Abstract
Objective
The purposes of this feasibility study are to assess: (1) the potential utility of early brain magnetic resonance imaging (MRI) in asphyxiated newborns treated with hypothermia; (2) whether early MRI predicts later brain injury observed in these newborns after hypothermia is completed; and (3) whether early MRI indicators of brain injury in these newborns represent reversible changes.
Patients and Methods
All consecutive asphyxiated term newborns meeting the criteria for therapeutic hypothermia were enrolled prospectively. Each of them underwent 1–2 “early” MRI scans while receiving hypothermia, on day of life (DOL) 1 and DOL 2–3, and also 1–2 “late” MRI scans on DOL 8–13 and at 1 month of age.
Results
Thirty-seven MRI scans were obtained in twelve asphyxiated neonates treated with induced hypothermia. Four newborns did develop MRI evidence of brain injury, already visible on early MRI scans. The remaining eight newborns did not develop significant MRI evidence of brain injury on any of the MRI scans. In addition, two patients displayed unexpected findings on early MRIs, leading to early termination of hypothermia treatment.
Conclusions
MRI scans obtained on DOL 2–3 during hypothermia seem to predict later brain injuries in asphyxiated newborns in this feasibility study. Brain injuries identified during this early time appear to represent irreversible changes. Early MRI scans might also be useful to demonstrate unexpected findings not related to hypoxic-ischemic encephalopathy, which could potentially be exacerbated by induced hypothermia. Additional studies with larger numbers of patients will be useful to more definitively confirm these results.
Keywords: hypoxic-ischemic encephalopathy, newborn brain, hypothermia, magnetic resonance imaging
INTRODUCTION
Induced hypothermia has a strong safety and efficacy record for the treatment of hypoxic ischemic encephalopathy (HIE), suggesting decreased death and disability at 12–18 months [1–8]. While this therapy is increasingly accepted, many questions remain unanswered [9–11] including issues pertaining to brain magnetic resonance imaging (MRI). One such question is when the optimal timing of brain imaging is to most accurately define the degree of brain injury sustained and predict the neurologic prognosis [9–11]. The optimal earliest timing of imaging has been extensively studied in asphyxiated newborns before the cooling era [12–14], but it is currently not clear if expected neurologic outcome extrapolated from early MRI in the pre-cooling era can accurately be applied to the newborn treated with induced hypothermia [10,15–17]. The minimal literature regarding brain imaging in newborns treated with induced hypothermia [15–17] does not address this issue, because brain MRIs have usually been performed after completion of induced hypothermia [15–17]. There are also no published studies that address the evolution of brain MRI findings within the first month of life in these newborns.
This feasibility study was designed to assess: (1) the potential utility of early brain imaging to define as early as possible the degree of brain injury sustained in asphyxiated newborns treated with induced hypothermia, (2) whether early MRI findings in term asphyxiated newborns being treated with induced hypothermia predict later brain injury observed on MRI in these patients after completion of induced hypothermia, and (3) whether or not early MRI indicators of brain injury seen in the first days of life in these newborns represent reversible changes.
PATIENTS AND METHODS
We conducted a prospective cohort study of consecutive term asphyxiated newborns admitted to the neonatal intensive care unit from June 2008 to August 2009 meeting the criteria for induced hypothermia: (1) gestational age ≥ 36 weeks and birth weight ≥ 2000 g; (2) evidence of fetal distress, e.g. history of acute perinatal event, biophysical profile < 6/10 within 6 hours of birth, or cord pH ≤ 7.0; (3) evidence of neonatal distress, such as Apgar score ≤ 5 at 10 minutes or postnatal blood gas pH obtained within the first hour of life ≤ 7.0, or continued need for ventilation initiated at birth and continued for at least 10 minutes; (4) evidences of neonatal encephalopathy by physical exam; and (5) abnormal amplitude-integrated electroencephalogram (aEEG) background pattern. Newborns who met all five of the above criteria received whole-body cooling to an esophageal temperature of 33.5°C, initiated ideally by 6 hours of life, continued for 72 hours (unless contraindications developed), and then slowly rewarmed [3].
Neonates were categorized according to their initial background pattern of aEEG into two categories: moderately or severely abnormal [2,18]. Initial background pattern of aEEG was assessed during a recording of at least 20 minutes within 7 hours of life [2]. aEEG recording was started as soon as the patient was admitted to the neonatal intensive care unit; to avoid delay, hypothermia treatment was usually initiated slowly at the same time. Seizures evident clinically or identified by aEEG or standard EEG were also recorded. Other variables associated with neonatal brain injury, including resuscitation score [19], encephalopathy score [20], and seizure score [19], were also collected prospectively.
Sequential MRI studies were planned in order to clarify the time course of brain changes during the first month of life. Specifically, the newborns underwent 1–2 “early” MRI scans while they were receiving hypothermia, including a first scan on day of life (DOL) 1 and a second on DOL 2–3. The timing of these MRI scans were chosen to ensure that there was no older brain injury (DOL 1) and according to the expected time of peak visibility of brain injury on diffusion-weighted imaging (DWI) and spectroscopy in the pre-cooling era (DOL 2–3). Then they underwent 1–2 “late” MRI scans, including a third scan on DOL 8–13 and a fourth at 1 month of age, in order to evaluate definitive T2-weighted MRI changes. Each enrolled newborn underwent all scans unless determined to be too unstable to tolerate the study safely. Patients receiving hypothermia treatment had the therapy maintained during the MRI scan without any adverse events. Any ventilation, pressor support or sedation was also maintained during the MRI scanning process; additional sedation was avoided. MRI scans were performed using a 3T Siemens Symphony (Siemens, Erlangen, Germany) scanner. Each MRI study included anatomic T1- and T2-weighted imaging, DWI and spectroscopy. Induced hypothermia was continued during the early brain MRI scans. The protocol was approved by the Institutional Review Board and parental consent was obtained for each MRI scan. MRI images were interpreted by neuroradiologists, who were blinded to the clinical condition of the infants. Each MRI was scored using an MRI scoring system [21], consisting of a basal ganglia (BG) injury scale, a watershed (W) pattern injury scale and a basal ganglia/watershed (BG/W) pattern injury scale. This MRI assessment provided a quantitative description of the distribution and extent of injury
A focused neurologic examination assessing changes of tone and/or reflexes, power in trunk and extremities, and presence or absence of cranial nerve involvement permitted calculation of a neuromotor outcome score [22] on DOL 10 or at discharge from NICU, whichever came first. It was calculated again at each neurology follow up visit through one year of age, typically at 2, 6 and 12 months of age. This score has been validated in asphyxiated newborns not treated with induced hypothermia. Though it has not been specifically re-validated in those treated with induced hypothermia, we selected it to provide a feasible, objective clinical comparison between the newborns. The scores were calculated from neurologists’ evaluations by a single investigator.
