Abstract
There is now a growing body of evidence supporting the use of fluorodeoxyglucose (FDG)-positron emission tomography (PET)/computed tomography (CT) in gynaecological malignancies. Although this molecular imaging technique is becoming increasingly available, PET/CT remains an expensive imaging tool. It is essential to be familiar with the circumstances in which FDG-PET/CT can add value and contribute to patient management and indeed to know when it is unlikely to be of benefit. It is also important to understand and recognize the potential pitfalls. FDG-PET/CT has been most widely adopted for staging patients with suspected advanced disease or in suspected recurrence, offering a whole-body imaging approach. However, there is great potential for this technique to act as a predictive biomarker of response to treatment, as well as a prognostic biomarker. In addition, FDG-PET images may now be incorporated into radiotherapy planning in order to refine the delineation of dose according to metabolically active sites of disease. This article reviews the literature that provides the evidence for the use of FDG-PET in gynaecological malignancies, identifies areas of real benefit and future potential, and highlights circumstances where there is limited value.
Keywords: PET, FDG-PET, PET/CT, FDG-PET/CT, gynaecological cancers, diagnosis, staging, treatment monitoring, ovarian cancer, endometrial cancer, cervix cancer, vulval cancer
Introduction
Gynaecological malignancies are predominantly staged according to the FIGO (International Federation of Gynaecology and Obstetrics) classification, using clinical examination in the case of cervical cancer and surgical-pathological findings in the case of endometrial, ovarian and vulvar cancers. However, following extensive publications evaluating the role of magnetic resonance imaging (MRI) and computed tomography (CT), the recent revision of the FIGO staging classification recognizes the benefits of preoperative imaging assessment with CT and MRI in treatment planning in cervical cancer[1]. CT is widely used in evaluation of patients with ovarian cancer to predict the feasibility of primary cytoreductive surgery. There is more debate concerning the role of preoperative imaging in patients with endometrial cancer, as primary surgical treatment and staging is the mainstay of patient management. Cross-sectional imaging is also widely used in the follow-up of gynaecological malignancies to assess treatment response and detect recurrent disease. However, there are well-recognized limitations of anatomic cross-sectional imaging, including difficulties in the detection of nodal metastases and in evaluating the extent of diffuse peritoneal disease in ovarian cancer. In addition, there is growing interest in using imaging as a biomarker of treatment response. Thus, the question of the role of functional imaging using positron emission tomography (PET) has been the subject of extensive research.
Until relatively recently, [18F]fluorodeoxyglucose (FDG)-PET and PET/CT have had only a limited role in the diagnosis and staging of disease in patients presenting with gynaecological malignancy. The strength of PET lies in its ability to identify abnormal biological processes associated with cancer, such as increased glucose metabolism using FDG. In addition, PET/CT provides precise whole-body assessment, including the primary tumour site, lymph node spread and distant metastases except in the brain[2]. FDG-PET/CT can also contribute to more accurate staging and thus influence the therapeutic decision. FDG-PET/CT is also a valuable tool for assessing the extent of recurrent disease and may be critical in defining the optimal treatment.
FDG-PET/CT has also been evaluated in assessment of response to treatment; it is generally a valuable tool for assessment of treatment response after completion of therapy in patients with metastatic gynaecological malignancies, particularly in identifying residual viable tumour tissue by its increased metabolic activity. Prediction of treatment response early in the course of therapy by comparing the tumour metabolic activity after one or two cycles of treatment with baseline is a promising indication but needs further validation in clinical trials.
This article reviews the literature and the current use of FDG-PET/CT in patients with gynaecological malignancy, including established indications as well as areas of current research interest.
Technique
Gynaecological malignancies are typically characterized by increased glucose metabolism and therefore present with increased FDG uptake, whereas benign tumours are usually negative on PET. However, common pitfalls include increased FDG uptake in normal ovaries during ovulation, as well as normal physiologic activity in bowel, endometrium, and blood vessels, focal retained activity in ureters, bladder diverticula, pelvic kidneys, and urinary diversions[3–5]. The co-registration of CT images with the PET images has overcome many of these difficulties.
