Abstract
Disruption of the blood-brain barrier (BBB) is a serious complication frequently encountered in neurodegenerative disorders. Infantile neuronal ceroid lipofuscinosis (INCL) is a devastating childhood neurodegenerative lysosomal storage disorder caused by palmitoyl-protein thioesterase-1 (PPT1) deficiency. It remains unclear whether BBB is disrupted in INCL and if so, what might be the molecular mechanism(s) of this complication. We previously reported that the Ppt1-knockout (Ppt1-KO) mice that mimic INCL manifest high levels of oxidative stress and neuroinflammation. Recently, it has been reported that CD4+ T-helper 17 (TH17) lymphocytes may mediate BBB disruption and neuroinflammation, although the precise molecular mechanism(s) remain unclear. We sought to determine: (i) whether the BBB is disrupted in Ppt1-KO mice, (ii) if so, do TH17-lymphocytes underlie this complication, and (iii) how might TH17 lymphocytes breach the BBB. Here, we report that the BBB is disrupted in Ppt1-KO mice and that TH17 lymphocytes producing IL-17A mediate disruption of the BBB by stimulating production of matrix metalloproteinases (MMPs), which degrade the tight junction proteins essential for maintaining BBB integrity. Importantly, dietary supplementation of resveratrol (RSV), a naturally occurring antioxidant/anti-inflammatory polyphenol, markedly reduced the levels of TH17 cells, IL-17A and MMPs, and elevated the levels of tight junction proteins, which improved the BBB integrity in Ppt1-KO mice. Intriguingly, we found that RSV suppressed the differentiation of CD4+ T lymphocytes to IL-17A-positive TH17 cells. Our findings uncover a mechanism by which TH17 lymphocytes mediate BBB disruption and suggest that small molecules such as RSV that suppress TH17 differentiation are therapeutic targets for neurodegenerative disorders such as INCL.
INTRODUCTION
The blood-brain barrier (BBB) is a selective permeability barrier between the blood and the brain. It restricts the entry of plasma components, including red blood cells and leukocytes into the brain, which can damage neurons. In several conditions, such as ischemic injury, trauma, cerebral hemorrhage, neurodegeneration and neuroinflammation, the BBB may be compromised. Thus, a breach of the BBB allows immune cells to freely migrate to the brain damaging the neurons. Indeed, in chronic neurodegenerative diseases, disruption of the BBB is a frequent complication and the development of effective treatment remains challenging. The endothelial cells of the microvasculature and tight junction proteins between adjacent endothelial cells are the major constituents of the BBB (1). Previous work has shown that CD4+ T-helper 17 (TH17) lymphocytes (2) expressing retinoic acid receptor-related orphan receptor γt (RORγt) (3) mediate BBB disruption and neuroinflammation (4). However, it is unclear how these immune cells disrupt the BBB.
Lysosomal storage disorders (LSDs) affect 1 in 5000 live-born infants and neurodegeneration is a devastating manifestation in the majority of the more than 50 LSDs (5). Neuronal Ceroid lipofuscinoses (NCLs) commonly known as Batten disease represent a group of common (1 in 12500 births) neurodegenerative LSDs that mostly affect children (6,7). Mutations in at least eight different genes cause various types of NCLs (8). The infantile form of NCL (INCL) (6,7) is a devastating childhood neurodegenerative storage disease caused by inactivating mutations in the palmitoyl-protein thioesterase-1 (PPT1) gene (9). The Ppt1-knockout (Ppt1-KO) mice (10) recapitulate virtually all clinical and pathological features of INCL (10,11), providing a reliable animal model of a neurodegenerative disease to explore the mechanism(s) of BBB disruption and to develop novel therapeutic strategies.
In this study, we sought to determine whether BBB is disrupted in Ppt1-KO mice and if so what might be the mechanism of BBB disruption in this animal model of INCL. Our results showed that BBB is disrupted in Ppt1-KO mice and IL-17A, produced by TH17 lymphocytes mediate BBB disruption by stimulating production of matrix metalloproteinases (MMPs), which degrade the tight junction proteins that are essential for maintaining BBB integrity. Most importantly, we found that dietary supplementation of resveratrol (RSV), an anti-oxidant and anti-inflammatory polyphenol, prevented disruption of the BBB by suppressing the differentiation of naïve CD4+ T cells to TH17 lymphocytes, which reduced production of IL-17-mediated production of MMPs. We propose that small molecules such as RSV that suppress TH17 differentiation are therapeutic targets for neurodegenerative disorders, such as INCL.
RESULTS
BBB integrity in Ppt1-KO mice evaluated by gadolinium-enhanced magnetic resonance imaging
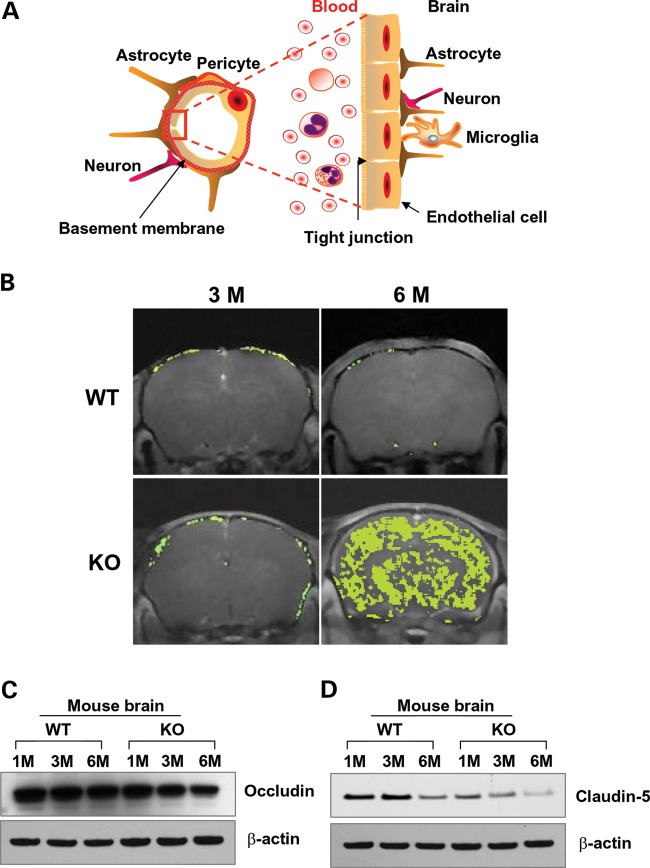
The endothelial cells lining the lumen of the cerebral microvasculature and the tight junction proteins between adjacent endothelial cells maintain the integrity of the BBB (Fig. 1A). To determine whether the BBB is disrupted in Ppt1-KO mice, we performed brain magnetic resonance imaging (MRI) of 3- and 6-month-old Ppt1-KO mice and their wild-type (WT) littermates following intravenous infusion of gadolinium (Gd), a contrast medium, which does not infiltrate the normal brain parenchyma due to an intact BBB (12). The results showed that there was no Gd infiltration in the brains of 3-month-old WT mice (Fig. 1B, upper left panel) as well as in those of their Ppt1-KO littermates (Fig. 1B, lower left panel). However, while the 6-month-old WT mice had no detectable Gd infiltration (Fig. 1B, upper right panel), there was a massive infiltration of Gd into the brain of their Ppt1-KO littermates (Fig. 1B, lower right panel). Taken together, these results clearly demonstrated that while the BBB was intact in WT mice and in their 3-month-old Ppt1-KO littermates, it was breached in 6-month-old Ppt1-KO mice. Remarkably, coinciding with BBB disruption, the 6-month-old Ppt1-KO mice also manifested signs of neurological deterioration (10).