Our relatively small sample size does not permit statistical testing. Therefore our results are reported descriptively.
RESULTS
Twelve asphyxiated term neonates receiving therapeutic hypothermia were enrolled in the study (TABLE 1), four of whom had an initial moderately abnormal aEEG, and eight who had an initial severely abnormal one. Whole-body cooling was initiated at an average of 4.4 hours of life (range: 2.6–6.6 hours of life). All the newborns had a very similar elevated encephalopathy score. Three patients (# 10, 11 and 12) died (on DOL 4, 4 and 13 respectively, after 68.5, 48.6 and 72 hours of hypothermia respectively) from complications of HIE with no improvement in their severely abnormal neurological clinical exam and EEG. This represents 25% of the total patients and 38% of the patients with initial severely abnormal aEEGs.
Table 1.
Clinical data.
| Patient No | Patient 1 †† | Patient 2 | Patient 3 | Patient 4 | Patient 5 ‡‡ | Patient 6 | Patient 7 | Patient 8 | Patient 9 | Patient 10 | Patient 11 | Patient 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gender | male | male | female | male | male | male | male | male | male | female | female | male |
| Gestational age (weeks) | 39.0 | 40.7 | 39.9 | 39.0 | 36.3 | 38.6 | 39.4 | 39.1 | 39.1 | 39.4 | 39.1 | 39.7 |
| Birthweight (kg) | 2510 | 4180 | 3735 | 3500 | 3100 | 3570 | 3390 | 4060 | 3500 | 3700 | 3600 | 3970 |
| Head circumference (cm) | 31.0 | 33.0 | 36.5 | 34.5 | 35.0 | 35.5 | 35.5 | 36.0 | 35.0 | 36.0 | 37.5 | 36.5 |
| Prolonged rupture of membranes (> 24h) | + | − | − | − | − | − | − | − | − | − | − | + |
| Acute perinatal event * | + | + | + | + | + | + | + | + | + | + | + | + |
| Mode of delivery | cesarean | vaginal | vaginal | cesarean | cesarean | cesarean | vaginal | cesarean | cesarean | cesarean | cesarean | vaginal |
| Apgar score | 2/4/5 | 0/4/6 | 2/6/8 | 1/2/5 | 8/8/- | 2/3/4 | 2/5/6 | 0/0/4 | 1/4/- | 0/1/2 | 0/0/0 | 0/0/3 |
| Umbilical artery pH | 6.9 | 6.9 | 6.9 | 6.9 | 7.3 | 6.9 | 6.8 | not known | 7.0 | 6.6 | not known | 7 |
| Umbilical venous pH | 7.3 | 7.2 | 7.0 | 7.2 | 7.4 | 7.2 | not known | not known | 7.3 | 6.8 | 7.3 | 7.1 |
| First postnatal blood gas pH | 7.0 | 7.2 | 7.4 | 6.9 | 7.1 | 7.2 | 7.3 | 6.8 | 7.1 | 7.2 | 6.6 | 7.1 |
| Resuscitation score † | 5 | 6 | 5 | 6 | 1 | 5 | 5 | 6 | 5 | 6 | 6 | 6 |
| Encephalopathy score ‡ | 5 | 5 | 5 | 6 | 5 | 5 | 5 | 6 | 6 | 6 | 6 | 6 |
| Seizures score § | 0 | 0 | 0 | 6 | 0 | 0 | 7 | 6 | 7 | 6 | 7 | 5 |
| Initial aEEG pattern || | moderate | moderate | moderate | moderate | severe | severe | severe | severe | severe | severe | severe | severe |
| Clinical seizures | − | − | − | + | − | − | + | + | + | + | + | + |
| Onset of seizures (hours after birth) | − | − | − | 3.5 | − | − | 6.0 | 4.0 | 1.0 | 2.0 | 2.0 | 34.2 |
| Onset of hypothermia (hours after birth) | 5.3 | 4.4 | 3.7 | 4.6 | 6.6 | 3.9 | 2.6 | 3.4 | 5.6 | 2.9 | 5.3 | 4.4 |
| Duration of hypothermia | 38.2 | 72.0 | 72.0 | 72.0 | 72.0 | 72.0 | 72.0 | 24.7 | 72.0 | 68.5 | 48.6 | 72.0 |
| Outcome | alive | alive | alive | alive | alive | alive | alive | alive | alive | dead | dead | dead |
| Neuromotor outcome score ** on DOL10 (or at discharge) | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 5 | N/A | N/A | 5 |
| Neuromotor outcome score ** at 1.5–2.5 months | 0 | 0 | 0 | 1 | N/A | 1 | 1 | 1 | 5 | N/A | N/A | N/A |
| Neuromotor outcome score ** at 5–9 months | 0 | N/A | N/A | 1 | N/A | 1 | 1 | N/A | 5 | N/A | N/A | N/A |
| Neuromotor outcome score ** at 12 months | N/A | N/A | N/A | N/A | N/A | N/A | 1 | N/A | 5 | N/A | N/A | N/A |
Including: e.g. abruptio placentae, cord prolapse, severe fetal heart rate abnormality, variable or late decelerations
According to Miller et al. [19]
According to Miller et al. [20]
According to Miller et al. [19]
Categorized as moderately abnormal (upper margin > 10 μV and lower margin <5 μV) or severely abnormal (upper margin <10 μV), according to Gluckman et al. [2,18]
According to Hajnal et al. [22]
Met the criteria for induced hypothermia because of history of acute perinatal event, low cord pH, low Apgar scor at 10 minutes, low blood gas pH within the first hour of life, continuous need for ventilatory support after birth and evidence of encephalopathy on exam, in the context of intrauterine growth restriction, with associated uteroplacental insufficiency and signs of acute chorioamnionitis
Met the criteria for induced hypothermia because of history of acute perinatal event, continuous need for ventilatory support after birth and evidence of encephalopathy on exam in the context of severe anemia. His mother presented the day of birth with decreased fetal movements and a biophysical profile was calculated at 2/8 prompting an emergent c-section. He was noted to be very pale at birth, and developed respiratory distress quickly after birth, which worsened over the first 15 minutes of life, requiring intubation and mechanical ventilation. His neurological status and his aEEG on admission to our neonatal intensive care unit was severely abnormal. His initial hematocrit was 14%, and the Kleihauer-Betke came back positive, suggesting the diagnosis of fetomaternal hemorrhage. His admission and the onset of hypothermia was slightly delayed in this case related to the ordering and the administration of a blood transfusion.
