Abstract
Retinal vascular diseases are a leading cause of blindness in the Western world. Advancement in the clinical management of these diseases has been fast-paced, with new treatments becoming available as well as license extensions of existing treatments. Vascular endothelial growth factor (VEGF) has been implicated in certain retinal vascular diseases, including wet age-related macular degeneration (AMD), diabetic macular oedema (DMO), and retinal vein occlusion (RVO). Treatment of wet AMD and visual impairment due to either DMO or macular oedema secondary to RVO with an anti-VEGF on an as needed basis, rather than a fixed schedule, allows an individualised treatment approach; providing treatment when patients are most likely to benefit from it, while minimising the number of unnecessary intravitreal injections. Thus, an individualised treatment regimen reduces the chances of over-treatment and under-treatment, optimising both the risk/benefit profile of the treatment and the efficient use of NHS resource. Streamlining of treatment for patients with wet AMD and visual impairment due to either DMO or macular oedema secondary to RVO, by using one treatment with similar posology across all three diseases, may help to minimise burden of clinic capacity and complexity and hence optimise patient outcomes. Informed treatment decisions and efficient clinic throughput are important for optimal patient outcomes in the fast-changing field of retinal vascular diseases.
Keywords: age-related macular degeneration, diabetic retinopathy, retinal vein occlusion, anti-VEGF therapy, individualised medicine
Introduction
Some of the most frequently occurring ocular diseases that cause certified visual loss are associated with pathological retinal neovascularisation and oedema. Of these diseases, wet (neovascular) age-related macular degeneration (AMD), diabetic retinopathy (proliferative diabetic retinopathy (PDR) and diabetic macular oedema (DMO)), and retinal vein occlusion (RVO) are of particular epidemiological importance as leading causes of blindness. In the United Kingdom, AMD is the leading cause of severe sight impairment (legal blindness) and partial sight certifications for all ages, accounting for over half of all visual impairment certifications.1 Diabetic retinopathy is the third most common cause of blindness and partial sight certifications for all age groups in the United Kingdom (6.3 and 7.6%, respectively);1 however, in people of working age (aged 16–64 years), diabetic retinopathy is the leading cause of severe sight impairment certification (17.7%).2 RVO is the second most common type of retinal vascular disease (after diabetic retinopathy) and includes branch RVO (BRVO) and central RVO (CRVO), accounting for <2% of all severe sight impairment and partial sight certifications in the United Kingdom.1
Vascular endothelial growth factor A (VEGF-A), a key regulator of angiogenesis and vascular permeability (reviewed by Ferrara et al3) has been implicated in the pathogenesis of retinal diseases associated with neovascularisation and oedema, including wet AMD,4, 5 diabetic retinopathy (particularly, PDR and DMO),6, 7 and RVO,6, 8, 9 as well as other ocular diseases such as retinopathy of prematurity.6 Although angiogenesis is the result of a highly complex molecular process involving several different receptors and ligands, VEGF-A seems to be a requirement for blood vessel growth in both normal and pathological angiogenesis. Consequently, several anti-VEGF agents have been developed for the treatment of these diseases.
Individualised treatment for retinal vascular diseases
In the years since the Human Genome Project10 was completed, huge progress has been made in unravelling the genetic basis of disease and understanding what drives diseases at a molecular level. By comparing patterns and frequencies of single-nucleotide polymorphisms (SNPs) in patients and controls, we have become able to identify which SNPs are associated with which diseases,11 helping drive the concept and clinical application of personalised medicine. Nowhere has this been better exemplified than in the field of oncology, where for example, only those patients who are most likely to respond are given a targeted treatment based on their genetic profile (eg, HER2/ErbB2 in breast cancer). Such is the potential for personalised medicine that the UK government's Technology Strategy Board has joined forces with Cancer Research UK and other bodies to fund the Stratified Medicines Programme,12 which they see as a significant step in making targeted therapies available for people with cancer in the United Kingdom. The benefit to patients of such an approach is clear. Individualised treatment allows the identification of patients who are most likely to benefit from the treatment. Tailoring treatment to the individual patient in this way should increase the chance of treatment success, while sparing patients from unnecessary drug exposure and risk of adverse events. Furthermore, avoiding unnecessary treatment also has the potential to improve the cost-effectiveness of treatment.
While treatment decisions for patients with retinal vascular diseases are not currently based on genetics, gene association work has already identified multiple genes that may be associated with AMD,13 and understanding how these are implicated in the pathogenesis of the disease opens up new research strategies based on specific pathways and molecules. In particular, dysregulation of the complement system has been shown to have a major part in the pathogenesis of wet and dry AMD,14 and a number of AMD-associated genetic loci have been identified.13 Numerous companies are currently developing genetically based and complement-targeted therapies with the goal of reducing complement-related AMD disease processes.14, 15
While in ophthalmology there is still some way to go before individualised treatment approaches that are based on genetics are available (as they are in certain cancers), it is already possible to begin to consider a similar patient-centred approach based on an individual's disease characteristics. It may be possible to use vision loss, visual acuity (VA) instability, or other signs of an active disease state as markers for requiring treatment, rather than using fixed dosing schedules. This type of approach should reduce the risks associated with over-treatment and under-treatment, thereby optimising the risk/benefit profile of the treatment and the efficient use of NHS resource. There is evidence to support this principle for the treatment of wet AMD, and visual impairment due to either DMO or macular oedema secondary to RVO with ranibizumab, as I will discuss later in this article.
Current management and recent therapeutic developments
Diagnosis
Wet AMD. Early diagnosis and treatment are vital for vision preservation in retinal vascular diseases, particularly wet AMD, because of the rapidly progressive nature of the disease. Patients with wet AMD typically present (to a general practitioner, optometrist, or local eye unit/eye casualty)16 with distortion, blurring, or loss of vision with a rapid onset. Some patients with unilateral wet AMD may be asymptomatic or report mild vision distortion and only be detected in a routine assessment.17 The Royal College of Ophthalmologists (RCOphth) recommends that suspected cases of wet AMD should be referred directly to the nearest AMD centre, eye casualty, or eye clinic, due to the aggressive nature of the disease within 1 week of initial presentation, with no more than 1 week between evaluation and treatment.
DMO. DMO can arise as early as the mild non-proliferative or as late as in the severe proliferative stages of diabetic retinopathy.18 DMO was defined by the Early Treatment of Diabetic Retinopathy Study (ETDRS) group as being clinically significant macular oedema when there is retinal thickening and/or hard exudates within 500 μm of the fovea or when there is a zone of oedema of at least 1 disc diameter in width and part of which is within 1 disc diameter from the fovea.19 DMO appears as retinal thickening on binocular stereoscopic slit-lamp examination and can be confirmed with retinal imaging techniques such as optical coherence tomography (OCT).20 Macular oedema may also be present in wet AMD and RVO, as well as other ocular diseases; however, the natural history of DMO distinguishes it from the other instances of macular oedema. In an attempt to reduce diabetes-related visual impairment in England, the English National Screening Programme for Diabetic Retinopathy (ENSPDR) was set up and provides annual photographic screening for every diabetic patient (over the age of 12 years) in England. All patients identified by screening as having sight-threatening diabetic retinopathy are referred to ophthalmology clinics.21, 22
RVO. Patients with RVO (including BRVO and CRVO) typically present with painless loss of vision.23, 24 BRVO (located in one of the branches of the central vein) is more common than CRVO (located in the central vein and affecting most of the retina) and usually occurs at sites where arterioles cross over veins.25, 26 Retinal imaging with fluorescein angiography is crucial for diagnosis and prognosis, allowing the identification of the specific type of RVO (eg, perfused vs non-perfused and BRVO vs CRVO), the identification of macular oedema (if present), its extent, persistence, regression, and degree of ischaemia.27 OCT provides additional information such as quantitative and qualitative assessment of retinal thickness and the exact location of the accumulated fluid (within the retinal layer vs the subretinal space).27 Clinical features that may be apparent at presentation—such as haemorrhage, cotton wool spots, and macular oedema27—overlap with those of other retinal vascular diseases, including diabetic retinopathy, hypertensive retinopathy, and retinopathy related to blood dyscrasias; thus, differential diagnosis is important.28
Management
Wet AMD. Treatment modalities for wet AMD have improved dramatically over the past decade, prior to which laser photocoagulation was the only available treatment option.29 Photodynamic therapy with verteporfin was licensed for wet AMD with predominantly classic subfoveal choroidal neovascularisation (CNV) in 2000,30 and recommended by the National Institute for Health and Clinical Excellence (NICE) in 2003 for patients with a confirmed diagnosis of classic subfoveal CNV with no sign of occult lesions.31 However, it was only with the emergence of anti-VEGFs that an effective treatment became available for patients with wet AMD, regardless of lesion type. Licensed anti-VEGFs for wet AMD include pegaptanib (Macugen®), which was authorised in the EU in 2006,32 and ranibizumab (Lucentis®▾), authorised in 2007.33 Ranibizumab is now considered the standard of care for wet AMD. This is because, in addition to demonstrating efficacy in preventing visual loss in large, randomised, controlled, clinical trials in the majority of patients,34, 35, 36, 37 ranibizumab has also been shown, on average, to provide significant gains in VA.34, 35, 36
The approved posology of ranibizumab for wet AMD consists of intravitreal injection (0.5 mg) given monthly and continued until maximum VA is achieved (defined as stable VA for 3 consecutive monthly assessments while on treatment). Subsequently, patients are monitored monthly for VA, and upon detection of reduced VA due to wet AMD, treatment is resumed until stable VA is reached.38 Notably, the current approved posology for ranibizumab represents an evolution in the treatment dosing paradigm for wet AMD, towards an individualised treatment approach, whereby injections are only administered at times of VA instability, during which patients are most likely to benefit; hence, minimising the chances of both under-treating or over-treating.