Figure 1.
Evaluation of the BBB integrity in Ppt1-KO mice by MRI. (A) Schematic of the BBB, which is a selective barrier between the blood and the brain. It is formed primarily by endothelial cells lining the cerebral microvasculature and the tight junction proteins between adjacent endothelial cells that play critical roles in maintaining the integrity of the BBB. (B) The status of the BBB in WT and Ppt1-KO mice at 3 and 6 months of age was evaluated by MRI with Gadolinium (Gd) infusion. The WT mice aged 3 and 6 months did not show any evidence of Gd infiltration (upper panels). While Gd infiltration was not detectable in the brain of the 3-month-old Ppt1-KO mouse (lower left panel), high level of infiltration was readily detectable in the brain of 6-month-old Ppt1-KO littermate (lower right panel). Western blot analyses of lysates of brain tissues (total protein loaded per lane is 20 µg) showed a markedly lower level of occludin (C) and claudin-5 (D) in the Ppt1-KO mouse brain.
Reduced levels of tight junction proteins in the brain of Ppt1-KO mice
The tight junctions between adjacent endothelial cells provide a metabolic and physical barrier, which selectively allows movement of macromolecules between the blood and the brain (13,14) in order to maintain homeostasis. The tight junction proteins including occludin, claudin and junctional adhesion molecule-1 (JAM1) play critical roles in maintaining the BBB integrity, and degradation of the tight junction allows free movement of molecules and immune cells from the blood to the brain and vice versa. In addition, abnormal angiogenesis, regression of blood vessels, hypoperfusion of the brain and neuroinflammation may contribute to a ‘vicious circle’ that eventually leads to synaptic as well as neuronal dysfunction and progression of neurodegeneration. We previously reported that Ppt1-KO mice suffer from neuroinflammation, which may contribute to BBB disruption. Thus, we determined the levels of tight junction proteins, occludin and claudin-5, in cortical tissues from 1-, 3- and 6-month-old Ppt1-KO mice and those of their WT littermates by western blot analysis. The results showed that the levels of occludin (Fig. 1C) as well as claudin-5 (Fig. 1D) declined with increasing age of the Ppt1-KO mice. Consistent with these findings, the levels of other tight junction proteins such as JAM1 and claudin-1 were also diminished in the brain of Ppt1-KO mice (Supplementary Material, Fig. S1). These findings strongly suggested that BBB integrity in Ppt1-KO mice is breached, at least in part, due to reduced levels of tight junction proteins.
Elevated levels of TH17 lymphocytes and IL-17A in Ppt1-KO mice
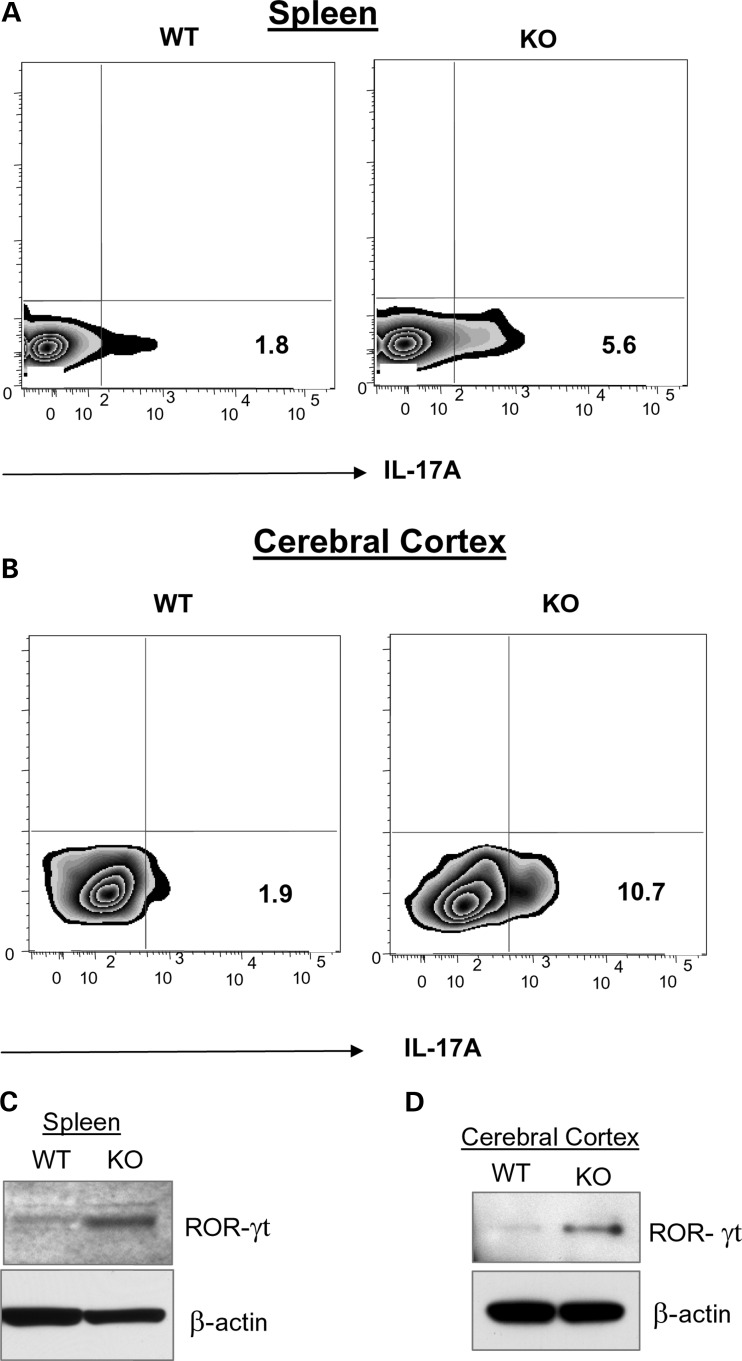
While TH17 lymphocytes have been implicated in mediating BBB disruption and neuroinflammation (4), the molecular mechanism(s), until now, remain unclear. To understand how TH17 lymphocytes may mediate BBB disruption, we sought to determine whether the levels of these immune cells in lymphoid organs and in the brains of Ppt1-KO mice are elevated compared with those of their WT littermates. Accordingly, we performed fluorescence-activated cell sorting (FACS) analysis of IL-17A-positive TH17 lymphocytes in the spleen and in the brain of WT mice and in those of their Ppt1-KO littermates. The results showed that compared with the levels of IL-17A-positive cells in the spleens of WT mice (Fig. 2A, left panel), those in the spleens of Ppt1-KO littermates (Fig. 2A, right panel) were markedly elevated. Similarly, compared with the levels of IL-17A-positive cells in the brains of WT mice (Fig. 2B, left panel), those in the brains of their Ppt1-KO littermates (Fig. 2B, right panel) were also significantly elevated. Since development and function IL-17A-producing TH17 lymphocytes (3) depend on RORγt (reviewed in 15), we first determined the levels of RORγt protein in the spleen and brain tissues from perfused Ppt1-KO mice and those of their WT littermates by western blot analysis. The results showed that compared with the levels of RORγt protein in the spleens of WT mice (Fig. 2C, left lane), those of their Ppt1-KO littermates (Fig. 2C, right lane) were markedly elevated. Similarly, compared with the levels of RORγt protein in the cerebral cortex of WT mice (Fig. 2D, left lane), those of the Ppt1-KO littermates (Fig. 2D, right lane) were also appreciably higher. We then determined the mRNA levels of IL-17A and RORγt in the spleens of WT mice and in that of their Ppt1-KO littermates. The results showed that mRNA levels of both IL-17A (Supplementary Material, Fig. S2A) and RORγt (Supplementary Material, Fig. S2B) were higher in the spleens of Ppt1-KO mice. Moreover, compared with the levels of RORγt-mRNA and RORγt-positive cells in the brain of WT mice, those in the brains of Ppt1-KO littermates were significantly higher (Supplementary Material, Fig. S3A and B).
