Abstract
A computed tomography (CT) incidentaloma is an incidentally detected and previously unsuspected finding or abnormality that is not related to the indication for obtaining the CT examination. The aim of this article is to review the frequency of incidentalomas on chest CT scans, discuss the potential clinical significance of the findings, and suggest guidelines for reporting, further evaluation, and follow-up, with particular focus on thyroid lesions, enlarged mediastinal lymph nodes and lung nodules.
Keywords: Incidentaloma, incidental finding, chest CT, thyroid lesions, lung nodule, enlarged mediastinal lymph nodes
Introduction
A computed tomography (CT) incidentaloma is an incidentally detected and previously unsuspected finding or abnormality that is not related to the indication for obtaining the CT examination. Chest CT incidentalomas are pandemic in modern health care due to many factors, including the explosion in the use of chest CT scanning, particularly in the emergency department, in oncology centers, and in screening for coronary artery disease (CAD) and lung cancer. Multidetector CT results in huge numbers of thin CT sections with excellent spatial resolution, leading to the detection of many incidental findings.
Incidentalomas on chest CT scans may represent normal findings, normal variants, or abnormal findings. Abnormal findings may be of little or no clinical significance (e.g. a calcified lung granuloma), requiring no further evaluation, or they may be highly significant, requiring immediate treatment (e.g. a large, central pulmonary embolus). Commonly, however, incidentalomas are of questionable clinical significance (e.g. a thyroid nodule), necessitating further evaluation via clinical correlation, follow-up and/or additional testing.
How common are chest CT incidentalomas?
A systematic review of 11 chest CT studies performed for CAD and lung cancer screening revealed that the proportion of patients with at least one incidental imaging abnormality requiring follow-up varied from 3% to 41.5%; the wide variation was largely due to considerable differences in follow-up recommendations for incidental findings across studies[1]. Overall, 7.7% and 14.2% of participants in CAD or lung cancer screening studies, respectively, had further investigations of incidental findings[1]. Other individual CAD screening studies found major extracardiac incidentalomas in 5–16% of patients[2–4]. A lung cancer screening study involving 5200 participants discovered incidental findings in 3%, leading to 12 invasive procedures; however, only 8 of these were clinically relevant[5]. The NELSON lung cancer screening trial found that 129/1929 (7%) CT examinations revealed clinically relevant incidental findings, resulting in 118 additional imaging tests; furthermore, 8 of these showed new incidental findings[6]. Eleven patients underwent more than one additional imaging study, and ultimately, only 15 patients needed clinical treatment or further follow-up of their incidental findings. Only one incidental cancer was detected (pancreatic cancer), which was incurable due to the presence of metastatic disease. Based on these results, the authors advised against systematically searching for and reporting of incidental findings on lung cancer screening studies, as this practice may lead to additional costs, patient anxiety, and risk of iatrogenic injury[6].
Despite the conclusions of the authors of the NELSON study, most radiologists feel obligated to report incidental findings and give suggestions regarding the clinical significance and appropriate work-up of such findings. For some findings, there are well established clinical guidelines, whereas, for others, no specific guidelines exist. The following sections give suggested guidelines for reporting incidental findings, based on the best evidence that is currently available in the medical literature.
Incidental thyroid lesions
Thyroid abnormalities, such as nodules (solitary or multiple), cystic lesions, calcifications and diffuse glandular enlargement, are often detected on chest CT examinations (Fig. 1). Thyroid nodules are found in approximately 16% of chest CT scans, more commonly in women. Most incidentally found thyroid lesions are benign, with an approximately 9–11% prevalence of malignant lesions[7,8]. The differential diagnosis for an incidental thyroid abnormality in euthyroid individuals includes nontoxic multinodular goiter, follicular adenoma, thyroiditis, simple cyst, and carcinoma (primary or secondary). Clinical features that correlate with malignancy include: age (younger than 35 years or older than 70 years); size (>2.5 cm); presence of dysphagia or hoarseness; history of external neck radiation in childhood or adolescence; firm, irregular and/or fixed nodule at physical examination; presence of cervical lymph node enlargement; multiple endocrine neoplastic syndromes; personal or family history of thyroid malignancy; and possibly male gender[9].
Figure 1.
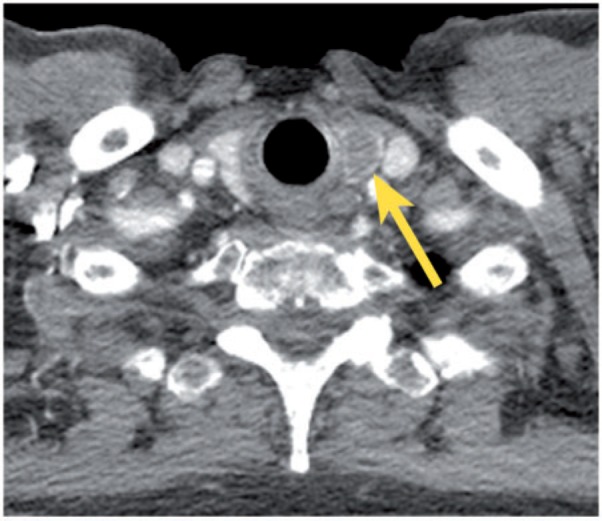
Incidental nodule in the left lobe of the thyroid gland (arrow).
