Abstract
Until now, brain magnetic resonance imaging (MRIs) in asphyxiated neonates receiving therapeutic hypothermia have been performed after treatment is complete. However, there is increasing interest in early brain MRI while hypothermia is still being provided, in order to rapidly understand the degree of brain injury and possibly refine neuroprotective strategies. This study was designed to assess whether therapeutic hypothermia can be maintained while performing a brain MRI. Twenty MRI scans were obtained in twelve asphyxiated neonates while they were treated with hypothermia. Median difference between esophageal temperature on NICU departure and return was 0.1°C (range: −0.8 to 0.8°C). In conclusion, therapeutic hypothermia can be safely and reproducibly maintained during a brain MRI. Hypothermia treatment should not prevent obtaining an early brain MRI if clinically indicated.
Keywords: hypoxic-ischemic encephalopathy, newborn, perinatal asphyxia, hypothermia, magnetic resonance imaging
INTRODUCTION
Induced hypothermia is a treatment for neonatal hypoxic-ischemic encephalopathy (HIE) with an accumulating safety and efficacy profile [1-6]. Currently, brain magnetic resonance imaging (MRI) has routinely been performed on day of life 4 to 7 in neonates receiving therapeutic hypothermia [7-9]. This timing falls in the convenient window after induced hypothermia is complete, and before transfer to another care center. This timing is also based on the idea that induced hypothermia might delay the appearance of brain injury and that early imaging while the asphyxiated newborn is treated with hypothermia might not capture the full extent of brain injury [10]. However, there is increasing interest in brain MRI during the first 3 days of life while hypothermia treatment is still being provided, in order to rapidly understand the degree of brain injury, screen for risks of complications that may be exacerbated by induced hypothermia (e.g. intracranial hemorrhage) and possibly refine neuroprotective strategies [11-13]. Such early neuroimaging would need to be performed while assuring maintenance of the hypothermia. This study was designed to assess whether hypothermia treatment for asphyxiated neonates can be maintained safely and reproducibly while performing brain MRIs.
TECHNIQUE
We conducted a prospective cohort study of consecutive term neonates with HIE admitted to the neonatal intensive care unit (NICU) and meeting the criteria for induced hypothermia [2-3,5-6]. Eligible patients received whole-body cooling to a goal esophageal temperature of 33.5°C with an acceptable range of 32.5-34.5°C per our NICU guidelines adapted from Shankaran et al. [3]. Induced hypothermia was initiated by 6 hours of life, continued for 72 hours (unless contraindications, such as significant hemorrhages or thromboses in the setting of a clinical coagulopathy, developed), and then followed by a slow rewarming [3]. As part of the research protocol [14], sequential MRI studies were planned in order to clarify the evolution of brain injury during the first month of life and compare the results of early versus late imaging in this patient population. If clinically stable, each enrolled neonate underwent one or two “early” brain MRIs during the first 3 days after birth while receiving the hypothermia treatment unless determined to be too unstable to tolerate the study safely. Then they underwent 1-2 “late” MRI scans, including a third scan on DOL 8-13 and a fourth at 1 month of age. MRI scans were performed, using a 3T Siemens Magnetom Trio (Siemens HealthCare, Erlangen, Germany), using preferentially a 32-channel head coil (Siemens HealthCare, Erlangen, Germany) [15] otherwise a standard 12-channel head coil. Each MRI study included anatomic T1- and T2-weighted imaging, diffusion-weighted imaging, spectroscopy and perfusion-weighted imaging. The protocol was approved by the Institutional Review Board and parental consent was obtained.
