Abstract
BACKGROUND
Although myeloperoxidase (MPO) monitoring is predictive for cardiovascular outcomes in suspected acute coronary syndromes, the value of serial testing is unknown.
METHODS
We investigated the relationship between serial MPO concentrations in 490 individuals with acute chest pain and incident major adverse cardiac events (MACE) during 6 months of follow-up. We measured MPO with the CardioMPO assay, and cardiac troponin I (cTnI), with the Abbott Architect assay.
RESULTS
Plasma MPO concentrations during the first 16 h were higher in individuals who experienced MACE. Higher MPO quartiles predicted a greater likelihood of 6-month MACE at baseline [OR (95% CI), 2.4 (1.4 – 4.1), P = 0.001 for highest vs lowest quartile] and all subsequent time points, with strongest predictive ability found in 16-h postbaseline samples [9.9 (4.7–20.9), P < 0.001 for highest vs lowest quartile]. MPO was predictive for MACE among individuals whose cTnI remained within reference intervals (<0.028 μg/L). The lowest rate of missed cases was found when MPO was <640 pmol/L at baseline and all other time points. Serial MPO monitoring predicted MACE risk better than baseline MPO measurements alone (c statistic 0.813 vs 0.602; P = 0.002), including in individuals whose cTnI remained within reference intervals (c statistic 0.903; P = 0.009). Combined serial cTnI and MPO testing improved accuracy for predicting 6-month MACE, reduced the number of missed MACE events from cTnI testing alone, and improved risk classification in 26.1% of patients.
CONCLUSIONS
MPO concentrations are predictive of outcome up to 16 h after presentation with chest pain and predict events missed by cTnI testing, supporting a potential role in rapid patient triage.
Clinical factors associated with an increased risk of atherosclerotic cardiovascular disease have been used for risk prediction algorithms. Conventional models for prediction of cardiovascular risk, however, are limited in their ability to accurately predict clinical events in all individuals. This limitation persists despite the increasing use of the most recent high-sensitivity assays of myocardial necrosis (1). Furthermore, clinical events occur in individuals who have no traditional risk factors. As a result, considerable interest has focused on the role of other factors involved in the progression and rupture of atherosclerotic plaque to help predict the likelihood of a near-term adverse cardiovascular outcome.
Increasing evidence has highlighted the role of inflammation in all stages of atherosclerotic cardiovascular disease (2). The role of inflammation in the translation of disease to clinical events is supported by the observation that increased systemic concentrations of the inflammatory biomarker, C-reactive protein (CRP),4 predict prospective cardiovascular risk in the settings of both primary and secondary prevention (3). It remains uncertain, however, whether CRP plays a direct pathologic role in atherosclerosis or whether it is simply a marker of inflammatory activity (4). Indeed, recent Mendelian randomization studies show a lack of concordance between the effect on coronary athero-sclerotic heart disease risk of CRP genotypes and CRP concentrations, arguing against a causal role for CRP in atherosclerotic cardiovascular disease (5). Identification of an inflammatory mediator involved in disease pathogenesis might enhance prediction of cardiovascular risk.
Numerous lines of evidence suggest a direct role of the leukocyte-derived enzyme, myeloperoxidase (MPO), in the pathogenesis of atherosclerosis. MPO and its oxidant products have been identified in atherosclerotic plaque and promote a number of pathological events that participate in plaque formation and rupture (6). MPO catalytically consumes nitric oxide in vitro and in vivo (7) and promotes endothelial dys-function (8), lipid peroxidation (9), activation of pro-tease cascades involved in plaque fissuring or rupture (10), induction of endothelial cell tissue factor expression (11), oxidative conversion of LDL into a high-uptake form for macrophages (9), and modification of apolipoprotein A-I of HDL, impairing its ability to promote cholesterol efflux (12, 13) and other athero-protective functions (14). Finally, genetic studies support a mechanistic contribution of MPO in atherosclerosis, because patients with either MPO deficiency or polymorphisms associated with diminished expression of MPO appear to be protected from cardiovascular disease (15–20), and overexpression of the human MPO transgene in mice induces accelerated atherosclerosis (21–23).
Multiple studies show that circulating concentrations of MPO are predictive of adverse clinical outcomes (24–30). MPO concentrations strongly correlate with the degree of endothelial dysfunction (31). Systemic MPO concentrations have been demonstrated to independently predict the prospective risk of adverse cardiac events in cohorts of patients who are asymptomatic (24), have stable coronary artery disease (25, 26), or present with acute coronary syndromes (27–29) or to the emergency room for the assessment of chest pain (30). In these latter cohorts who present acutely, the ability of MPO concentrations to predict cardiovascular outcome is observed in the absence of detectable levels of myocardial necrosis as indicated by concentrations of cardiac troponins or cardiac isoform of creatine kinase within the reference (28, 30). Although many patients who are evaluated for acute chest pain undergo serial assessment with cardiac markers of necrosis, the value of measuring MPO concentrations at multiple time points has not been systematically investigated. The aim of the current study was to determine the clinical utility of serial measurements of systemic concentrations of MPO to predict cardiovascular outcome in patients who present for assessment of suspected cardiac chest pain.
