Abstract
The ability of an implanted ear to integrate multiple pulses, as measured by the slopes of detection threshold level (T level) versus pulse rate functions, may reflect cochlear health in the cochlea, as suggested by previous animal studies (Kang et al., 2010; Pfingst et al., 2011). In the current study, we examined the slopes of T level versus pulse rate functions in human subjects with cochlear implants. Typically, T levels decrease as a function of pulse rate, consistent with a multipulse integration mechanism. The magnitudes of the slopes of the T level versus pulse rate functions obtained from the human subjects were comparable to those reported in the animal studies. The slopes varied across stimulation sites, but did not change systematically along the tonotopic axis. This suggests that the slopes are dependent on local conditions near the individual stimulation sites. The characteristics of these functions were also similar to those found in animals in that the slopes for higher pulse rates were steeper than those for the lower pulse rates, consistent with a combined effect of multipulse integration and cumulative partial depolarization mechanisms at rates above 1000 pps. The maximum comfortable loudness level (C level) versus pulse rate functions were also examined to determine the effect of level on the slopes. Slopes of C-level functions were shallower than those for the T-level functions and were not correlated with those of the T-level functions, so the mechanisms underlying these two functions are probably not identical. The slopes of the T- or C-level functions were not dependent on stimulus-current level. Based on these results, we suggest that slopes of T level versus pulse rate functions might be a useful measure for estimating nerve survival in the cochlea in regions close to the stimulation sites.
Keywords: cochlear implant, pulse rate, psychophysical detection threshold, maximum comfortable loudness level, across-site variation, human
1. Introduction
Most present-day processors for cochlear implant auditory prostheses encode important features of speech signals by amplitude modulation of carrier pulse trains using the low-frequency envelope of the speech waveform. The pulse rate in these carrier pulse trains is a variable of interest because of its potential to affect transmission of the envelope information. There are several theoretical advantages of using high pulse rates, including finer resolution of details in the envelope waveforms. High carrier pulse rates have also been postulated to evoke a more stochastic response pattern across the array of activated auditory neurons, resulting in a more normal encoding of auditory information (Rubinstein et al., 1999). On the other hand, high carrier pulse rates have been shown to degrade the sensitivity to low-frequency envelope modulations (Galvin and Fu, 2005; 2009; Pfingst et al., 2007) and they have not produced the expected improvements in speech recognition with cochlear implants relative to results with low carrier rates (Loizou et al., 2000; Vandali et al., 2000; Holden et al., 2002; Verschuur, 2005; Plant et al., 2007; Shannon et al., 2011). A better understanding of how carrier rates affect system responses to electrical stimulation provides guidance for optimizing implant performance.
Some insight into these mechanisms has been gained by neurophysiological and psychophysical studies examining detection of pulse trains as a function of pulse rate. In many cases, detection threshold levels (T levels) decrease as a function of pulse rate, and the rates of decrease (i.e., the slopes of the threshold versus pulse rate functions) seem to depend on two mechanisms. Specifically, the decrease of T levels with pulse rate is consistent with a multipulse integration mechanism, similar to temporal integration. Temporal integration is defined as increases in detectability of a stimulus with increases in stimulus duration, which typically occurs in a time window of 300 ms for both electrical and acoustic stimuli (Gerken et al., 1990; Donaldson et al., 1997). In a constant-duration stimulus, as pulse rate increases, the number of pulses also increases. The increased stimulus power produces a lower threshold. We call this multipulse integration. The decrease of T levels as a function of pulse rate becomes faster at rates above 1000 pps, probably due to an additional mechanism involving pulse interactions as described by Middlebrooks (2004), where threshold can be further lowered by the cumulative effect of residual partial depolarizations following subthreshold pulses. The increase in the slopes of the T level versus pulse rate functions above 1000 pps has been reported in both psychophysical (Kang et al., 2010; Pfingst et al., 2011) and neurophysiological studies (Middlebrooks, 2004). Importantly, psychophysical studies in guinea pigs have shown a correlation between the slopes of T level versus pulse rate functions at rates below 1000 pps and the numbers of surviving hair cells and auditory neurons as well as ensemble spontaneous activity that we refer to as cochlear health (Kang et al., 2010; Pfingst et al., 2011).
A primary objective of this study was to determine whether the characteristics of the pulse rate functions from animals reported in Kang et al. (2010) and Pfingst et al. (2011) are also present in human subjects, for the potential of using this measure as a non-invasive method for estimating neural status along the electrode arrays in humans. We expect to find variation in these functions in humans, because cochlear neural pathology is known to vary across patients and along the electrode array within patients. Also, if the two hypothesized mechanisms of multipulse integration and cumulative partial depolarization suggested in the animal studies hold true for human subjects, the slope of the functions in humans would be expected to be steeper at high rates. A previous study has reported T level versus pulse rate functions in humans up to 6500 pps (Kreft et al., 2004a), but it was not clear from the group-mean data reported for that study if the slopes changed systematically across subjects at high rates.
Another objective of this study was to determine the effects of pulse rate on hearing at higher current levels. It is known that maximum comfortable loudness levels (C levels) decrease as a function of pulse rate but that the slopes of the C level versus pulse rate functions are shallower than the slopes of the T level versus pulse rate functions. This results in a larger dynamic range at high pulse rates relative to that at low pulse rates (Kreft et al., 2004a; Galvin and Fu, 2005; 2009; Pfingst et al., 2007). However, the reasons for this difference in slopes are not known. Is this just a matter of the use of a logarithmic (dB) scale for stimulus current? That is, are the functions for C levels shallower simply because they occur at higher current levels? We used two methods to address this question. First, we asked if the slopes of T level or C level versus pulse rate functions were correlated across subjects and across stimulation sites with the absolute levels of those functions, which vary across subjects and stimulation sites. Second, we compared slopes of these functions for bipolar (BP) stimulation, which requires relatively high current levels, with those for monopolar (MP) stimulation, which requires relatively low current levels.