Induced hypothermia was continued during the early brain MRI scans (FIGURE 1). During the 3-day hypothermia treatment neonates were maintained on the Gelli-roll® hypothermia blanket and Blanketrol® III hypothermia system (Cincinnati Sub-Zero Products, Inc.) on the radiant warmer with the heat source off. During transport to MRI, the hypothermia system was unplugged while the neonate remained on the hypothermia blanket. Upon arrival to the MRI receiving area the hypothermia system was plugged back in to maintain the temperature of the hypothermia blanket while the neonate was in the MRI scanner. Neither the hypothermia blanket nor the hypothermia unit are MRI-compatible, therefore they had to remain outside the MRI suite. Neonates were wrapped with 1-2 thin blankets and placed on a Vac-Fix® MRI-compatible pillow containing Styrofoam, which had been stored for one hour prior to the exam in a 4°C refrigerator, and carried to MRI table. The blankets and pillow were kept dry to avoid skin injury at the site of skin contact. Ears were covered with earmuffs, in order to reduce noise exposure. The MRI-incompatible esophageal probe was removed and an MRI-compatible temperature skin probe was attached to monitor continuously skin temperature during the MRI exam. The strategy in place was to add or remove a blanket if the temperature dropped or increased more that 1°C compared to the baseline skin temperature just before MRI. Supportive therapies including mechanical ventilation, vasoactive infusions and sedation were maintained throughout the study per current clinical practice. Additional sedation was administered only if deemed clinically necessary, and was rarely required. Once the neonate was placed in the MRI scanner, the air in the MRI-compatible pillow was removed by suction in order to mould the shape of the pillow to the infant’s head and body, and further reduce motion artifacts. At the end of the MRI study, the neonate was removed from the MRI-compatible pillow and blankets, placed back on the hypothermia blanket and brought back to the NICU. Time and esophageal temperature were measured at the time of NICU departure and return.
FIGURE 1.
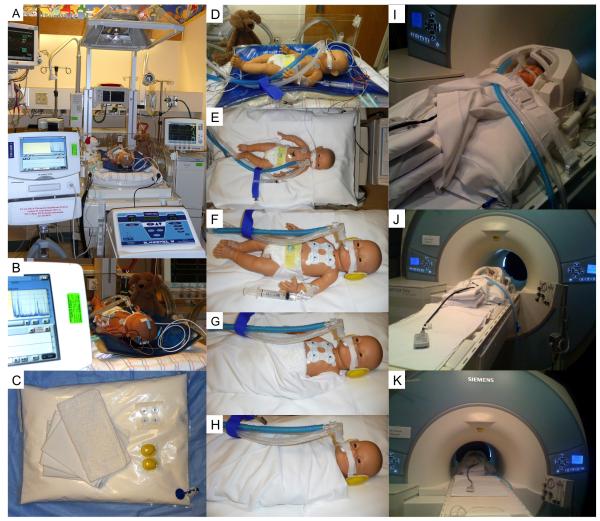
Procedure for performing brain MRI in neonates with hypoxic-ischemic encephalopathy treated with induced hypothermia. A-B: During hypothermia treatment neonates are maintained on the hypothermia blanket and hypothermia system in the incubator with the canopy up and the heat source off. During the whole treatment, neonates are monitored by amplitude-integrated electroencephalogram. C. Materials: a MRI-compatible pillow containing Styrofoam and 1-2 thin blankets, stored for a few hours prior to the exam in a 4°C fridge, as well as earmuffs and complete MRI-compatible cardiovascular monitoring. D-H: Neonates are wrapped with 1-2 thin blankets and placed on a MRI-compatible pillow containing Styrofoam. Ears are covered with earmuffs. The MRI-incompatible esophageal probe is removed. Complete MRI-compatible cardiovascular monitoring is placed. I-K: Once the neonate is in place in the MRI scanner, the air in the MRI-compatible pillow is suctioned, and imaging process starts.
Complete imaging and data were available for 20 MRI studies in 12 term asphyxiated neonates treated with induced hypothermia. No adverse events were recorded. Median difference between esophageal temperature on NICU departure and return was 0.1°C (FIGURE 2), with a range of -0.8 to 0.8°C, remaining in the acceptable range of 32.5-34.5°C for all scans, except four. Of note, in the patients in whom temperature was outside the acceptable range (first scan for Patient # 2, 3, 5 and 8), the temperature was already inadvertently out of range before NICU departure, and temperature was readjusted after the MRI. Also of note, skin temperature measured by the MRI-compatible temperature skin probe remained stable throughout all studies.
FIGURE 2.