Materials and Methods
The selection of individuals for inclusion in the study has been presented in detail (30). In brief, we recruited patients from a cohort that participated in a study to compare cardiac troponin T (cTnT) and creatine kinase MB in the diagnosis of myocardial infarction. For the present analyses, we evaluated patients who presented to the emergency room within 4 h of onset of acute chest pain of suspected cardiac etiology and continued to require evaluation to determine cardiovascular risk. Accordingly, patients who presented with ST-segment elevation were not included in the analysis.
Myocardial infarction was defined as a cardiac troponin I (cTnI) concentration, as measured by a sensitive assay, of at least 0.028 μg/L (99th percentile of asymptomatic apparently healthy population, n = 300). Unstable angina was defined as the presence of angina at rest, a sudden increase in episodes of angina, ST-segment depression, or T-wave inversion. Acute coronary syndrome was defined as myocardial infarction or unstable angina at presentation. History of hyperlipidemia was defined as history of total cholesterol concentrations >200 mg/dL (5.17 mmol/L) or triglyceride concentrations >200 mg/dL (2.26 mmol/L). All clinical diagnoses and electrocardiographic data were confirmed by an investigator who was unaware of the patients’ diagnoses and outcomes.
Plasma samples were collected from patients at the time of presentation to the emergency room (baseline) and 4, 8, and 16 h later. MPO concentrations were measured by use of an ELISA (CardioMPO Test, Cleveland Heart Labs) cleared by the US Food and Drug Administration (FDA). The intraday assay and interday assay variabilities were 2% and 6%, respectively. The lower detection limit was 13 pmol/L, and the upper limit of the analytical measurement range was 5223 pmol/L, >10 000 pmol/L with sample dilution. In a cohort of apparently healthy middle-aged individuals (n = 300), we observed a mean (SD) MPO concentration of 204 (139) pmol/L (lithium heparin plasma samples), with upper reference limit of 640 pmol/L (95th percentile from healthy individuals). Concentrations of cTnI in plasma (also collected in lithium heparin tubes) were measured by using the STAT Troponin I assay (Abbott Laboratories) in a research-based immunoanalyzer that provides a 3–decimal point readout. This assay provides highly sensitive analytical measurement of cTnI with a limit of detection at 0.009 μg/L reported in the literature, and an upper reference limit of 0.028 μg/L (defined 99th percentile cutoff) (1). High-sensitivity CRP concentrations were measured on the Abbott Architect platform (Abbott Laboratories) in a central clinical laboratory.
Patients who had a baseline MPO measurement and at least 1 postbaseline measurement were included in the analysis. Baseline patient characteristics were expressed as median and interquartile range for continuous data and percentages for categorical data. MPO values at each time point were categorized into quartiles. We calculated unadjusted odds ratios (ORs) and 95% CIs using logistic regression to assess the risk of major adverse cardiac events (MACE: nonfatal myocardial infarction, coronary revascularization, or death) at 6 months for each MPO quartile compared with the lowest quartile at each time point. We also performed this analysis for the group of patients who demonstrated cTnI persistently within the reference interval (<0.028 μg/L). We generated a multivariable logistic regression model to determine whether serial MPO measurements were independently predictive of 6-month MACE. We used bootstrapping methods to ascertain which variables were predictive of the endpoint and stepwise selection methods to select variables that were significant; those that had a P value <0.05 remained in the final model. MPO at baseline and the average of the postbaseline measures were included in the model as continuous variables. Both these MPO variables were logarithmically transformed. Whereas CRP was not a significant predictor on univariate analysis, it was included in the multivariable model for adjustment.
To determine whether MPO has an additional predictive value compared to a model including traditional risk factors, we calculated the concordance indexes (c statistics) with and without MPO. The traditional risk factor model included age, sex, race, history of smoking, diabetes mellitus, hypertension, hypercholesterolemia, coronary artery bypass graft, and percutaneous transluminal coronary angioplasty. The c statistic is the probability that, for 2 randomly selected patients, the patient with higher predicted risk actually experienced the MACE event (32). We assessed the improvement in predictability by calculating the difference in the c statistic, which was bias corrected. Bootstrapping was used to generate 95% CIs. A 1-sample t-test was performed to determine if the difference was significantly different from zero.
To quantify the improvement in classifying patients at higher risk of MACE with the serial MPO measurements, net classification improvement (33) was calculated and tested to determine if the improvement was significantly different from zero. ROC curves were created to graphically demonstrate the discriminatory ability of (a) baseline MPO only; (b) baseline MPO plus the average postbaseline MPO values; and (c) CRP for the entire cohort. ROC curves were also created for these tests in individuals whose cTnI was persistently within reference intervals. All statistical analyses were performed using SAS version 9.1 (SAS Institute). P values <0.05 were considered significant.
Results
The clinical and laboratory characteristics of the study participants, stratified according to the presence of an MPO concentration that was increased or within the reference interval at baseline, are summarized in Table 1. Patients with an increased MPO at baseline were older and more likely to be male and have a history of smoking, diabetes, myocardial infarction, and peripheral vascular disease. There was no difference in medication use between the groups. The presence of an increased MPO concentration at baseline was associated with white blood cell count and CRP. Although MPO concentrations were greater postbaseline in both groups, concentrations were higher at all time points in patients with an increased MPO at baseline. The index presentation to the emergency room resulted in a diagnosis of myocardial infarction in 23.6% and acute coronary syndrome in 40.5%. Incident MACE were observed in 43.2% of patients at 6 months.