2. Methods
2.1. Subjects
Eleven adult post-lingually deaf subjects participated in the study. Nine subjects were implanted with the Nucleus CI24R (CS) contour device and two were implanted with the Nucleus CI24M straight array. The subjects’ ages at the time of these experiments varied from 37 to 77 years (mean = 60; S.D. = 11 years). All subjects had at least one year of experience with the implant prior to data collection for this experiment. The demographic details of the subjects are summarized in Table 1. The use of human subjects was reviewed and approved by the University of Michigan Medical School Institutional Review Board.
Table 1.
Demographic information for the cochlear implant participants.
| Subject # | Age at testing (yrs) | Implant type | Processing strategy | Age at onset of profound deafness | Etiology | Ear |
|---|---|---|---|---|---|---|
| S45 | 48 | 24R(CS) | ACE | 44 | Accident & congenital | L |
| S57 | 66 | 24M | SPEAK | 60 | Meniere’s | R |
| S58 | 55 | 24M | SPEAK | 42 | Autoimmune | L |
| S60 | 65 | 24R(CS) | ACE | 62 | Familial | L |
| S63 | 77 | 24R(CS) | ACE | Unknown | Unknown | R |
| S65 | 36 | 24R(CS) | ACE | 35 | Familial | R |
| S71 | 59 | 24R(CS) | ACE | 57 | Familial | L |
| S72 | 66 | 24R(CS) | ACE | Birth | Congenital | R |
| S73 | 64 | 24R(CS) | ACE | 50 | Unknown | R |
| S74 | 66 | 24R(CS) | ACE | 61 | Familial | R |
| S75 | 58 | 24R(CS) | ACE | 55 | Noise Exposure | L |
2.2 Stimuli and test procedures
Stimuli were trains of symmetric biphasic pulses. The pulse duration was 40 μs/phase with an interphase interval of 8 μs and the pulse-train duration was 600 ms. The tested pulse rates were 156, 313, 625, 1250, 2500, and 5000 pps.
The implanted electrode array in the scala tympani consisted of 22 electrodes numbered 1 to 22 from base to apex. T and C levels were measured at three stimulation sites (4, 11, and 18) covering the basal, middle, and apical locations in the electrode array and using both monopolar and bipolar electrode configurations. For monopolar stimulation, the number of the stimulation site corresponds to the number of the stimulated electrode. For bipolar (BP+0) stimulation, the number of the stimulation site corresponds to the number of the basal member of the electrode pair, e.g., bipolar site 4 utilized electrodes 4 and 5. All subjects completed tests using MP stimulation, and only eight subjects participated in tests using BP stimulation.
A laboratory-owned Freedom processor (Cochlear Corporation, Englewood, CO) was used for the psychophysical testing. The factors of stimulation site, electrode configuration, and pulse rate were fully randomized. For each condition, T level was obtained first, followed by the C level. The 600 ms pulse trains were presented repeatedly with a 600 ms inter-stimulus interval as the subject adjusted the levels to determine the T and C levels. Method of adjustment was used for measuring the T levels, where the subjects had access to two sets of buttons, one set of which allowed adjustment in a step size of 5 current level units (CUs) and the other for a finer adjustment in 1 CU. The subjects were first instructed to use the larger step size by clicking the up or down buttons in 5 CUs to approximate the threshold region and then fine tune the stimulus level using the smaller step size (1 CU) until the level at which the signal was just audible was determined. After determining and recording the T level, the subjects were instructed to continue increasing the stimulus level until they found the loudest level at which they could comfortably listen for an extended period of time. This procedure was repeated three times for each experimental condition for both T and C levels. For each condition, the mean of the closest two values was taken as the final measurement. In cases where the level exceeded the highest current level available (255 CUs), the data point was excluded. 10.50% of the C levels and 0.11% of the T levels were excluded for this reason.
The implant receiver-stimulator delivered current in 256 steps in CUs. The CU range of 0–255 corresponded to approximately 10 μApeak to 1750 μApeak. Each current level step corresponded to 2.046% (0.176 dB) of current change. The T and C levels obtained in CUs were first converted to μA and then expressed in dB relative to 1 mA. Slopes of the level versus pulse rate functions were calculated separately for pulse rates below 1000 pps and above 1000 pps. The slopes were calculated using least-squares linear regression to fit the level versus pulse rate functions where the T and C levels were in dB re 1 mA and pulse rates were also expressed in a log scale (log2). The goodness of fit of these linear functions was then determined.
2.3. Audiometric data
Preoperative acoustic thresholds were obtained from the audiometric records for 6 of the 11 subjects (S60, S65, S72, S73, S74, and S75) for estimating their hair cell survival status near the tested sites. Those unaided acoustic thresholds had been obtained in the clinic using narrowband-noise stimuli with center frequencies at octave intervals between 250 Hz and 8000 Hz. The purpose of estimating the subjects’ hair cell survival status was to determine if multipulse integration similar to that observed in the animals with good hair cell and spiral ganglion cell survival (Kang et al., 2010; Pfingst et al., 2011) could be achieved in the absence of hair cells.
3. Results
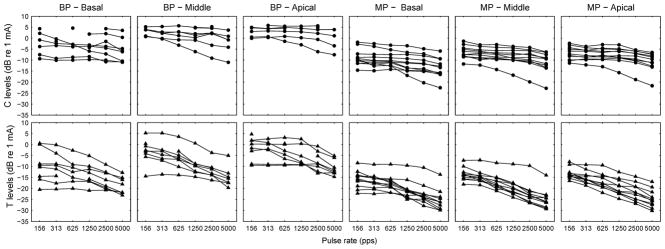
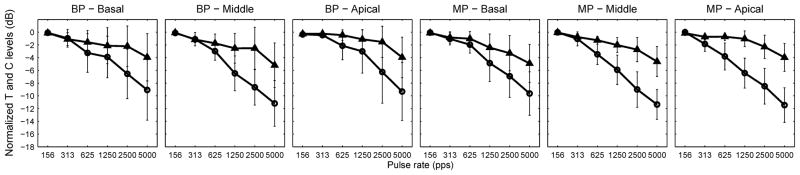
T and C levels as a function of pulse rate are shown in Figure 1 for individual subjects. Figure 2 shows the group-mean normalized data for both T and C functions. Each T level versus pulse rate function was normalized by subtracting the highest threshold from the function to better elucidate the effect of pulse rate across conditions. The highest threshold in most cases was the data point measured at the lowest pulse rate (156 pps), or in a few cases, the next to the lowest pulse rate (312 pps). The same normalization procedure was performed for the C level versus pulse rate functions.