DOL = day of life; N/A = not available
Thirty-seven MRI scans were obtained in these twelve patients. Among the four patients with initial moderately abnormal aEEGs, none developed significant MRI evidence of brain injury by early or late brain MRI scans (TABLE 2). Among the eight patients with initial severely abnormal aEEGs, four did not develop any clear MRI evidence of HIE by early or late brain MRI scans, and four developed MRI evidence of brain injury, already visible on early MRI scans (TABLE 3). Three of the four patients who developed brain injury displayed severe injury of the basal ganglia (# 9, 10, and 11). One patient (# 12) developed more extensive injury, with lesions of the basal ganglia, white matter, cortex, pons and cerebellum (FIGURE 1).
Table 2.
Brain MRI findings in newborn infants with initial moderately abnormal aEEG pattern.
| Patient | MRI evidence for brain injury *
|
Other | |||
|---|---|---|---|---|---|
| Basal ganglia (BG) | Watershed (W) | ||||
| Patient 1 | |||||
| DOL 1 not available | - | - | - | ||
| DOL 2 (39.0h of life / 33.7h after starting hypothermia) | BG 0 |
|
W 0 |
|
|
| DOL 10 | BG 0 | W 0 | |||
| 1M of age (DOL 31) | BG 0 | W0 | |||
| Patient 2 | |||||
| DOL 1 (19.9h of life / 15.5h after starting hypothermia) | BG 0 | W 0 |
|
||
| DOL 2 (43.9h of life / 39.5h after starting hypothermia) | BG 0 | W 0 | |||
| DOL 10 | BG 0 | W 0 | |||
| 1M of age (DOL 31) | BG 0 | W 0 | |||
| Patient 3 | |||||
| DOL 1 (6.0h of life / 2.3h after starting hypothermia) | BG 0 | W 2 |
|
|
|
| DOL 2 (43.0h of life / 39.3h after starting hypothermia) | BG 0 | W 2 | |||
| DOL 10 | BG 0 | W 2 | |||
| 1M of age (DOL 32) | BG 0 | W 2 | |||
| Patient 4 | |||||
| DOL 1 (12.0h of life / 7.4h after starting hypothermia) | BG 0 | W 2 |
|
|
|
| DOL 2 (36.0h of life / 31.4h after starting hypothermia) | BG 0 | W 2 | |||
| DOL 13 | BG 0 | W 2 | |||
| 1M of age (DOL 34) | BG 0 | W 2 | |||
Table 3.
Brain MRI findings in newborn infants with initial severely abnormal aEEG pattern.
| Patient | MRI evidence for brain injury *
|
Other | |||
|---|---|---|---|---|---|
| Basal ganglia (BG) | Watershed (W) | ||||
| Patient 5 | |||||
| DOL 1 not available | - | - | - | ||
| DOL 3 (54.0h of life / 47.4h after starting hypothermia) | BG 0 | W 2 |
|
|
|
| DOL 8 | BG 0 | W 2 | |||
| 1M of age not available | - | - | - | ||
| Patient 6 | |||||
| DOL 1 (24.0h of life / 20.1h after starting hypothermia) | BG 0 | W 0 |
|
||
| DOL 2 (48.0h of life / 44.1h after starting hypothermia) | BG 0 | W 0 | |||
| DOL 10 | BG 0 | W 0 | |||
| 1M of age (DOL 31) | BG 0 | W 0 | |||
| Patient 7 | |||||
| DOL 1 not available | - | - | - | ||
| DOL 2 (34.0h of life / 31.4h after starting hypothermia) | BG 0 | W 2 |
|
||
| DOL 10 | BG 0 | W 2 | |||
| 1M of age not available | - | - | |||
| Patient 8 | |||||
| DOL 1 (15.0h of life / 11.7h after starting hypothermia) | BG 0 | W 0 |
|
||
| DOL 2 (41.0h of life / hypothermia stopped) | BG 0 | W 0 | |||
| DOL 11 | BG 0 | W 0 | |||
| 1M of age (DOL 31) | BG 0 | W 0 | |||
| Patient 9 | |||||
| DOL 1 (7.0h of life / 1.4h after starting hypothermia) | BG 0 |
|
W 0 |
|
|
| DOL 2 (29.5h of life / 23.9h after starting hypothermia) | BG 4 | W 0 | |||
| DOL 10 | BG 4 | W 0 | |||
| 1M of age (DOL 32) | BG 4 | W 0 | |||
| Patient 10 | |||||
| DOL 1 (11.0h of life / 8.1h after starting hypothermia) | BG 0 | W 0 |
|
||
| DOL 2 (41.0h of life / 38.1h after starting hypothermia) | BG 4 |
|
W 0 | ||
| DOL 10 not available | - | - | - | ||
| 1M of age not available | - | - | - | ||
| Patient 11 | |||||
| DOL 1 (8.5h of life / 3.3h after starting hypothermia) | BG 0 |
|
W 0 |
|
|
| DOL 2 (35.5h of life / 29.7h after starting hypothermia) | BG 4 | W 0 | |||
| DOL 10 not available | - | - | - | ||
| 1M of age not available | - | - | - | ||
| Patient 12 | |||||
| DOL 1 (6.5h of life / 2.1h after starting hypothermia) | BG 0 | W 2 |
|
|
|
| DOL 2 (26.0h of life / 21.6h after starting hypothermia) | BG 4 |
|
W 5 | ||
| DOL 10 not available | - | - | - | ||
| 1M of age not available | - | - | - | ||
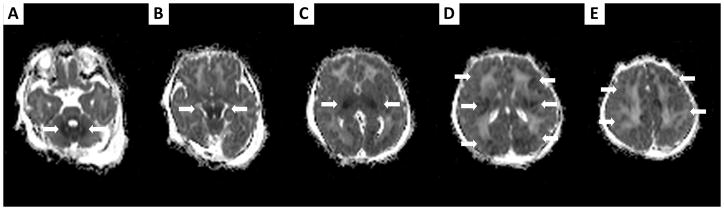
FIGURE 1.