Table 1.
Clinical characteristics and biochemical parameters of patients.a
| Parameter | Baseline MPO ≤640 pmol/L | Baseline MPO >640 pmol/L | P |
|---|---|---|---|
| n | 69 | 421 | |
| Age, years | 59.6 (13.9) | 63.4(13.8) | 0.04 |
| Males | 43.5 | 61.5 | 0.005 |
| History of smoking | 52.4 | 65.1 | 0.05 |
| Current smoker | 37.5 | 38.9 | 0.88 |
| White | 46.0 | 59.7 | 0.04 |
| Black | 50.8 | 36.1 | 0.03 |
| History of coronary artery disease | 58.7 | 48.6 | 0.14 |
| History of revascularization | 33.3 | 36.4 | 0.64 |
| History of diabetes | 13.6 | 27.7 | 0.015 |
| History of hypertension | 66.7 | 64.8 | 0.77 |
| History of hyperlipidemia | 46.2 | 50.6 | 0.51 |
| History of myocardial infarction | 23.8 | 39.7 | 0.02 |
| History of peripheral vascular disease | 4.8 | 13.3 | 0.05 |
| Aspirin use | 32.2 | 38.2 | 0.37 |
| Statin use | 13.6 | 12.9 | 0.89 |
| Angiotensin-converting enzyme inhibitor use | 28.8 | 26.3 | 0.69 |
| β-Blocker use | 30.5 | 23.3 | 0.23 |
| White blood cell | 6.2 (5.1–8.2) | 7.3 (6.2–0.1) | 0.02 |
| CRP | 0.3 (0.1–0.7) | 0.6 (0.2-1.2) | 0.003 |
| MPO | |||
| Baseline | 454 (397–559) | 1945 (1199–3127) | <0.001 |
| 4h | 1051 (593–1750) | 2030 (1163–3263) | <0.001 |
| 8h | 921 (602–1887) | 2325 (1286–3644) | <0.001 |
| 16h | 927 (594–1539) | 2018 (1160–3503) | <0.001 |
Data are mean (SD), %, or median (interquartile range).
Table 2 summarizes the risk of MACE during 6-month follow-up, stratified according to increasing quartiles of plasma MPO concentrations in samples obtained at different time points in relation to initial time of presentation for assessment of chest pain. Compared with the lowest MPO quartile, clinical events were more common in patients with higher MPO concentrations at all time points. Increasing quartiles of MPO concentrations predicted a greater likelihood of 6-month MACE when measured at baseline [OR (95% CI), 2.4 (1.4–4.1), P = 0.001 for comparison between highest vs lowest MPO quartile], as well as all subsequent time points, with strongest prediction in postbaseline samples. For example, the OR (95% CI) was 5.9 (3.2–11.1) and 9.9 (4.7–20.9) (P < 0.001) for fourth vs first quartile samples measured at 4 h and 16 h, respectively. Similar findings were also observed in patients in whom cTnI concentrations were persistently within reference intervals (Table 2). After adjustment for other predictors of clinical events, increased MPO concentrations (P < 0.001) continued to predict clinical outcome at all time points beyond presentation to the emergency room in both the entire cohort and those for whom serial cTnI testing gave results persistently within the reference interval (Table 2).
Table 2.
Ability of quartiles of MPO, compared with the first quartile, for the prediction of major adverse cardiac events in samples collected from subjects at different time points in relation to presentation with chest pain.