In order to reduce errors of interpretation, specific emphasis needs to be directed towards good technique when undertaking pelvic PET/CT imaging. Ideally, the bladder should be empty to avoid artefacts on PET images from high radioactivity concentration in the bladder. It is suggested that the patient is scanned in a caudal to cranial direction, thus imaging the pelvis at the beginning of the study. The CT portion of PET/CT is often helpful to identify bladder diverticula and focal retained activity in ureters. Pelvic imaging can be improved by intravenous injection of a diuretic agent to reduce tracer retention in the urinary system. A technique of hydration, diuretic administration and pre-imaging voiding obviates the need for invasive procedures such as bladder drainage in most cases.
The CT portion of PET/CT is also helpful in identifying normal physiologic FDG uptake in the bowel, endometrium, and blood vessels as well as demonstrating pathologies that are not demonstrated on the PET portion, such as pleural effusions, ascites or predominantly cystic/necrotic lesions. Bowel preparation can be performed with oral hydration as well and some groups recommend the use of oral contrast[5]. The administration of butylscopolamine (20–40 mg) at the time of FDG injection has been reported to reduce FDG uptake in the bowel[6].
Limitations of FDG-PET
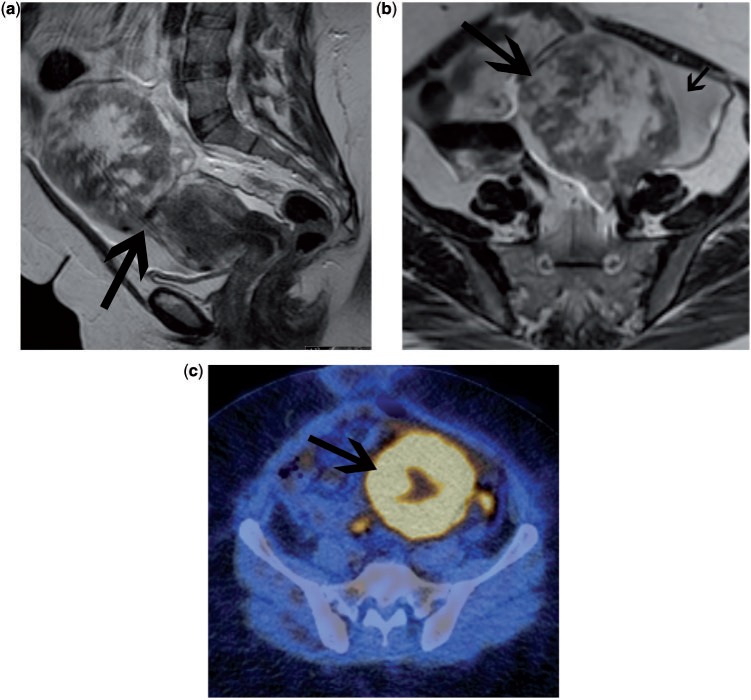
FDG-PET has well-recognized limitations in identifying metastatic disease. It is not possible to reliably detect small lung deposits, small nodal deposits or diffuse peritoneal carcinomatosis; conversely false-positive findings occur in acute inflammation and granulomatous processes such as tuberculosis and sarcoidosis. Certain benign tissue masses may be of relatively high FDG avidity, such as certain fibroids (Fig. 1).
Figure 1.
Patient being investigated for a uterine mass. Sagittal T2 (a) and axial T2 (b) MRI demonstrate a mass arising from the uterus (arrow). The mixed high signal intensity of the mass raised the possibility of a leiomyosarcoma. FDG-PET/CT (c) demonstrated marked FDG uptake within the mass with some central necrosis. After surgical resection, the mass was confirmed to be a benign fibroid that had undergone degenerative changes.