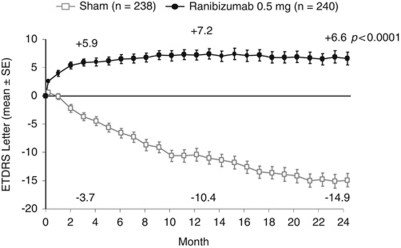
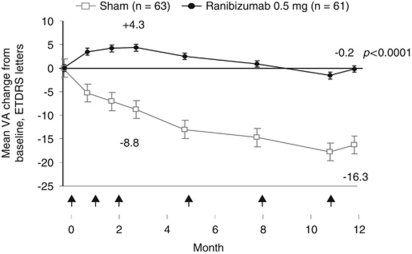
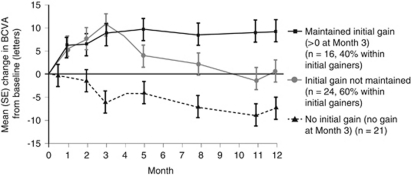
The initial marketing authorisation for ranibizumab was based on the pivotal trials MARINA36 and ANCHOR,34 which investigated a monthly dosing regimen, and a study which investigated a quarterly regimen, PIER.37 Almost all of the patients receiving monthly 0.5-mg ranibizumab injections in MARINA and ANCHOR maintained their VA at 1 year (94.6 and 96.4%, vs 62.2 and 64.3% of controls, respectively; P<0.001 for both), at least a third of patients (33.8% MARINA, 40.3% ANCHOR) gained 15 or more letters of VA (vs 5.0 and 5.6% of controls, respectively; P<0.001 for both comparisons) and, on average in both trials, there was an improvement in VA (+7.2 letters and +11.3 letters in MARINA and ANCHOR, respectively; Figure 1).34, 37 These outcomes were also maintained to 24 months.35, 36 However, in the PIER study, on average, patients who received fixed quarterly injections of 0.5 mg ranibizumab after a loading phase of 3 monthly injections, did not maintain the initial gain in VA seen at 3 months (mean of +4.3 letters vs baseline) at the 12-month time point (mean change from baseline −0.2 letters), although the difference compared with sham remained statistically significant at year 1 (Figure 2).37 Although, on average, quarterly injections were not frequent enough to maintain the initial gains in VA, an exploratory analysis of the ranibizumab group in the PIER study showed that patients could be stratified depending on their initial increase in VA and whether they were able to maintain this initial gain. Of the 40 patients (66%) who showed an initial increase in VA, 16 patients (40%) were ‘sustained responders' (ie, had initial VA gains during the 3 monthly ranibizumab loading phase injections that were sustained to 1 year with subsequent quarterly injections), whereas the remainder of the patients showed a gradual decline in VA from month 4 (with or without initial VA gain), and may have benefitted if treated more frequently (Figure 3).39 This subanalysis showed that different patients needed different frequency of treatment to maintain initial gain.39 Therefore, the originally approved posology for ranibizumab included a loading phase of 3 monthly doses followed by an individualised pro re nata (PRN) maintenance phase with a VA-based retreatment criteria (treatment was resumed upon a VA loss of >5 letters), because on average, VA appeared to plateau after 3 consecutive monthly injections in MARINA, ANCHOR and, PIER.40
Figure 1.
Mean changes from baseline in VA with monthly 0.5 mg ranibizumab in patients with wet AMD with minimally classic/occult CNV, in the MARINA study. Only data from the licensed dose of ranibizumab (0.5 mg) are shown. P-value shown is for difference in mean change in VA vs sham at 24 months. ETDRS, Early Treatment Diabetic Retinopathy Study. Adapted from Rosenfeld et al.36
Figure 2.
Mean changes from baseline in VA in patients with wet AMD with quarterly 0.5 mg ranibizumab following a loading phase of 3 monthly injections, in the PIER study. Only data from the licensed dose of ranibizumab (0.5 mg) are shown. Arrows indicate injection time point. P-value shown is for difference in mean change VA vs sham at 12 months. ETDRS, Early Treatment Diabetic Retinopathy Study. Adapted from Regillo et al,37 Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER study year 1. Am J Ophthalmol 2008; 145: 239–248, with permission from Elsevier © 2008.
Figure 3.
Mean changes from baseline in VA in wet AMD in three subgroups of patients receiving 0.5 mg ranibizumab in the PIER study. Adapted from Holz et al,39 The effects of a flexible visual acuity-driven ranibizumab treatment regimen in age-related macular degeneration: outcomes of a drug and disease model. Invest Ophthalmol Vis Sci 2010; 51(1): 405–412, with permission from Association for Research in Vision and Ophthalmology © 2010.
Since these initial trials, further evidence has emerged which suggested that an individualised patient-centred approach would be more suitable. In a retrospective analysis of data from studies evaluating monthly ranibizumab injections including the MARINA and ANCHOR trials as well as from the ranibizumab monotherapy arm in the DENALI study (a Phase IIIb study conducted in the United States and Canada),41 the time course of vision stability was evaluated.42 This retrospective analysis demonstrated that <20% of wet AMD patients reached VA stability by month 3; however, nearly 80% of patients reached stability within the first year of ranibizumab treatment. Furthermore, the analysis showed that once VA stability is achieved, the incremental VA benefit of continued monthly injections was minimal (the mean absolute change between the visit in which visual stability was achieved and the subsequent visit was ⩽0.3 letters in the MARINA, ANCHOR, and DENALI studies).42 The impact of treatment interruptions in ranibizumab monotherapy arms in trials that used PRN or quarterly dosing regimens including EXCITE,43 SUSTAIN,44 and MONT BLANC was also explored.45 The analysis demonstrated that once VA stability is achieved, the majority (60–80%) of patients have stable VA 2 months after last injection.42 Furthermore, the analysis demonstrated a consistent trend for better VA outcomes when treatment is resumed when VA is unstable (mean change after treatment re-initiation following a visit in which unstable VA was identified was +2.7, +4.3, and +3.0 letters in the EXCITE, SUSTAIN, and MONT BLANC studies, respectively).42
On the basis of these data, a revised ranibizumab wet AMD posology was approved by the EMA in September 2011.40 Ranibizumab can now be administered using a three-step individualised PRN treatment regimen: (1) treatment is initiated by monthly injections until maximum VA is achieved (defined as stable VA for 3 consecutive assessments while on ranibizumab); (2) treatment is then interrupted and patients are monitored monthly; (3) treatment is resumed if monitoring indicates loss of VA associated with active wet AMD and is continued monthly until VA is stable. This approach allows patients the opportunity to receive enough injections to gain maximum VA (at treatment initiation), prevents unnecessary injections (by treatment interruption once maximum VA has been achieved), and allows patients to receive injections when they are most likely to benefit from them (at treatment re-initiation). With the original posology, there was no opportunity for further injections unless >5 letter VA loss was observed. Waiting for a >5 letter VA drop may allow vision to be lost that cannot be regained.
Bevacizumab, which is unlicensed for any ocular use, is also sometimes used for treating wet AMD in clinical practice. Bevacizumab is a humanised full-length anti-VEGF monoclonal antibody that differs from ranibizumab in a number of its properties, including molecular structure, size and design, systemic pharmacokinetics, and formulation. It is licensed only for intravenous administration for the treatment of colorectal cancer and certain cases of breast, renal, lung, ovarian, fallopian tube, and peritoneal cancer.46 Some differences between the ocular and systemic safety profiles of bevacizumab and ranibizumab have been suggested, but despite the widespread use of bevacizumab for wet AMD in clinical practice, evidence from large-scale randomised trials were lacking until recently.