Fig 1.
Psychophysical detection thresholds (Ts) and maximum comfortable levels (Cs) as a function of pulse rate. Top panels: Raw data of C levels as a function of pulse rate measured at three stimulation sites with two electrode configurations. Bottom panels: Raw data of T levels as a function of pulse rate measured at three stimulation sites with two electrode configurations. Data points indicate the mean of two repeated measures. Data points equal to the maximum deliverable current (255 CU) were removed from data analysis.
Fig 2.
Group means of normalized T levels and C levels as a function of pulse rate. The raw data (shown in Figure 1) were normalized against the highest T or C level for the two functions respectively. Error bars represent one standard deviation. The T-level functions are plotted using circles and C-level functions are plotted using triangles.
Both T and C levels decreased as a function of pulse rate in most cases. The rate of decrease was quantified by fitting a linear regression line to the level versus pulse rate functions for pulse rates below 1000 pps and those above 1000 pps. Chi-square Goodness of Fit values of the T level versus pulse rate functions ranged from ~0 to 0.75 with a median of 0.03. Chi-square Goodness of Fit values of the C level versus pulse rate functions ranged from ~0 to 1 with a median of 0.06. Thus, all Chi-square values were smaller than 1, indicating good fit. The breakpoint of 1000 pps was chosen based on the theoretical consideration that pulse interaction could occur when interpulse interval becomes smaller than 1 ms. Although the breakpoint might not necessarily occur exactly at 1000 pps for all functions, slopes tended to become steeper at high rates (Fig. 1 and 2). A comparison of the slopes for pulse rates below and above 1000 pps can adequately capture the effect of pulse interaction as shown below.
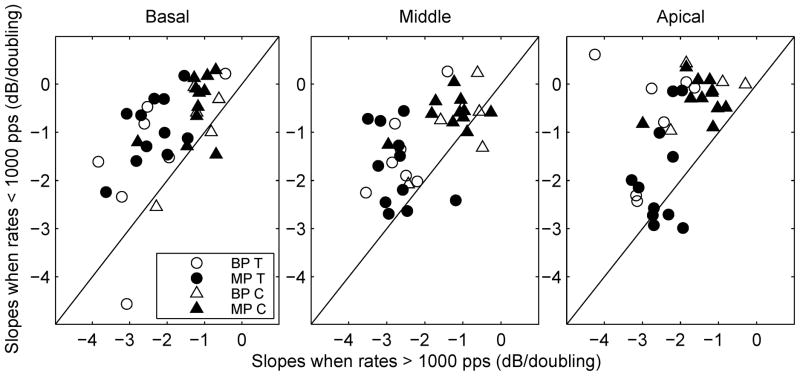
On average, slopes for T level versus pulse rate functions above 1000 pps were steeper than those below 1000 pps by 1.24 dB, 1.07 dB, and 1.16 dB per doubling of pulse rate, respectively for the basal, middle and apical sites (Fig. 3). These differences were statistically significant (Table 2). Slopes for the C-level functions were significantly steeper above 1000 pps by 0.65 dB, 0.54 dB, and 1.19 dB/doubling for three sites (Table 2), although the increase in slopes was smaller in magnitude for the basal and middle sites than that for the T-level functions. After the removal of the subject who showed steeper C-level slopes than the rest of the group, the mean slope increase for the C-level functions remained statistically significant for all three sites, although p value slightly increased to 0.009 for the middle site.
Fig 3.
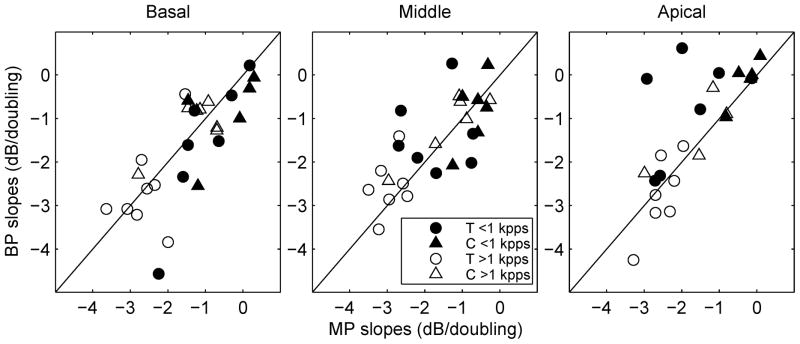
Scatter plot of slopes for pulse rates below 1000 pps versus slopes for pulse rates above 1000 pps. Circles are used to represent slopes for the T-level functions and triangles are used to represent slopes for the C-level functions. Open symbols are for BP stimulation and filled symbols are for MP stimulation.
Table 2.
Differences in threshold versus pulse rate function slopes for pulse rates below 1000 pps versus those above 1000 pps and results of corresponding t-tests.
| T levels | C levels | |||||||
|---|---|---|---|---|---|---|---|---|
| difference (dB/dblg) | t | n | p | difference (dB/dblg) | t | n | p | |
| Basal | 1.24 | 5.57 | 17 | < 0.001 | 0.65 | 4.00 | 15 | 0.001 |
| Middle | 1.07 | 4.63 | 17 | < 0.001 | 0.54 | 3.47 | 16 | 0.003 |
| Apical | 1.17 | 3.69 | 17 | 0.001 | 1.19 | 6.78 | 14 | < 0.001 |
Slopes did not vary consistently across stimulation sites. Of the 8 conditions (2 electrode configurations × 2 pulse rate conditions × 2 levels), the effect of stimulation site was statistically significant in only 2: C-level slopes at < 1000 pps in BP configuration [F (2, 3) = 7.30, p = 0.025] and T- level slopes at < 1000 pps in MP configuration [F (2, 10) = 9.05, p = 0.002]. Furthermore, for these two conditions, the slopes did not change systematically or consistently along the tonotopic axis.