ADC maps of patient # 12, performed on DOL 2 while the patient is treated with induced hypothermia, showing extensive brain injury (thick arrows), with lesions of the cerebellum (A), pons (B), basal ganglia (C), white matter and cortex (D–E).
Due to clinical instability, not all newborn infants were able to receive the full sequence of four scans. MRIs on DOL 1 were performed in nine of twelve patients, at a mean of 12.2 hours of life (range: 6.0–24.0 hours) and at a mean of 8.0 hours after initiation of induced hypothermia (range: 1.4–20.1 hours). In the four patients developing MRI evidence of brain injury, these first MRIs were initially read as negative; subtle abnormalities were only retrospectively identified.
MRIs on DOL 2–3 were performed in all 12 patients at a mean of 39.2 hours of life (range: 26.0–54.0 hours), and at a mean of 34.6 hours after initiation of induced hypothermia (range: 21.6–47.4 hours). Brain lesions were unequivocal in four patients (# 9, 10, 11 and 12), visible on all MRI modalities especially DWI and spectroscopy. The remaining patients did not demonstrate any MRI evidence of brain injury on their MRIs obtained on DOL 2–3. MRIs on DOL 8–13 were performed on nine of twelve patients, and MRIs at 1 month of age were performed on seven of twelve patients. These late brain imaging studies did not reveal any new MRI evidence of brain injury that had not been seen on early MRI scans. Late imaging confirmed the extent of brain lesions identified early in patient # 9 (FIGURE 2), despite having completed the full 72-hour induced hypothermia. Autopsy was performed in patient # 11, and also confirmed the extent of the brain injury identified early on DOL 2.
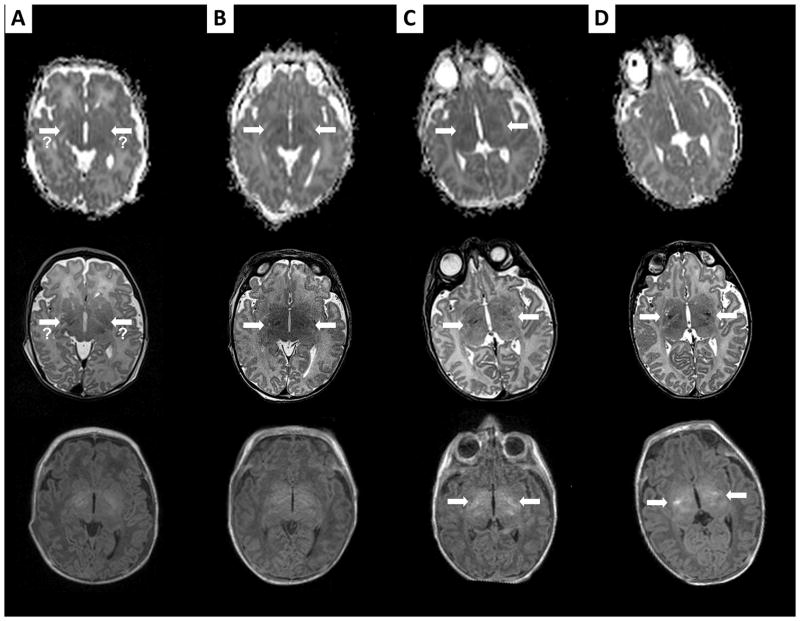
FIGURE 2.
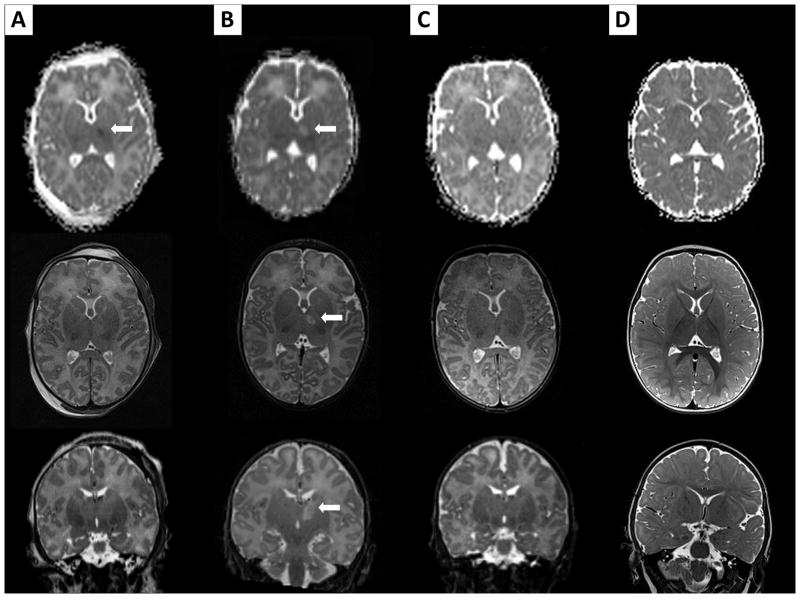
Brain MRIs of patient # 9, performed on DOL 1 (A), on DOL 2 (B), on DOL 10 (C), and at 1 month of age (D). For each MRI exam, the first line shows ADC maps, the second axial T2-weighted imaging, and the last axial T1-weighted imaging at that time. The MRI on DOL 1 in this patient shows a questionable very mild restricted diffusion involving the corticospinal tracts, with questionable T2 signal abnormality (thick arrows). The MRI on DOL 2 definitely confirms these findings, with clear restricted diffusion and T2 signal abnormalities in these areas (thick arrows). ADC maps subsequently “normalize”, while findings become more and more evident on subsequent T2-weighted imaging (thick arrows). Initial T1-weighted imaging shows normal posterior limbs of internal capsule. However, the T1-weighted signal associated with the posterior limbs of internal capsule nearly disappears on DOL 2, and then appears again on late imaging, but brighter than normal (thick arrows).