The head-to-head study of ranibizumab and bevacizumab, CATT, is a 2-year, randomised, prospective single-blind, non-inferiority trial to evaluate the comparative safety and efficacy of the two agents. In the 1-year primary end point analysis, while the non-inferiority limit was met for monthly bevacizumab compared with monthly ranibizumab, the non-inferiority limit (5 letters difference) was not met for PRN bevacizumab (n=300) compared with either monthly bevacizumab (n=286) or monthly ranibizumab (n=298). From an anatomical perspective, 4 weeks after their first injection, no fluid was seen on the OCT in 17.3% of bevacizumab-treated patients, while 27.5% of ranibizumab-treated patients were dry on OCT (P<0.001).47 The mean number of injections required in the bevacizumab PRN arm was significantly higher than the number of PRN ranibizumab injections required (7.7±3.5 and 6.9±3.0, respectively; P<0.003).47 Consistent with the move towards individualised treatment with ranibizumab, PRN ranibizumab was non-inferior to monthly ranibizumab dosing.47 Importantly, the observed mean VA gain from baseline to 1 year in the ranibizumab PRN arm of the CATT study (6.8 letters)47 represents the best outcome in randomised controlled trials for 1-year VA gain, with less than monthly ranibizumab dosing in comparison with the relevant arms of PIER (mean gain of 4.3 letters in the 0.5-mg quarterly group),37 EXCITE (mean gain of 3.8 letters in the 0.5-mg quarterly group),43 SUSTAIN (mean gain of 3.6 letters using a VA/OCT-based PRN regimen),44 and SAILOR (a phase IIIb study in which cohort 1 evaluated 0.3 and 0.5 mg ranibizumab using VA/OCT-based PRN regimen; mean gain of 2.3 letters in the 0.5-mg group).48 Thus, the findings of the CATT study supports the currently approved posology for ranibizumab, by suggesting that a more refined individualised regimen may optimise clinical outcomes while reducing the number of injections.
While not powered to identify rare but serious adverse events, safety differences were also identified in the CATT study, despite the population of the study being relatively fit due to patients being excluded from entering the study if they had significant concomitant medical conditions. No significant differences were observed between ranibizumab and bevacizumab in rates of death (1.3 and 1.4% for the ranibizumab and bevacizumab monthly groups, respectively; 1.7 and 3.7% for the respective PRN groups; P=0.18 for comparing all groups, P=0.22 for between drug comparison), nonfatal myocardial infarction (0.7% for both the ranibizumab and bevacizumab monthly groups; 1.0 and 0.3% for the respective PRN groups; P=0.78 for comparing all groups, P=0.73 for between drug comparison), or nonfatal stroke (1.0 and 0.7% for the ranibizumab and bevacizumab monthly groups, respectively; 0.3 and 0.7% for the respective PRN groups; P=0.88 for comparing all groups, P=1.0 for between drug comparison).47 However, comparing rates of serious systemic adverse events associated with hospitalisation between ranibizumab- and bevacizumab-treated patients (combining dosing-regimen groups) demonstrated a statistically significant higher rate with bevacizumab (24.1 (141) vs 19.0% (114); RR 1.29; 95% CI 1.01–1.66; P=0.04).47 Intravitreal bevacizumab also led to a significantly higher incidence of gastrointestinal disorders (including haemorrhage) compared with ranibizumab (2.6 vs 0.8% P=0.02). These observed adverse events are consistent with those noted in the Summary of Product Characteristics for bevacizumab and are known to be potential risks related to systemic exposure of anti-VEGFs.46
DMO. The standard of care in DMO consisted of focal/grid laser photocoagulation (laser treatment) since the trial of the ETDRS in 1985, which demonstrated that this treatment substantially reduced the risk of visual loss.49 Some recent trials showed that laser treatment may also improve vision. For example, the Diabetic Retinopathy Clinical Research Network (DRCR.net) randomised study compared focal/grid photocoagulation to intravitreal administration of the corticosteroid triamcinolone acetonide (IVTA). The study showed that from baseline to year 3, laser treatment was associated with a mean gain of 5 letters and with an improved VA by ⩾10 letters in 44% of patients (vs worsening VA by ⩾10 letters in 12% of patients).50
Pharmacotherapeutic options in DMO were very limited until recently. Unlicensed and contraindicated use of IVTA is widespread and its rationale is based on the anti-angiogenic properties of corticosteroids (possibly due to downregulation of VEGF).51, 52 Notably, in the IVTA vs laser DRCR.net study, at 4 months, VA in the 4-mg IVTA group was superior to that in the 1-mg IVTA group (mean difference between the groups adjusted for baseline VA and prior macular photocoagulation, 3.6 letters; P=0.001) and in the laser-treated group (mean difference between the groups adjusted for baseline VA and prior macular photocoagulation, 3.8 letters; P<0.001); however, the VA differences between the groups disappeared by the end of year 1. By year 3, laser treatment conferred better VA outcomes compared with IVTA treatment (mean difference between laser and 1-mg IVTA groups adjusted for baseline VA and prior macular photocoagulation, 5.6 letters; 95% CI, 0.8–10.4; respective difference between laser and 4-mg IVTA groups, 4.7 letters; 95% CI, 0.0–9.5).50, 53 Furthermore, IVTA may increase the risk for secondary glaucoma and secondary cataracts.50
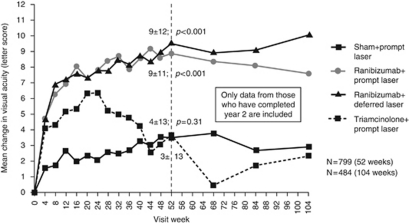
Ranibizumab was authorised in the EU for the treatment of visual impairment due to DMO in 2011,40 thereby significantly expanding the treatment armamentarium for DMO. Phase II studies such as READ-2 and RESOLVE demonstrated efficacy and tolerability of ranibizumab in DMO.54, 55 Phase III studies of ranibizumab in DMO further supported its utility in this indication. The independent DRCR.net Protocol I study was a 4-arm trial (854 eyes) evaluating ranibizumab plus prompt laser to ranibizumab plus deferred laser (⩾24 weeks), IVTA plus prompt laser, and sham injection plus prompt laser.56 Ranibizumab (or sham) injections were administered monthly for the first 3 months (totalling four injections) followed by PRN dosing based on VA/OCT criteria.56 The Protocol I study showed that at 1 year, ranibizumab plus prompt or deferred laser was superior to sham plus laser (mean increase of 9 letters for both ranibizumab groups vs 3 letters for the sham plus laser group; P<0.001 for comparisons vs sham), whereas IVTA plus laser was comparable to sham plus laser (mean increase of 4 letters for the IVTA plus laser group; P=0.31 vs sham plus laser).56 Two-year VA outcomes were similar to 1-year outcomes (Figure 4).56 The RESTORE phase III study (N=345) compared ranibizumab monotherapy (plus sham laser) to ranibizumab plus laser to laser alone (plus sham injections).57 Ranibizumab (0.5 mg) regimen included a loading phase of 3 monthly injections followed by PRN dosing (based on VA stability criteria).57 The mean number of ranibizumab injections in the ranibizumab and ranibizumab/laser groups was 7.0±2.8 and 6.8±3.0, respectively.57 The mean average change in best corrected visual acuity (BCVA) letter score from baseline to month 1 through month 12 was significantly superior with ranibizumab and ranibizumab plus laser vs laser alone (6.1, 5.9, and 0.8 letters, respectively; P<0.0001 for both comparisons vs laser alone); the difference between the two ranibizumab groups was not significant (P=0.61).57 The safety profile of ranibizumab in DMO trials was consistent with that of ranibizumab in wet AMD.56, 57 In addition to the DRCR.net and RESTORE studies, data from two other Phase III studies (RISE, RIDE) have strengthened the evidence for ranibizumab in DMO.58, 59
Figure 4.
Mean changes from baseline in VA in patients with DMO in the DRCR.net Protocol I study. No equivalent to the unpreserved triamcinolone formulation used in this study is currently available in the United Kingdom. P-values shown are for difference in mean change in VA vs sham plus prompt laser at 52 weeks. Adapted from Elman et al,56 Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 2010; 117(6): 1064–1077, e1035, with permission from Elsevier © 2010.
RVO. The standard of care for macular oedema secondary to BRVO consisted of grid laser photocoagulation since the Branch Vein Occlusion Study demonstrated the efficacy of this treatment approach (over no treatment).60 In contrast, as the Central Vein Occlusion Study showed no VA benefit for grid laser photocoagulation (over no treatment) in patients with macular oedema secondary to CRVO at any follow-up point,61 the standard of care for CRVO was observation until the recent development of pharmacotherapeutic options.