Ovarian cancer
FDG-PET/CT in primary ovarian cancer
Current guidelines for the investigation of an ovarian mass include cancer antigen 125 (CA-125) measurement and ultrasonography usually via a transvaginal route. Cross-sectional imaging modalities such as CT and MRI have been investigated in the diagnosis of ovarian cancer. Studies have found sensitivities and specificities for diagnosis of ovarian cancer by ultrasonography to range from 90% to 92% and 53% to 61%, respectively[7–11]. For MRI, the sensitivity has been found to be 83% and specificity 84% for diagnosis of ovarian cancer[8,9,11].
Most studies assessing the role of FDG-PET in the detection of primary ovarian cancer date back 10–15 years. Consequently, these were undertaken with a previous generation of PET technology with lower sensitivity and spatial resolution and no direct co-registration with CT. Nevertheless, data are still comparable with more recent reports using PET/CT. In 2000 and 2002, 2 studies assessing women with asymptomatic adnexal masses found FDG-PET to have a sensitivity of 58% and specificity of 76–80%[8,9]. Similarly, 2 further studies assessed FDG-PET for characterization of a suspicious pelvic mass on ultrasonography and found sensitivities and specificities to range from 58% to 78% and from 78% to 87%, respectively[11,12]. Despite the relatively low sensitivity of FDG-PET in the detection of ovarian cancer, all of the false-negative results were either invasive stage I tumours or tumours of low malignant potential (borderline ovarian tumours). More advanced stages of ovarian cancer demonstrated intensely increased FDG uptake and were well visualized. However, the cellular composition of tumours has a significant effect on the level of FDG uptake. Abdominal or pelvic masses containing large cystic components as well as mucinous tumours will often not be metabolically active.
Recently, a study of 50 patients with a suspicious pelvic lesion found the sensitivity and specificity of FDG-PET/CT in the diagnosis of ovarian cancer to be 87% and 100%, respectively[7]. Regarding the extent of disease, they reported 69% concordance between FDG-PET/CT staging and surgical staging. The authors concluded that the use of FDG-PET/CT can improve the specificity and accuracy of the pretreatment diagnosis and staging of ovarian cancer in comparison with contrast-enhanced CT alone. Risum et al.[13] evaluated FDG-PET/CT in 97 patients with suspected pelvic malignancy with a risk of malignancy index of >150, based on CA-125, ultrasonographic findings and menopausal status. They reported a sensitivity of 100% and specificity of 92.5% for detecting primary ovarian cancer. This was higher than all other imaging modalities including ultrasonography, CT, MRI and FDG-PET alone.
Generally, the number of false-positive FDG-PET findings in the abdomen and pelvis is low. However, in patients with suspicious ovarian masses, a number of benign conditions can cause a significant false-positive rate. For example, benign cystadenomas, teratomas, schwannomas, endometriomas and inflammatory processes were found to exhibit increased glucose metabolism. Increased FDG uptake in the pelvis has also been found in healthy premenopausal women. Two studies reported that most premenopausal women show FDG uptake in the ovaries and uterine endometrium in the late follicular and early luteal phases of the menstrual cycle[14,15]. Lerman et al.[16] confirmed that in premenopausal women with a normal menstrual cycle, the ovarian glucose metabolism was statistically higher during menstruation and midcycle than during the proliferative or secretory phases. The standardized uptake value (SUV) as a semi-quantitative measure of FDG uptake separated benign from malignant ovarian uptake with a sensitivity of only 57% but with a high specificity of 95% (cut-off threshold SUV 7.9).
Most cases of newly diagnosed ovarian cancer present with advanced stages of disease. Therefore, the standard treatment for primary ovarian cancer is debulking surgery followed by chemotherapy. It is widely accepted that optimal debulking is associated with improved survival outcome. In many institutions, a large number of patients, particularly where the imaging suggests that optimal debulking is unlikely to be achieved, undergo neoadjuvant (preoperative) chemotherapy. It is therefore important to identify the exact extent of disease preoperatively in order to select patients for the most appropriate primary treatment.