No CT feature is reliable in distinguishing benign from malignant thyroid lesions. Furthermore, it has been reported that CT is unreliable in distinguishing simple cysts, complex cysts, and solid nodules[8]. CT underestimates the number of nodules relative to sonography, suggesting that sonography is a useful adjunctive test after the incidental detection of a thyroid abnormality on CT; furthermore, current management guidelines for thyroid nodules are based on morphologic sonographic characteristics[8,10]. Although sonography is sensitive in detecting thyroid nodules, it is not accurate in diagnosing malignancy; its major usefulness is in guiding thyroid biopsies.
Once an incidental thyroid nodule is found, referral to a thyroid specialist may be the most cost-effective option for further evaluation, although ultrasonography may also be suggested. According to the guidelines of the American Thyroid Association, generally, only nodules >1 cm need further evaluation, because they have a greater potential to represent clinically significant cancers compared with lesions <1 cm, and because of cost/benefit considerations regarding the work-up of all small nodules[11]. However, it is probably prudent to correlate even small nodules with physical examination and assessment of individual risk factors[12].
Incidental mediastinal lymph node enlargement in congestive heart failure
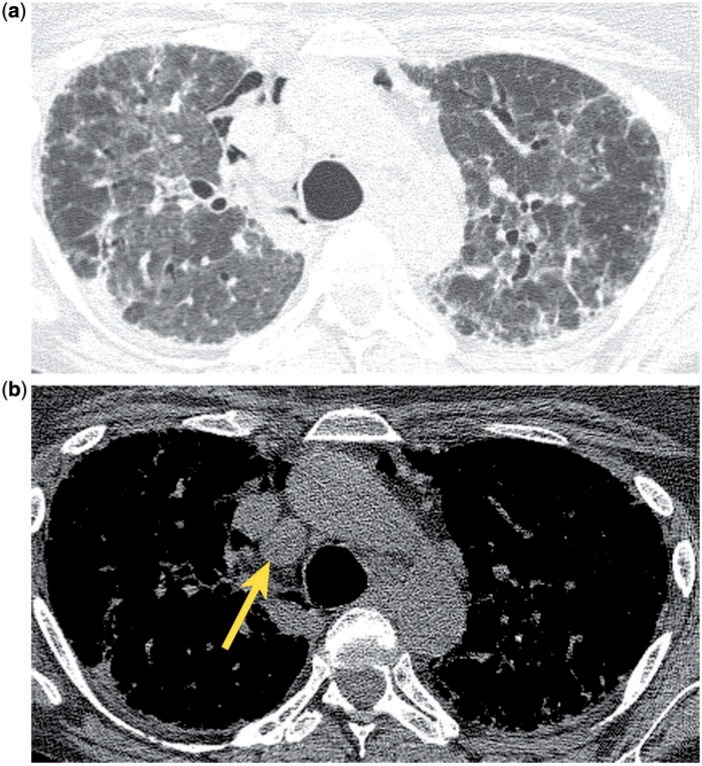
Mildly enlarged mediastinal lymph nodes are commonly seen in patients with congestive heart failure (CHF) (Fig. 2), and they should be recognized as a manifestation of this disease process, rather than as a marker of potential malignancy. In one study of 31 patients with subacute CHF, 42% (13/31) showed enlarged mediastinal lymph nodes; in 8/13 patients the lymph node enlargement regressed within 1 week after treatment of the heart failure. The lymph nodes were mildly to moderately enlarged, measuring ≤2 cm in short axis diameter, and were generally located in the right and left paratracheal, subcarinal, anterior mediastinal and precarinal regions. The severity of left heart decompensation correlated with the frequency of lymph node enlargement. Other correlative findings included inhomogeneity of the mediastinal fat, consistent with edema, and ill-defined lymph node margins[13]. Lymph node enlargement has also been reported for acute CHF[14]; CT findings of acute failure such as ground glass opacities, smooth septal thickening, fissural and peribronchovascular thickening, air space consolidation, perihilar parenchymal disease, pleural and pericardial effusions, and cardiomegaly may help to establish the diagnosis of CHF, and concomitant, enlarged mediastinal lymph nodes are to be expected. Enlarged lymph nodes are also seen in association with chronic CHF[15]; in these patients the myriad manifestations of acute failure may not be present, and therefore the cause of the nodal enlargement may not be immediately obvious without clinical information regarding cardiac function. A proposed mechanism for nodal enlargement postulates that elevated left ventricular filling pressure leads to decreased pulmonary drainage and the accumulation of excessive interstitial fluid, with dilatation of lymphatics draining the lungs and heart, resulting in lymph node enlargement[16].
Figure 2.
Incidental mediastinal lymph node enlargement in a patient with CHF. CT shows ground glass pulmonary opacities, parenchymal consolidation and smooth septal thickening (a), as well as bilateral pleural effusions, consistent with CHF. There are transvenous pacemaker leads in the superior vena cava and enlarged mediastinal lymph nodes (b, arrows).