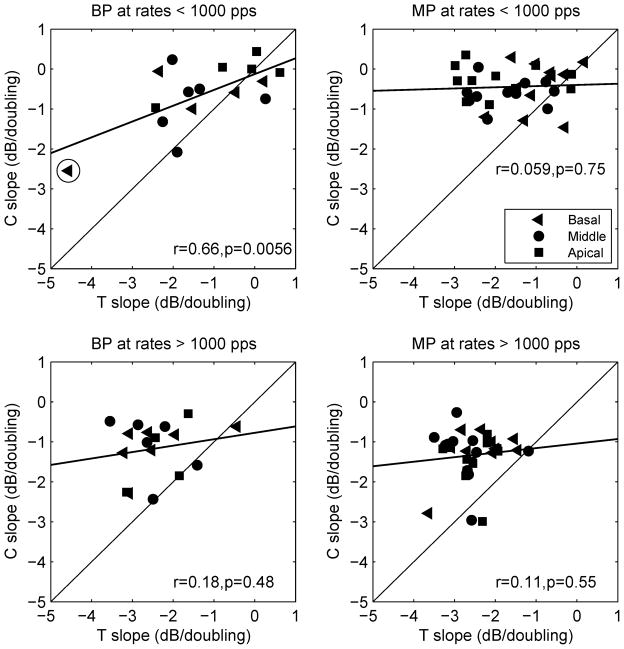
Figure 4 compares the slopes of C level versus pulse rate functions to those of T level versus pulse rate functions for two electrode configurations and for pulse rates below and above 1000 pps. C levels decreased more gradually with pulse rate compared to T levels, giving rise to shallower slopes in most cases (points above the diagonal in Fig. 4). The differences in the slopes between the T and C level functions resulted in larger dynamic ranges at higher pulse rates. In BP configuration, the averaged dynamic range across subjects increased from 7.26 dB to 13.21 dB as pulse rate increased from 156 pps to 5000 pps, corresponding to an increase in dynamic range of 1.19 dB/doubling of pulse rate. In MP configuration, dynamic range across subjects increased from 6.77 dB to 13.17 dB as pulse rate increased from 156 pps to 5000 pps, corresponding to an increase of 1.28 dB/doubling of pulse rate.
Fig 4.
Scatter plot of slopes for C level versus pulse rate functions against slopes for T level versus pulse rate functions. Slopes obtained from three different stimulation sites are shown in different symbols. The regression lines represent the linear fit to the data. Correlation coefficients (r) and p values are shown in each panel.
An important and unexpected finding was that the slopes for C level versus pulse rate functions were not significantly correlated with the slopes of the T level versus pulse rate functions in most cases. One exception was the BP configuration at < 1000 pps (r = 0.66, p < 0.01). However, after the one outlier data point (circled in Fig. 4, upper left panel) was removed from the analysis, the correlation in this case was not significant (r = 0.42, p = 0.12). Given the general lack of correlation between the slopes of the functions for C and T levels, the slope of one function cannot be used to predict the slope of the other function for a given stimulation site in a given subject.
The sample variance (s2) in the slopes across subjects and sites was greater for T levels than for C levels for BP (s2T = 1.84; s2C = 0.67) and MP stimulation (s2T = 0.88; s2C = 0.22) at pulse rates below 1000 pps, and for BP stimulation (s2T = 0.61; s2C = 0.46) at pulse rates above 1000 pps, but was comparable for MP stimulation at pulse rates above 1000 pps (s2T = 0.33; s2C = 0.37).
Effects of current level on slopes of T level versus pulse rate functions were examined in two ways. First, correlations were examined between the slopes of T level versus pulse rate functions and the T levels measured at the lowest tested pulse rate (156 pps), and between the slopes of C level versus pulse rate functions and the C levels measured at 156 pps. Lower thresholds at 156 pps did not predict steeper slopes for T level versus pulse rate function with one exception for BP stimulation at < 1000 pps. Nor was there a predictive relationship between C levels and the slopes of C-level functions.
To further explore the relationship between current levels and slopes, electrode configuration (BP versus MP) was used to test slopes at different current levels. T level currents across subjects and pulse rates averaged 7.17 dB, 11.23 dB, and 13.72 dB higher for BP stimulation compared to those measured with MP stimulation for the basal, middle, and apical sites, respectively. C level currents for BP stimulation were higher on average by 8.16 dB, 10.77 dB, and 11.27 dB than those for MP stimulation for the three stimulation sites respectively. In spite of these differences in levels of Ts and Cs for the two electrode configurations, slopes for T and C level versus pulse rate functions were similar across the two electrode configurations [basal: t (14) =1.08, p = 0.29; middle: t (13) = −1.42, p = 1.78; apical: t (13) = −1.50, p = 0.16 ], as shown in Figure 5.
Fig 5.
Scatter plots of slopes of level versus pulse rate functions measured using bipolar versus monopolar stimulation. Circles are used to represent slopes for the T-level functions and triangles are used to represent slopes for the C-level functions. Open symbols are for slopes at > 1000 pps and filled symbols are for slopes at < 1000 pps.