The rationale for using corticosteroids for macular oedema secondary to RVO is based on their anti-angiogenic and anti-inflammatory properties.51, 52, 62 The SCORE-BRVO study—a randomised trial (N=411) comparing IVTA (1 and 4 mg) to standard-of-care (grid laser photocoagulation that may be prompt or deferred depending on the absence/presence of dense macular haemorrhage) in patients with macular oedema secondary to BRVO—failed to demonstrate an advantage for IVTA over prompt/deferred laser.63 In this study, the mean letter gain from baseline to 1 year was not statistically different across the treatment groups (4.2, 5.7, and 4.0 letters for the standard care, 1-mg IVTA, and 4-mg IVTA groups, respectively; P=0.70), and neither was the difference in the proportion of patients who gained ⩾15 letters from baseline to 1 year (28.9, 25.6, and 27.2% for the standard care, 1-mg IVTA, and 4-mg IVTA groups, respectively; P=0.89 for all comparisons).63 Rates of elevated intraocular pressure (IOP) and cataracts were comparable in the standard-of-care and 1-mg IVTA groups and higher in the 4-mg IVTA group. There was, however, a dose-dependent higher frequency initiation of IOP-lowering medications and an increase in lens capacity onset/progression in the IVTA groups compared with the standard care group.63 In contrast to the SCORE-BRVO study, the complementary SCORE-CRVO trial—a randomised trial (N=271) comparing IVTA (1 and 4 mg) to observation in patients with macular oedema secondary to CRVO—demonstrated a superiority for IVTA over observations in these patients.64 In SCORE-CRVO study, from baseline to 1 year, IVTA-treated patients lost, on average, fewer letters compared with the observation group (loss of 12.1, 1.2, and 1.2 letters for the observation, 1-mg IVTA, and 4-mg IVTA groups, respectively; P=0.004); similarly, a higher proportion of patients in the IVTA groups gained ⩾15 letters from baseline to 1 year (6.8, 26.5, and 25.6% for the observation, 1-mg IVTA, and 4-mg IVTA groups, respectively; P=0.001 for both comparisons vs observation).64 Similar to the SCORE-CRVO findings, rates of IOP and cataracts were comparable in the observation and 1-mg IVTA groups and higher in the 4-mg IVTA group, and there was a dose-dependent higher frequency initiation of IOP-lowering medications in the IVTA groups compared with the observation group.64
Another corticosteroid that has been investigated and is now authorised for the treatment of RVO is dexamethasone intravitreal implant (DEX implant; Ozurdex®). The GENEVA studies (N=1267) were randomised trials comparing DEX implant (0.35 or 0.7 mg) with sham treatment in patients with macular oedema secondary to BRVO or CRVO at 6 months, followed by an open-label 6-month extension phase in which patients could receive a second DEX implant (0.7 mg) based on BCVA and retinal thickness.65, 66 The studies demonstrated that from baseline to 6 months, mean VA improvement was better in the DEX groups than in the sham group (P⩽0.006); the greatest between-group difference was at day 60 (∼7 letters).65 From day 30 to 90 (but not later), the proportion of patients with ⩾15 letter gain was significantly greater in the DEX groups (P<0.001); at day 60, 29% of patients in both DEX groups gained ⩾15 letters compared with 11% of the sham group (P<0.001),65 and this was maintained at 12 months in patients who received two 0.7-mg DEX implants (30 and 32%, 60 days after the first and second implant, respectively).66 Furthermore, patients in the DEX groups achieved the 15-letter gain faster than those in the sham group (P<0.001 vs sham).65 Rates of elevated IOP were overall higher in the DEX groups than the sham group (P⩽0.002) and the percentage of eyes receiving IOP-lowering medication increased in the DEX implant treatment groups from ∼6% at the beginning of the study to ∼24% by day 180, whereas there was no change in the sham group. By day 180, there was no difference in the rates of elevated IOP between the DEX groups and sham.65 Rates of cataracts were not significantly different between the DEX (7.3% in 0.7-mg group, 4.1% in 0.35-mg group) and sham (4.5%) groups at 6 months.65 However, at 12 months, patients who received two 0.7-mg DEX implants had a higher rate of cataract progression compared with sham (29.8% of phakic eyes vs 5.7% of phakic eyes, respectively).66
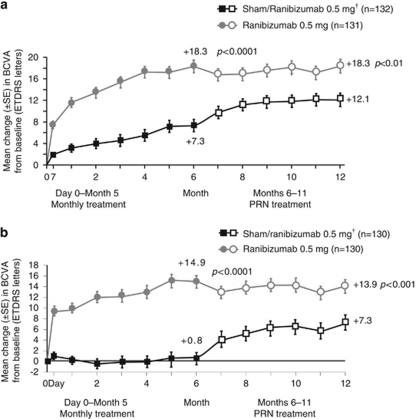
Ranibizumab was authorised in the EU for the treatment of visual impairment due to macular oedema secondary to RVO in 2011,40 thereby expanding the treatment options for RVO using an anti-VEGF approach. Pilot studies of ranibizumab in RVO provided preliminary proof for the potential utility of ranibizumab in this disease67, 68, 69 and provided the rationale for the two Phase III 12-month studies, BRAVO (in patients with macular oedema secondary to BRVO; N=397)25, 70 and CRUISE (in patients with macular oedema secondary to CRVO; N=392).71, 72 In both studies participants were randomised to receive monthly intravitreal ranibizumab (0.3 or 0.5 mg) or sham injections from day 0 to month 5 (thereafter, all patients with study eye BCVA of ⩽20/40 or central retinal thickness of ⩾250 μm received ranibizumab PRN).25, 70, 71, 72 In the BRAVO study, patients could receive rescue laser treatment once during the treatment period and once during the observation period if criteria were met.70 In both studies, the mean letter gain from baseline to month 6 was superior with ranibizumab compared with sham group. In BRAVO, patients gained 18.3 and 7.3 letters in the 0.5-mg group and sham group, respectively (P<0.0001 for ranibizumab vs sham),25 and in CRUISE, the respective values were 14.9 and 0.8 letters (P<0.0001 for ranibizumab vs sham).72 The treatment benefits were maintained through month 12 on a PRN regimen (Figure 5). Also, in both studies, the proportion of patients with ⩾15 letter gain from baseline was significantly higher in the ranibizumab groups (vs sham) at 6 months (BRAVO: 61.1 and 28.8% of patients in the 0.5 mg and sham groups; P<0.0001 for ranibizumab vs sham;25 CRUISE: 47.7 and 16.9% for the respective groups; P<0.0001).72 These proportions were maintained in both studies from month 6 through 12 when ranibizumab was given PRN. A total number of 2.7 (BRAVO) and 3.3 (CRUISE) 0.5-mg ranibizumab injections were needed to maintain patients visual stability from months 6 to 12.70, 71 Safety profile of ranibizumab in these trials was consistent with that found in other studies.70, 71
Figure 5.
Mean changes from baseline in VA in patients with macular oedema secondary to RVO. Mean changes from baseline in VA in patients with (a) BRVO in the BRAVO study (adapted from Brown et al,70 Sustained benefits from ranibizumab for macular edema following branch retinal vein occlusion: 12-month outcomes of a phase III study. Ophthalmology 2011; 118(8): 1594–1602, with permission from Elsevier © 2011) and (b) CRVO in the CRUISE study (adapted from Campochiaro et al,71 Sustained benefits from ranibizumab for macular edema following central retinal vein occlusion: twelve-month outcomes of a phase III study. Ophthalmology 2011; 118(10): 2041–2049, with permission from Elsevier © 2011). Only data from the licensed dose of ranibizumab (0.5 mg) are shown. †Sham patients received 0.5 mg ranibizumab PRN treatment from months 6 to 11. P-values shown are for difference in mean change VA vs sham/ranibizumab. ETDRS, Early Treatment Diabetic Retinopathy Study.
The viability of a stability-based individualised approach for ranibizumab in RVO was evaluated in a retrospective analysis conducted using data from the ranibizumab arms (pooled doses) in the Phase III BRAVO and CRUISE trials (in which patients received monthly injections from day 0 to month 5 and were dosed PRN thereafter (months 6–11)). The analysis demonstrated that 59 and 53% of ranibizumab-treated patients in the BRAVO and CRUISE trials, respectively, reached VA stability (⩽3 letters in 3 consecutive monthly visits with treatment at the first two visits) up to month 6.73 The mean VA change 1 month after ranibizumab treatment at a VA stability visit was small (BRAVO, 0.8 letters; CRUISE, 1.5 letters). During the PRN period, 1 month after ranibizumab re-initiation, mean VA gains were clinically relevant (BRAVO, 7.1 letters; CRUISE, 9.3 letters).73 Thus, this retrospective analysis is consistent with that conducted for the wet AMD trials and supports the currently approved posology.
Notably, the approved posology for all the ranibizumab indications is similar (the exception is that for visual impairment due to DMO or macula oedema secondary to RVO, further treatment is not recommended if there is no response after the first three injections; this is not the case for wet AMD).38 This harmonisation facilitates patient flow as it allows all patients with wet AMD, DMO, or macular oedema secondary to RVO to be treated using the same approach.
The impact of anti-VEGF treatments for DMO and RVO on clinic capacity
Following the introduction of anti-VEGF treatment for wet AMD, there was a consequent rise in the number of wet AMD patients, potentially suitable for treatment as well as the number (frequency) of follow-up appointments. The associated increase in clinical workload has been substantial and there is concern that the introduction of anti-VEGF treatments for DMO and RVO could further exacerbate pressure on clinic capacity in the hospital eye service.