If incidental, mild mediastinal lymph node enlargement is seen in the setting of CHF, no further work-up is necessary, unless there are other clinical or imaging features to suggest an underlying malignancy.
Incidental mediastinal lymph node enlargement in interstitial lung disease
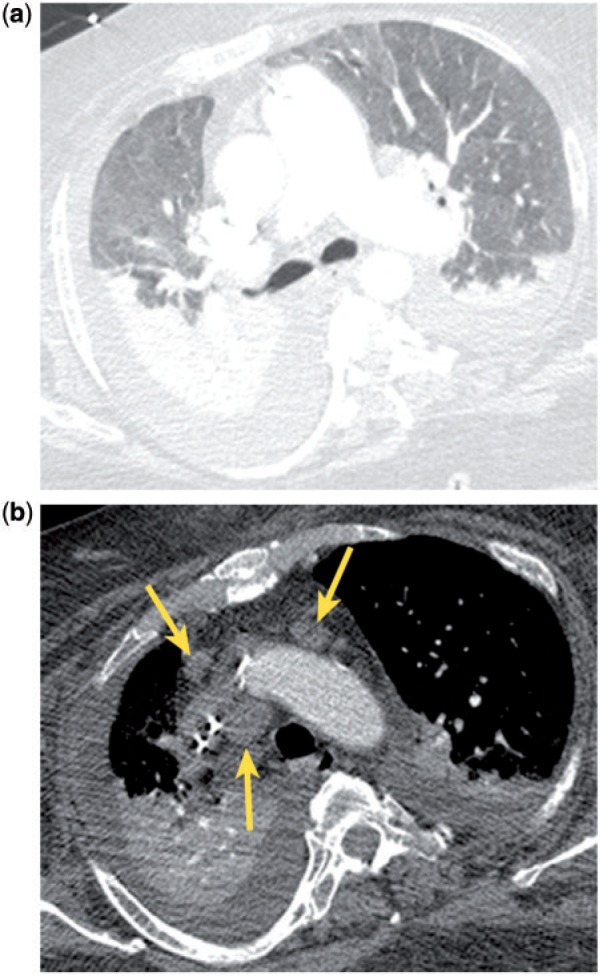
Mediastinal lymph node enlargement is common in various types of interstitial lung disease (ILD), including usual interstitial pneumonitis (UIP), nonspecific interstitial pneumonitis (NSIP), collagen vascular disease (CVD), sarcoidosis, extrinsic allergic alveolitis (EAA), respiratory bronchiolitis, and desquamative interstitial pneumonitis (DIP) (Fig. 3)[17–19]. In one study, the prevalence of enlarged nodes was 84% (46/55) in sarcoidosis, 67% (41/61) in idiopathic pulmonary fibrosis (IPF), 70% (14/20) in CVD, 53% (9/17) in EAA, and 36% (8/22) in cryptogenic organizing pneumonia (COP), previously known as idiopathic bronchiolitis obliterans organizing pneumonia (BOOP)[18]. The prevalence and extent of enlarged mediastinal lymph nodes correlate with disease severity[17,19]. Involved nodes typically occur in the right lower paratracheal and subcarinal regions, and enlargement is typically mild (the mean size of the largest lymph node was 1.4 cm in one study)[17]. Similar to the situation for incidental, mild mediastinal lymph node enlargement in CHF, no further work-up is indicated in the absence of other clinical or imaging features that are suggestive of a coexistent malignancy.
Figure 3.
Incidental mediastinal lymph node enlargement (b, arrow) in a patient with pulmonary interstital fibrosis (a).