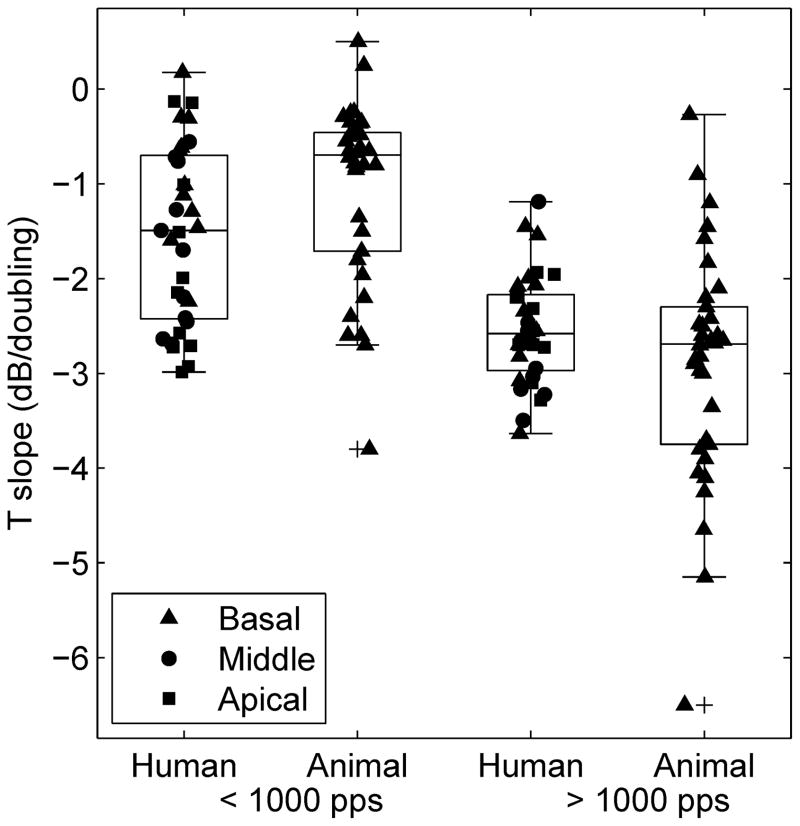
The slopes of T level versus pulse rate functions for MP stimulation for our human subjects (11 subjects × 3 sites) are compared in Figure 6 with those obtained from 34 guinea pigs with a large range of cochlear health conditions near their (basal) stimulation sites (data from Kang et al., 2010 and Pfingst et al., 2011, re-plotted). In both the humans and the guinea pigs, the slopes of the functions above 1000 pps were steeper than those below 1000 pps. For pulse rates below 1000 pps, the ranges of the slopes for the animal and human groups were similar and the means were not statistically significantly different [t test for means: t (32) = 0.71, p = 0.10]. For pulse rates above 1000 pps, range of the slopes from the animals was larger than that for the humans, but the means were not significantly different [t (31) = −1.63, p = 0.11].
Fig 6.
Box plot for T-level slopes for MP stimulation from 11 humans times 3 stimulation sites (from this paper) and 34 animal subjects (basal site) re-plotted from Kang et al. (2010) and Pfingst et al. (2011) for pulse rates below 1000 pps and above 1000 pps. Each box depicts the median and interquartile range. The outliers (+ symbols) are data points that fall more than 1.5 box-lengths away from 25th or 75th percentile. The whiskers show the range of the rest of the data.
Pre-implant acoustic thresholds were used to estimate hair cell survival status near the tested sites. The averaged pre-implant acoustic thresholds across the 6 human subjects tested at octave intervals between 250 Hz and 8000 Hz are shown in Table 3. Assuming a relatively deep insertion depth of 25 mm (Skinner et al., 2002), the characteristic frequencies corresponding to stimulation sites 4, 11, and 18, are 4076 Hz, 1888 Hz, and 829 Hz, respectively (Greenwood, 1990). Therefore, the middle (i.e., electrode 11) and basal (i.e., electrode 4) stimulation sites that were tested should be located in the cochlear regions well above 1000 Hz place. The thresholds suggest that it is unlikely these subjects had residual hearing or hair cells at the middle and basal stimulation sites.
Table 3.
Unaided acoustic thresholds for narrow-band noise stimuli obtained in the clinic prior to implantation.
| Center frequency (Hz) | 250 | 500 | 1000 | 2000 | 4000 | 8000 |
|---|---|---|---|---|---|---|
| Upper limit of audiometer (dB HL) | 105 | 110 | 115 | 115 | 115 | 85 |
| Hearing threshold (dB HL) | 65 | 83 | 103 | 102 | n/a* | n/a* |
| Standard deviation (dB HL) | 10 | 8 | 12 | 17 | n/a* | n/a* |
Thresholds not measurable
4. Discussion
4.1. Comparison of human and animal data
Results of the present study showed that slopes of the T-level functions in human subjects were similar to those found in guinea pigs (Fig. 6). Kang et al. (2010) and Pfingst et al. (2011) showed that the variation in the slopes of these functions especially for pulse rates below 1000 pps was related to the health of the implanted guinea pig cochleae near the sites of stimulation. In these animal studies, cochlear health was defined in terms of numbers of surviving hair cells and spiral ganglion cells near the implant and the amount of ensemble spontaneous activity recorded from the implant, which presumably depends on the presence of hair cells. Since the two anatomical variables were highly correlated with each other it was not possible to conclude which elements (hair cells or neurons or both) were responsible for the psychophysical results. Hair cells could potentially contribute to the observed differences in threshold versus pulse rate function slope through electrophonic mechanisms or direct electrical activation of inner hair cells. However, our data from human subjects showed that the slopes of the pulse rate functions varied in cochlear regions where the subjects probably had minimal or no residual hair cells as estimated based on their preoperative audiometric data. This result suggests that hair cells were not responsible for the variability in slopes of the T level versus pulse rate functions, leaving the likely possibility that variability in the number of surviving spiral ganglion cells accounted for the variability in the multipulse integration. We suggest that T levels might be more sensitive to pulse rate in a healthy cochlea because more fibers are available to respond to each pulse in the pulse train. Survival of spiral ganglion neurons in the absence of hair cells is typically higher in humans than in guinea pigs, but it is variable across subjects and along the cochlear length within subjects (Hinojosa and Lindsay, 1980; Johnsson et al., 1981; Nadol, 1997). Because the range of slopes below 1000 pps found in the human subjects was similar to that found in animals, the T level versus pulse rate functions in humans can potentially be used as a non-invasive predictive measure of the neural survival status near the stimulation sites.
