacter DL. Correlated low-frequency BOLD fluctuations in the resting human brain are modulated by recent experience in category-preferential visual regions. Cereb Cortex. 2009;20:1997–2006. doi: 10.1093/cercor/bhp270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewis CM, Baldassarre A, Committeri G, Romani GL, Corbetta M. Learning sculpts the spontaneous activity of the resting human brain. Proc Natl Acad Sci U S A. 2009;106:17558–17563. doi: 10.1073/pnas.0902455106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lohmann G, Hoehl S, Brauer J, Danielmeier C, Bornkessel-Schlesewsky I, Bahlmann J, et al. Setting the frame: the human brain activates a basic low-frequency network for language processing. Cereb Cortex. 2010;20:1286–1292. doi: 10.1093/cercor/bhp190. [DOI] [PubMed] [Google Scholar]
- 13.Hasson U, Nusbaum HC, Small SL. Task-dependent organization of brain regions active during rest. Proc Natl Acad Sci U S A. 2009;106:10841–10846. doi: 10.1073/pnas.0903253106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 15.Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: Evidence of underconnectivity. Brain. 2004;127:1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- 16.Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Functional and anatomical cortical underconnectivity in autism: Evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cereb Cortex. 2007;17:951–961. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mason RA, Williams DL, Kana RK, Minshew NJ, Just MA. Theory of Mind disruption and recruitment of the right hemisphere during narrative comprehension in autism. Neuropsychologia. 2008;46:269–280. doi: 10.1016/j.neuropsychologia.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kleinhans NM, Richards T, Sterling L, Stegbauer KC, Mahurin R, Johnson LC, et al. Abnormal functional connectivity in autism spectrum disorders during face processing. Brain. 2008;131:1000–1012. doi: 10.1093/brain/awm334. [DOI] [PubMed] [Google Scholar]
- 19.Mostofsky SH, Powell SK, Simmonds DJ, Goldberg MC, Caffo B, Pekar JJ. Decreased connectivity and cerebellar activity in autism during motor task performance. Brain. 2009;132:2413–2425. doi: 10.1093/brain/awp088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Welchew DE, Ashwin C, Berkouk K, Salvador R, Suckling J, Baron-Cohen S, et al. Functional disconnectivity of the medial temporal lobe in Asperger's syndrome. Biol Psychiatry. 2005;57:991–998. doi: 10.1016/j.biopsych.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 21.Noonan SK, Haist F, Müller R-A. Aberrant functional connectivity in autism: Evidence from low-frequency BOLD signal fluctuations. Brain Res. 2009;1262:48–63. doi: 10.1016/j.brainres.2008.12.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koshino H, Carpenter PA, Minshew NJ, Cherkassky VL, Keller TA, Just MA. Functional connectivity in an fMRI working memory task in high-functioning autism. Neuroimage. 2005;24:810–821. doi: 10.1016/j.neuroimage.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 23.Shih P, Shen M, Ottl B, Keehn B, Gaffrey MS, Müller R-A. Atypical network connectivity for imitation in autism spectrum disorder. Neuropsychologia. 2010;48:2931–2939. doi: 10.1016/j.neuropsychologia.2010.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mizuno A, Villalobos ME, Davies MM, Dahl BC, Müller R-A. Partially enhanced thalamocortical functional connectivity in autism. Brain Res. 2006;1104:160–174. doi: 10.1016/j.brainres.2006.05.064. [DOI] [PubMed] [Google Scholar]
- 25.Beauchamp MS, Nath AR, Pasalar S. FMRI-guided transcranial magnetic stimulation reveals that the superior temporal sulcus is a cortical locus of the McGurk effect. J Neurosci. 2010;30:2414–2417. doi: 10.1523/JNEUROSCI.4865-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allison T, Puce A, McCarthy G. Social perception from visual cues: Role of the STS region. Trends Cogn Sci. 2000;4:267–278. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- 27.Redcay E. The superior temporal sulcus performs a common function for social and speech perception: Implications for the emergence of autism. Neurosci Biobehav Rev. 2008;32:123–142. doi: 10.1016/j.neubiorev.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 28.Pelphrey KA, Carter EJ. Charting the typical and atypical development of the social brain. Dev Psychopathol. 2008;20:1081–1102. doi: 10.1017/S0954579408000515. [DOI] [PubMed] [Google Scholar]