The cysterna chyli simulating mediastinal lymph node enlargement
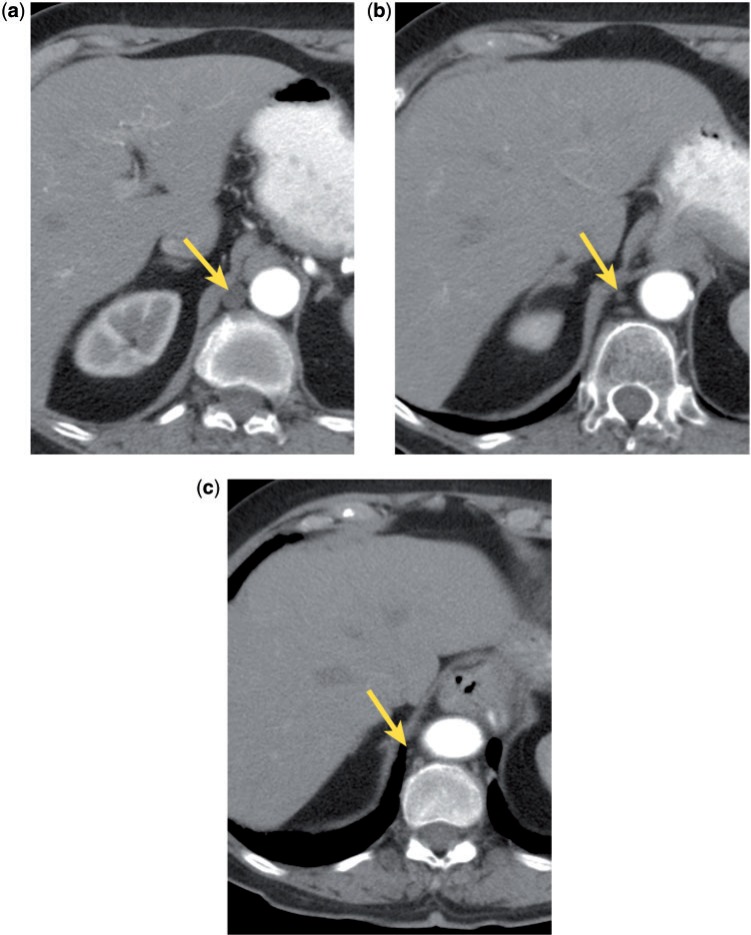
The cisterna chyli (CC) is a bulbous convergence of lymphatic channels formed by the right and left lumbar trunks and the intestinal trunk; it is often mistaken for an enlarged lymph node on cross-sectional imaging, either as an incidental finding, or in the setting of cancer staging (Fig. 4). The key to correct identification includes knowledge of the anatomy and morphology of the structure. The CC is located in the retrocrural region at the level of T11–L2, usually on the right, between the aorta and azygos vein. It is continuous with the thoracic duct, which ascends in the right side of the mediastinum medial to the azygos vein, eventually crossing to the left and emptying into the confluence of the left subclavian and jugular veins[20,21]. The CC may demonstrate a tubular, rounded, oval, or plexiform appearance. It is generally approximately 7 mm in axial cross-sectional diameter and 15–30 mm long; it has been suggested that diameter larger than 10 mm correlates with the presence of an underlying malignancy[22]. Although the CC usually shows the attenuation of water (average ∼5 HU[22]), the attenuation may be elevated due to increased protein content or after the injection of intravenous contrast (particularly in patients with renal failure). Fatty attenuation may occasionally be seen, most likely due to dietary fat intake; a fat-fluid level may rarely be detected. The CC may vary in size depending on the phase of respiration, degree of hydration, and the peristalsis of the muscular lower one-third of the thoracic duct[23]. The structure is visible on approximately 16% of CT scans[22] and can usually be differentiated from an abnormal lymph node by its characteristic appearance and location.
Figure 4.
The CC (a, arrow) may simulate an abnormal retrocrural lymph node. The CC is continuous with the thoracic duct (b and c, arrows).
Incidental lung nodules
Results of CAD and lung cancer screening studies have shown a high prevalence (25–53%) of lung nodules on chest CT examinations[1,24–27]. Nodules representing granulomas are particularly common in endemic areas for histoplasmosis, such as the central United States. Approximately half of smokers older than age 50 years have small nodules on thin-section CT, and most of these nodules are benign[28]. When assessing an indeterminate nodule for possible malignant potential, the first rule of thumb is to look for comparison examinations. A solid nodule that is stable for at least 2 years is presumed to be benign, and no further follow-up is advocated. If comparison images are not available, size and morphologic characteristics may be helpful. The likelihood of malignancy is directly correlated with nodule size; <1% of nodules <4 mm in diameter in smokers turn into lethal cancers, compared with approximately 10–20% of nodules that are about 8 mm[28]. Benign morphologic features include certain types of calcification (laminated, concentric, dense central nidus, popcorn) and the presence of fat (consistent with a hamartoma). A feeding artery and a draining vein indicate an arteriovenous malformation. Smooth nodule margins, polygonal shape, and subpleural location are also suggestive of benignancy[29]. Incidental perifissural nodules are extremely common; these are generally well circumscribed, smooth, triangular or oval, contacting or closely related to a pleural fissure, occurring below the level of the carina and often showing a septal attachment to the pleural surface (Figs. 5 and 6). These nodules probably represent lymph nodes, and it has been reported that they have low or no chance of malignancy in the setting of lung cancer screening[30].
Figure 5.
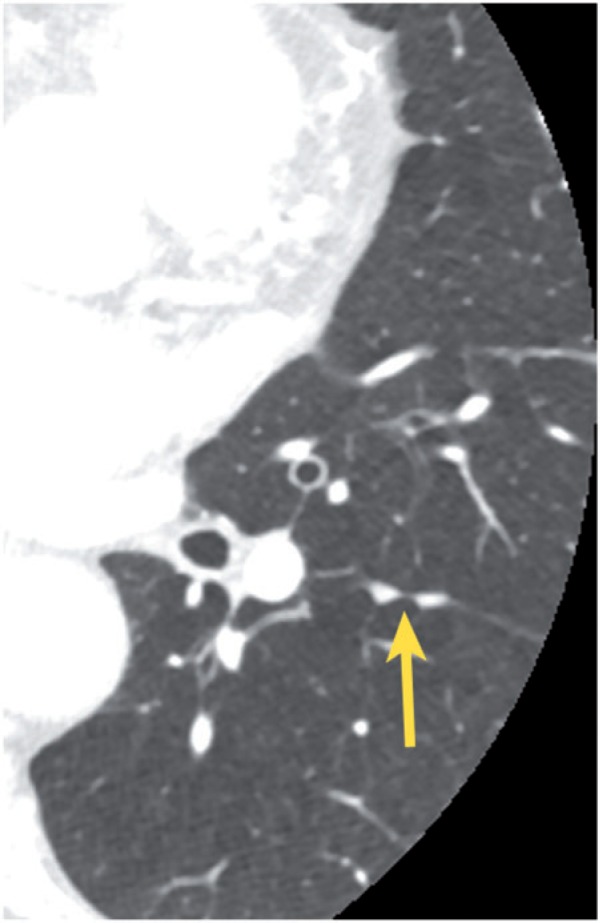
Small, incidental perifissural nodules (arrow) associated with the left major fissure.