Staging of ovarian cancer is conventionally undertaken using CT. In a small number of patients, Yoshida et al.[17] concluded that the addition of FDG-PET to CT improved the accuracy in preoperative staging of ovarian cancer to 87% compared with 53% with CT alone.
In a more recent series, 40 patients with ovarian cancer underwent FDG-PET/CT with intravenous contrast-enhanced CT for staging before primary debulking surgery[18]. An increase in sensitivity (from 37.6% to 69.4%) and accuracy (from 89.7% to 94.0%) and stable specificity (from 97.1% to 97.5%) was found with FDG-PET/CT compared with contrast-enhanced CT alone. FDG-PET/CT findings and the final pathologic staging were in concordance in 75% of cases. A further study in 133 women with suspected ovarian cancer reported a concordance between FDG-PET/CT findings and the final pathologic staging of 78%[10]. FDG-PET/CT identified unexpected nodal metastases in 15 of 95 patients confirmed to have ovarian cancer. They also reported that FDG-PET/CT was superior to CT and MRI in discriminating between cases of benign and borderline/malignant ovarian tumours.
In summary, compared with conventional imaging, most studies found an improvement in diagnostic accuracy using FDG-PET/CT for staging of ovarian cancer before surgery. PET/CT provides an accurate assessment of the extent of disease, particularly in areas difficult to assess for metastases by CT and MRI such as the mediastinum and supraclavicular region. However, given the limited access to FDG-PET/CT and the higher cost, the overall benefit for individual patients remains to be proved.