- 29.Groen WB, Zwiers MP, van der Gaag R-JJ, Buitelaar JK. The phenotype and neural correlates of language in autism: an integrative review. Neurosci Biobehav Rev. 2008;32:1416–1425. doi: 10.1016/j.neubiorev.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 30.Klin A, Lin DJ, Gorrindo P, Ramsay G, Jones W. Two-year-olds with autism orient to non-social contingencies rather than biological motion. Nature. 2009;459:257–261. doi: 10.1038/nature07868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carper RA, Moses P, Tigue ZD, Courchesne E. Cerebral lobes in autism: Early hyperplasia and abnormal age effects. Neuroimage. 2002;16:1038–1051. doi: 10.1006/nimg.2002.1099. [DOI] [PubMed] [Google Scholar]
- 32.Hardan AY, Muddasani S, Vemulapalli M, Keshavan MS, Minshew NJ. An MRI study of increased cortical thickness in autism. Am J Psychiatry. 2006;163:1290–1292. doi: 10.1176/appi.ajp.163.7.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hazlett HC, Poe MD, Gerig G, Smith RG, Piven J. Cortical gray and white brain tissue volume in adolescents and adults with autism. Biol Psychiatry. 2006;59:1–6. doi: 10.1016/j.biopsych.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 34.Casanova MF, Buxhoeveden DP, Switala AE, Roy E. Minicolumnar pathology in autism. Neurology. 2002;58:428–432. doi: 10.1212/wnl.58.3.428. [DOI] [PubMed] [Google Scholar]
- 35.Castelli F, Frith C, Happe F, Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 2002;125:1839–1849. doi: 10.1093/brain/awf189. [DOI] [PubMed] [Google Scholar]
- 36.Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. Atypical frontal-posterior synchronization of Theory of Mind regions in autism during mental state attribution. Soc Neurosci. 2009;4:135–152. doi: 10.1080/17470910802198510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Monk CS, Peltier SJ, Wiggins JL, Weng S-J, Carrasco M, Risi S, et al. Abnormalities of intrinsic functional connectivity in autism spectrum disorders. Neuroimage. 2009;47:764–772. doi: 10.1016/j.neuroimage.2009.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gilbert SJ, Dumontheil I, Simons JS, Frith CD, Burgess PW. Comment on "Wandering minds: the default network and stimulus-independent thought". Science. 2007;317:43. doi: 10.1126/science.317.5834.43. author reply 43. [DOI] [PubMed] [Google Scholar]
- 39.Harrison BJ, Pujol J, Ortiz H, Fornito A, Pantelis C, Yücel M. Modulation of brain resting-state networks by sad mood induction. PLoS ONE. 2008;3:e1794. doi: 10.1371/journal.pone.0001794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waites AB, Stanislavsky A, Abbott DF, Jackson GD. Effect of prior cognitive state on resting state networks measured with functional connectivity. Hum Brain Mapp. 2005;24:59–68. doi: 10.1002/hbm.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frith U, Happé F. Theory of mind and self-consciousness: What is it like to be autistic? Mind & Language. 1999;14:82–89. [Google Scholar]
- 42.Hurlburt RT, Happé F, Frith U. Sampling the form of inner experience in three adults with Asperger syndrome. Psychol Med. 1994;24:385–395. doi: 10.1017/s0033291700027367. [DOI] [PubMed] [Google Scholar]
- 43.Kennedy DP, Courchesne E. Functional abnormalities of the default network during self- and other-reflection in autism. Soc Cogn Affect Neurosci. 2008;3:177–190. doi: 10.1093/scan/nsn011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kennedy DP, Redcay E, Courchesne E. Failing to deactivate: Resting functional abnormalities in autism. Proc Natl Acad Sci U S A. 2006;103:8275–8280. doi: 10.1073/pnas.0600674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arfanakis K, Cordes D, Haughton VM, Moritz CH, Quigley MA, Meyerand ME. Combining independent component analysis and correlation analysis to probe interregional connectivity in fMRI task activation datasets. Magn Reson Imaging. 2000;18:921–930. doi: 10.1016/s0730-725x(00)00190-9. [DOI] [PubMed] [Google Scholar]