The prevalence of diabetes (particularly type II) is increasing in the Western world with the ageing population. In the United Kingdom (in 2009), it was estimated that 2.6 million people were diagnosed with diabetes and that ∼500 000 more people had undiagnosed diabetes; the prevalence of diabetes in the adult population ranged from 3.9 (Scotland) to 5.1% (England).74 In an epidemiologic study of patients with type I diabetes (in the United States), the 14-year rates of progression to proliferative retinopathy and incidence of macular oedema were 37 and 26%, respectively.75 In a cohort study evaluating patients with type II diabetes in the United Kingdom, the cumulative 5-year incidence of developing sight-threatening diabetic retinopathy in patients without retinopathy at baseline was 3.9%.76 In contrast to diabetes and its associated retinal morbidities, RVO is relatively infrequent. In an epidemiologic study in the United States, the prevalence of BRVO was 0.6% and the prevalence of CRVO was 0.1%.77 In a recent analysis of pooled data from population studies worldwide, the overall RVO prevalence was 0.52% (0.44% BRVO, 0.08% CRVO), translating to ∼16 million individuals worldwide affected by RVO.78
The authorisation of anti-VEGF therapy for DMO and macular oedema secondary to RVO represented an increase in the number of patients eligible for anti-VEGF therapy, an important factor with respect to clinic capacity pressures. The RCOphth issued a preferred practice guidelines addressing the diabetic retinopathy screening and ophthalmology clinic set up in England as well as a guidance for management of RVO addressing patient pathways.20, 21 Ideally, referrals of patients with diabetic retinopathy should come through ENSPDR; whereas referrals of patients with RVO are likely to come from an optometrist, general practitioner, or other health workers.20, 21 In both cases, referral pathways should be streamlined to ensure timely treatments. Intravitreal injection facilities may be integrated into the patient pathways (similar to the way laser clinics are integrated in the guidance for diabetic retinopathy patients), so that patients could benefit from effective monitoring and care.21
Intravitreal injection facilities may be shared with wet AMD services. Importantly, the unified ranibizumab posology for wet AMD, DMO, and macular oedema secondary to RVO should streamline treatment of all these indications, thereby maximising clinic capacity. Conversely, utilising different treatments for each of these diseases may impact negatively on the capacity burden in specialist retinal clinics.
Summary
Treatment for retinal vascular diseases including wet AMD, DMO, and RVO has improved dramatically in recent years, due primarily, to the development and authorisation of anti-VEGF therapy. Treatment regimens have evolved through experience gained in clinical trials and clinical practice. The current treatment regimen for ranibizumab across these indications reflects an individualised treatment approach designed to treat patients when they could benefit the most while minimising the number of unnecessary intravitreal injections, and hence the risk of adverse events. To maximise patient outcomes, care should be taken to integrate intravitreal injection facilities into the patient pathways for DMO and RVO (similar to the approach taken for wet AMD); the new unified posology for ranibizumab may streamline the treatment of these patients, thereby minimising impact on clinic capacity and optimising patient outcomes.
Acknowledgments
This study was supported by Novartis Pharmaceuticals Limited (UK). Novartis Pharmaceuticals Limited (UK) has had the opportunity to comment on the medical content and accuracy of the article; however, final editorial content resides with the author and the journal. We thank Sue Harris from ApotheCom, who provided medical writing support on behalf of Novartis Pharmaceuticals.
Appendix
Lucentis®▾ (ranibizumab), ABBREVIATED UK PRESCRIBING INFORMATION
Please refer to the SmPC before prescribing Lucentis 10 mg/ml solution for injection.
Presentation: A glass single-use vial containing 0.23 ml solution containing 2.3 mg of ranibizumab (10 mg/ml).
Indications: The treatment in adults of neovascular (wet) age-related macular degeneration (AMD), the treatment of visual impairment due to diabetic macular oedema (DMO), or the treatment of visual impairment due to macular oedema secondary to retinal vein occlusion (branch RVO or central RVO).
Administration and dosage: Single-use vial for intravitreal use only. Lucentis must be administered by a qualified ophthalmologist experienced in intravitreal injections under aseptic conditions. The recommended dose is 0.5 mg (0.05 ml).
For treatment of wet AMD: Treatment is given monthly and continued until maximum visual acuity is achieved, i.e., the patient's visual acuity is stable for three consecutive monthly assessments performed while on ranibizumab. Thereafter, patients should be monitored monthly for visual acuity. Treatment is resumed when monitoring indicates loss of visual acuity due to wet AMD. Monthly injections should then be administered until stable visual acuity is reached again for three consecutive monthly assessments (implying a minimum of two injections). The interval between two doses should not be shorter than 1 month.
For treatment of visual impairment due to either DMO or macular oedema secondary to RVO: Treatment is given monthly and continued until maximum visual acuity is achieved, i.e., the patient's visual acuity is stable for three consecutive monthly assessments performed while on ranibizumab treatment. If there is no improvement in visual acuity over the course of the first three injections, continued treatment is not recommended. Thereafter, patients should be monitored monthly for visual acuity. Treatment is resumed when monitoring indicates loss of visual acuity due to DMO or to macular oedema secondary to RVO. Monthly injections should then be administered until stable visual acuity is reached again for three consecutive monthly assessments (implying a minimum of two injections). The interval between two doses should not be shorter than 1 month.
Lucentis and laser photocoagulation in DMO and in macular oedema secondary to BRVO: When given on the same day, Lucentis should be administered at least 30 minutes after laser photocoagulation. Lucentis can be administered in patients who have received previous laser photocoagulation. Before treatment, evaluate the patient's medical history for hypersensitivity. The patient should also be instructed to self-administer anti-microbial drops, 4 times daily for 3 days before and following each injection.
Children and adolescents: Not recommended for use in children and adolescents due to a lack of data.
Elderly: No dose adjustment is required in the elderly. There is limited experience in patients older than 75 years with DMO.
Hepatic and renal impairment: Dose adjustment is not needed in these populations.
Contraindications: Hypersensitivity to the active substance or excipients. Patients with active or suspected ocular or periocular infections. Patients with active severe intraocular inflammation.
Special warnings and precautions for use: Lucentis is for intravitreal injection only. Intravitreal injections have been associated with endophthalmitis, intraocular inflammation, rhegmatogenous retinal detachment, retinal tear, and iatrogenic traumatic cataract. Monitor during week following injection for infections. Patients should be instructed to report symptoms suggestive of any of the above without delay. Transient increases in intraocular pressure (IOP) within 1 hour of injection and sustained IOP increases have been identified. Both IOP and perfusion of the optic nerve head should be monitored and managed appropriately. Concurrent use in both eyes has not been studied and could lead to an increased systemic exposure. There is a potential for immunogenicity with Lucentis, which may be greater in subjects with DMO. Patients should report an increase in severity of intraocular inflammation. Lucentis should not be administered concurrently with other anti-VEGF agents (systemic or ocular). Withhold dose and do not resume treatment earlier than the next scheduled treatment in the event of the following: a decrease in best corrected visual acuity of (BCVA) ⩾30 letters compared with the last assessment of visual acuity; an intraocular pressure of ⩾30 mm Hg; a retinal break; a subretinal haemorrhage involving the centre of the fovea, or if the size of the haemorrhage is ⩾50% of the total lesion area; performed or planned intraocular surgery within the previous or next 28 days. Risk factors associated with the development of a retinal pigment epithelial (RPE) tear after anti-VEGF therapy for wet AMD include a large and/or high pigment epithelial retinal detachment. When initiating Lucentis therapy, caution should be taken in patients with these risk factors for RPE tears. Discontinue treatment in cases of rhegmatogenous retinal detachment or stage 3 or 4 macular holes. There is only limited experience in the treatment of subjects with DMO due to type I diabetes. Lucentis has not been studied in patients who have previously received intravitreal injections, in patients with active systemic infections, proliferative diabetic retinopathy, or in patients with concurrent eye conditions such as retinal detachment or macular hole. There is also no experience of treatment with Lucentis in diabetic patients with an HbA1c over 12% and uncontrolled hypertension. There are limited data on safety in the treatment of DMO and macular oedema due to RVO patients with prior history of stroke or transient ischaemic attacks. Since there is a potential risk of arterial thromboembolic events following intravitreal use of VEGF (vascular endothelial growth factor) inhibitors, caution should be exercised when treating such patients. There is limited experience with treatment of patients with prior episodes of RVO and of patients with ischaemic BRVO and CRVO. Treatment is not recommended in RVO patients presenting with clinical signs of irreversible ischaemic visual function loss.
Interactions: No formal interaction studies have been performed. In wet AMD adjunctive use of verteporfin photodynamic therapy (PDT) and Lucentis in an open study showed an incidence of intraocular inflammation following initial combination treatment of 6.3% (2 of 32 patients). In DMO and BRVO adjunctive use of laser therapy and Lucentis was not associated with any new ocular or non-ocular safety findings.
Pregnancy and lactation: Women of childbearing potential should use effective contraception during treatment. No clinical data on exposed pregnancies are available. Ranibizumab should not be used during pregnancy unless the expected benefit outweighs the potential risk to the foetus. For women who wish to become pregnant and have been treated with ranibizumab, it is recommended to wait at least 3 months after the last dose of ranibizumab before conceiving. Breast-feeding is not recommended during the use of Lucentis.
Driving and using machines: The treatment procedure may induce temporary visual disturbances, and patients who experience these signs must not drive or use machines until these disturbances subside.