Figure 6.
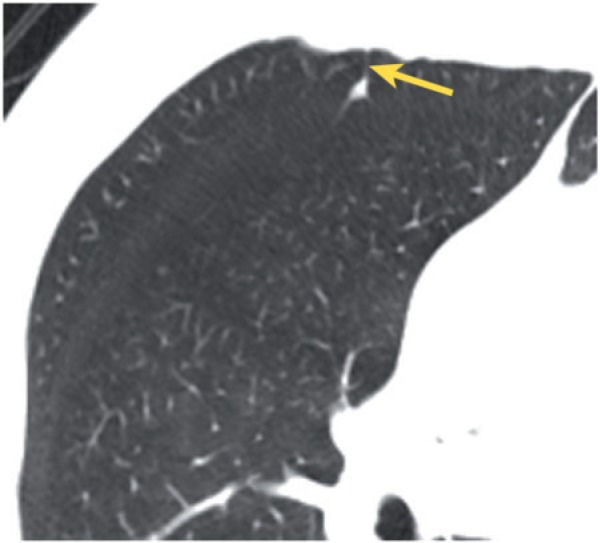
Small, incidental perifissural nodule associated with the minor fissure and showing a septal attachment (arrow) to the anterior pleural surface.
When an incidental, indeterminate lung nodule is encountered (without established long-term stability or clearly benign morphologic features), the Fleischner Society guidelines should be utilized in recommending further work-up or imaging follow-up[28]. These guidelines stratify patients according to clinical risk for developing lung cancer and nodule size. Although most radiologists are familiar with the guidelines, the latter are often applied inappropriately or incorrectly[31]. For example, the guidelines only apply to incidental nodules, meaning that the nodules are unrelated to any known underlying disease[28]. It is inappropriate to use the guidelines for patients with a known primary neoplasm that may metastasize to the lungs or for patients with an unexplained fever, in whom a nodule may represent infection. In addition, the guidelines only apply to patients 35 years of age or older, because primary lung cancer is rare in young patients, and this population is at greater risk from radiation exposure compared with older patients[28]. According to the guidelines, no follow-up is recommended for a small nodule (≤4 mm) in a low-risk patient, because the risk of malignancy is less than 1%; yet many radiologists incorrectly recommend follow-up for such a nodule, perhaps due to disagreement with the guidelines, medicolegal concerns, or, more likely, lack of familiarity with the details of the guidelines. Furthermore, it should be remembered that conservative management of incidentally found lung nodules is often appropriate for very elderly patients and patients with major co-morbid disease and shorter life expectancy[28].
Pure ground glass nodules should be approached differently than solid nodules. Most small (<5 mm) ground glass nodules probably represent foci of atypical adenomatous hyperplasia (AAH), although the differential diagnosis includes infection, inflammation, fibrosis, organizing pneumonia, and hemorrhage (Fig. 7). Although AAH lesions are felt to be pre-malignant, they tend to grow extremely slowly, and therefore follow-up is not recommended for such small lesions (<5 mm)[32,33]. On the other hand, a 3- to 6-month follow-up CT may be recommended for a pure ground glass nodule that measures 5–10 mm; a persistent nodule likely represents AAH or adenocarcinoma in situ (previously called bronchioloalveolar cell carcinoma). These nodules should be followed with serial CT examinations for at least 3 years in order to document long-term stability. For ground glass nodules >10 mm, a 3-month follow-up examination may be optimal; persistence suggests the presence of adenocarcinoma in situ or invasive adenocarcinoma. These nodules can be followed to assess for long-term stability, although a more aggressive approach, including biopsy or resection, may be chosen[32,33]. Features that correlate with malignancy include increase in nodule size, increase in overall attenuation, and development of a solid component (Figs. 8 and 9).
Figure 7.