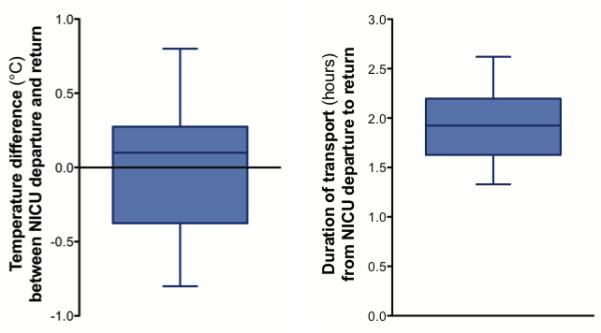
Duration of total transport and difference between esophageal temperature on NICU departure and return in term asphyxiated newborns treated with induced hypothermia undergoing brain MRI. Box and whisker plots (median, 25th and 75th percentiles, minimum and maximum) representation.
Median duration of the total transport from NICU departure to return was 1.9 hours (FIGURE 2), with a range of 1.3-2.6 hours. This included the transport time to and from the MRI area, the time to prepare and transfer the neonate into the MRI scanner and then back in the incubator on the cooling blanket, and the MRI scan time. The MRI scan time was approximately 1 hour.
As described in another communication [14], brain injuries were already visible on these early MRI scans in some of the asphyxiated patients while they were still treated with induced hypothermia (FIGURE 3). No significant motion artifacts were present.
FIGURE 3.
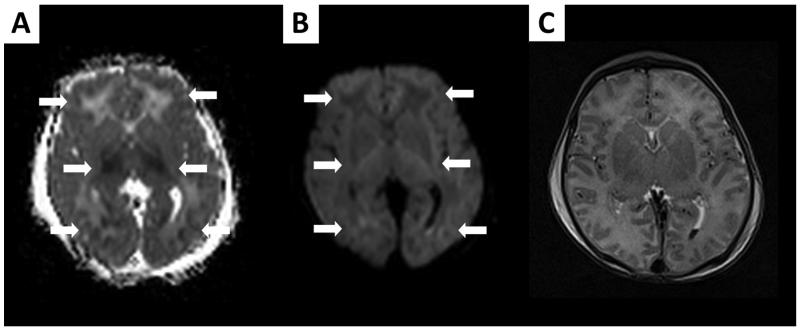
Brain MRI performed on day of life 2 in a newborn demonstrating total cortical injury pattern while he is still receiving therapeutic hypothermia; (A) ADC map, (B) DWI images, and (C) T2-weighted imaging. Clear diffusion abnormalities are present in the cortex, white matter and basal ganglia of this newborn as seen on ADC map and DWI images (arrows), while findings are not so evident on concomitant T2-weighted imaging.
DISCUSSION
For practical reasons, performing brain MRIs is more convenient after induced hypothermia is complete. However, it should not be delayed if an early MRI may be of significant clinical value: for example if there is a suspicion of a complication that may be exacerbated by induced hypothermia (e.g. intracranial hemorrhage). We found that therapeutic hypothermia with a goal core temperature of 33.5°C and an acceptable range of 32.5-34.5°C can be safely and reproducibly maintained during an MRI in term asphyxiated neonates. Hypothermia treatment should not prevent obtaining an early brain imaging, as hypothermia can be maintained safely during the entire imaging process.
It is unknown when the optimal timing is for brain imaging of term asphyxiated neonates treated with induced hypothermia to accurately define their brain injuries and predict their neurologic function [11-13]. In the era before induced hypothermia was widely offered, the day of life 2-3 window was considered ideal to understand early potential brain injury [16-17]. However, it has been hypothesized that induced hypothermia might delay the appearance of brain injury and that early imaging while the asphyxiated newborn is treated with hypothermia might not capture the full extent of brain injury [10]. In our feasibility study, we found that MRI scans obtained on DOL 2-3 during hypothermia still seem to predict later brain injuries in asphyxiated newborns and that the brain injuries identified during this early time appear to represent irreversible changes [14]. The late brain imaging studies did not reveal any new brain injuries that were not seen on DOL 2-3, but also did not show that the brain injuries, if present, were underestimated on these early scans [14]. Larger studies would be useful to determine whether early MRI’s obtained during hypothermia treatment might allow the refinement of induced hypothermia or suggest the addition of other neuroprotective strategies for preventing further brain injury. In the meantime, in clinical settings where only one brain imaging may be obtained, it is certainly reasonable to delay the imaging to the second week after delivery, when the lesions are clearly visible on conventional imaging [14]. Median duration of the total transport from NICU departure to NICU return was nearly 2 hours. One contribution to our successful maintenance of hypothermia during this prolonged period was our team approach during the entire process. This team included a neuroradiologist and MRI technician [18] as well as a neonatologist or a neonatal nurse practitioner, NICU nurse and a respiratory therapist, who exclusively cared for the neonate from NICU departure to return. The team was trained in critical neonatal transport to MRI and was aware of the details of providing care for asphyxiated neonates treated with induced hypothermia. Collaboration between neonatology and neuroradiology was focused on minimizing the time outside of the NICU. The imaging protocol should include all the essential MRI sequences to evaluate brain injury in term asphyxiated neonates [including high spatial resolution T1- and T2-weighted imaging, diffusion-weighted imaging, spectroscopy and perfusion-weighted imaging) [19], while avoiding prolonged time in the MRI scanner, preferably less than one hour.