Undesirable effects: wet AMD population: Serious adverse events related to the injection procedure included endophthalmitis, rhegmatogenous retinal detachment, retinal tear, and iatrogenic traumatic cataract. Other serious ocular events among Lucentis-treated patients included intraocular inflammation and increased intraocular pressure. The safety data below includes all adverse events suspected to be due to the injection procedure or medicinal product in the wet AMD trial population.
Very common: Intraocular pressure increased, headache, vitritis, vitreous detachment, retinal haemorrhage, visual disturbance, eye pain, vitreous floaters, conjunctival haemorrhage, eye irritation, foreign body sensation in eyes, lacrimation increased, blepharitis, dry eye, ocular hyperaemia, eye pruritus, arthralgia, nasopharyngitis.
Common: Anaemia, retinal degeneration, retinal disorder, retinal detachment, retinal tear, detachment of the retinal pigment epithelium, retinal pigment epithelium tear, visual acuity reduced, vitreous haemorrhage, vitreous disorder, uveitis, iritis, iridocyclitis, cataract, cataract subcapsular, posterior capsule opacification, punctuate keratitis, corneal abrasion, anterior chamber flare, vision blurred, injection site haemorrhage, eye haemorrhage, conjunctivitis, conjunctivitis allergic, eye discharge, photopsia, photophobia, ocular discomfort, eyelid oedema, eyelid pain, conjunctival hyperaemia, cough, nausea, allergic reactions, hypersensitivity, and anxiety.
DMO and RVO populations: Ocular and non-ocular events in the DMO and RVO trials were reported with a frequency and severity similar to those seen in the wet AMD trials with the addition of urinary tract infection, which was found to be ‘common' in the DMO population.
Product-class-related adverse reactions: There is a theoretical risk of arterial thromboembolic events following intravitreal use of VEGF inhibitors. A low-incidence rate of arterial thromboembolic events was observed in the Lucentis clinical trials in patients with AMD and DMO and RVO, and there were no major differences between the groups treated with ranibizumab compared to control. Please refer to the SmPC for full listing of all undesirable effects.
For United Kingdom: Adverse events should be reported. Reporting forms and information can be found at www.yellowcard.mhra.gov.uk. Adverse events should also be reported to Novartis Pharmaceuticals UK Ltd on (01276) 698370.
Legal category: POM, UK Basic NHS cost: £742.17. Marketing authorisation number: EU/1/06/374/001
Marketing authorisation holder: Novartis Europharm Limited, Wimblehurst Road, Horsham, West Sussex RH12 5AB, UK. Full prescribing information, including SmPC, is available from: Novartis Pharmaceuticals, Frimley Business Park, Frimley, Camberley, Surrey GU16 7SR, UK. Tel: 01276 692255; Fax: 01276 692508.
Date of PI preparation: September 2011.
VISUDYNE® (verteporfin), ABBREVIATED UK PRESCRIBING INFORMATION
Presentation: Glass vial containing 15 mg of verteporfin as powder. Indications: Treatment of age-related macular degeneration (AMD) in adult patients with predominantly classic subfoveal choroidal neovascularisation or subfoveal choroidal neovascularisation secondary to pathological myopia.
Dosage and administration: A 10-minute intravenous infusion of Visudyne (30 ml solution) at a dose of 6 mg/m2 body surface area. This is followed by the activation of Visudyne 15 minutes after the start of the infusion using a diode laser generating non-thermal red light (wavelength 689 nm (±3 nm). At the recommended light intensity of 600 mW/cm2, it takes 83 seconds to deliver the required light dose of 50 J/cm2. Reevaluate every 3 months; if recurrent CNV leakage occurs, Visudyne therapy may be given up to 4 times per year.
Contraindications: Porphyria, known hypersensitivity to verteporfin or to any of the excipients, or severe hepatic impairment.
Precautions: Due to photosensitivity, avoid exposure of unprotected skin, eyes, or other body organs to direct sunlight or bright indoor light for 48 hours after infusion. UV sunscreens are not effective at protecting against photosensitivity reactions. Exercise caution in moderate hepatic impairment, biliary obstruction, and treatment under general anaesthesia. If severe decrease of vision (equivalent to 4 lines or more) occurs within 1 week after treatment, do not re-treat at least until vision completely recovers to pretreatment level. If extravasation occurs, stop infusion immediately. Protect the affected area thoroughly from bright direct light until swelling and discolouration have disappeared. Visudyne contains small amounts of butylated hydroxytoluene that may be irritant to eyes, skin, and mucous membranes, it should be washed off extensively with water in the event of direct contact. Patients should be under close medical supervision during Visudyne infusion. Chest pain, vasovagal reactions (posture-related), and hypersensitivity reactions have been reported.
Interactions: No specific drug–drug interaction studies have been conducted in humans. Concomitant use of other photosensitising agents (eg, tetracyclines, sulphonamides, phenothiazines, sulphonylurea, hypoglycaemic agents, thiazide diuretics, and griseofulvin) could increase the potential for photosensitivity reactions.
Pregnancy and lactation: Visudyne should be used in pregnant women only if the benefit justifies the potential risk to the foetus. Do not administer to nursing mothers or stop breast-feeding for 48 hours after administration.
Effects on ability to drive and use machines: Do not drive or use machines as long as symptoms such as abnormal vision persist.
Undesirable effects: Most adverse reactions were mild to moderate, transient in nature, and similar in patients with either pathological myopia or AMD.
Reported frequency of ocular adverse reactions: Common (⩾1/100 to <1/10): Severe reduced visual acuity, visual impairment such as reduced visual acuity, blurred, fuzzy vision, or photopsia as well as visual field defect such as scotoma, grey or dark haloes, and black spots. Uncommon (⩾1/1000 to <1/100): Retinal detachment (non-rhegmatogenous), subretinal/retinal haemorrhage, vitreous haemorrhage. Rare (⩾1/10 000 to <1/1000): Retinal or choroidal vessel non-perfusion. Frequency not known: Retinal pigment epithelial tear. Reported frequency of systemic adverse reactions: Common (⩾1/100 to <1/10): Hypercholesteraemia, nausea, photosensitivity reaction, injection site pain, injection site oedema, injection site inflammation, injection site extravasation, asthenia, infusion-related reaction primarily presented as back pain. Uncommon (⩾1/1000 to <1/100): Hyperaesthesia, hypertension, injection site hypersensitivity, injection site haemorrhage, injection site discolouration, pyrexia, pain. Frequency not known: Hypersensitivity, vasovagal reactions, myocardial infarction, injection site blister, infusion-related chest pain.
Prescribers should consult the Summary of Product Characteristics for full information about other side effects.
Legal category: POM. Packaging quantities: Each vial containing 15 mg verteporfin. Price: UK £850. Marketing authorisation number: EU/1/00/140/001.
Marketing authorisation holder: Novartis Europharm Limited, Wimblehurst Road, Horsham, West Sussex RH12 5AB, UK.
Date of preparation: 14 May 2010. Visudyne is a registered trade mark. Full prescribing information, including SmPC, is available from Novartis Pharmaceuticals, Frimley Business Park, Frimley, Camberley, Surrey GU16 7SR, UK. Tel: 01276 692255. Fax: 01276 692508.
Adverse events should be reported. Reporting forms and information can be found at www.yellowcard.mhra.gov.uk. Adverse events should also be reported to Novartis Pharmaceuticals UK Ltd on (01276) 698370.
Job code: LUC12-C005.
Date of preparation: January 2012.
CS Brand has received payment from Allergan, Bayer, Novartis Pharmaceuticals, and Pfizer for attendance at advisory board meetings and consultation fees over the past 2 years. He has received payment for giving lectures and talks on behalf of: Bausch & Lomb, Eli Lilly, Merck, Sharpe & Dohme, Novartis Pharmaceuticals, Pfizer, and Spectrum Thea. The department at the Royal Hallamshire Hospital, Sheffield, where CS Brand works has received funding to conduct research on behalf of: Allergan, Novartis Pharmaceuticals, and Pfizer. He has received remuneration for his work on this article.
Footnotes
Please note that prescribing information is available at the end of this article as an Appendix.