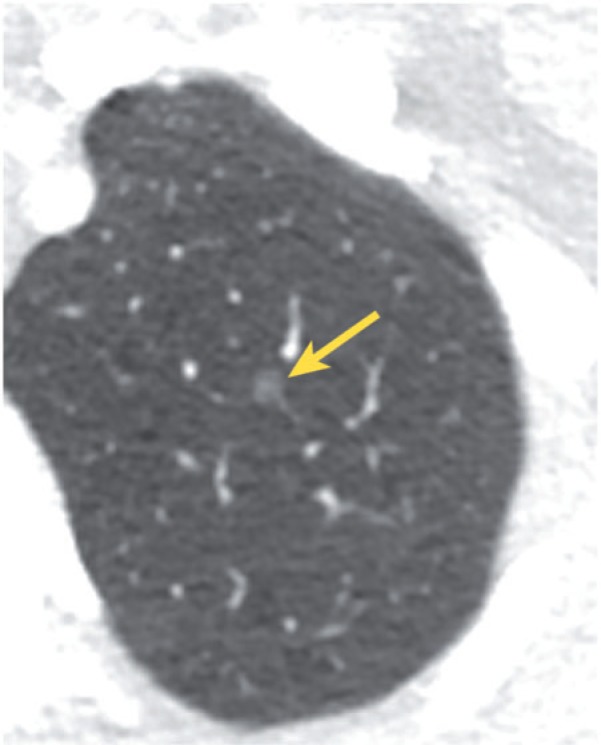
Small, incidental ground glass nodule (arrow) that likely represents a focus of AAH.
Figure 8.
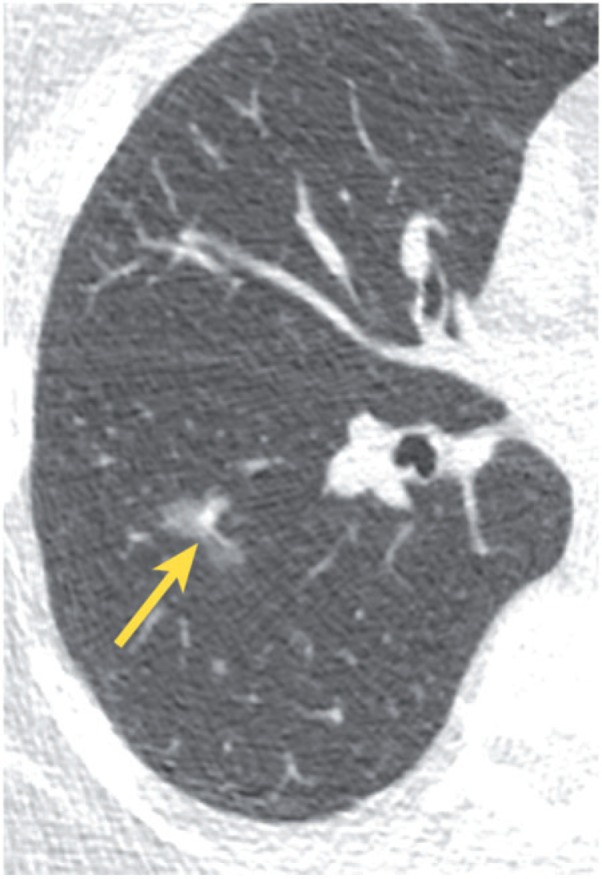
Incidentally found, lobulated ground glass nodule (arrow). Pathologic analysis of the resected specimen showed adenocarcinoma in situ.
Figure 9.
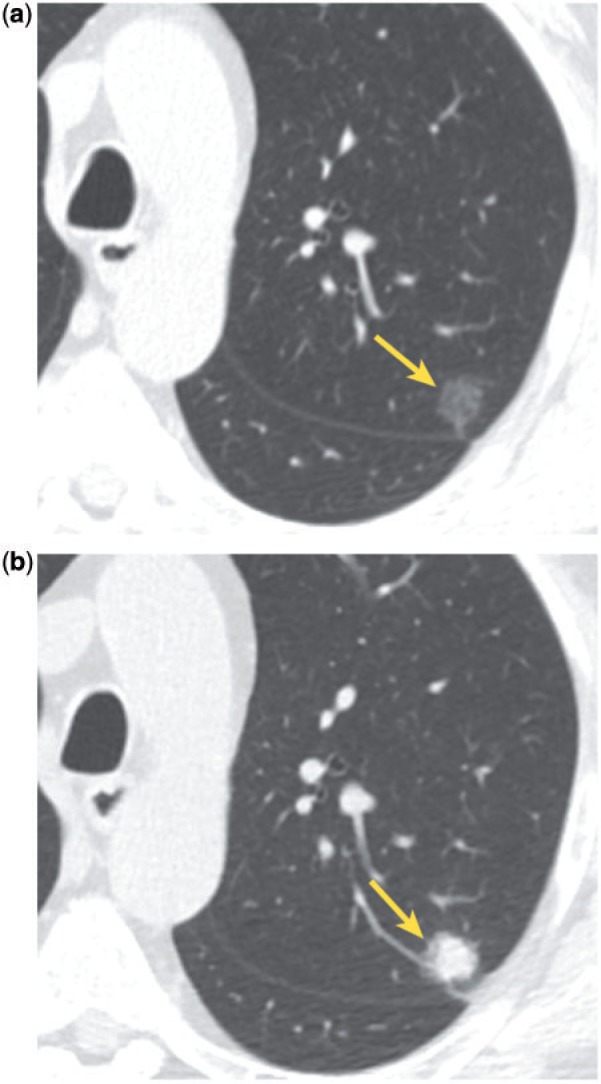
Incidental ground glass nodule (a, arrow) grew into a mixed solid and ground glass nodule 4 years later (b, arrow). Pathologic analysis of the resected specimen showed invasive adenocarcinoma.