| Quartile 1 |
Quartile 2 |
Quartile 3 |
Quartile 4 |
|||||
|---|---|---|---|---|---|---|---|---|
| N | OR | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Baseline | 490 | 1.0 | 1.3 (0.8–2.2) | 0.31 | 2.0 (1.2–3.3) | 0.01 | 2.4 (1.4–4.1) | 0.001 |
| 4h | 423 | 1.0 | 2.6 (1.4–5.0) | 0.003 | 5.1 (2.7–9.5) | <0.001 | 5.9 (3.2–11.1) | <0.001 |
| 8h | 387 | 1.0 | 1.7 (0.9–3.3) | 0.09 | 3.4 (1.8–6.4) | <0.001 | 5.2 (2.8–9.8) | <0.001 |
| 16h | 372 | 1.0 | 3.4 (1.6–7.3) | 0.002 | 6.7 (3.2–14.2) | <0.001 | 9.9 (4.7–20.9) | <0.001 |
| Patients with cTnl persistently within reference intervals | ||||||||
| Baseline | 251 | 1.0 | 1.5(0.7–3.4) | 0.33 | 1.6 (0.7–3.8) | 0.24 | 1.7 (0.7–4.0) | 0.23 |
| 4h | 217 | 1.0 | 3.0 (0.9–10.1) | 0.08 | 6.0 (1.8–20.1) | 0.004 | 8.3 (2.5–26.9) | <0.001 |
| 8h | 202 | 1.0 | 6.1 (1.3–29.2) | 0.02 | 13.1 (2.8–61.4) | 0.001 | 17.6 (3.8–81.9) | <0.001 |
| 16h | 195 | 1.0 | 4.0 (1.0–16.1) | 0.048 | 5.9 (1.5–22.9) | 0.01 | 7.9 (2.0–31.3) | 0.003 |
For clinical applications, the impact of specific MPO cutpoints on clinical outcomes is of interest. At all subsequent time points during the next 16 h of hospitalization (4, 8, and 16 h), an increased MPO concentration (>640 pmol/L) was observed in >90% of patients who experienced MACE (6 months). By comparison, a positive cTnI (≥0.028 μg/L) concentration at baseline presentation was observed only among 55.6% of those that experienced MACE over the ensuing 6-month period. Serial cTnI testing increased this percentage to 72.4% of MACE identified (i.e., approximately 27% of MACE over the ensuing 6-month period were missed, which constituted predominantly revascularization). Remarkably, the combination of MPO and cTnI testing helped to capture all subsequent MACE within the cohort, as illustrated by the percentage of MACE observed stratified by MPO testing in those who had cTnI concentrations persistently within the reference interval. In a similar fashion, the finding of MPO within the reference interval (i.e., less than the upper limit of the reference interval, 640 pmol/L) at time points following presentation was associated with improved risk prediction, with significantly less MACE missed (Table 4). For example, 44.4% of MACE (6 months) experienced by the entire cohort were missed by cTnI within the normal reference interval at baseline. In contrast, using an MPO cutoff of the upper limit of normal (<640 pmol/L), the number of MACE missed was reduced nearly 80% to 9.3%. Finally, baseline MPO and cTnI concentrations that were within reference intervals resulted in missing only 5.6% of MACE (i.e., if baseline testing showed cTnI and MPO concentrations within reference intervals the risk of missing a MACE event was lowered by nearly 90%). A negative MPO at baseline and at least 1 other time point was associated with the lowest rate of missed cardiovascular events (Table 3).
Table 4.
Major adverse cardiovascular events stratified according to presence of MPO in the reference interval (<640 pmol/L) or increased from baseline to different time points.
| Baseline within reference interval |
Baseline increased |
|||||
|---|---|---|---|---|---|---|
| Time point | Within reference interval/ within reference interval | Within reference interval/increased | P | Increased/within reference interval | Increased/increased | P |
| 4h | 10.0(0–23.1) | 36.7 (23.2–50.2) | 0.03 | 11.5(0–23.8) | 48.4 (43.4–53.3) | <0.001 |
| 8h | 15.4(0–35) | 32.1 (19.9–44.4) | 0.32 | 8.3(0–19.4) | 48.4 (43.4–53.3) | <0.001 |
| 16 h | 0 | 37.7 (24.7–5038) | 0.003 | 15.0(0–30.6) | 47.6 (42.7–52.5) | 0.004 |
Data are % (95% CI).
Table 3.
Major adverse cardiovascular events missed when MPO or troponin was within the reference interval (MPO <640 pmol/L or cTnI <0.028 μg/L) at different time points.
| Time point | MACE missed |
|---|---|
| MPO negative | |
| Baseline | 9.3 (5.1–13.2) |
| 4h | 2.3 (0.3–4.4) |
| 8 h | 1.9 (0.1–3.7) |
| 16 h | 1.4 (0–3.0) |
| cTnI negative | |
| Baseline | 44.4 (37.7–51.0) |
| 4h | 34.1 (27.8–40.5) |
| 8 h | 31.8 (25.5–38.0) |
| 16h | 32.2 (26–38.5) |
| MPO and cTnI both negative | |
| Baseline | 5.6 (2.5–8.7) |
| Any other time point | 1.9 (0.1–3.7) |
Data are % (95% CI).
Serial monitoring of MPO was further investigated by characterizing the percentage change in MPO from baseline to subsequent time points in patients who experienced a MACE vs those who did not. The presence of MACE during 6-month follow-up was associated with increases (mean increase of 15% or greater) in MPO concentrations from baseline to later time points during the initial monitoring period after presentation (Fig. 1). In contrast, patients who did not experience MACE did not demonstrate increases in MPO concentrations from baseline with serial testing during the clinical evaluation period. Furthermore, serial changes in status of MPO positivity predicted likelihood of subsequent MACE (Table 4). For example, the finding of a low MPO concentration at any time point after presentation was associated with a lower clinical risk, regardless of the whether MPO concentrations were increased at baseline. In contrast, development of an increased MPO concentration predicted an adverse outcome in patients with low concentrations at baseline. These findings were observed regardless of the timing of the postbaseline sample measured or the MPO cutoff used to define positivity. In general, these findings support an increased likelihood of MACE with increasing MPO concentrations. On multivariable analysis, factors independently associated with a greater likelihood of MACE during the next 6 months included increased MPO concentration on at least 1 occasion on serial evaluation of MPO (P < 0.001), cTnI persistently within the reference interval (P < 0.001), diagnosis of unstable angina at the time of the index presentation (P < 0.001), white race (P = 0.001), history of smoking (P = 0.008), and requirement for treatment with β-blockers (P = 0.009). The paradoxical finding of a relationship between cTnI concentrations persistently within reference intervals and subsequent cardiovascular outcomes may reflect patients who are less likely to receive risk factor intervention; however, this requires further investigation. Of particular interest, measurement of MPO at baseline alone did not predict events (P = 0.36) when serial evaluation was also included in the model. In contrast, CRP concentrations did not independently predict MACE in this cohort in models with MPO testing at any time point (P = 0.19).