Figure 2.
Increased TH17 cells in Ppt1-KO spleen and brain. FACS analysis of IL-17A-positive lymphocytes in the spleen (A) and brain (B) of WT and Ppt1-KO mice. Cells isolated from the spleen and brain of WT and Ppt1-KO mice were stimulated with the leukocyte activation cocktail for 6h, fixed, permeabilized and incubated with Fc block to minimize non-specific binding of antibodies followed by staining with PE-anti-IL-17A antibodies before analysis. Quadrants were drawn based on the staining with isotype controls (data not shown) and the percentages of cells staining positive are displayed. Data shown are the mean of two representative mice each of three experiments. Markedly elevated level of the RORγt protein was detected in Ppt1-KO mouse spleen (C) and brain (D) by western blot analysis. Total protein loaded per lane is 20 µg.
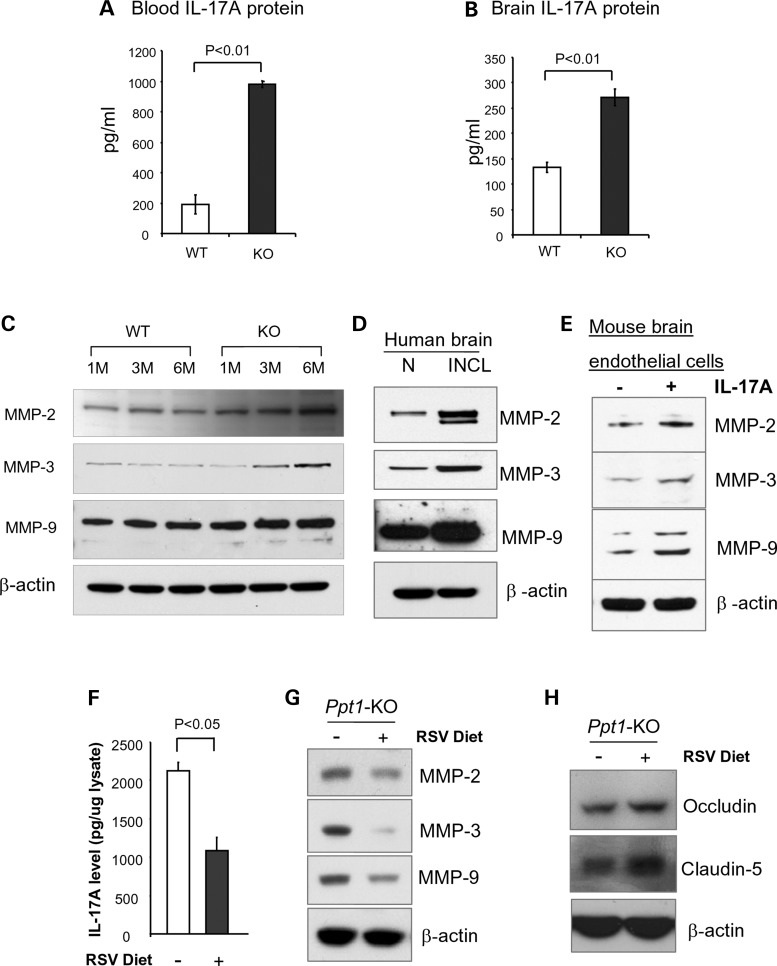
Since IL-17A is produced by TH17 lymphocytes (1,16,17), we determined IL-17A levels in the blood and in the brains of WT mice and those of their Ppt1-KO littermates. The results showed that compared with the levels of IL-17A in the blood of WT mice (Fig. 3A, left bar), those of the Ppt1-KO littermates (Fig. 3A, right bar) were significantly higher. Consistent with these results, the levels of IL-17A in brain tissues of Ppt1-KO mice (Fig. 3B, right bar) were markedly higher than those of the WT littermates (Fig. 3B, left bar). In addition, increased levels of IL-17 receptor (IL-17R) mRNA (Supplementary Material, Fig. S4A) and protein (Supplementary Material, Fig. S4B) were detected in brain tissues of Ppt1-KO mice when compared with those of their WT littermates. Consistent with these findings, immunohistochemical analyses of brain tissues from WT mice and those of their Ppt1-KO littermates showed elevated levels of IL-17R over the cerebral microvasculature of Ppt1-KO mice (Supplementary Material, Fig. S4C). Taken together, these results confirmed that the levels of TH17 lymphocytes in the spleen and in the cerebral cortex as well as IL-17A levels in the same organs of Ppt1-KO mice are significantly higher compared with those of their WT littermates.
Figure 3.
RSV diet suppresses IL-17A and MMPs and increases tight junction proteins. Increased levels of the IL-17A protein in the blood (A) and in the brain (B) of Ppt-1 KO mice. IL-17A was determined by enzyme linked immunosorbent assay (ELISA) and expressed as pg/ml of brain lysates, which contained 0.5 mg/ml of total protein. Elevated levels of MMP-2, MMP-3 and MMP-9 were detected by western blot analysis using lysates of brain tissues from Ppt1-KO mice and those of their WT littermates (C). Lysates of postmortem brain tissues from an INCL patient and those of an age-matched normal subject were analyzed by western blot (D). Markedly elevated levels of MMP-2, MMP-3 and MMP-9 in murine brain endothelial cells treated with IL-17A were detected (E). Ppt1-KO mice were fed with either control or RSV diet. The concentration of RSV in the diet was 2 mg of RSV/1 g of the diet, which provided a dose of 600 mg RSV/day/kg body weight of a mouse. The levels of IL-17A, MMP-2, MMP-3 and MMP-9 as well as the tight junction proteins (occludin and claudin-5) were determined. While the levels of IL-17A (F) and MMPs (G) were reduced in Ppt1-KO mice on RSV diet, those of the tight junction proteins (H) were appreciably increased.
MMPs in the brain of Ppt1-KO mice and in that of postmortem INCL patient
How might an increase in TH17 lymphocytes mediate BBB disruption? One possibility is that IL-17A produced by TH17 lymphocytes (3) stimulates or activates proteases that catalyze the degradation of tight junction proteins, essential for maintaining the integrity of the BBB. MMPs have been reported to have multiple roles in a variety of physiological as well as pathological conditions (18), including neurodegenerative (19) and neuroinflammatory diseases (20). Moreover, MMPs have been reported to degrade tight junction proteins such as occludin (21). Therefore, we sought to test the hypothesis that IL-17A produced by TH17 lymphocytes promotes the expression of MMPs, which are reported to play critical roles in neurodegenerative and neuroinflammatory diseases (19–21). Accordingly, we performed western blot analysis of brain tissue lysates from Ppt1-KO mice and those of their WT littermates using antibodies against MMP-2, MMP-3 and MMP-9. The results showed that protein levels of all three MMPs were elevated in the brain of Ppt1-KO mice in an age-dependent manner (Fig. 3C). We also determined the MMP-protein levels in postmortem brain tissues from an INCL patient and those of an age-matched normal individual by western blot analysis. The results showed that MMP-2-, MMP-3- and MMP-9-protein levels are markedly elevated in the brain tissues of the INCL patient (Fig. 3D), although whether increased levels of MMPs in brain tissues of Ppt1-KO mice and in those of the INCL patient were mediated by IL-17A still remained unclear.