Conclusions
Incidental findings are frequently found on chest CT examinations. Although most of these findings are not clinically significant, some may represent incidentally discovered malignancies. Detection of incidentalomas may lead to a large number of expensive follow-up examinations, extra radiation in healthy people, unnecessary invasive procedures and patient anxiety. When reporting an incidental finding, the radiologist should indicate the probability of the importance of the finding, and should offer evidence-based guidelines for further work-up or follow-up when necessary.
Footnotes
This paper is available online at http://www.cancerimaging.org. In the event of a change in the URL address, please use the DOI provided to locate the paper.
References
- 1.Jacobs PC, Mali WP, Grobbee DE, van der Graaf Y. Prevalence of incidental findings in computed tomographic screening of the chest: a systematic review. J Comput Assisted Tomogr. 2008;32:214–21. doi: 10.1097/RCT.0b013e3181585ff2. [DOI] [PubMed] [Google Scholar]
- 2.Haller S, Kaiser C, Buser P, Bongartz G, Bremerich J. Coronary artery imaging with contrast-enhanced MDCT: extracardiac findings. AJR Am J Roentgenol. 2006;187:105–10. doi: 10.2214/AJR.04.1988. [DOI] [PubMed] [Google Scholar]
- 3.Mueller J, Jeudy J, Poston R, White CS. Cardiac CT angiography after coronary bypass surgery: prevalence of incidental findings. AJR Am J Roentgenol. 2007;189:414–9. doi: 10.2214/AJR.06.0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson KM, Dennis JM, Dowe DA. Extracardiac findings on coronary CT angiograms: limited versus complete image review. AJR Am J Roentgenol. 2010;195:143–8. doi: 10.2214/AJR.08.1050. [DOI] [PubMed] [Google Scholar]
- 5.Veronesi G, Bellomi M, Spaggiari L. The rate of incidental findings in lung cancer screening trials is not negligible. Eur Radiol. 2008;18:529. doi: 10.1007/s00330-007-0797-5. [DOI] [PubMed] [Google Scholar]
- 6.van de Wiel JC, Wang Y, Xu DM, et al. Neglectable benefit of searching for incidental findings in the Dutch-Belgian lung cancer screening trial (NELSON) using low-dose multidetector CT. Eur Radiol. 2007;17:1474–82. doi: 10.1007/s00330-006-0532-7. [DOI] [PubMed] [Google Scholar]
- 7.Yoon DY, Chang SK, Choi CS, et al. The prevalence and significance of incidental thyroid nodules identified on computed tomography. J Comput Assisted Tomogr. 2008;32:810–5. doi: 10.1097/RCT.0b013e318157fd38. [DOI] [PubMed] [Google Scholar]
- 8.Shetty SK, Maher MM, Hahn PF, Halpern EF, Aquino SL. Significance of incidental thyroid lesions detected on CT: correlation among CT, sonography, and pathology. AJR Am J Roentgenol. 2006;187:1349–56. doi: 10.2214/AJR.05.0468. [DOI] [PubMed] [Google Scholar]
- 9.Green D. Incidental findings computed tomography of the thorax. Semin Ultrasound CT MR. 2005;26:14–9. doi: 10.1053/j.sult.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Frates MC, Benson CB, Charboneau JW, et al. Management of thyroid nodules detected at US: Society of Radiologists in Ultrasound consensus conference statement. Radiology. 2005;237:794–800. doi: 10.1148/radiol.2373050220. [DOI] [PubMed] [Google Scholar]
- 11.Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 12.Ahmed S, Horton KM, Jeffrey RB, Jr, Sheth S, Fishman EK. Incidental thyroid nodules on chest CT: review of the literature and management suggestions. AJR Am J Roentgenol. 2010;195:1066–71. doi: 10.2214/AJR.10.4506. [DOI] [PubMed] [Google Scholar]
- 13.Chabbert V, Canevet G, Baixas C, et al. Mediastinal lymphadenopathy in congestive heart failure: a sequential CT evaluation with clinical and echocardiographic correlations. Eur Radiol. 2004;14:881–9. doi: 10.1007/s00330-003-2168-1. [DOI] [PubMed] [Google Scholar]
- 14.Slanetz PJ, Truong M, Shepard JA, Trotman-Dickenson B, Drucker E, McLoud TC. Mediastinal lymphadenopathy and hazy mediastinal fat: new CT findings of congestive heart failure. AJR Am J Roentgenol. 1998;171:1307–9. doi: 10.2214/ajr.171.5.9798869. [DOI] [PubMed] [Google Scholar]