Fig. 1.
Mean (95% CI) percentage change in MPO concentrations from baseline to each additional time point in patients stratified according to the presence (■) or absence (▲) of a major adverse cardiovascular event during 6 months of follow-up.
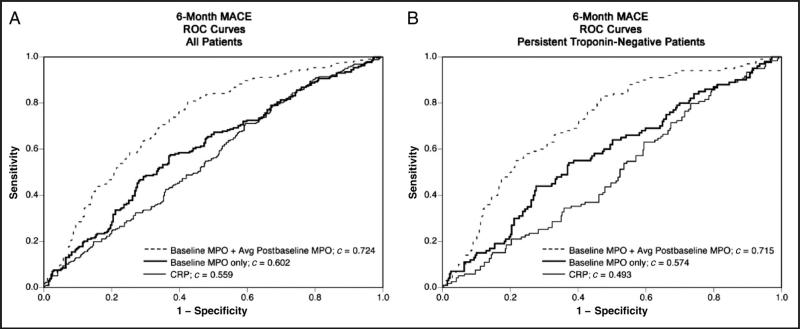
The ability of serial measurements of MPO to predict subsequent cardiovascular events during the next 6 months was evaluated by plotting ROC curves. The c statistic for prediction of MACE during 6 months of follow-up was greater for use of the combination of MPO concentrations at baseline with a second time point (data shown are for the mean of postbaseline values, c = 0.724, P < 0.001) in comparison with use of baseline MPO alone (c = 0.602, P < 0.001) and CRP (c = 0.559, P < 0.001). This finding was observed in the entire cohort, and in those patients with cTnI persistently within the reference interval (Fig. 2). After adjustment for clinical characteristics, a greater c-statistic value was observed for serial MPO evaluation in the entire cohort (c = 0.813, P = 0.002) and those with cTnI persistently within the reference interval (c = 0.903, P = 0.009) compared with baseline MPO alone. Furthermore, calculation of the net reclassification index revealed that serial monitoring of MPO to detect either the presence or absence of increased MPO (>640 pmol/L) resulted in an improvement in classification of risk in 26.1% of patients (P < 0.001), compared with assessment of clinical characteristics and troponin alone. Having an MPO concentration greater than the median (3080 pmol/L) was associated with sensitivity 66.4%, specificity 62.7%, positive predictive value 58.0%, and negative predictive value 70.6% for prediction of MACE. The negative predictive value (87.2%) was greater in patients with cTnI persistently within the reference interval.
Fig. 2.
ROC curves for ability of baseline MPO only, baseline MPO plus the mean postbaseline MPO, and CRP to predict MACE during 6 months of follow-up in the entire cohort (A) and those patients whose cTnI was persistently within the reference interval (B).
Discussion
Increasing evidence suggests a role for MPO and its oxidant products in the formation and propagation of atherosclerotic cardiovascular disease (6). Systemic concentrations of MPO have been reported to predict prospective clinical risk in patients who present for assessment of chest pain of suspected cardiac etiology (30). The current findings use an assay cleared by the FDA and extend observations from early reports from investigators who used a research-grade MPO assay (28, 30), as well as building on subsequent studies demonstrating the incremental prognostic value of a single measure of plasma MPO compared with cardiac troponin measurements (34), to suggest that serial monitoring of MPO concentrations provides incremental ability to predict the risk of cardiovascular events. The present observations also confirm previous findings of a rapid increase in MPO concentrations in patients presenting with acute coronary syndromes (35). Although some groups have failed to observe a relationship between MPO concentrations and cardiovascular risk in patients presenting with an acute coronary syndrome (36–38), this is the first investigation to our knowledge that, using an FDA-cleared assay, shows the potential utility of serial MPO measures in the patient undergoing evaluation for acute chest pain. This has important implications for the prognostic evaluation of the patient who presents with the assessment of acute chest pain.
These observations provide further evidence linking inflammatory cascades that promote MPO release from activated leukocytes, MPO activity, and acute ischemic events (39). They are also consistent with the demonstration that MPO promotes endothelial dys-function and apoptosis of endothelial cells, factors that participate in breakdown of the barrier between atherosclerotic plaque and circulating blood and acute ischemia. Furthermore, the findings support the association of mediators of oxidative stress with atherosclerotic cardiovascular disease.
Several important points should be noted. This is a retrospective analysis of a study of patients presenting to the emergency room with chest pain of suspected cardiac origin, and the study used samples that had been in long-term storage, using new-generation assays for both MPO and troponin. Although a multivariate analysis was performed to control for differences in clinical characteristics, it is possible that residual confounding was present. The current findings do not provide evidence that MPO plays a direct causative role in the adverse clinical outcome of patients presenting for assessment of chest pain. In addition, the cost effectiveness of this approach has not been defined.