References
- Bunce C, Xing W, Wormald R. Causes of blind and partial sight certifications in England and Wales: April 2007–March 2008. Eye (Lond) 2010;24 (11:1692–1699. doi: 10.1038/eye.2010.122. [DOI] [PubMed] [Google Scholar]
- Bunce C, Wormald R. Causes of blind certifications in England and Wales: April 1999–March 2000. Eye (Lond) 2008;22 (7:905–911. doi: 10.1038/sj.eye.6702767. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9 (6:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- Kliffen M, Sharma HS, Mooy CM, Kerkvliet S, de Jong PT. Increased expression of angiogenic growth factors in age-related maculopathy. Br J Ophthalmol. 1997;81 (2:154–162. doi: 10.1136/bjo.81.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez PF, Sippy BD, Lambert HM, Thach AB, Hinton DR. Transdifferentiated retinal pigment epithelial cells are immunoreactive for vascular endothelial growth factor in surgically excised age-related macular degeneration-related choroidal neovascular membranes. Invest Ophthalmol Vis Sci. 1996;37 (5:855–868. [PubMed] [Google Scholar]
- Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331 (22:1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- Adamis AP, Miller JW, Bernal MT, D'Amico DJ, Folkman J, Yeo TK, et al. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994;118 (4:445–450. doi: 10.1016/s0002-9394(14)75794-0. [DOI] [PubMed] [Google Scholar]
- Boyd SR, Zachary I, Chakravarthy U, Allen GJ, Wisdom GB, Cree IA, et al. Correlation of increased vascular endothelial growth factor with neovascularization and permeability in ischemic central vein occlusion. Arch Ophthalmol. 2002;120 (12:1644–1650. doi: 10.1001/archopht.120.12.1644. [DOI] [PubMed] [Google Scholar]
- Noma H, Minamoto A, Funatsu H, Tsukamoto H, Nakano K, Yamashita H, et al. Intravitreal levels of vascular endothelial growth factor and interleukin-6 are correlated with macular edema in branch retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol. 2006;244 (3:309–315. doi: 10.1007/s00417-004-1087-4. [DOI] [PubMed] [Google Scholar]
- The Human Genome Project Available at: http://www.ornl.gov/sci/techresources/ Human_Genome/project/about.shtml (accessed on 9 January 2011).
- Chakravarti A. To a future of genetic medicine. Nature. 2001;409 (6822:822–823. doi: 10.1038/35057281. [DOI] [PubMed] [Google Scholar]
- Cancer Research UK Stratified Medicines ProgrammeAvailable at: http://science.cancerresearchuk.org/research/how-we-deliver-our-research/ others/by-programme/stratified-medicine-programme/ (accessed on 9 January 2011).
- Chen Y, Bedell M, Zhang K. Age-related macular degeneration: genetic and environmental factors of disease. Mol Interv. 2010;10 (5:271–281. doi: 10.1124/mi.10.5.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa PC, Change NV, Scholl HPN. The significance of the complement system for the pathogenesis of age-related macular degeneration – current evidence and translation into clinical application. Graefes Arch Clin Exp Ophthalmol. 2011;249:163–174. doi: 10.1007/s00417-010-1568-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrs KM, Jackson JR, Brown EN, Allikmets R, Hageman GS. Complement, age-related macular degeneration and a vison of the future. Arch Ophthalmol. 2010;128 (3:349–358. doi: 10.1001/archophthalmol.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Royal College of Ophthalmologists Age-related macular degeneration guidelines for management 2009. Update. Available at: http://www.rcophth.ac.uk/page.asp?section= 451§ionTitle=Clinical+Guidelines (accessed on 9 January 2012).
- Cook HL, Patel PJ, Tufail A. Age-related macular degeneration: diagnosis and management. Br Med Bull. 2008;85:127–149. doi: 10.1093/bmb/ldn012. [DOI] [PubMed] [Google Scholar]
- Boscia F. Current approaches to the management of diabetic retinopathy and diabetic macular oedema. Drugs. 2010;70 (16:2171–2200. doi: 10.2165/11538130-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Early photocoagulation for diabetic retinopathy. ETDRS report number 9. Early Treatment Diabetic Retinopathy Study Research Group. 1991;98 (5 Suppl:766–785. [PubMed] [Google Scholar]
- The Royal College of Ophthalmologists Guidelines for diabetic retinopathy 2005. Available at: http://www.rcophth.ac.uk/page.asp?section= 451§ionTitle=Clinical+Guidelines (accessed on 9 January 2011).
- The Royal College of Ophthalmologists Preferred practice guidelines. Diabetic retinopathy screening (DRS) and the ophthalmology clinic set up in England 2010. Available at: http://www.rcophth.ac.uk/page.asp?section=451 §ionTitle=Clinical+Guidelines (accessed on 9 January 2012).
- The English National Screening Programme for Diabetic Retinopathy (ENSPDR) websiteAvailable at: http://retinalscreening.nhs.uk/pages/default.asp (accessed on 9 January 2012).
- Aref AA, Scott IU. Management of macular edema secondary to central retinal vein occlusion: an evidence-based. Adv Ther. 2011;28 (1:40–50. doi: 10.1007/s12325-010-0088-4. [DOI] [PubMed] [Google Scholar]
- Aref AA, Scott IU. Management of macular edema secondary to branch retinal vein occlusion: an evidence-based update. Adv Ther. 2011;28 (1:28–39. doi: 10.1007/s12325-010-0089-3. [DOI] [PubMed] [Google Scholar]
- Campochiaro PA, Heier JS, Feiner L, Gray S, Saroj N, Rundle AC, et al. Ranibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a phase III study Ophthalmology 2010117(61102–1112.e1101. [DOI] [PubMed] [Google Scholar]
- Lattanzio R, Torres Gimeno A, Battaglia Parodi M, Bandello F. Retinal vein occlusion: current treatment. Ophthalmologica. 2011;225 (3:135–143. doi: 10.1159/000314718. [DOI] [PubMed] [Google Scholar]
- Jonas J, Paques M, Mones J, Glacet-Bernard A. Retinal vein occlusions. Dev Ophthalmol. 2010;47:111–135. doi: 10.1159/000320076. [DOI] [PubMed] [Google Scholar]
- Kanski JJ.Retinal vascular disease Clinical Ophthalmology5th edn.Butterworth-Heinemann: London; 2006438–486. [Google Scholar]
- Treatment of senile disciform macular degeneration: a single-blind randomised trial by argon laser photocoagulation. The Moorfields Macular Study Group. Br J Ophthalmol. 1982;66 (12:745–753. doi: 10.1136/bjo.66.12.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Medicines Agency Visudyne authorisation letterAvailable at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/ human/medicines/000305/human_med_001146.jsp&mid=WC0b01ac058001d124 (accessed on 9 January 2012).
- TA68 Macular degeneration (age related)—photodynamic therapy: Guidance, 2003Available at: : http://www.nice.org.uk/nicemedia/ pdf/68_PDTGuidance.pdf (accessed on 9 January 2011).
- European Medicines Agency Macugen authorisation letterAvailable at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000620/ human_med_000898.jsp&mid=WC0b01ac058001d124 (accessed on 9 January 2012).
- European Medicines Agency Lucentis authorisation letterAvailable at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000715/ human_med_000890.jsp&mid=WC0b01ac058001d124 (accessed on 9 January 2012).
- Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355 (14:1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T.Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two-year results of the ANCHOR study Ophthalmology 2009116(157–65.e55. [DOI] [PubMed] [Google Scholar]
- Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355 (14:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- Regillo CD, Brown DM, Abraham P, Yue H, Ianchulev T, Schneider S, et al. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER Study year 1. Am J Ophthalmol. 2008;145 (2:239–248. doi: 10.1016/j.ajo.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Lucentis Summary of product characteristicsAvailable at: http://www.medicines.org.uk/emc/medicine/19409 (accessed on 9 January 2012).
- Holz FG, Korobelnik JF, Lanzetta P, Mitchell P, Schmidt-Erfurth U, Wolf S, et al. The effects of a flexible visual acuity-driven ranibizumab treatment regimen in age-related macular degeneration: outcomes of a drug and disease model. Invest Ophthalmol Vis Sci. 2010;51 (1:405–412. doi: 10.1167/iovs.09-3813. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency Lucentis procedural steps taken and scientific information after the authorisationAvailable at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Procedural_steps_taken_and_ scientific_information_after_authorisation/human/000715/WC500043552.pdf (accessed on 9 January 2012).
- DENALI study descriptionAvailable at: http://www.clinicaltrials.gov/ct2/show/record/ NCT00436553?term=NCT00436553&rank=1 (accessed on 9 January 2012).
- Novartis Data on file, LUCDOF12-001. 2012.
- Schmidt-Erfurth U, Eldem B, Guymer R, Korobelnik JF, Schlingemann RO, Axer-Siegel R, et al. Efficacy and safety of monthly versus quarterly ranibizumab treatment in neovascular age-related macular degeneration: the EXCITE study. Ophthalmology. 2011;118 (5:831–839. doi: 10.1016/j.ophtha.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Holz FG, Amoaku W, Donate J, Guymer RH, Kellner U, Schlingemann RO, et al. Safety and efficacy of a flexible dosing regimen of ranibizumab in neovascular age-related macular degeneration: the SUSTAIN study. Ophthalmology. 2011;118 (4:663–671. doi: 10.1016/j.ophtha.2010.12.019. [DOI] [PubMed] [Google Scholar]
- MONT BLANC study descriptionAvailable at: http://clinicaltrials.gov/ct2/show/study/NCT0043301 (accessed on 9 January 2012).
- Avastin Summary of product characteristicsAvailable at: http://www.medicines.org.uk/EMC/medicine/15748/SPC/ Avastin+25mg+ml+concentrate+for+solution+for+infusion/ (accessed on 9 January 2012).
- Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364 (20:1897–1908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer DS, Heier JS, Brown DM, Francom SF, Ianchulev T, Rubio RG. A Phase IIIb study to evaluate the safety of ranibizumab in subjects with neovascular age-related macular degeneration. Ophthalmology. 2009;116 (9:1731–1739. doi: 10.1016/j.ophtha.2009.05.024. [DOI] [PubMed] [Google Scholar]
- Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol. 1985;103 (12:1796–1806. [PubMed] [Google Scholar]
- Beck RW, Edwards AR, Aiello LP, Bressler NM, Ferris F, Glassman AR, et al. Three-year follow-up of a randomized trial comparing focal/grid photocoagulation and intravitreal triamcinolone for diabetic macular edema. Arch Ophthalmol. 2009;127 (3:245–251. doi: 10.1001/archophthalmol.2008.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauck M, Karakiulakis G, Perruchoud AP, Papakonstantinou E, Roth M. Corticosteroids inhibit the expression of the vascular endothelial growth factor gene in human vascular smooth muscle cells. Eur J Pharmacol. 1998;341 (2–3:309–315. doi: 10.1016/s0014-2999(97)01464-7. [DOI] [PubMed] [Google Scholar]
- Nauck M, Roth M, Tamm M, Eickelberg O, Wieland H, Stulz P, et al. Induction of vascular endothelial growth factor by platelet-activating factor and platelet-derived growth factor is downregulated by corticosteroids. Am J Respir Cell Mol Biol. 1997;16 (4:398–406. doi: 10.1165/ajrcmb.16.4.9115750. [DOI] [PubMed] [Google Scholar]
- Diabetic Retinopathy Clinical Research Network A randomized trial comparing intravitreal triamcinolone acetonide and focal/grid photocoagulation for diabetic macular edema Ophthalmology 2008115(91447–1449.1449; e1441–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen QD, Shah SM, Heier JS, Do DV, Lim J, Boyer D, et al. Primary end point (six months) results of the Ranibizumab for Edema of the mAcula in Diabetes (READ-2) study Ophthalmology 2009116(112175–2181.e2171. [DOI] [PubMed] [Google Scholar]
- Massin P, Bandello F, Garweg JG, Hansen LL, Harding SP, Larsen M, et al. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care. 2010;33 (11:2399–2405. doi: 10.2337/dc10-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman MJ, Aiello LP, Beck RW, Bressler NM, Bressler SB, Edwards AR, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema Ophthalmology 2010117(61064–1077.e1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P, Bandello F, Schmidt-Erfurth U, Lang GE, Massin P, Schlingemann RO, et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118 (4:615–625. doi: 10.1016/j.ophtha.2011.01.031. [DOI] [PubMed] [Google Scholar]
- Boyer DS, Sy J, Rundle AC, Hopkins JJ, Ehrlich JS.RIDE and RISE Research GroupRanibizumab (anti-VEGF) for vision loss due to diabetic macular edema—results of two phase III randomized trials. American Diabetes Association 71st Scientific Sessions. 2011. 133-LBOR.
- Patel S, Wong P, Hopkin JJ.RISE and RIDE trials of ranibizumab for diabetic macular edema: pooled efficacy and safety analysesAmerican Academy of Ophthalmology. Annual Meeting, 2011. PA043.
- The Branch Vein Occlusion Study Group Argon laser photocoagulation for macular edema in branch vein occlusion. Am J Ophthalmol. 1984;98 (3:271–282. doi: 10.1016/0002-9394(84)90316-7. [DOI] [PubMed] [Google Scholar]
- The Central Vein Occlusion Study Group M report Evaluation of grid pattern photocoagulation for macular edema in central vein occlusion. Ophthalmology. 1995;102 (10:1425–1433. doi: 10.1016/s0161-6420(95)30849-4. [DOI] [PubMed] [Google Scholar]
- Lee HB, Pulido JS, McCannel CA, Buettner H. Role of inflammation in retinal vein occlusion. Can J Ophthalmol. 2007;42 (1:131–133. doi: 10.1139/i06-101. [DOI] [PubMed] [Google Scholar]
- Scott IU, Ip MS, VanVeldhuisen PC, Oden NL, Blodi BA, Fisher M, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with standard care to treat vision loss associated with macular edema secondary to branch retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 6. Arch Ophthalmol. 2009;127 (9:1115–1128. doi: 10.1001/archophthalmol.2009.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip MS, Scott IU, VanVeldhuisen PC, Oden NL, Blodi BA, Fisher M, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 5. Arch Ophthalmol. 2009;127 (9:1101–1114. doi: 10.1001/archophthalmol.2009.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller JA, Bandello F, Belfort R, Jr, Blumenkranz MS, Gillies M, Heier J, et al. Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion Ophthalmology 2010117(61134–1146.e1133. [DOI] [PubMed] [Google Scholar]
- Haller JA, Bandello F, Belfort Jr R, Blumenkranz MS, Gillies M, et al. Dexamethasone intravitreal implant in patients with macular edema related to branch or central retinal vein occlusion. Twelve month study results. Ophthalmology. 2011;118 (12:2453–2460. doi: 10.1016/j.ophtha.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Campochiaro PA, Hafiz G, Shah SM, Nguyen QD, Ying H, Do DV, et al. Ranibizumab for macular edema due to retinal vein occlusions: implication of VEGF as a critical stimulator. Mol Ther. 2008;16 (4:791–799. doi: 10.1038/mt.2008.10. [DOI] [PubMed] [Google Scholar]
- Spaide RF, Chang LK, Klancnik JM, Yannuzzi LA, Sorenson J, Slakter JS, et al. Prospective study of intravitreal ranibizumab as a treatment for decreased visual acuity secondary to central retinal vein occlusion. Am J Ophthalmol. 2009;147 (2:298–306. doi: 10.1016/j.ajo.2008.08.016. [DOI] [PubMed] [Google Scholar]
- Pieramici DJ, Rabena M, Castellarin AA, Nasir M, See R, Norton T, et al. Ranibizumab for the treatment of macular edema associated with perfused central retinal vein occlusions. Ophthalmology. 2008;115 (10:e47–e54. doi: 10.1016/j.ophtha.2008.06.021. [DOI] [PubMed] [Google Scholar]
- Brown DM, Campochiaro PA, Bhisitkul RB, Ho AC, Gray S, Saroj N, et al. Sustained benefits from ranibizumab for macular edema following branch retinal vein occlusion: 12-month outcomes of a phase III study. Ophthalmology. 2011;118 (8:1594–1602. doi: 10.1016/j.ophtha.2011.02.022. [DOI] [PubMed] [Google Scholar]
- Campochiaro PA, Brown DM, Awh CC, Lee SY, Gray S, Saroj N, et al. Sustained benefits from ranibizumab for macular edema following central retinal vein occlusion: twelve-month outcomes of a phase III study. Ophthalmology. 2011;118 (10:2041–2049. doi: 10.1016/j.ophtha.2011.02.038. [DOI] [PubMed] [Google Scholar]
- Brown DM, Campochiaro PA, Singh RP, Li Z, Gray S, Saroj N, et al. Ranibizumab for macular edema following central retinal vein occlusion: six-month primary end point results of a phase III study Ophthalmology 2010117(61124–1133.e1121. [DOI] [PubMed] [Google Scholar]
- Pearce I, Gerding H, Burian G, Pilz S.Evaluation of ranibizumab treatment concept based on stability and deterioration of visual acuity in BRAVO and CRUISE patientsEuropean Society of Retina Specialists—11th EURETINA Congress 2011; FP-2362.
- Diabetes UK Diabetes in the UK 2010: key statistics on diabetesAvailable at: http://www.diabetes.org.uk/Documents/Reports/ Diabetes_in_the_UK_2010.pdf (accessed on 9 January 2012).
- Klein R, Klein BE, Moss SE, Cruickshanks KJ. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XVII. The 14-year incidence and progression of diabetic retinopathy and associated risk factors in type 1 diabetes. Ophthalmology. 1998;105 (10:1801–1815. doi: 10.1016/S0161-6420(98)91020-X. [DOI] [PubMed] [Google Scholar]
- Younis N, Broadbent DM, Vora JP, Harding SP. Incidence of sight-threatening retinopathy in patients with type 2 diabetes in the Liverpool Diabetic Eye Study: a cohort study. Lancet. 2003;361 (9353:195–200. doi: 10.1016/s0140-6736(03)12267-2. [DOI] [PubMed] [Google Scholar]
- Klein R, Klein BE, Moss SE, Meuer SM.The epidemiology of retinal vein occlusion: the Beaver Dam Eye Study Trans Am Ophthalmol Soc 200098133–141.discussion 141–133. [PMC free article] [PubMed] [Google Scholar]
- Rogers S, McIntosh RL, Cheung N, Lim L, Wang JJ, Mitchell P, et al. The prevalence of retinal vein occlusion: pooled data from population studies from the United States, Europe, Asia, and Australia Ophthalmology 2010117(2313–319.e311. [DOI] [PMC free article] [PubMed] [Google Scholar]