- 15.Erly WK, Borders RJ, Outwater EK, Zaetta JM, Borders GT. Location, size, and distribution of mediastinal lymph node enlargement in chronic congestive heart failure. J Comput Assisted Tomogr. 2003;27:485–9. doi: 10.1097/00004728-200307000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Ardekani MS, Issa M, Green L. Diagnostic and economic impact of heart failure induced mediastinal lymphadenopathy. Int J Cardiol. 2006;109:137–8. doi: 10.1016/j.ijcard.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 17.Attili AK, Kazerooni EA, Gross BH, Flaherty KR, Martinez FJ. Thoracic lymph node enlargement in usual interstitial pneumonitis and nonspecific-interstitial pneumonitis: prevalence, correlation with disease activity and temporal evolution. J Thoracic Imaging. 2006;21:288–92. doi: 10.1097/01.rti.0000213562.55914.9a. [DOI] [PubMed] [Google Scholar]
- 18.Niimi H, Kang EY, Kwong JS, Carignan S, Muller NL. CT of chronic infiltrative lung disease: prevalence of mediastinal lymphadenopathy. J Comput Assisted Tomogr. 1996;20:305–8. doi: 10.1097/00004728-199603000-00026. [DOI] [PubMed] [Google Scholar]
- 19.Souza CA, Muller NL, Lee KS, Johkoh T, Mitsuhiro H, Chong S. Idiopathic interstitial pneumonias: prevalence of mediastinal lymph node enlargement in 206 patients. AJR Am J Roentgenol. 2006;186:995–9. doi: 10.2214/AJR.04.1663. [DOI] [PubMed] [Google Scholar]
- 20.Schnyder P, Hauser H, Moss A, et al. CT of the thoracic duct. Eur J Radiol. 1983;3:18–23. [PubMed] [Google Scholar]
- 21.Smith TR, Grigoropoulos J. The cisterna chyli: incidence and characteristics on CT. Clin Imaging. 2002;26:18–22. doi: 10.1016/S0899-7071(01)00358-8. [DOI] [PubMed] [Google Scholar]
- 22.Feuerlein S, Kreuzer G, Schmidt SA, et al. The cisterna chyli: prevalence, characteristics and predisposing factors. Eur Radiol. 2009;19:73–8. doi: 10.1007/s00330-008-1116-5. [DOI] [PubMed] [Google Scholar]
- 23.Gollub MJ, Castellino RA. The cisterna chyli: a potential mimic of retrocrural lymphadenopathy on CT scans. Radiology. 1996;199:477–80. doi: 10.1148/radiology.199.2.8668798. [DOI] [PubMed] [Google Scholar]
- 24.Horton KM, Post WS, Blumenthal RS, Fishman EK. Prevalence of significant noncardiac findings on electron-beam computed tomography coronary artery calcium screening examinations. Circulation. 2002;106:532–4. doi: 10.1161/01.CIR.0000027136.56615.DE. [DOI] [PubMed] [Google Scholar]
- 25.Onuma Y, Tanabe K, Nakazawa G, et al. Noncardiac findings in cardiac imaging with multidetector computed tomography. J Am Coll Cardiol. 2006;48:402–6. doi: 10.1016/j.jacc.2006.04.071. [DOI] [PubMed] [Google Scholar]
- 26.Schragin JG, Weissfeld JL, Edmundowicz D, Strollo DC, Fuhrman CR. Non-cardiac findings on coronary electron beam computed tomography scanning. J Thoracic Imaging. 2004;19:82–6. doi: 10.1097/00005382-200404000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Gil BN, Ran K, Tamar G, Shmuell F, Eli A. Prevalence of significant noncardiac findings on coronary multidetector computed tomography angiography in asymptomatic patients. J Computer Assisted Tomogr. 2007;31:1–4. doi: 10.1097/01.rct.0000233125.83184.33. [DOI] [PubMed] [Google Scholar]
- 28.MacMahon H, Austin JH, Gamsu G, et al. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology. 2005;237:395–400. doi: 10.1148/radiol.2372041887. [DOI] [PubMed] [Google Scholar]
- 29.Xu DM, van der Zaag-Loonen HJ, Oudkerk M, et al. Smooth or attached solid indeterminate nodules detected at baseline CT screening in the NELSON study: cancer risk during 1 year of follow-up. Radiology. 2009;250:264–72. doi: 10.1148/radiol.2493070847. [DOI] [PubMed] [Google Scholar]
- 30.Ahn MI, Gleeson TG, Chan IH, et al. Perifissural nodules seen at CT screening for lung cancer. Radiology. 2010;254:949–56. doi: 10.1148/radiol.09090031. [DOI] [PubMed] [Google Scholar]
- 31.Eisenberg RL, Bankier AA, Boiselle PM. Compliance with Fleischner Society guidelines for management of small lung nodules: a survey of 834 radiologists. Radiology. 2010;255:218–24. doi: 10.1148/radiol.09091556. [DOI] [PubMed] [Google Scholar]
- 32.Alpert JB, Naidich DP. Imaging of incidental findings on thoracic computed tomography. Radiol Clin North Am. 2011;49:267–89. doi: 10.1016/j.rcl.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 33.Godoy MC, Naidich DP. Subsolid pulmonary nodules and the spectrum of peripheral adenocarcinomas of the lung: recommended interim guidelines for assessment and management. Radiology. 2009;253:606–22. doi: 10.1148/radiol.2533090179. [DOI] [PubMed] [Google Scholar]