In conclusion, therapeutic hypothermia can be safely and reproducibly maintained during brain MRI in term asphyxiated neonates. This treatment should not prevent obtaining an early brain MRI if it is thought to be of important clinical value. Additional studies are needed in these patients to determine the full prognostic value of early imaging during hypothermia treatment.
ACKNOWLEDGMENTS
Pia Wintermark receives generous research grant funding from the William Randolph Hearst Fund Award and the Thrasher Research Fund Early Career Award Program. The work of Simon K. Warfield is supported by NIH grants R01 RR021885, R01 GM074068, R03 EB008680 and P30 HD018655. The authors thank the families and their neonates in participating in the study. A special thank you is also expressed to the NICU clinicians and the MRI technicians, who have made this study possible.
Financial disclosure: Pia Wintermark receives generous research grant funding from the William Randolph Hearst Fund Award and the Thrasher Research Fund Early Career Award Program. The work of Simon K. Warfield is supported by NIH grants R01 RR021885, R01 GM074068, R03 EB008680 and P30 HD018655.
Abbreviations
- HI
Hypoxic-Ischemic
- DWI
Diffusion-Weighted Imaging
- HIE
Hypoxic-Ischemic Encephalopathy
- MRI
Magnetic Resonance Imaging
- NICU
Neonatal Intensive Care Unit
Footnotes
CONFLICT OF INTEREST.
No conflict of interest. The mention of specific vendors for equipment is solely reflective of equipment usage in our unit. We do not receive any financial or other compensation from any of the vendors mentioned in this review. We realize that there are other vendors who manufacture MR-compatible equipment.
REFERENCES
- 1.Eicher DJ, Wagner CL, Katikaneni LP, Hulsey TC, Bass WT, Kaufman DA, Horgan MJ, Languani S, Bhatia JJ, Givelichian LM, Sankaran K, Yager JY. Moderate hypothermia in neonatal encephalopathy: efficacy outcomes. Pediatr Neurol. 2005;32:11–17. doi: 10.1016/j.pediatrneurol.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 2.Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, Polin RA, Robertson CM, Thoresen M, Whitelaw A, Gunn AJ. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365:663–670. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 3.Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, Fanaroff AA, Poole WK, Wright LL, Higgins RD, Finer NN, Carlo WA, Duara S, Oh W, Cotten CM, Stevenson DK, Stoll BJ, Lemons JA, Guillet R, Jobe AH, National Institute of Child Health and Human Development Neonatal Research Network Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs S, Hunt R, Tarnow-Mordi W, Inder T, Davis P. Cooling for newborns with hypoxic-ischaemic encephalopathy. Cochrane Database Syst Rev. 2007;4:CD003311. doi: 10.1002/14651858.CD003311.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Azzopardi D, Brocklehurst P, Edwards D, Halliday H, Levene M, Thoresen M, Whitelaw A, TOBY Study Group. The TOBY Study Whole body hypothermia for the treatment of perinatal asphyxial encephalopathy: a randomised controlled trial. BMC Pediatr. 2008;8:17. doi: 10.1186/1471-2431-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azzopardi DV, Strohm B, Edwards AD, Dyet L, Halliday HL, Juszczak E, Kapellou O, Levene M, Marlow N, Porter E, Thoresen M, Whitelaw A, Brocklehurst P, TOBY Study Group Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361:1349–1358. doi: 10.1056/NEJMoa0900854. [DOI] [PubMed] [Google Scholar]