Two patients displayed unexpected findings not related to hypoxic-ischemic encephalopathy on early MRIs, leading to early termination of induced hypothermia. On his MRI on DOL 2, patient # 1 demonstrated a left parietal epidural hematoma, bilateral parietal and left frontal subpial hemorrhages, cerebellar hemorrhages and a large subgaleal hematoma (FIGURE 3). On his MRI on DOL 1, patient # 8 demonstrated a non-occlusive dural venous sinus thrombosis involving the bilateral transverse and superior sagittal sinuses (FIGURE 4). Furthermore, both patients demonstrated abnormal hematological and coagulation studies while receiving induced hypothermia, requiring red blood cells, fresh frozen plasma and platelets transfusions. Because of the hemorrhages and thromboses seen on MRI in the setting of the clinical coagulopathy, induced hypothermia was terminated early in these two patients, after 38.2 and 24.7 hours of treatment for patients # 1 and 8 respectively. Neither of these two patients developed significant MRI evidence of brain hypoxic-ischemic injury, despite receiving only a partial course of induced hypothermia.
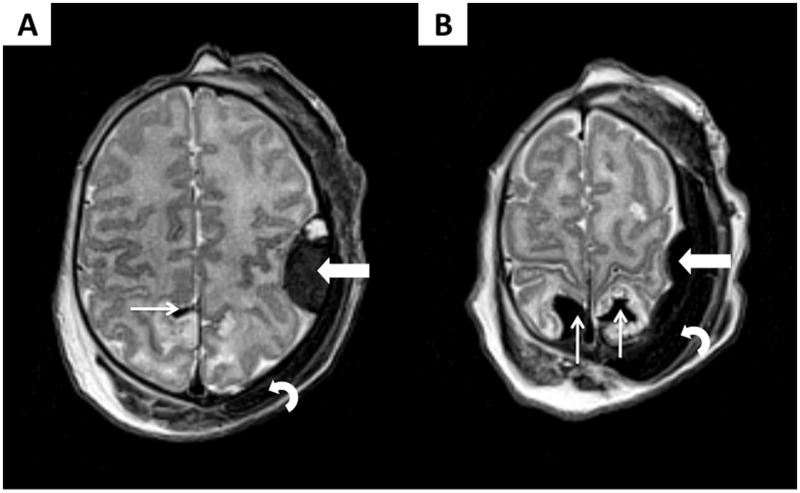
FIGURE 3.
Axial T2-weighted imaging of patient # 1, performed on DOL 2 while the patient is treated with induced hypothermia, showing a left parietal epidural hematoma (thick arrows), some bilateral parietal and left frontal subpial hemorrhages (thin arrows), and a large subgaleal hematoma (curved arrow). Two views (A–B) are presented for better delineation of the lesions.
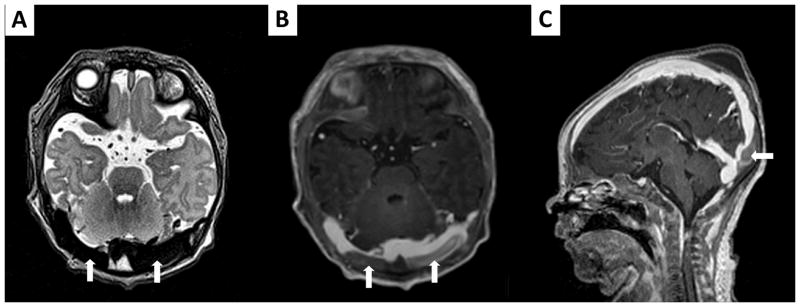
FIGURE 4.
Cerebral MRI on DOL 1 in patient # 8 demonstrates a non-occlusive dural venous sinus thrombosis involving the bilateral transverse and superior sagittal sinuses (thick arrows). Injection of gadolinium permits to visualize it more precisely on axial (B) and sagittal (C) T1-weighted imaging, compared to axial T2-weighted imaging (A) before contrast injection.
Of note, on his MRI scan on DOL 2, patient # 1 also displayed a unilateral focus of restricted diffusion involving the left thalamus, with a concomitant normal MRS study in that area (FIGURE 5). This lesion was still visible on DOL 10, but completely resolved at 1 month of age (FIGURE 5), and was no longer visible on an MRI scan at 9 months of age. The patient was ultimately diagnosed with a venous infarct because of the unilaterality and the resolution of the lesion.
FIGURE 5.
Brain MRIs of patient # 1, performed on DOL 2 (A), on DOL 10 (B), at 1 month of age (C) and at 9 months of age (D). For each MRI exam, the first line shows ADC maps, the second axial T2-weighted imaging, and the last coronal T2-weighted imaging at that timing. A unilateral focus of restricted diffusion involving the left thalamus (thick arrow) is identified on MRI obtained on DOL 2. This lesion is still visible on DOL 10 on ADC maps (thick arrow) and T2-weighted imaging (thick arrow), but completely resolves at 1 month of age, and is no longer visible at 9 month of age. Venous infarct was diagnosed rather than HIE, because of the unilaterality of the lesion and its resolution.