IL-17A stimulates production of MMPs in cultured brain endothelial cells
To determine whether IL-17A stimulated the expression of the MMPs, we performed in vitro experiments in which cultured murine brain endothelial cells were treated with IL-17A and determined the levels of MMPs by western blot analysis. The untreated endothelial cells served as controls. Our results showed that the levels of MMP-2, MMP-3 and MMP-9 were all elevated in IL-17A-treated cultured murine brain endothelial cells (Fig. 3E). Since the astrocyte population in the brain of Ppt1-KO mice increase with age contributing to neuroinflammation (22), we also sought to determine whether these cells express IL-17A receptor (IL-17R) and are responsive to IL-17A treatment increasing the levels of MMPs. Accordingly, we determined the levels of IL-17R and MMP-2, MMP-3 and MMP-9 in untreated and IL-17A-treated cultured astrocytes from Ppt1-KO mice. The results showed that the cultured astrocytes from Ppt1-KO mice expressed IL-17R, which was further augmented by IL-17A treatment (Supplementary Material, Fig. S5A). Moreover, treatment of these cells with IL-17A also stimulated the production of MMP-2, MMP-3 and MMP-9 (Supplementary Material, Fig. S5B). Although these results strongly suggest that TH17 lymphocytes mediate the disruption of the BBB via IL-17A-stimulated production of MMPs by brain endothelial cells and astrocytes, we cannot rule out the possibility that other pro-inflammatory mediators causing neuroinflammation (23,24) do not also contribute to the BBB disruption in these mice.
RSV protects the BBB in Ppt1-KO mice
Previous reports have indicated that oxidative stress is prevalent in cultured cells as well as postmortem brain tissues from INCL patients (25). MoreoverPpt1-KO mice (10) reliably recapitulate INCL phenotype (11) and manifest increased oxidative stress (25–27) as well as neuroinflammation (24). Furthermore, it has been reported that both oxidative stress (28) and neuroinflammation (22) contribute to BBB disruption (29). Additionally, we have reported that in Ppt1-KO mice, disruption of adaptive energy metabolism contributed to neurodegeneration and dietary supplementation of RSV ameliorated this abnormality (27). Recent reports also indicate that RSV improves mitochondrial function (30), which is also abnormal in Ppt1-KO mice (27). Thus, we sought to determine whether compounds such as RSV with anti-oxidant and anti-inflammatory properties may protect the BBB. Accordingly, we fed Ppt1-KO mice with either control diet or RSV diet and determined the levels of IL-17A, MMPs and tight junction proteins. Our results showed that compared with Ppt1-KO mice on control diet, their Ppt1-KO littermates on RSV diet showed markedly decreased levels of IL-17A (Fig. 3F) as well as MMP-2, MMP-3 and MMP-9 (Fig. 3G) and elevated the levels of occludin as well as claudin-5 (Fig. 3H). Taken together, these results strongly suggested that RSV treatment has protective effects on the BBB of Ppt1-KO mice.
RSV suppresses differentiation of TH17 cells from naïve CD4+ T cells
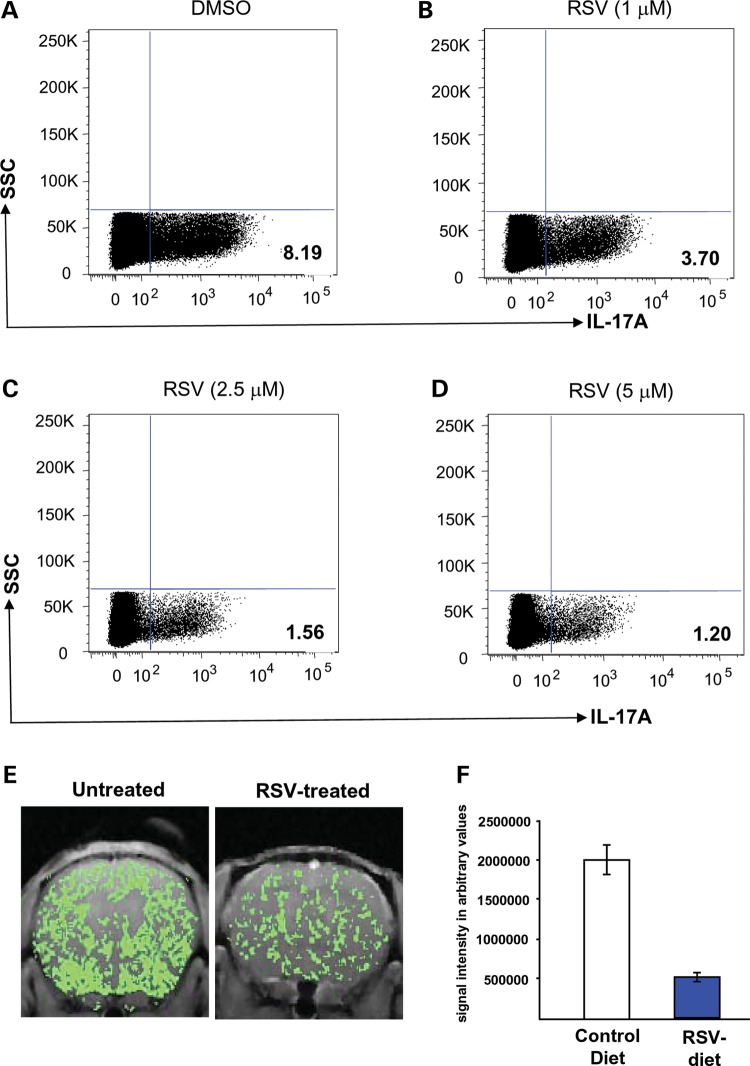
How might RSV mediate improvement of the BBB in the Ppt1-KO mice? Recent reports indicate that oxidative stress has deleterious effects, which enhances permeability of the BBB (31). Moreover, disruption of the BBB and oxidative stress has been reported to be involved in the pathogenesis of inflammatory brain disease (32). Further, TH17 lymphocytes have been suggested to disrupt the BBB and cause neuroinflammation (4). We have previously reported that Ppt1-KO mice, which develop neuroinflammation and manifest high levels of oxidative stress, when treated with RSV had improvement of these pathological conditions (27). Thus, we rationalized that due to its anti-oxidative and anti-inflammatory properties, RSV may also suppress the differentiation of TH17 cells from naïve CD4+ T cells and consequently, reduce the levels of pro-inflammatory IL-17A levels in Ppt1-KO mice. We therefore evaluated the effect of RSV on the differentiation of TH17 phenotype from naïve (CD4+CD25−CD62LhiCD44lo) T cells. We isolated and cultured these naïve CD4+ T cells from the spleens of Ppt1-KO mice under TH17 polarizing conditions and analyzed for IL-17A expression on day 5 of culture. The results showed that compared with the dimethylsulfoxide (DMSO)-treated controls, the treatment of naïve (CD4+CD25−CD62LhiCD44lo) T cells with varying doses of RSV significantly inhibited TH17 differentiation (Fig. 4A–D). Moreover, compared with the level of TH17 cells in the spleen of WT mice (Fig. 2A), RSV treatment in a dose-dependent manner normalized the number of these cells in the spleen of Ppt1-KO mice (Fig. 4B–D). Finally, we maintained Ppt1-KO mice (n = 6) on RSV diet for 2 months starting at 4 months of age before evaluation of the BBB status by Gd-enhanced MRI. The Ppt1-KO mice (n = 6) on diet without RSV served as controls. The results showed that compared with the Ppt1-KO mice on diet without RSV (Fig. 4E), the Ppt1-KO mice on RSV diet showed significantly improved BBB status (Fig. 4F). Taken together, these results showed that RSV protects the BBB in the Ppt1-KO mice most likely through its anti-oxidative and anti-inflammatory properties as well as by its heretofore unknown property as suppressor of TH17 differentiation from naïve CD4+ T cells.