- 7.Inder TE, Hunt RW, Morley CJ, Coleman L, Stewart M, Doyle LW, Jacobs SE. Randomized trial of systemic hypothermia selectively protects the cortex on MRI in term hypoxic-ischemic encephalopathy. J Pediatr. 2004;145:835–837. doi: 10.1016/j.jpeds.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 8.Rutherford MA, Azzopardi D, Whitelaw A, Cowan F, Renowden S, Edwards AD, Thoresen M. Mild hypothermia and the distribution of cerebral lesions in neonates with hypoxic-ischemic encephalopathy. Pediatrics. 2005;116:1001–1006. doi: 10.1542/peds.2005-0328. [DOI] [PubMed] [Google Scholar]
- 9.Rutherford M, Ramenghi LA, Edwards AD, Brocklehurst P, Halliday H, Levene M, Strohm B, Thoresen M, Whitelaw A, Azzopardi D. Assessment of brain tissue injury after moderate hypothermia in neonates with hypoxic-ischaemic encephalopathy: a nested substudy of a randomised controlled trial. Lancet Neurol. 2010;9:39–45. doi: 10.1016/S1474-4422(09)70295-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neil J. Is MRI still cool after hypothermia? Lancet Neurol. 2010;9:19–20. doi: 10.1016/S1474-4422(09)70302-3. [DOI] [PubMed] [Google Scholar]
- 11.Higgins RD, Raju TN, Perlman J, Azzopardi DV, Blackmon LR, Clark RH, Edwards AD, Ferriero DM, Gluckman PD, Gunn AJ, Jacobs SE, Eicher DJ, Jobe AH, Laptook AR, LeBlanc MH, Palmer C, Shankaran S, Soll RF, Stark AR, Thoresen M, Wyatt J. J Pediatr; Hypothermia and perinatal asphyxia: executive summary of the National Institute of Child Health and Human Developmental workshop; 2006; pp. 170–175. [DOI] [PubMed] [Google Scholar]
- 12.Barks JD. Current controversies in hypothermic neuroprotection. Semin Fetal Neonatal Med. 2008;13:30–34. doi: 10.1016/j.siny.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Higgins RD, Shankaran S. Hypothermia for hypoxic ischemic encephalopathy in infants > or =36 weeks. Early Hum Dev. 2009;85:S49–52. doi: 10.1016/j.earlhumdev.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wintermark P, Hansen A, Soul J, Labrecque M, Robertson RL, Warfield SK. Early versus late MRI in asphyxiated newborns treated with hypothermia. Accepted for publication in Archives of Disease in Childhood. 2010 doi: 10.1136/adc.2010.184291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiggins GC, Triantafyllou C, Potthast A, Reykowski A, Nittka M, Wald LL. 32-channel 3 Tesla receive-only phase-array head coil with soccer-ball element geometry. Magn Reson Med. 2006;56:216–223. doi: 10.1002/mrm.20925. [DOI] [PubMed] [Google Scholar]
- 16.Barkovich AJ. MR imaging of the neonatal brain. Neuroimaging Clin N Am. 2006;16:117–35. viii–ix. doi: 10.1016/j.nic.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Rutherford M, Srinivasan L, Dyet L, Ward P, Allsop J, Counsell S, Cowan F. Magnetic resonance imaging in perinatal brain injury: clinical presentation, lesions and outcome. Pediatr Radiol. 2006;36:582–592. doi: 10.1007/s00247-006-0164-8. [DOI] [PubMed] [Google Scholar]
- 18.Mathur AM, Neil JJ, McKinstry RC, Inder TE. Transport, monitoring, and successful brain MR imaging in unsedated neonates. Pediatr Radiol. 2008;38:260–264. doi: 10.1007/s00247-007-0705-9. [DOI] [PubMed] [Google Scholar]
- 19.Barkovich AJ, Miller SP, Bartha A, Newton N, Hamrick SE, Mukherjee P, Glenn OA, Xu D, Partridge JC, Ferriero DM, Vigneron DB. MR imaging, MR spectroscopy, and diffusion tensor imaging of sequential studies in neonates with encephalopathy. AJNR Am J Neuroradiol. 2006;27:533–547. [PMC free article] [PubMed] [Google Scholar]