All patients without MRI evidence of HIE had a low neuromotor score on DOL 10 or at discharge from NICU. In these patients, the score remained unchanged or slightly improved (in patient # 2) on follow-up. In contrast, patients with evidence of brain injury on MRI (patient # 9 and # 12) had a high score on DOL 10, and the score remained elevated in patient # 9 on follow-up. Patient # 12 did not have any further follow-up, as he died on DOL 13. Patients # 10 and 11 who died on DOL 4 prior to formal neuromotor scoring would likely have had elevated scores given their documented abnormal neurological examinations including severe encephalopathy.
DISCUSSION
Minimal literature exists regarding brain imaging in newborns treated with induced hypothermia. It mainly addresses the incidence of brain tissue injury following treatment with induced hypothermia and the predictive value of MRIs performed after completion of hypothermia for subsequent neurological impairment [15–17]. Therapeutic hypothermia decreases brain tissue injury in asphyxiated newborns [15–17], as demonstrated by less cortical gray matter lesions [15–16], and less basal ganglia and thalamic lesions on MRI [16], especially in infants with initial moderately abnormal aEEG. The predictive value of these MRI scans for subsequent neurological impairment does not seem to be affected by therapeutic hypothermia [17].
However, it is currently not known when is the optimal timing of brain imaging for term asphyxiated newborns treated with induced hypothermia to accurately define their brain injuries as early as possible and predict their neurologic function [9–11]. In this population of patients, a brain MRI has typically been performed on DOL 4 to 7 [15–17]. This timing usually falls in the practical window after induced hypothermia is complete, and before transfer to another care center. However, in the era before induced hypothermia was widely offered, this day 4–7 window was not considered the ideal timing to obtain brain imaging in newborns with HIE; abnormalities of DWI may be less evident than in the first few days of life and abnormalities on anatomical imaging may still be difficult to visualize, especially in mild forms of neonatal HIE [13–14].
In our feasibility study, we found that asphyxiated infants treated with induced hypothermia develop some of the same sequential MRI changes described in those not treated [12–14]. In cases of severe HIE, diffusion-weighted changes are subtle in the first 24 hours of life, but become more apparent and better defined on DOL 2–3. Proton MRI spectroscopy remains a sensitive technique for the identification of brain injury, showing an elevation of lactate in the injured areas in the first 24 hours. We also found that all brain injuries are already visible on early MRI scans. The late brain imaging studies do not reveal any new brain injuries that were not seen on DOL 2–3, but also do not show that the brain injuries, if present, were underestimated on these early scans. Induced hypothermia does not appear to mask the appearance of brain injury lesions on MRI. Our findings suggest the value of adding early brain MRIs to hypothermia protocols, in order to optimize the prompt understanding of potential brain injury. MRI on DOL 2–3 may be effective in accurately defining brain injuries as early as possible in asphyxiated newborns treated with induced hypothermia. This may be helpful from a clinical perspective, especially in severe cases where some parents choose palliative care rather than pursuing intensive care treatment. From a research perspective, this may also in the future enable early identification of newborns, who might benefit from adjunctive neuroprotective therapies.
However, the numbers of studied patients in the present feasibility study is too small to give enough power to these results and to advice for generalization of early brain MRI in this population of newborns. Additional studies with larger numbers of patients will be useful to confirm these results. In clinical settings where only one brain imaging may be obtained, it is certainly reasonable and practical to delay the imaging to the second week after delivery, when the lesions are clearly visible on conventional imaging as described in the era before induced hypothermia [13–14].
Questions remain regarding whether changes seen in the first days of life in asphyxiated infants treated with induced hypothermia may represent reversible changes in evolution [9–11]. Our study does not support this idea. Severe HI injuries did not resolve over time in our four patients with brain injury detected on early MRIs. Late MRIs in patient # 9 confirm the pattern and the extent of injury identified on early MRI scans, despite completion of a 72-hour course of induced hypothermia. Autopsy was performed in patient # 11, and also confirmed the extent of the brain injury identified early on DOL 2. Though he did not survive long enough to receive late MRI’s, patient # 12 with extensive brain injury on early MRI scans never showed any signs of improvement of his neurological clinical exam or his EEG, even after finishing 72 hours of induced hypothermia. The only brain lesion that resolved in these twelve patients was the unilateral focus of restricted diffusion involving the left thalamus observed on early MRI scans in patient # 1, ultimately diagnosed to be a venous infarct. However, again considering the small sample size, additional studies with larger numbers of patients will be needed to confirm this finding.
We also found early MRI to be useful in this population to demonstrate unexpected findings, which could potentially be exacerbated by induced hypothermia. We discovered such findings in two patients during induced hypothermia: one (patient # 1) with intracranial hemorrhage and the other (patient # 8) with a non-occlusive cerebral venous sinus thrombosis. Both of these findings most likely occured perinatally [23–25], given the accompanying coagulopathies. We do not have clear evidence that these lesions were caused by the induced hypothermia [26]. Yet since induced hypothermia has documented side effects including coagulopathy, the overall risk:benefit analysis argued in favor of early termination of the hypothermia [6,27–28]. These patients did not demonstrate early or late MRI evidence of HIE despite their incomplete treatment.
Areas of mild T2 prolongation were noted in the white matter of some of the newborns enrolled in the study. Some authors have questioned the pathological nature of these signal abnormalities observed on T2-weighted imaging in other populations of newborns [29–32]. In our study, when these T2 signal abnormalities were noted in the white matter, they remained present on all subsequent MRI scans (DOL 1, 2–3, 8–13 and at 1 month of age) without showing any evolution. They were never associated with abnormalities of T1-weighted imaging, DWI or spectroscopy. Thus, we do not consider them to be MRI evidence of perinatal HI injury. It is also possible that these signal abnormalities may be signal artifact due to field inhomogeneity in term newborns scanned with the 3T MRI. Further studies are needed to correlate these T2 signal abnormalities with the mild abnormalities of tone detected in a few of these patients and long-term neurodevelopmental outcomes. If they represent an antenatal brain injury, they may identify a patient population vulnerable to additional perinatal injury.
In this study, the main outcome variable used to confirm brain injury in these newborns was late MRI evidence of brain hypoxic-ischemic injury. However, we recognize that neurodevelopmental outcome for survivors and autopsy confirmation of injury for infants that died would have been the gold standard. Autopsy results were available only in of the three patients who died, but confirmed the early MRI results in this patient. We used the neuromotor outcome score [22] to permit objective clinical comparisons of the neurological status and to correlate with the sequential MRI findings. The degree to which this score can predict future neurodevelopmental outcome needs to be validated with a larger number of asphyxiated infants treated with hypothermia.