- 46.Fair DA, Schlaggar BL, Cohen AL, Miezin FM, Dosenbach NUF, Wenger KK, et al. A method for using blocked and event-related fMRI data to study "resting state" functional connectivity. Neuroimage. 2007;35:396–405. doi: 10.1016/j.neuroimage.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rogers BP, Gore JC. Empirical comparison of sources of variation for fMRI connectivity analysis. PLoS ONE. 2008;3:e3708. doi: 10.1371/journal.pone.0003708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kandel ER, Jessell TM, Sanes JR. Sensory experience and the fine tuning of synaptic connections. In: Kandel ER, Schwartz JH, Jessell TM, editors. Principles of Neural Science. 4th ed. New York, NY: Elsevier; 2000. pp. 1115–1130. [Google Scholar]
- 49.Johnson MH. Sensitive periods in functional brain development: Problems and prospects. Dev Psychobiol. 2005;46:287–292. doi: 10.1002/dev.20057. [DOI] [PubMed] [Google Scholar]
- 50.Friston KJ, Price CJ. Dynamic representations and generative models of brain function. Brain Res Bull. 2001;54:275–285. doi: 10.1016/s0361-9230(00)00436-6. [DOI] [PubMed] [Google Scholar]
- 51.Passingham RE, Stephan KE, Kötter R. The anatomical basis of functional localization in the cortex. Nat Rev Neurosci. 2002;3:606–616. doi: 10.1038/nrn893. [DOI] [PubMed] [Google Scholar]
- 52.Wechsler D. Wechsler abbreviated scale of intelligence (WASI) San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- 53.Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 54.Rutter M, Le Couteur A, Lord C. Autism diagnostic interview-revised. Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- 55.Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, et al. The Autism Diagnostic Observation Schedule-Generic: A standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–223. [PubMed] [Google Scholar]
- 56.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 57.Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, et al. Frequencies contributing to functional connectivity in the cerebral cortex in "resting-state" data. AJNR Am J Neuroradiology. 2001;22:1326–1333. [PMC free article] [PubMed] [Google Scholar]
- 58.Gavrilescu M, Stuart GW, Rossell S, Henshall K, Mckay C, Sergejew AA, et al. Functional connectivity estimation in fMRI data: Influence of preprocessing and time course selection. Hum Brain Mapp. 2008;29:1040–1052. doi: 10.1002/hbm.20446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.He BJ, Snyder AZ, Vincent JL, Epstein A, Shulman GL, Corbetta M. Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron. 2007;53:905–918. doi: 10.1016/j.neuron.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 60.Jones TB, Bandettini PA, Kenworthy LE, Case LK, Milleville SC, Martin A, et al. Sources of group differences in functional connectivity: An investigation applied to autism spectrum disorder. Neuroimage. 2010;49:401–414. doi: 10.1016/j.neuroimage.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fischl B, van der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- 62.Van Dijk KRA, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. Intrinsic functional connectivity as a tool for human connectomics: Theory, properties, and optimization. J Neurophysiol. 2010;103:297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system: An approach to cerebral imaging. New York, NY: Thieme; 1988. [Google Scholar]
- 64.Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 65.Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 66.Cronbach L. Coefficient alpha and the internal structure of tests. Psychometrika. 1951;16:297–334. [Google Scholar]
- 67.Müller R-A, Shih P, Keehn B, Deyoe J, Leyden KM, Shukla DK. Underconnected, but how? A survey of functional connectivity MRI studies in autism spectrum disorders. Cereb Cortex. doi: 10.1093/cercor/bhq296. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hadjikhani N, Joseph RM, Snyder J, Tager-Flusberg H. Anatomical differences in the mirror neuron system and social cognition network in autism. Cereb Cortex. 2006;16:1276–1282. doi: 10.1093/cercor/bhj069. [DOI] [PubMed] [Google Scholar]
- 69.Stevens MC, Pearlson GD, Calhoun VD. Changes in the interaction of resting-state neural networks from adolescence to adulthood. Hum Brain Mapp. 2009;30:2356–2366. doi: 10.1002/hbm.20673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Supekar K, Musen M, Menon V. Development of large-scale functional brain networks in children. PLoS Biology. 2009;7:e1000157. doi: 10.1371/journal.pbio.1000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fair DA, Cohen AL, Power JD, Dosenbach NUF, Church JA, Miezin FM, et al. Functional brain networks develop from a "local to distributed" organization. PLoS Comput Biol. 2009;5:e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fair DA, Cohen AL, Dosenbach NUF, Church JA, Miezin FM, Barch DM, et al. The maturing architecture of the brain's default network. Proc Natl Acad Sci U S A. 2008;105:4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Watanabe H, Homae F, Taga G. General to specific development of functional activation in the cerebral cortexes of 2- to 3-month-old infants. Neuroimage. 2010;50:1536–1544. doi: 10.1016/j.neuroimage.2010.01.068. [DOI] [PubMed] [Google Scholar]