4.2. Effects of interpulse interaction
The decrease of T level as a function of pulse rate below 1000 pps is well known for pulse trains with short phase durations (Shannon, 1985, 1989, Skinner et al., 2000; Vandali et al., 2000; Kreft et al., 2004a; Pfingst et al., 1993) and can be attributed to temporal multipulse integration. However, the steeper slopes of the T-level functions at high rates (Fig. 3) have not been reported before in humans, but are consistent with what has been reported in animal psychophysical (Kang et al., 2010; Pfingst et al., 2011) and cortical-neural studies (Middlebrooks, 2004). The mechanism for the faster decrease of T levels, as pulse rate increases above 1000 pps, is attributed to pulse interactions. In addition to temporal integration of multiple pulses, the model suggests that threshold is further lowered by the cumulative effect of residual partial depolarization following subthreshold pulses (Middlebrooks, 2004). This interpulse interaction would occur only when the interpulse interval is small enough (i.e., 1 ms or shorter) that the neurons do not completely recover from the partial depolarization in response to preceding subthreshold pulses.
The slopes of C-level functions also increased at pulse rates greater than 1000 pps, but the magnitude of increase was smaller compared to that of the T-level functions for the basal and middle sites (Table 2). The residual partial depolarization mechanism proposed for T levels above 1000 pps could also apply to C levels. Although many neurons are firing at well above threshold at the C level, there still could be a small population of fibers at the periphery of the neural excitation field that are below threshold and are only partially depolarized by the stimulus. Based on the McKay et al. (1998, 2001) model, loudness of electrical stimulation is dependent on the total neural excitation regardless of the location of the active fibers. If higher pulse rate helps recruit more neural fibers at the periphery of the excitation field, it would be reasonable to expect a faster decrease of C level as a function of pulse rate at rates above 1000 pps compared to that below 1000 pps. The number of these sub-threshold fibers at suprathreshold level however should be small compared to those recruited at T levels. This might account for the smaller magnitude of slope change above 1000 pps seen for the C-level functions.
The effect of interpulse interaction can be a detrimental factor for coding temporal information in cochlear implant users. In a multichannel implant, high pulse rates produce relatively short interpulse intervals between adjacent channels, which introduces channel interaction (Middlebrooks, 2004). Channel interaction could result in masking of temporal modulation and poor modulation sensitivity (Chatterjee, 2003; Middlebrooks, 2008).
4.3. Relationship between the T- and C-level functions
In most cases, the slopes of C-level functions were shallower and less variable than the slopes of T-level functions both below and above 1000 pps (Fig. 1 and Fig. 4). In addition, the slopes of the C-level functions were not correlated across stimulation sites and subjects with those of the T-level functions (Fig. 4). This suggests that mechanisms that account for the rate of decrease for the C-level functions may be different from those for the T-level functions. Previous research has demonstrated that loudness grows as an exponential function of current (Shannon, 1985, Chatterjee et al., 2000). It is also known that the loudness growth functions are dependent on pulse rate, with loudness growing faster at low pulse rates (Zeng and Shannon, 1994; Fu, 2005; Galvin and Fu, 2009). This, in turn, would result in a smaller difference in C levels between different pulse rates compared to the differences in T levels across rates, giving rise to the shallower slopes of C level versus pulse rate functions. Furthermore, loudness growth functions have been shown to vary across subjects and across stimulation sites within individuals (Fu, 2005; Garadat and Pfingst, 2011). If the slopes of C level versus pulse rate functions are at least in part dependent on loudness growth, then across-subject and across-site variation in loudness growth could account for the lack of correlation between the slopes of T- and C-level functions.
The shallower slopes of the C-level functions relative to the T- level functions produce larger dynamic ranges at higher pulse rates, which is well known (e.g., Kreft et al., 2004a). The larger dynamic range produced by high rates however does not help improve temporal modulation sensitivity (e.g., Galvin and Fu, 2005; 2009; Pfingst et al., 2007). This is probably due to the fact that high pulse rates do not produce a larger number of just noticeable differences in amplitude within the dynamic range (Kreft et al., 2004b).
Given the greater complexity of mechanisms that may determine the slopes of C-level functions, the lack of correlation of C- level function slopes with T-level function slopes, and the smaller variation in C-level slopes across subjects and stimulation sites, we suggest that the slopes of T level versus pulse rate functions would be the better measure for assessing the health of the cochlea near the individual stimulation sites.
4.4. Relationship between slopes of pulse rate functions and level
In the guinea pig studies (Kang et al., 2010; Pfingst et al., 2011), thresholds for animals with steep slopes of T level versus pulse rate functions were often lower than those with shallow slopes. The opposite trend was observed in the human subjects for T-level functions in the present study, but it was not statistically significant in most cases. Furthermore, the slopes of C-level functions were not significantly correlated with C levels in our human subjects.
To further examine the relationship between slopes of the T and C level versus pulse rate functions and absolute current level, we compared slopes for BP stimulation with those for MP stimulation. It is known that thresholds for BP stimulation are typically higher than those for MP stimulation. However, slopes of the T- level and C-level functions were not systematically shallower for BP stimulation (Fig. 5), although both T and C levels were higher for BP stimulation by approximately 10 dB. This provides further evidence that the slopes of the pulse rate functions are not necessarily dependent on stimulus-current level. In addition to poor neural survival, stimulus-current level can be influenced by distance from electrode to modiolus and other factors limiting current flow to the neural tissue (Pfingst and Xu, 2004; Long et al., 2010). Distance from modiolus was probably relatively uniform in the guinea pigs because the implant tended to fill the whole scala (Kang et al., 2010; Pfingst et al., 2011), but more variable in humans (Roland et al., 2000; Tykocinski et al., 2000; Saunders et al., 2002), which probably affected the thresholds but not the slopes of the threshold versus pulse rate functions. These complexities might explain the poor correlations between level and slope found in human subjects. Further studies are needed to determine which conditions near the stimulation sites affect threshold levels and which affect the slopes.