In conclusion, despite the current practice for induced hypothermia in which brain MRI’s are obtained DOL 4 to 7, MRIs obtained on DOL 2–3 appear to be a powerful indicator of long-term brain injuries in asphyxiated newborns, especially in severe cases. The brain injury identified on early MRI scans of asphyxiated newborns treated with induced hypothermia do not appear to represent reversible changes, and seem to follow the same MRI evolution pattern described in these patients before the cooling era. As information accumulates regarding the timing and interpretation of MRIs in newborns who have been treated with induced hypothermia, the degree to which MRI findings within the first month of life correlate with and are predictive of neurodevelopmental outcome will be better understood. Patients with moderately abnormal aEEG pattern may have especially subtle MRI changes that require more detailed scans.
“WHAT IS ALREADY KNOWN ON THIS TOPIC”
Induced hypothermia has a strong safety and efficacy record for the treatment of hypoxic-ischemic encephalopathy, suggesting decreased death and disability at 12–18 months.
Many questions remain unanswered including issues pertaining to brain MRI findings: e.g. when is the optimal earliest timing of brain imaging to most accurately define the degree of brain injury sustained?
The minimal literature regarding brain imaging in newborns treated with induced hypothermia does not address this issue, because MRIs have usually been performed after completion of induced hypothermia.
“WHAT THIS STUDY ADDS”
This feasibility study addresses three currently unanswered issues: (1) the potential utility of early brain magnetic resonance imaging (MRI) in asphyxiated newborns treated with hypothermia; (2) whether early MRI predicts later brain injury observed in these newborns after hypothermia is completed; and (3) whether early MRI indicators of brain injury in these newborns represent reversible changes.
This study demonstrates that MRI scans obtained on DOL 2–3 during hypothermia may predict later brain injuries in asphyxiated newborns.
Brain injuries identified during this early time appear to represent irreversible changes.
Acknowledgments
Pia Wintermark receives research grant funding from the William Randolph Hearst Fund Award and the Thrasher Research Fund Early Career Award Program. The work of Simon K. Warfield is supported by NIH grants R01 RR021885, R01 GM074068, R03 EB008680 and P30 HD018655. The authors thank the families and their newborns in participating in the study. A special thank is also expressed to the NICU nurses and the MRI technicians, who have made this study possible.
FUNDING:
Pia Wintermark receives research grant funding from the William Randolph Hearst Fund Award and the Thrasher Research Fund Early Career Award Program. The work of Simon K. Warfield is supported by NIH grants R01 RR021885, R01 GM074068, R03 EB008680 and P30 HD018655.
Abbreviations
- HIE
Hypoxic-Ischemic Encephalopathy
- MRI
Magnetic Resonance Imaging
- aEEG
amplitude-integrated ElectroEncephaloGram
- HI
Hypoxic-Ischemic
- DWI
Diffusion-Weighted Imaging
- DOL
Day Of Life
- N/A
Not Available
Footnotes
COMPETING INTERESTS: No conflict of interest.
COPYRIGHT LICENSE STATEMENT:
“I, Pia Wintermark, the Corresponding Author of this article has the right to grant on behalf of all authors and does grant on behalf of all authors, a licence to the BMJ Publishing Group Ltd and its licensees, to permit this Contribution (if accepted) to be published in Archives of Disease in Childhood (ADC) and any other BMJ Group products and to exploit all subsidiary rights, as set out in our licence set out at: (http://adc.bmj.com//ifora/licence.pdf)
References
- 1.Eicher DJ, Wagner CL, Katikaneni LP, et al. Moderate hypothermia in neonatal encephalopathy: efficacy outcomes. Pediatr Neurol. 2005;32(1):11–17. doi: 10.1016/j.pediatrneurol.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 2.Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365(9460):663–670. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 3.Shankaran S, Laptook AR, Ehrenkranz RA, et al. National Institute of Child Health and Human Development Neonatal Research Network. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353(15):1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 4.Edwards AD, Azzopardi DV. Therapeutic hypothermia following perinatal asphyxia. Arch Dis Child Fetal Neonatal Ed. 2006;91(2):F127–131. doi: 10.1136/adc.2005.071787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah PS, Ohlsson A, Perlman M. Hypothermia to treat neonatal hypoxic ischemic encephalopathy: systematic review. Arch Pediatr Adolesc Med. 2007;161(10):951–8. doi: 10.1001/archpedi.161.10.951. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs S, Hunt R, Tarnow-Mordi W, et al. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. 2007;(4):CD003311. doi: 10.1002/14651858.CD003311.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Azzopardi D, Brocklehurst P, Edwards D, et al. TOBY Study Group. The TOBY Study. Whole body hypothermia for the treatment of perinatal asphyxial encephalopathy: a randomised controlled trial. BMC Pediatr. 2008;8:17. doi: 10.1186/1471-2431-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azzopardi DV, Strohm B, Edwards AD, et al. TOBY Study Group. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361(14):1349–1358. doi: 10.1056/NEJMoa0900854. [DOI] [PubMed] [Google Scholar]