Figure 4.
RSV suppresses TH17 differentiation and protects BBB integrity in Ppt1-KO mice. To determine how RSV might protect the BBB, we investigated the effect of RSV on the differentiation of naïve CD4+T cells from the spleen of Ppt1-KO mice to IL-17A-positive TH17 cells in vitro. Flow cytometry of intracellular staining of IL-17A in sorted naïve T cells polarized under TH17-polarizing conditions described under Materials and Methods. Dimethylsulfoxide (DMSO) or RSV was added on day 0 and day 3 of culture at indicated concentrations. On day 5, the cells were stimulated with the leukocyte activation cocktail for 6h, fixed, permeabilized and incubated with PE-anti-IL-17A antibody before FACS analysis. Quadrants were drawn based on the staining with isotype controls (data not shown) and displayed the percentages of cells staining positive for IL-17A. Intriguingly, RSV suppressed the differentiation of cultured naïve CD4+ T cells to IL-17A-positive TH17 cells (A–D). Representative FACS plots from one of two independent experiments are shown in this panel. (E) Status of BBB in untreated and RSV-treated 6-month-old Ppt1-KO mice was evaluated by gadolinium-enhanced brain MRI. The results revealed a markedly lower level of infiltration of gadolinium (green stain) in the brain of RSV-treated Ppt-1 KO mouse (right panel) when compared with untreated Ppt1-KO mouse brain (left panel). (F) Graphical presentation of intensity of gadolinium infiltration in the brain of mice fed with normal or RSV diet. Data presented as mean ± SD (n = 3).
DISCUSSION
In this study, we have determined that BBB is disrupted in the Ppt1-KO mice, a reliable animal model of INCL. Moreover, we show that elevated levels of RORγt-positive TH17 cells are present in the brain as well as in the spleen of these mice. We further demonstrate that IL-17A, produced by TH17 cells, stimulates the production of MMPs, which are reported to degrade tight junction proteins, which are essential for maintaining the integrity of the BBB. Importantly, we found that Ppt1-KO mice put on a diet containing RSV, a polyphenol present in most plants including red grapes that has anti-oxidant and anti-inflammatory properties, ameliorate the abnormalities that can disrupt the BBB. Most unexpectedly, RSV was found to suppress TH17 differentiation, which at least in part, one of its effects that may protect the BBB.
We previously reported that the Ppt1-KO mice suffer from endoplasmic reticulum (33,34) as well as oxidative stress (25–27), which contribute to neuropathology in these mice. We also reported that the deficiency of Ppt1 in these mice most likely impairs dynamic palmitoylation of proteins that are critical for synaptic vesicle recycling and regeneration (35), causing progressive decline in SV pool at the neuronal synapses (35,36). The results of our present experiments indicate that oxidative stress caused by the deficiency of PPT1 may contribute to many abnormalities, including disruption of the BBB. This assumption may be supported by the fact that RSV appears to ameliorate this abnormality at least in part due to its anti-oxidant property.
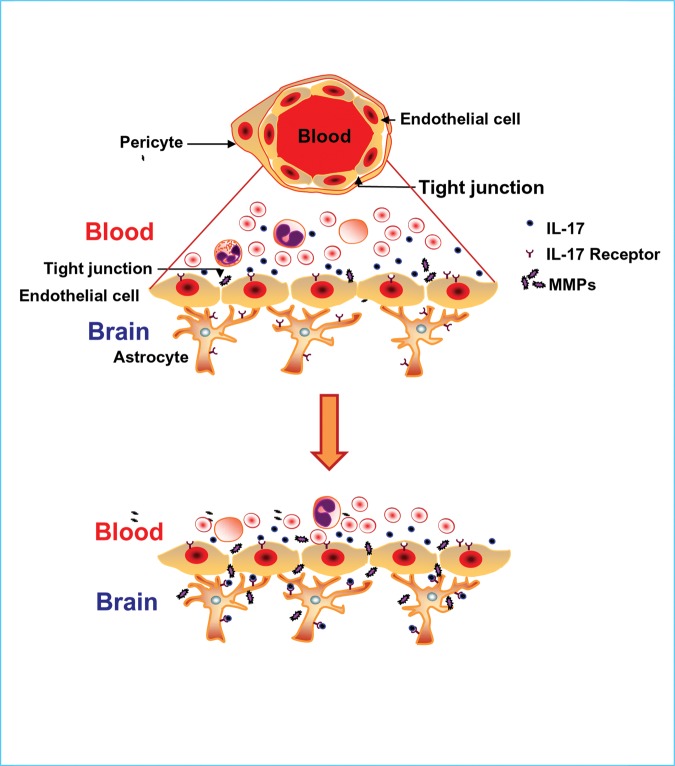
The disruption of the BBB is a serious complication in numerous neurodegenerative diseases, although to our knowledge this complication in INCL has not been reported previously. It allows blood components including immune cells to freely enter the brain. Some of these components may cause neuronal death facilitating rapid progression of the disease. A clear understanding of the mechanism(s) by which the BBB is disrupted in a rare neurodegenerative disease such as INCL may have implications for other more common neurodegenerative disorders, such as Alzheimer's and Parkinson's diseases. Such understanding may facilitate not only the development of effective therapies to protect the BBB but also the delivery of potentially therapeutic and diagnostic compounds to specific areas of the brain (37). On the basis of our findings in this study, we present a model (Fig. 5) to explain a possible mechanism of BBB disruption in neurodegenerative disorders, including INCL. In this model, we propose that increased IL-17A levels in the blood of Ppt1-KO mice stimulate the endothelial cells of the brain microvasculature (constituent of the BBB) expressing IL-17R to produce high levels of MMPs. The MMPs then mediate degradation of the tight junction proteins that are essential for maintaining the BBB integrity. Following this event, the blood components including IL-17A enter the brain and stimulate the astrocytes that also express IL-17R to produce more MMPs, which in turn further degrade the tight junction proteins disrupting the BBB. Despite the fact that disruption of the BBB is a serious complication frequently encountered in chronic neurodegenerative and neuroinflammatory diseases, the development of effective treatment remains challenging. Our findings define a mechanism by which TH17 lymphocytes via induction of MMPs mediate BBB disruption and provide the proof of principle that small molecules such as RSV, which reduce oxidative stress and suppress TH17 lymphocyte differentiation, may have therapeutic potential for protecting the BBB in neurodegenerative disorders, including INCL.