5. Conclusions
Slopes of the T level versus pulse rate functions obtained from our human subjects showed variation across subjects and across stimulation sites similar to that found in guinea pigs, where the slopes were related to hair-cell and nerve survival. Since our human subjects showed little or no residual hearing in the tested regions, it seems likely that the variation in slopes of the T-level functions was due to variation in nerve survival in the absence of hair cells. Steeper slopes of T and C level versus pulse rate functions were observed above 1000 pps, probably due to the cumulative effects of residual partial depolarization at high pulse rates. The slopes of the C-level functions were shallower than and not predictive of those of the T level functions. The lack of correlation between the slopes of T and C level versus pulse rate functions is likely due to the differences in loudness growth at different pulse rates. The slopes of the T- or C-level functions were not dependent on stimulus-current level, indicating that the mechanisms affecting T and C levels are different from those affecting the slopes of the functions. Based on these results and previous studies in animals, we suggest that slopes of T level versus pulse rate functions might be a useful measure for estimating the nerve survival in the cochlea in regions close to the stimulation sites.
Highlights.
Slopes of T- and C-level versus pulse rate functions increase above 1000 pps.
These slopes are stimulation-site specific, suggesting a dependence on pathology.
Slopes for humans are similar to those for animals with varying nerve survival.
Slopes for C levels are shallower than and not correlated with those for T levels.
Acknowledgments
We express appreciation to Catherine Thompson for assistance with subject recruitment and data collection and to our research subjects for their cheerful participation in this work. The work was supported by NIH/NIDCD grants R01 DC004312, R01 DC010786 and T32 DC00011.
Abbreviations
- T level
threshold level
- C level
maximum comfortable level
- MP
monopolar
- BP
bipolar
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Chatterjee M. Modulation masking in cochlear implant listeners: envelope versus tonotopic components. J Acoust Soc Am. 2003;95:2042–2053. doi: 10.1121/1.1555613. [DOI] [PubMed] [Google Scholar]
- Chatterjee M, Fu QJ, Shannon RV. Effects of phase duration and electrode separation on loudness growth in cochlear implant listeners. J Acoust Soc Am. 2000;107:1637–1644. doi: 10.1121/1.428448. [DOI] [PubMed] [Google Scholar]
- Cullen JK, Thompson CL, Hughes LF, Berlin C, Samson DS. Brain and Language. 1974;1:307–322. [Google Scholar]
- Donaldson GS, Viemeister NF, Nelson DA. Psychometric functions and temporal integration in electric hearing. J Acoust Soc Am. 1997;101:3706–3721. doi: 10.1121/1.418330. [DOI] [PubMed] [Google Scholar]
- Fu QJ. Loudness growth in cochlear implants: effect of stimulation rate and electrode configuration. Hear Res. 2005;202:55–62. doi: 10.1016/j.heares.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Galvin JJ, III, Fu QJ. Effects of stimulation rate, mode and level on modulation detection by cochlear implant users. J Assoc Res Otolaryngol. 2005;6:269–279. doi: 10.1007/s10162-005-0007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvin JJ, III, Fu QJ. Influence of stimulation rate and loudness growth on modulation detection and intensity discrimination in cochlear implant users. Hear Res. 2009;250:46–54. doi: 10.1016/j.heares.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garadat SN, Pfingst BE. Relationship between gap detection thresholds and loudness in cochlear-implant users. Hear Res. 2011;275:130–138. doi: 10.1016/j.heares.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerken GM, Bhat VKH, Hutchison-Clutter M. Auditory temporal integration and the power function model. J Acoust Soc Am. 1990;88:767–778. doi: 10.1121/1.399726. [DOI] [PubMed] [Google Scholar]
- Greenwood DD. A cochlear frequency-position function for several species-29 years later. J Acoust Soc Am. 1990;87:2592–2605. doi: 10.1121/1.399052. [DOI] [PubMed] [Google Scholar]
- Hinojosa R, Lindsay JR. Profound deafness. Associated sensory and neural degeneration. Arch Otolaryngol. 1980;106:193–209. doi: 10.1001/archotol.1980.00790280001001. [DOI] [PubMed] [Google Scholar]
- Holden LK, Skinner MW, Holden TA, Demorest ME. Effects of stimulation rate with the Nucleus 24 ACE speech coding strategy. Ear Hear. 2002;23:463–476. doi: 10.1097/00003446-200210000-00008. [DOI] [PubMed] [Google Scholar]
- Johnsson LG, Hawkins JE, Jr, Kingsley TC, Black FO, Matz GJ. Aminoglycoside-induced cochlear pathology in man. Acta Otolaryngol Suppl. 1981;383:1–19. [PubMed] [Google Scholar]
- Kang SY, Colesa DJ, Swiderski DL, Su GL, Raphael Y, Pfingst BE. Effects of hearing preservation on psychophysical responses to cochlear implant stimulation. J Assoc Res Otolaryngol. 2010;11:245–265. doi: 10.1007/s10162-009-0194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreft HA, Donaldson GS, Nelson DA. Effects of pulse rate on threshold and dynamic range in Clarion cochlear-implant users (L) J Acoust Soc Am. 2004a;115:1885–1888. doi: 10.1121/1.1701895. [DOI] [PubMed] [Google Scholar]
- Kreft HA, Donaldson GS, Nelson DA. Effects of pulse rate and electrode array design on intensity discrimination in cochlear implant users. J Acoust Soc Am. 2004b;116:2258–2268. doi: 10.1121/1.1786871. [DOI] [PubMed] [Google Scholar]
- Loizou PC, Poroy O, Dorman M. The effect of parametric variations of cochlear implant processors on speech understanding. J Acoust Soc Am. 2000;108:790–802. doi: 10.1121/1.429612. [DOI] [PubMed] [Google Scholar]