- 9.Higgins RD, Raju TN, Perlman J, et al. Hypothermia and perinatal asphyxia: executive summary of the National Institute of Child Health and Human Development workshop. J Pediatr. 2006;148(2):170–175. doi: 10.1016/j.jpeds.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Barks JD. Current controversies in hypothermic neuroprotection. Semin Fetal Neonatal Med. 2008;13(1):30–34. doi: 10.1016/j.siny.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Higgins RD, Shankaran S. Hypothermia for hypoxic ischemic encephalopathy in infants > or =36 weeks. Early Hum Dev. 2009;85(10 Suppl):S49–S52. doi: 10.1016/j.earlhumdev.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barkovich AJ, Miller SP, Bartha A, et al. MR imaging, MR spectroscopy, and diffusion tensor imaging of sequential studies in neonates with encephalopathy. AJNR Am J Neuroradiol. 2006;27(3):533–547. [PMC free article] [PubMed] [Google Scholar]
- 13.Barkovich AJ. MR imaging of the neonatal brain. Neuroimaging Clin N Am. 2006;16(1):117–135. doi: 10.1016/j.nic.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Rutherford M, Srinivasan L, Dyet L, et al. Magnetic resonance imaging in perinatal brain injury: clinical presentation, lesions and outcome. Pediatr Radiol. 2006;36(7):582–592. doi: 10.1007/s00247-006-0164-8. [DOI] [PubMed] [Google Scholar]
- 15.Inder TE, Hunt RW, Morley CJ, et al. Randomized trial of systemic hypothermia selectively protects the cortex on MRI in term hypoxic-ischemic encephalopathy. J Pediatr. 2004;145(6):835–837. doi: 10.1016/j.jpeds.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 16.Rutherford MA, Azzopardi D, Whitelaw A, et al. Mild hypothermia and the distribution of cerebral lesions in neonates with hypoxic-ischemic encephalopathy. Pediatrics. 2005;116(4):1001–1006. doi: 10.1542/peds.2005-0328. [DOI] [PubMed] [Google Scholar]
- 17.Rutherford M, Ramenghi LA, Edwards AD, Brocklehurst P, Halliday H, Levene M, Strohm B, Thoresen M, Whitelaw A, Azzopardi D. Assessment of brain tissue injury after moderate hypothermia in neonates with hypoxic-ischaemic encephalopathy: a nested substudy of a randomised controlled trial. Lancet Neurol. 2010;9(1):39–45. doi: 10.1016/S1474-4422(09)70295-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.al Naqeeb N, Edwards AD, Cowan FM, et al. Assessment of neonatal encephalopathy by amplitude-integrated electroencephalography. Pediatrics. 1999;103(6 Pt 1):1263–1271. doi: 10.1542/peds.103.6.1263. [DOI] [PubMed] [Google Scholar]
- 19.Miller SP, Weiss J, Barnwell A, et al. Seizure-associated brain injury in term newborns with perinatal asphyxia. Neurology. 2002;58(4):542–548. doi: 10.1212/wnl.58.4.542. [DOI] [PubMed] [Google Scholar]
- 20.Miller SP, Latal B, Clark H, et al. Clinical signs predict 30-month neurodevelopmental outcome after neonatal encephalopathy. Am J Obstet Gynecol. 2004;190(1):93–99. doi: 10.1016/s0002-9378(03)00908-6. [DOI] [PubMed] [Google Scholar]
- 21.Barkovich AJ, Hajnal BL, Vigneron D, Sola A, Partridge JC, Allen F, Ferriero DM. Prediction of neuromotor outcome in perinatal asphyxia: evaluation of MR scoring systems. AJNR Am J Neuroradiol. 1998;19(1):143–149. [PMC free article] [PubMed] [Google Scholar]
- 22.Hajnal BL, Sahebkar-Moghaddam F, Barnwell AJ, et al. Early prediction of neurologic outcome after perinatal depression. Pediatr Neurol. 1999;21(5):788–793. doi: 10.1016/s0887-8994(99)00101-0. [DOI] [PubMed] [Google Scholar]
- 23.Shroff M, deVeber G. Sinovenous thrombosis in children. Neuroimaging Clin N Am. 2003;13(1):115–138. doi: 10.1016/s1052-5149(02)00064-3. [DOI] [PubMed] [Google Scholar]
- 24.Huang AH, Robertson RL. Spontaneous superficial parenchymal and leptomeningeal hemorrhage in term neonates. AJNR Am J Neuroradiol. 2004;25(3):469–75. Erratum in: AJNR Am J Neuroradiol 2004, 35, 4, 666. [PMC free article] [PubMed] [Google Scholar]
- 25.Teksam M, Moharir M, Deveber G, et al. Frequency and topographic distribution of brain lesions in pediatric cerebral venous thrombosis. AJNR Am J Neuroradiol. 2008;29(10):1961–1965. doi: 10.3174/ajnr.A1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Azzopardi D, Robertson NJ, Cowan FM, et al. Pilot study of treatment with whole body hypothermia for neonatal encephalopathy. Pediatrics. 2000;106(4):684–694. doi: 10.1542/peds.106.4.684. [DOI] [PubMed] [Google Scholar]
- 27.Valeri CR, Feingold H, Cassidy G, et al. Hypothermia-induced reversible platelet dysfunction. Ann Surg. 1987;205(2):175–181. doi: 10.1097/00000658-198702000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valeri CR, MacGregor H, Cassidy G, et al. Effects of temperature on bleeding time and clotting time in normal male and female volunteers. Crit Care Med. 1995;23(4):698–704. doi: 10.1097/00003246-199504000-00019. [DOI] [PubMed] [Google Scholar]
- 29.Miller SP, McQuillen PS, Hamrick S, et al. Abnormal brain development in newborns with congenital heart disease. N Engl J Med. 2007;357(19):1928–1938. doi: 10.1056/NEJMoa067393. [DOI] [PubMed] [Google Scholar]
- 30.Counsell SJ, Allsop JM, Harrison MC, et al. Diffusion-weighted imaging of the brain in preterm infants with focal and diffuse white matter abnormality. Pediatrics. 2003;112(1 Pt 1):1–7. doi: 10.1542/peds.112.1.1. [DOI] [PubMed] [Google Scholar]
- 31.Counsell SJ, Shen Y, Boardman JP, et al. Axial and radial diffusivity in preterm infants who have diffuse white matter changes on magnetic resonance imaging at term-equivalent age. Pediatrics. 2006;117(2):376–386. doi: 10.1542/peds.2005-0820. [DOI] [PubMed] [Google Scholar]
- 32.Hagmann CF, De Vita E, Bainbridge A, et al. T2 at MR imaging is an objective quantitative measure of cerebral white matter signal intensity abnormality in preterm infants at term-equivalent age. Radiology. 2009;252(1):209–217. doi: 10.1148/radiol.2522080589. [DOI] [PubMed] [Google Scholar]