The association between systemic concentrations of MPO and prospective cardiovascular risk has important implications for the approach to the patient who presents for evaluation of chest pain. The present results suggest that rapid risk stratification may be achieved by addition of MPO testing to cTnI testing, because an increased MPO concentration helped to identify individuals at risk, even in those for whom cTnI testing results were not yet positive (or remained persistently within the reference interval). It is important to note that the current findings were observed with a more sensitive cTnI assay than in the initial evaluation of baseline measures of MPO in this cohort, which has been recently reported to result in improved diagnostic accuracy in risk assessment (1). More accurate risk stratification at earlier time points is a clinical goal. Among individuals who presented within 4 h of onset of chest pain, baseline and 4-h testing with cTnI and MPO in combination was a powerful prognostic pair for identifying those at risk for MACE over the ensuing 6-month interval. Furthermore, although cTnI testing defines the presence of myocardial infarction, it is clear that numerous individuals whose cTnI remained persistently within the reference interval during evaluation of acute chest pain experienced a MACE over the ensuing 6-month interval. This continues to be demonstrated with use of newer-generation, more-sensitive assays (1). A remarkable feature of the prognostic value of MPO is its ability to identify risks within patients who have cTnI concentrations persistently within the reference interval. Finally, another remarkable finding in the present studies was the ability of MPO testing to help rapidly define those who are at low risk for MACE, another feature that may allow for cost-efficient and rapid triage. Indeed, the combined presence of negative MPO and cTnI concentrations at baseline resulted in substantially fewer missed MACE than serial cTnI testing alone, the current standard of care for cardiac risk assessment in this clinical situation.
Acknowledgments
Research Funding: Reagents for cardiac troponin I and high sensitivity C-reactive protein assays were provided by Abbott Laboratories. S.J. Nicholls, grant from the Cleveland Clinic Foundation General Clinical Research Center (M01 RR018390); W.H.W. Tang, research grant support from Abbott Laboratories and grants from the NIH (NHLBI) (2P01HL076491, 1P01HL098055, and 1R01HL103931); S.L. Hazen, Abbott, Cleveland Heart Lab, Esperion, LipoScience, and the NIH (NHLBI) (grants 2P01HL076491, 1P01HL098055, and 1R01HL103931).
Role of Sponsor: The funding organizations played no role in the design of study, choice of enrolled patients, review and interpretation of data, or preparation or approval of manuscript.
Footnotes
Nonstandard abbreviations: CRP, C-reactive protein; MPO, myeloperoxidase; cTnT, cardiac troponin T; cTnI, cardiac troponin I; FDA, US Food and Drug Administration; OR, odds ratio; MACE, major adverse cardiac events.
Author Contributions: All authors confirmed they have contributed to the intellectual content of this paper and have met the following 3 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; and (c) final approval of the published article.
Authors’ Disclosures or Potential Conflicts of Interest: Upon manuscript submission, all authors completed the Disclosures of Potential Conflict of Interest form. Potential conflicts of interest:
Employment or Leadership: None declared.
Consultant or Advisory Role: S.L. Hazen, Abbott, Cleveland Heart Lab, Lilly, LipoScience, Merck, and Pfizer.
Stock Ownership: S.L. Hazen, Cleveland Heart Lab.
Honoraria: S.L. Hazen, Abbott, Cleveland Heart Lab, Lilly, LipoScience, Merck, and Pfizer.
Expert Testimony: None declared.
Other Remuneration: M.-L. Brennan, coinventor on patents filed by the Cleveland Clinic that refer to the use of biomarkers in inflammatory and cardiovascular disorders; S.L. Hazen, coinventor on patents filed by the Cleveland Clinic that refer to the use of biomarkers in inflammatory and cardiovascular disorders, right to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics from Cleveland Heart Lab, Abbott Laboratories, Inc., Frantz Biomarkers, LLC, and Siemens.