- 74.Hebb DO. The organization of behavior. New York, NY: John Wiley & Sons; 1949. [Google Scholar]
- 75.Saxe R, Kanwisher N. People thinking about thinking people. The role of the temporo-parietal junction in "theory of mind". Neuroimage. 2003;19:1835–1842. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- 76.Happé F, Ronald A, Plomin R. Time to give up on a single explanation for autism. Nat Neurosci. 2006;9:1218–1220. doi: 10.1038/nn1770. [DOI] [PubMed] [Google Scholar]
- 77.Tager-Flusberg H, Joseph RM. Identifying neurocognitive phenotypes in autism. Philos Trans R Soc Lond B Biol Sci. 2003;358:303–314. doi: 10.1098/rstb.2002.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Courchesne E, Pierce KL, Schumann CM, Redcay E, Buckwalter JA, Kennedy DP, et al. Mapping early brain development in autism. Neuron. 2007;56:399–413. doi: 10.1016/j.neuron.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 79.Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tamnes CK, Ostby Y, Fjell AM, Westlye LT, Due-Tønnessen P, Walhovd KB. Brain maturation in adolescence and young adulthood: Regional age-related changes in cortical thickness and white matter volume and microstructure. Cereb Cortex. 2010;20:534–548. doi: 10.1093/cercor/bhp118. [DOI] [PubMed] [Google Scholar]
- 81.Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, Tigue ZD, et al. Unusual brain growth patterns in early life in patients with autistic disorder: An MRI study. Neurology. 2001;57:245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- 82.Herbert MR, Ziegler DA, Makris N, Filipek PA, Kemper TL, Normandin JJ, et al. Localization of white matter volume increase in autism and developmental language disorder. Ann Neurol. 2004;55:530–540. doi: 10.1002/ana.20032. [DOI] [PubMed] [Google Scholar]
- 83.Raznahan A, Toro R, Daly E, Robertson D, Murphy C, Deeley Q, et al. Cortical anatomy in autism spectrum disorder: An in vivo MRI study on the effect of age. Cereb Cortex. 2010;20:1332–1340. doi: 10.1093/cercor/bhp198. [DOI] [PubMed] [Google Scholar]
- 84.Schumann CM, Bloss CS, Barnes CC, Wideman GM, Carper RA, Akshoomoff NA, et al. Longitudinal magnetic resonance imaging study of cortical development through early childhood in autism. J Neurosci. 2010;30:4419–4427. doi: 10.1523/JNEUROSCI.5714-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Johnson MH, Grossmann T, Cohen Kadosh K. Mapping functional brain development: Building a social brain through interactive specialization. Dev Psychol. 2009;45:151–159. doi: 10.1037/a0014548. [DOI] [PubMed] [Google Scholar]
- 86.Johnson MH. Interactive specialization: A domain-general framework for human functional brain development? Developmental Cognitive Neuroscience. 2011;1:7–21. doi: 10.1016/j.dcn.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Senju A, Johnson MH. Atypical eye contact in autism: Models, mechanisms and development. Neurosci Biobehav Rev. 2009;33:1204–1214. doi: 10.1016/j.neubiorev.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 88.Karmiloff-Smith A. Atypical epigenesis. Dev Sci. 2007;10:84–88. doi: 10.1111/j.1467-7687.2007.00568.x. [DOI] [PubMed] [Google Scholar]
- 89.Fox MD, Snyder AZ, Vincent JL, Raichle ME. Intrinsic fluctuations within cortical systems account for intertrial variability in human behavior. Neuron. 2007;56:171–184. doi: 10.1016/j.neuron.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 90.Quartz SR, Sejnowski TJ. The neural basis of cognitive development: A constructivist manifesto. Behav Brain Sci. 1997;20:537–596. doi: 10.1017/s0140525x97001581. [DOI] [PubMed] [Google Scholar]
- 91.Cowan WM, Fawcett JW, O'Leary DD, Stanfield BB. Regressive events in neurogenesis. Science. 1984;225:1258–1265. doi: 10.1126/science.6474175. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.