Figure 5.
A model explaining how TH17 lymphocytes may mediate BBB disruption. Under normal physiological conditions, the tight junction proteins between adjacent endothelial cells of the cerebral microvasculature maintain integrity of the selectively permeable barrier between the blood and the brain. This barrier prevents migration of blood components, including immune cells from freely entering the brain (upper panel). However, under pathological conditions such as the one exists in Ppt1-KO mice, increased IL-17A levels in the blood coming in contact with BBB endothelial cells, which express IL-17R, stimulate the production of MMPs. These MMPs then hydrolyze the tight junction proteins and the BBB becomes breached. This opening of the BBB allows immune cells, including TH17 lymphocytes expressing IL-17A to enter the brain where the astrocytes, which also express IL-17R, respond by increasing MMP levels further creating vicious cycle that not only damages the BBB but also viable neurons aggravating the neurodegenerative process.
MATERIALS AND METHODS
Animals
Ppt1-KO mice (a generous gift from Dr S.L. Hofmann, University of Texas Southwestern Medical Center, Dallas, TX, USA) were generated and characterized as previously described (10). These mice (generated using ES cells from 129 mouse strain) were backcrossed 10 generations with C57BL/6 strain in the laboratory of Dr M. S. Sands (Washington University School of Medicine, St Louis, MO,USA) until congenic C57BL/6 genetic background was reached. We are grateful to Dr Sands for providing a mating pair, which started our mouse colony. Herterozygous mice (Ppt1+/−) were mated and the progeny were genotyped. All mice were maintained and housed in a germ-free facility and animal procedures were carried out in accordance with institutional guidelines after approval of an animal study protocol by the NICHD Animal Care and Use Committee. The mice were fed standard normal NIH-31 diet (Zeigler Brothers, Gardner, PA, USA) or NIH-31 diet containing resveratrol (RSV diet) as previously described (27,30). Briefly, 4 ml of RSV stock solution (50 mg RSV/ml of ethanol) was sprayed onto 100 g of pulverized diet. The final concentration of RSV in the diet was 2 mg of RSV/gram of diet, which provided a dose of 600 mg of RSV/day/kg body weight.
Postmortem brain tissues from INCL patient and normal subject
A clinical protocol (#01-CH-0086) was approved by the institutional review board. Postmortem brain tissue samples were obtained from the Human Brain and Spinal Fluid Resource Center, VA West Los Angeles Healthcare Center, Los Angeles, CA 90073, USA, which is sponsored by NINDS/NIMH, National Multiple Sciences Society and Department of Veterans Affairs. We also obtained control autopsy brain tissue samples from the Brain and Tissue Bank for Developmental Disorders, University of Maryland, Baltimore, MD 21201, USA.
Western blot analysis and cytokine ELISA
Western blot analyses of proteins from mouse brain tissues were performed as previously described (25). The primary antibodies used are: anti-β-actin (1:5000, US Biological), anti-Claudin-1 (1:1000, Cell signaling Technology), anti-Claudin-5 (1:500, Invitrogen), anti-IL17R (1:500, Santacruz Biotech), anti-JAM-1(1:1000, Abcam), anti-MMP2 (1:1000, Abcam), anti-MMP3 (1:1000, Abcam), anti-MMP9 (1:1000, Cell Signaling Technology), anti-Occludin (1:500, Invitrogen) and anti-RORγτ (1:500, Abcam). The secondary antibodies used are: goat anti-rabbit IgG, donkey anti-goat IgG and goat anti-mouse IgG (Santa Cruz Biotechnology). Chemiluminescent detection was performed by using Supersignal west pico luminol/enhancer solution (Thermo scientific) according to the manufacturer's protocol. Amount of proteins from brain lysates and sera from WT mice as well as those of their Ppt1-KO littermates were adjusted to a concentration of 0.5 mg/ml and used for enzyme linked immunosorbent assay (ELISA) (Assaygate Inc.) for quantitating the IL-17A protein.
IL-17A treatment of cultured mouse brain endothelial cells
Immortalized mouse brain endothelial cells (bEnd.3) (American Type Culture Collection) were cultured in Dulbecco's minimal essential medium supplemented with 10% FBS at 37°C under humidified atmosphere containing 5% CO2. Day before treatment with IL-17A, cells were trypsinized and seeded in six-well plate at a density of 5 × 105 cells/well. Next day, cells were treated with or without 100 ng/ml of recombinant IL-17A protein (Biolegend, USA) for 18h. Protein samples were prepared from the treated as well as control untreated endothelial cells and were used for western blot analysis.
Evaluation of BBB status by gadolinium (Gd)-enhanced MRI
Age- and sex-matched Ppt1-KO mice and their WT littermates (n = 5), at age 3 and 6 months were investigated for the status of BBB Ppt1-KO mice. Mice were anesthetized with 1.5% isofluorane and a tail vein catheter was placed, for administering the contrast agent, Gd-DTPA (Bayer Pharmaceuticals Inc., NJ, USA) and positioned in a steriotaxic holder. The body core temperature was maintained at 37°C using a circulating water pad. MRI was performed on a horizontal 7T Bruker (Bruker Biospin Inc., Bellerica, MA, USA) Avance scanner with the brain centered in a 72/25 mm transmit/receive coil ensemble. T1-weighted axial images (matrix 256 × 256, number of averages = 8, echo train length = 8, repetition time (TR) = 300 ms and echo time (TE) = 6 ms, field of view = 1.92 cm, slice thickness = 1 mm), specified via a tri-axial pilot scan to encompass the whole brain, were acquired using a gradient echo pulse sequence. Post-contrast images, with identical parameters, were acquired using the same gradient echo sequence 5min after infusion of Gd DTPA (0.2 cm3/kg body weight). The images accentuating the relative contrast enhancement were evaluated using MATLAB (Mathworks Inc., Natick, MA, USA) software.
Cell purification and flow cytometry
Mice were anesthetized and perfused with cold phosphate buffered saline (PBS) to remove leukocytes from the microvasculature of the brain (constituent of the BBB). Brain tissues were manually minced in Hank's balanced salt solution (HBSS) (Mediatech Inc.), supplemented with 5% FBS (HyClone) and filtered through a 70 µm nylon mesh cell strainer using a rubber policeman. The resulting slurry was digested for 30 min at 37°C in HBSS supplemented with 2 mg/ml collagenase type I (Sigma-Aldrich) and 5000 U/ml DNase I (Invitrogen) to obtain a single-cell suspension. Cells were resuspended in 37% percoll and gently laid over 70% percoll in 15 ml tubes. Cells were centrifuged at 2400 rpm for 20 min in a swinging bucket rotor with centrifuge breaks turned off. After centrifugation, myelin debris was carefully aspirated and the ring containing mononuclear cells at the interface was collected, and washed extensively in HBSS. For analyzing IL-17A and transcription factor RORγt, cells were stimulated with Leukocyte Activation Cocktail, with GolgiPlus™ (BD Pharmingen) for 6 h at 37°C. Subsequently, cells were fixed, and permeabilized using Cytofix/CytoPerm Plus kit (BD Pharmingen) according to the manufacturer's protocol, and incubated with Fc block (BD Biosciences) to minimize non-specific antibodies binding followed by staining with PE-anti-IL-17A or PE-anti-RORγt (eBioscience). For isolation of splenocytes, spleen was harvested and perfused with PBS and cells were collected after centrifugation. RBCs were lysed with ACK lysis buffer at room temperature. Single-cell splenocyte suspensions were incubated with Fc block (BD Biosciences) to minimize non-specific antibodies binding followed by staining with FITC-anti-CD4 and Pacific blue-anti-CD3 for 30 min at room temperature. For analyzing IL-17A-producing cells from the spleen, cells were activated, fixed, permeabilized and stained with PE-anti-IL-17A antibody as stated above. Cells were analyzed on a FACSCalibur cytometer with CellQuest (BD Biosciences), and data were analyzed using FlowJO (Tree Star). Control-included cells stained with directly conjugated isotype control Abs to assess the degree of non-specific staining.