References
- 1.Tang WH, Wu Y, Nicholls SJ, Brennan DM, Pepoy M, Mann S, et al. Subclinical myocardial necrosis and cardiovascular risk in stable patients undergoing elective cardiac evaluation. Arterioscler Thromb Vasc Biol. 2010;30:634–40. doi: 10.1161/ATVBAHA.109.201210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM. Clinical applications of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107:363–9. doi: 10.1161/01.cir.0000053730.47739.3c. [DOI] [PubMed] [Google Scholar]
- 4.Pepys MB, Hawkins PN, Kahan MC, Tennent GA, Gallimore JR, Graham D, et al. Proinflammatory effects of bacterial recombinant human C-reactive protein are caused by contamination with bacterial products, not by C-reactive protein itself. Circ Res. 2005;97:e97–103. doi: 10.1161/01.RES.0000193595.03608.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elliott P, Chambers JC, Zhang W, Clarke R, Hopewell JC, Peden JF, et al. Genetic loci associated with C-reactive protein levels and risk of coronary heart disease. JAMA. 2009;302:37–48. doi: 10.1001/jama.2009.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicholls SJ, Hazen SL. Myeloperoxidase and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2005;25:1102–11. doi: 10.1161/01.ATV.0000163262.83456.6d. [DOI] [PubMed] [Google Scholar]
- 7.Abu-Soud HM, Hazen SL. Nitric oxide is a physiological substrate for mammalian peroxidases. J Biol Chem. 2000;275:37524–32. doi: 10.1074/jbc.275.48.37524. [DOI] [PubMed] [Google Scholar]
- 8.Eiserich JP, Baldus S, Brennan ML, Ma W, Zhang C, Tousson A, et al. Myeloperoxidase, a leukocyte-derived vascular NO oxidase. Science (Wash DC) 2002;296:2391–4. doi: 10.1126/science.1106830. [DOI] [PubMed] [Google Scholar]
- 9.Hazen SL, Zhang R, Shen Z, Wu W, Podrez EA, MacPherson JC, et al. Formation of nitric oxide-derived oxidants by myeloperoxidase in monocytes: pathways for monocyte-mediated protein nitration and lipid peroxidation in vivo. Circ Res. 1999;85:950–8. doi: 10.1161/01.res.85.10.950. [DOI] [PubMed] [Google Scholar]
- 10.Fu X, Kassim SY, Parks WC, Heinecke JW. Hypochlorous acid oxygenates the cysteine switch domain of pro-matrilysin (MMP-7): a mechanism for matrix metalloproteinase activation and athero-sclerotic plaque rupture by myeloperoxidase. J Biol Chem. 2001;276:41279–87. doi: 10.1074/jbc.M106958200. [DOI] [PubMed] [Google Scholar]
- 11.Sugiyama S, Kugiyama K, Aikawa M, Nakamura S, Ogawa H, Libby P. Hypochlorous acid, a macrophage product, induces endothelial apoptosis and tissue factor expression: involvement of myeloperoxidase-mediated oxidant in plaque erosion and thrombogenesis. Arterioscler Thromb Vasc Biol. 2004;24:1309–14. doi: 10.1161/01.ATV.0000131784.50633.4f. [DOI] [PubMed] [Google Scholar]
- 12.Zheng L, Nukuna B, Brennan ML, Sun M, Goormastic M, Settle M, et al. Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J Clin Invest. 2004;114:529–41. doi: 10.1172/JCI21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bergt C, Pennathur S, Fu X, Byun J, O'Brien K, McDonald TO, et al. The myeloperoxidase product hypochlorous acid oxidizes HDL in the human artery wall and impairs ABCA1-dependent cholesterol transport. Proc Natl Acad Sci U S A. 2004;101:13032–7. doi: 10.1073/pnas.0405292101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Undurti A, Huang Y, Lupica JA, Smith JD, DiDo-nato JA, Hazen SL. Modification of high density lipoprotein by myeloperoxidase generates a pro-inflammatory particle. J Biol Chem. 2009;284:30825–35. doi: 10.1074/jbc.M109.047605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kutter D, Devaquet P, Vanderstocken G, Paulus JM, Marchal V, Gothot A. Consequences of total and subtotal myeloperoxidase deficiency: risk or benefit ? Acta Haematol. 2000;104:10–5. doi: 10.1159/000041062. [DOI] [PubMed] [Google Scholar]
- 16.Nikpoor B, Turecki G, Fournier C, Theroux P, Rouleau GA. A functional myeloperoxidase polymorphic variant is associated with coronary artery disease in French-Canadians. Am Heart J. 2001;142:336–9. doi: 10.1067/mhj.2001.116769. [DOI] [PubMed] [Google Scholar]
- 17.Pecoits-Filho R, Stenvinkel P, Marchlewska A, Heimburger O, Barany P, Hoff CM, et al. A functional variant of the myeloperoxidase gene is associated with cardiovascular disease in end-stage renal disease patients. Kidney Int Suppl. 2003:S172–6. doi: 10.1046/j.1523-1755.63.s84.32.x. [DOI] [PubMed] [Google Scholar]
- 18.Asselbergs FW, Tervaert JW, Tio RA. Prognostic value of myeloperoxidase in patients with chest pain. N Engl J Med. 2004;350:516–8. author reply 8. [PubMed] [Google Scholar]
- 19.Asselbergs FW, Reynolds WF, Cohen-Tervaert JW, Jessurun GA, Tio RA. Myeloperoxidase polymorphism related to cardiovascular events in coronary artery disease. Am J Med. 2004;116:429–30. doi: 10.1016/j.amjmed.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 20.Rudolph V, Rudolph TK, Kubala L, Clauberg N, Maas R, Pekarova M, et al. A myeloperoxidase promoter polymorphism is independently associated with mortality in patients with impaired left ventricular function. Free Radic Biol Med. 2009;47:1584–90. doi: 10.1016/j.freeradbiomed.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Castellani LW, Chang JJ, Wang X, Lusis AJ, Reynolds WF. Transgenic mice express human MPO –463G/A alleles at atherosclerotic lesions, developing hyperlipidemia and obesity in –463G males. J Lipid Res. 2006;47:1366–77. doi: 10.1194/jlr.M600005-JLR200. [DOI] [PubMed] [Google Scholar]