- Long CJ, Holden TA, McClelland GH, Parkinson WS, Smith ZM. Towards a Measure of Neural Survival Using Psychophysics and CT Scans. Objective Measures in Auditory Implants, 6th International Symposium Abs; 2010. pp. 23–25. [Google Scholar]
- McKay CM, McDermott HJ. Loudness perception with pulsatile electrical stimulation: The effect of interpulse intervals. J Acoust Soc Am. 1998;104:1061–1074. doi: 10.1121/1.423316. [DOI] [PubMed] [Google Scholar]
- McKay CM, Remine MD, McDermott HJ. Loudness summation for pulsatile electrical stimulation of the cochlea: Effects of rate, electrode separation, level, and mode of stimulation. J Acoust Soc Am. 2001;110:1514–1524. doi: 10.1121/1.1394222. [DOI] [PubMed] [Google Scholar]
- Middlebrooks JC. Effects of cochlear-implant pulse rate and inter-channel timing on channel interactions and thresholds. J Acoust Soc Am. 2004;116:452–468. doi: 10.1121/1.1760795. [DOI] [PubMed] [Google Scholar]
- Middlebrooks JC. Cochlear-implant high pulse rate and narrow electrode configuration impair transmission of temporal information to the auditory cortex. J Neurophysiol. 2008;100:92–107. doi: 10.1152/jn.01114.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadol JB., Jr Patterns of neural degeneration in the human cochlea and auditory nerve: implications for cochlear implantation. Otolaryngol Head Neck Surg. 1997;117:220–228. doi: 10.1016/s0194-5998(97)70178-5. [DOI] [PubMed] [Google Scholar]
- Pfingst BE, Colesa DJ, Hembrador S, Kang SY, Middlebrooks JC, Raphael Y, Su GL. Detection of pulse trains in the electrically stimulated cochlea: Effects of cochlear health. J Acoust Soc Am. 2011;130:3954–3968. doi: 10.1121/1.3651820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfingst BE, Morris DJ. Stimulus features affecting psychophysical detection thresholds for electrical stimulation of the cochlea II: Frequency and interpulse interval. J Acoust Soc Am. 1993;94:1287–1294. doi: 10.1121/1.408155. [DOI] [PubMed] [Google Scholar]
- Pfingst BE, Xu L. Across-site variation in detection thresholds and maximum comfortable loudness levels for cochlear implants. J Assoc Res Otolaryngol. 2004;5:11–24. doi: 10.1007/s10162-003-3051-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfingst BE, Xu L, Thompson CS. Effects of carrier pulse rate on modulation detection in subjects with cochlear implants. J Acoust Soc Am. 2007;121:2236–2246. doi: 10.1121/1.2537501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant K, Holden LK, Skinner MW, Arcaroli J, Whitford L, Law MA, Nel E. Clinical Evaluation of Higher Stimulation Rates in the Nucleus Research Platform 8 System. Ear Hear. 2007;28:381–393. doi: 10.1097/AUD.0b013e31804793ac. [DOI] [PubMed] [Google Scholar]
- Roland JT, Fishman AJ, Alexiades G, Cohen N. Electrode to modiolus proximity: A fluoroscopic and histologic analysis. Am J Oto. 2000;21:218–225. doi: 10.1016/s0196-0709(00)80012-5. [DOI] [PubMed] [Google Scholar]
- Rubinstein JT, Wilson BS, Finley CC, Abbas PJ. Pseudospontaneous activity: stochastic independence of auditory nerve fibers with electrical stimulation. Hear Res. 1999;127:108–118. doi: 10.1016/s0378-5955(98)00185-3. [DOI] [PubMed] [Google Scholar]
- Saunders CE, Aschendorff A, Shapiro W, Knight M, Stecher M, Richter B, Walzman S, Tykocinski M, Roland T, Lasziq R, Cowan R. Threshold, comfortable level and impedance changes as a function of electrode-modiolar distance. Ear Hear. 2002;23:28S–40S. doi: 10.1097/00003446-200202001-00004. [DOI] [PubMed] [Google Scholar]
- Shannon RV. Threshold and loudness functions for pulsatile stimulation of cochlear implants. Hear Res. 1985;18:135–143. doi: 10.1016/0378-5955(85)90005-x. [DOI] [PubMed] [Google Scholar]
- Shannon RV. A model of threshold for pulsatile electrical stimulation of cochlear implants. Hear Res. 1989;40:197–204. doi: 10.1016/0378-5955(89)90160-3. [DOI] [PubMed] [Google Scholar]
- Shannon RV, Cruz RJ, Galvin JJ., III Effect of stimulation rate on cochlear implant users’ phoneme, word and sentence recognition in quiet and in noise. Audiol Neurotol. 2011;16:113–123. doi: 10.1159/000315115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner MW. Effect of stimulation rate on cochlear implant recipients’ thresholds and maximum acceptable loudness levels. J Am Acad Audiol. 2000;11:203–213. [PubMed] [Google Scholar]
- Skinner MW, Ketten DR, Holden LK, Harding GW, Smith PG, Gates GA, Neely JG, Kletzker GR, Brunsden B, Blocker B. CT-derived estimation of cochlear morphology and electrode array position in relation to word recognition in Nucleus-22 recipients. J Assoc Res Otolaryngol. 2002;3:332–350. doi: 10.1007/s101620020013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tykocinski M, Cohen LT, Pyman BC, Roland T, Jr, Treaba C, Palamara J, Dahm MC, Shepherd RK, Xu J, Cowan RS, Cohen NL, Clark GM. Comparison of electrode position in the human cochlea using various peri-modiolar electrode arrays. Am J Otol. 2000;21:205–211. doi: 10.1016/s0196-0709(00)80010-1. [DOI] [PubMed] [Google Scholar]
- Vandali AE, Whiteford LA, Plant KL, Clark GM. Speech perception as a function of electrical stimulation rate: using the Nucleus 24 cochlear implant system. Ear Hear. 2000;21:608–624. doi: 10.1097/00003446-200012000-00008. [DOI] [PubMed] [Google Scholar]
- Verschuur CA. Effect of stimulation rate on speech perception in adult users of the Med-El CIS speech processing strategy. Int J Audiol. 2005;44:58–63. doi: 10.1080/14992020400022488. [DOI] [PubMed] [Google Scholar]
- Zeng FG, Shannon RV. Loudness-coding mechanisms inferred from electric stimulation of human auditory system. Science. 1994;264:564–566. doi: 10.1126/science.8160013. [DOI] [PubMed] [Google Scholar]