Naïve CD4+ T cell isolation and in vitro polarization
Naïve CD4+ T cells were isolated from mouse spleen using CD4+ T cell isolation kit (Miltenyi Biotec) according to the manufacturer's protocol. Cells were stained with APCcy7-anti-CD4, PE-anti-CD25, FITC-anti-CD62L (BD Pharmingen) and eFluor 605NC-anti-CD44 (eBioscience). Cell sorting was performed with a FacsAria cell sorter (Becton Dickinson) to obtain a naïve cell population of CD4+CD25−CD62LhiCD44lo T cells. Naïve T cells (0.5 × 106 cells/well) were cultured in 48-well plate at 37°C and 5% CO2 in T cell media: RPMI-1640 (Invitrogen) supplemented with 10% (vol/vol) heat-inactivated FCS, 1% l-glutamine, 1% penicillin/streptomycin and 50 μm 2-mercaptoethenol. Cells were stimulated with anti-CD3/CD28 coated Dynabeads (Invitrogen) at a bead-to-cell ratio of 1:1. To polarize naïve cells to TH17 phenotype, cells were cultured for 5 days in IL-6 (20 ng/ml), IL-1β (10 ng/ml), TNF-α (10 ng/ml), IL-23 (20 ng/ml), TGF-β 1.2 (5 ng/ml), anti-IL-4 (0.4 μg/ml), anti-IL-12 (2 μg/ml) and anti-IFN-γ (8 μg/ml). To study the effect of RSV on TH17 differentiation, DMSO (control) or varying concentrations of RSV (1, 2.5 and 5 μm) were added to the culture media at day 0 and subsequently on alternate days. For IL-17A analysis, the cells were stimulated with Leukocyte Activation Cocktail, with GolgiPlus™ for 6 h at 37°C. Intracellular staining was performed according to manufacturer's protocol using Cytofix/CytoPerm Plus kit (BD Pharmingen) with PE-anti-IL-17A (eBioscience). An LSR II (BD Bioscience) and FlowJo software were used for flow cytometry and analysis.
Statistical analysis
Statistical analyses of the data were performed by Student's t-test using Excel Office 2000 (Microsoft) and a P-value of <0.05 was considered statistically significant.
SUPPLEMENTARY MATERIAL
AUTHOR CONTRIBUTIONS
A.S. and C.S. designed majority of the experiments. C.S. and S.P.S. designed and executed the FACS analyses for RORγt and TH17 lymphocytes. A.S., C.S. and S.P.S. analyzed the data. J.M., G.C. and Z.Z. executed the MRI experiments and analyzed the results. S.P., G.C. and E.K. performed the in vivo RSV experiments and analyzed the results. A.B.M. conceived the project, provided guidance for experimental design and analysis of data as well as wrote the manuscript with input from the co-authors.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr S.L. Hofmann for the generous gift of the Ppt1-KO mice (10) and Dr Mark Sands for providing a mating pair of congenic C57BL/6 Ppt1-KO mice, which were used to establish our Ppt1-KO mouse colony. We thank Dr J.M. Farber, Laboratory of Molecular Immunology, NIAID, NIH, for valuable discussions on the design of TH17-related experiments, helpful suggestions and critical review of the manuscript. We are also grateful to Drs S.W. Levin, I. Owens and J.Y. Chou for critical review of the manuscript as well as helpful suggestions and A.M. Heffer for her help in conducting the MRI experiments.
Conflict of Interest statement. None declared.
FUNDING
This research was supported in part by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development [NICHD], and by Intramural Research Program of the National Institute of Allergy and Infectious Diseases (NIAID), NIH.
References
- 1.Zlokovic B.V. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Wilson N.J., Boniface K., Chan J.R., McKenzie B.S., Blumenschein W.M., Mattson J.D., Basham B., Smith K., Chen T., Morel F., et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat. Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 3.Ivanov I.I., McKenzie B.S., Zhou L., Tadokoro C.E., Lepelley A., Lafaille J.J., Cua D.J., Littman D.R. The orphan nuclear receptor RORgamma directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 4.Kebir H., Kreymborg K., Ifergan I., Dodelet-Devillers A., Cayrol R., Bernard M., Giuliani F., Arbour N., Becher B., Prat A. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13:1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeyakumar M., Dwek R.A., Butters T.D., Platt F.M. Storage solutions: treating lysosomal disorders of the brain. Nat Rev Neurosci. 2005;6:713–725. doi: 10.1038/nrn1725. [DOI] [PubMed] [Google Scholar]
- 6.Goebel H.H., Wisniewski K.E. Current state of clinical and morphological features in human NCL. Brain Pathol. 2004;14:61–69. doi: 10.1111/j.1750-3639.2004.tb00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper J.D., Russell C., Mitchison H.M. Progress towards understanding disease mechanisms in small vertebrate models of neuronal ceroid lipofuscinoses. Biochim Biophys Acta. 2006;1762:873–889. doi: 10.1016/j.bbadis.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Jalanko A., Braulke T. Neuronal ceroid lipofuscinoses. Biochim Biophys Acta. 2009;1793:697–709. doi: 10.1016/j.bbamcr.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Vesa J., Hellsten E., Verkruyse L.A., Camp L.A., Rapola J., Santavuori P., Hofmann S.L., Peltonen L. Mutations in the palmitoyl protein thioesterase gene causing infantile neuronal ceroid lipofuscinosis. Nature. 1995;376:584–587. doi: 10.1038/376584a0. [DOI] [PubMed] [Google Scholar]
- 10.Gupta P., Soyombo A.A., Atashband A., Wisniewski K.E., Shelton J.M., Richardson J.A., Hammer R.E., Hofmann SL. Disruption of PPT1 or PPT2 causes neuronal ceroid lipofuscinosis in knockout mice. Proc. Natl Acad. Sci. USA. 2001;98:13566–13571. doi: 10.1073/pnas.251485198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bible E., Gupta P., Hofmann S.L., Cooper J.D. Regional and cellular neuropathology in the palmitoyl protein thioesterase-1 null mutant mouse model of infantile neuronal ceroid lipofuscinosis. Neurobiol. Dis. 2004;16:346–359. doi: 10.1016/j.nbd.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 12.Grossman R.I., Braffman B.H., Brorson J.R., Goldberg H.I., Silberberg D.H., Gonzalez-Scarano F. Multiple sclerosis serial study of gadolinium-enhanced MR imaging. Radiology. 1988;169:117–122. doi: 10.1148/radiology.169.1.3420246. [DOI] [PubMed] [Google Scholar]
- 13.Tsukita S., Furuse M., Itoh M. Multifunctional strands in tight junctions. Nature Rev Mol Cell Biol. 2001;2:285–293. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- 14.Persidsky Y., Ramirez S.H., Haorah J., Kanmogne G.D. Blood-brain barrier: structural components and function under physiologic and pathologic conditions. J Neuroimmune Pharmacol. 2006;1:223–236. doi: 10.1007/s11481-006-9025-3. [DOI] [PubMed] [Google Scholar]