- 22.McMillen TS, Heinecke JW, LeBoeuf RC. Expression of human myeloperoxidase by macrophages promotes atherosclerosis in mice. Circulation. 2005;111:2798–804. doi: 10.1161/CIRCULATIONAHA.104.516278. [DOI] [PubMed] [Google Scholar]
- 23.Wang Z, Nicholls SJ, Rodriguez ER, Kummu O, Horkko S, Barnard J, et al. Protein carbamylation links inflammation, smoking, uremia and athero-genesis. Nat Med. 2007 doi: 10.1038/nm1637. [DOI] [PubMed] [Google Scholar]
- 24.Meuwese MC, Stroes ES, Hazen SL, van Miert JN, Kuivenhoven JA, Schaub RG, et al. Serum myeloperoxidase levels are associated with the future risk of coronary artery disease in apparently healthy individuals: the EPIC-Norfolk Prospective Population Study. J Am Coll Cardiol. 2007;50:159–65. doi: 10.1016/j.jacc.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 25.Ndrepepa G, Braun S, Mehilli J, von Beckerath N, Schomig A, Kastrati A. Myeloperoxidase level in patients with stable coronary artery disease and acute coronary syndromes. Eur J Clin Invest. 2008;38:90–6. doi: 10.1111/j.1365-2362.2007.01908.x. [DOI] [PubMed] [Google Scholar]
- 26.Wong ND, Gransar H, Narula J, Shaw L, Moon JH, Miranda-Peats R, et al. Myeloperoxidase, sub-clinical atherosclerosis, and cardiovascular disease events. JACC Cardiovasc Imaging. 2009;2:1093–9. doi: 10.1016/j.jcmg.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 27.Cavusoglu E, Ruwende C, Eng C, Chopra V, Yanamadala S, Clark LT, et al. Usefulness of baseline plasma myeloperoxidase levels as an independent predictor of myocardial infarction at two years in patients presenting with acute coronary syndrome. Am J Cardiol. 2007;99:1364–8. doi: 10.1016/j.amjcard.2006.12.060. [DOI] [PubMed] [Google Scholar]
- 28.Baldus S, Heeschen C, Meinertz T, Zeiher AM, Eiserich JP, Munzel T, et al. Myeloperoxidase serum levels predict risk in patients with acute coronary syndromes. Circulation. 2003;108:1440–5. doi: 10.1161/01.CIR.0000090690.67322.51. [DOI] [PubMed] [Google Scholar]
- 29.Morrow DA, Sabatine MS, Brennan ML, de Lemos JA, Murphy SA, Ruff CT, et al. Concurrent evaluation of novel cardiac biomarkers in acute coronary syndrome: myeloperoxidase and soluble CD40 ligand and the risk of recurrent ischaemic events in TACTICS-TIMI 18. Eur Heart J. 2008;29:1096–102. doi: 10.1093/eurheartj/ehn071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brennan ML, Penn MS, Van Lente F, Nambi V, Shishehbor MH, Aviles RJ, et al. Prognostic value of myeloperoxidase in patients with chest pain. N Engl J Med. 2003;349:1595–604. doi: 10.1056/NEJMoa035003. [DOI] [PubMed] [Google Scholar]
- 31.Vita JA, Brennan ML, Gokce N, Mann SA, Goormastic M, Shishehbor MH, et al. Serum myeloperoxidase levels independently predict endothelial dysfunction in humans. Circulation. 2004;110:1134–9. doi: 10.1161/01.CIR.0000140262.20831.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harrell FE, Jr, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. JAMA. 1982;247:2543–6. [PubMed] [Google Scholar]
- 33.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–72. doi: 10.1002/sim.2929. discussion 207–12. [DOI] [PubMed] [Google Scholar]
- 34.Apple FS, Smith SW, Pearce LA, Schulz KM, Ler R, Murakami MM. Myeloperoxidase improves risk stratification in patients with ischemia and normal cardiac troponin I concentrations. Clin Chem. 2011;57:603–8. doi: 10.1373/clinchem.2010.158014. [DOI] [PubMed] [Google Scholar]
- 35.Goldmann BU, Rudolph V, Rudolph TK, Holle AK, Hillebrandt M, Meinertz T, Baldus S. Neutrophil activation precedes myocardial injury in patients with acute myocardial infarction. Free Radic Biol Med. 2009;47:79–83. doi: 10.1016/j.freeradbiomed.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 36.Eggers KM, Dellborg M, Johnston N, Oldgren J, Swahn E, Venge P, Lindahl B. Myeloperoxidase is not useful for the early assessment of patients with chest pain. Clin Biochem. 2010;43:240–5. doi: 10.1016/j.clinbiochem.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 37.Borges FK, Stella SF, Souza JF, Wendland AE, Werres Junior LC, Ribeiro JP, Polanczyk CA. Serial analyses of C-reactive protein and myeloperoxidase in acute coronary syndrome. Clin Cardiol. 2009;32:E58–62. doi: 10.1002/clc.20462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCann CJ, Glover BM, Menown IB, Moore MJ, McEneny J, Owens CG, et al. Prognostic value of a multimarker approach for patients presenting to hospital with acute chest pain. Am J Cardiol. 2009;103:22–8. doi: 10.1016/j.amjcard.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 39.Klebanoff SJ. A peroxidase-mediated antimicrobial system in leukocytes. J Clin Invest. 1967;46:1078–85. [Google Scholar]