- 15.Iwakura Y., Ishigame H., Saijo S., Nakae S. Functional specialization of interleukin-17 family members. Immunity. 2011;34:149–162. doi: 10.1016/j.immuni.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 16.Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat. Rev. Immunol. 2008;8:335–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 17.Zheng Y., Josefowicz S.Z., Kas A., Chu T.T., Gavin M.A. Genome-wide analysis of Foxo3 target genes in developing and mature regulatory T cells. Nature. 2007;445:936–940. doi: 10.1038/nature05563. [DOI] [PubMed] [Google Scholar]
- 18.Kaczmarek L., Lapinska-Dzwonek J., Szymczak S. Matrix metalloproteinases in the adult brain physiology: a link between c-Fos, AO-1 and remodeling of neuronal connections? EMBO J. 2002;21:6643–6648. doi: 10.1093/emboj/cdf676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenberg G.A. Matrix metalloproteinases and their multiple roles in neurodegenerative diseases. Lancet Neurol. 2009;8 doi: 10.1016/S1474-4422(09)70016-X. [DOI] [PubMed] [Google Scholar]
- 20.Rosenberg G.A. Matrix metalloproteinases in neuroinflammation. Glia. 2002;39:279–291. doi: 10.1002/glia.10108. [DOI] [PubMed] [Google Scholar]
- 21.Lischper M., Beuck S., Thanabalasundaram G., Pieper C., Galla H.J. Metalloproteinase mediated occludin cleavage in the cerebral microcapillary endothelium under pathological conditions. Brain Res. 2010;1326:114–127. doi: 10.1016/j.brainres.2010.02.054. [DOI] [PubMed] [Google Scholar]
- 22.Man S., Ubogu E.E., Ransohoff R.M. Inflammatory cell migration into the central nervous system: a few new twists on an old tale. Brain Pathol. 2007;17:243–250. doi: 10.1111/j.1750-3639.2007.00067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Z, Lee YC, Kim SJ, Choi MS, Tsai PC, Saha A, Wei H, Xu Y, Xiao YJ, Zhang P, Heffer A, Mukherjee AB. Production of lysophosphatidylcholine by cPLA2 in the brain of mice lacking PPT1 is a signal for phagocyte infiltration. Hum Mol Genet. 2007;16:837–847. doi: 10.1093/hmg/ddm029. [DOI] [PubMed] [Google Scholar]
- 24.Saha A., Kim S.J., Zhang Z., Lee Y.C., Sarkar C., Tsai P.C., Mukherjee A.B. RAGE signaling contributes to neuroinflammation in infantile neuronal ceroid lipofuscinosis. FEBS Lett. 2008;582:3823–3831. doi: 10.1016/j.febslet.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wei H., Kim S.J., Zhang Z., Tsai P.C., Wisniewski K.E., Mukherjee A.B. ER and oxidative stresses are common mediators of apoptosis in both neurodegenerative and non-neurodegenerative lysosomal storage disorders and are alleviated by chemical chaperones. Hum Mol Genet. 2008;17:469–477. doi: 10.1093/hmg/ddm324. [DOI] [PubMed] [Google Scholar]
- 26.Kim S.J., Zhang Z., Lee Y.C., Mukherjee A.B. Palmitoyl-protein thioesterase-1 deficiency leads to the activation of caspase-9 and contributes to rapid neurodegeneration in INCL. Hum Mol Genet. 2006;15:1580–1586. doi: 10.1093/hmg/ddl078. [DOI] [PubMed] [Google Scholar]
- 27.Wei H., Zhang Z., Saha A., Peng S., Chandra G., Quezado Z., Mukherjee A.B. Disruption of adaptive energy metabolism and elevated ribosomal p-S6K1 levels contribute to INCL pathogenesis: partial rescue by resveratrol. Hum Mol Genet. 2011;20:1111–1121. doi: 10.1093/hmg/ddq555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chrissobolis S., Faraci F.M. The role of oxidative stress and NADPH oxidase in cerebrovascular disease. Trends Mol Med. 2008;14:495–502. doi: 10.1016/j.molmed.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lochhead J.J., McCaffrey G., Quigley C.E., Finch J., DeMarco K.M., Nametz N., Davis T.P. Oxidative stress increases blood-brain barrier permeability and induces alterations in occludin during hypoxia-reoxygenation. J Cereb Blood Flow Metab. 2010;30:1625–1636. doi: 10.1038/jcbfm.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lagouge M., Argmann C., Gerhart-Hines Z., Meziane H., Lerin C., Daussin F., Messadeq N., Milne J., Lambert P., Elliott P., et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 31.Lagrange P., Romero I.A., Minn A., Revest P.A. Transendothelial permeability changes induced by free radicals in an in vitro model of the blood-brain barrier. Free Radic. Biol.Med. 1999;27:667–672. doi: 10.1016/s0891-5849(99)00112-4. [DOI] [PubMed] [Google Scholar]
- 32.Schreibelt G., Musters R.J., Reijerkerk A., de Groot L.R., van der Pol S.M., Hendrikx E.M., Döpp E.D., Dijkstra C.D., Drukarch B., de Vries H.E. Lipoic acid affects cellular migration into the central nervous system and stabilizes blood-brain barrier integrity. J . Immunol. 2006;177:2630–2637. doi: 10.4049/jimmunol.177.4.2630. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Z., Lee Y.C., Kim S.J., Choi M.S., Tsai P.C., Xu Y., Xiao Y.J., Zhang P., Heffer A., Mukherjee A.B. Palmitoyl-protein thioesterase-1 deficiency mediates the activation of the unfolded protein response and neuronal apoptosis in INCL. Hum Mol Genet. 2006;15:337–346. doi: 10.1093/hmg/ddi451. [DOI] [PubMed] [Google Scholar]
- 34.Kim S.J., Zhang Z., Hitomi E., Lee Y.C., Mukherjee A.B. Endoplasmic reticulum stress-induced caspase-4 activation mediates apoptosis and neurodegeneration in INCL. Hum Mol Genet. 2006;15:1826–1834. doi: 10.1093/hmg/ddl105. [DOI] [PubMed] [Google Scholar]
- 35.Kim S.J., Zhang Z., Sarkar C., Tsai P.C., Lee Y.C., Dye L., Mukherjee A.B. Palmitoyl protein thioesterase-1 deficiency impairs synaptic vesicle recycling at nerve terminals contributing to neuropathology in human and mice. J. Clin. Invest. 2008;118:3075–3086. doi: 10.1172/JCI33482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Virmani T., Gupta P., Liu X., Kavalali E.T., Hofmann S.L. Progressively reduced synaptic vesicle pool size in cultured neurons derived from neuronal ceroid lipofuscinosis-1 knockout mice. Neurobiol. Dis. 2005;20:314–323. doi: 10.1016/j.nbd.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 37.Neuwelt E.A., Bauer B., Fahlke C., Fricker G., Iadecola C., Janigro D., Leybaert L., Molnár Z., O'Donnell M.E., Povlishock J.T., et al. Engaging neuroscience to advance translational research in brain barrier biology. Nat Rev Neurosci. 2011;12:169–182. doi: 10.1038/nrn2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.