FDG-PET/CT in recurrent ovarian cancer
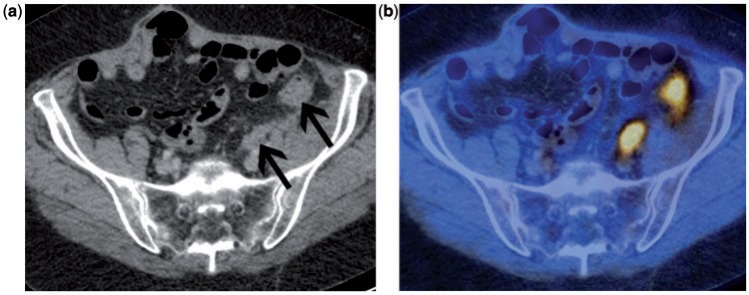
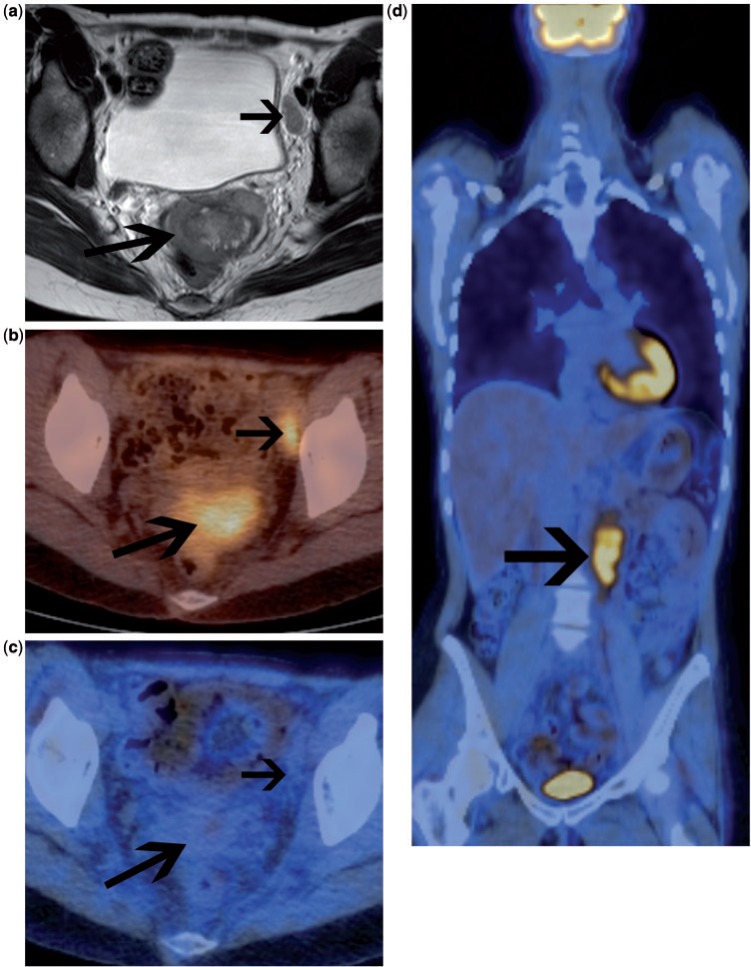
Most patients with advanced stage ovarian cancer have persistent disease or develop recurrent disease, even after complete clinical response after primary therapy. CA-125 is a membrane-associated mucin protein expressed on female reproductive tract epithelia and used as a tumour marker in recurrent ovarian cancer. However, CA-125 is not elevated in all patients with persistent or recurrent disease and does not reflect disease burden. Cross-sectional imaging, predominately contrast-enhanced CT is used to localize recurrent ovarian cancer although the identification of disease in the abdomen and pelvis is often difficult. A study compared CT with second look laparotomy in 58 women who were clinically disease free and found a sensitivity of only 47% for detection of recurrent disease (corresponding specificity 87%)[19]. It is particularly difficult to identify small tumour deposits adjacent to the bowel by CT (Figs. 2 and 3). It is often not easy to identify peritoneal and serosal tumour deposits using MRI and conventional MRI has a low accuracy for lesions <2 cm and metastatic lesions in the peritoneum and mesentery[20].
Figure 2.
Patient with suspected recurrent ovarian cancer. Contrast-enhanced CT (a) demonstrates 2 sites of recurrent disease along the bowel serosa (arrows) that are very difficult to identify with confidence. The accompanying fused FDG-PET/CT (b) clearly demonstrates the 2 highly metabolically active serosal deposits.
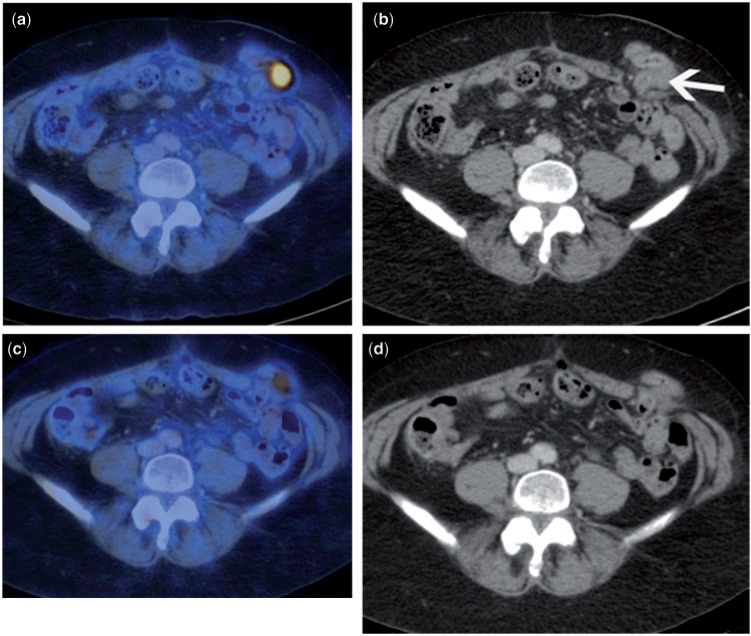
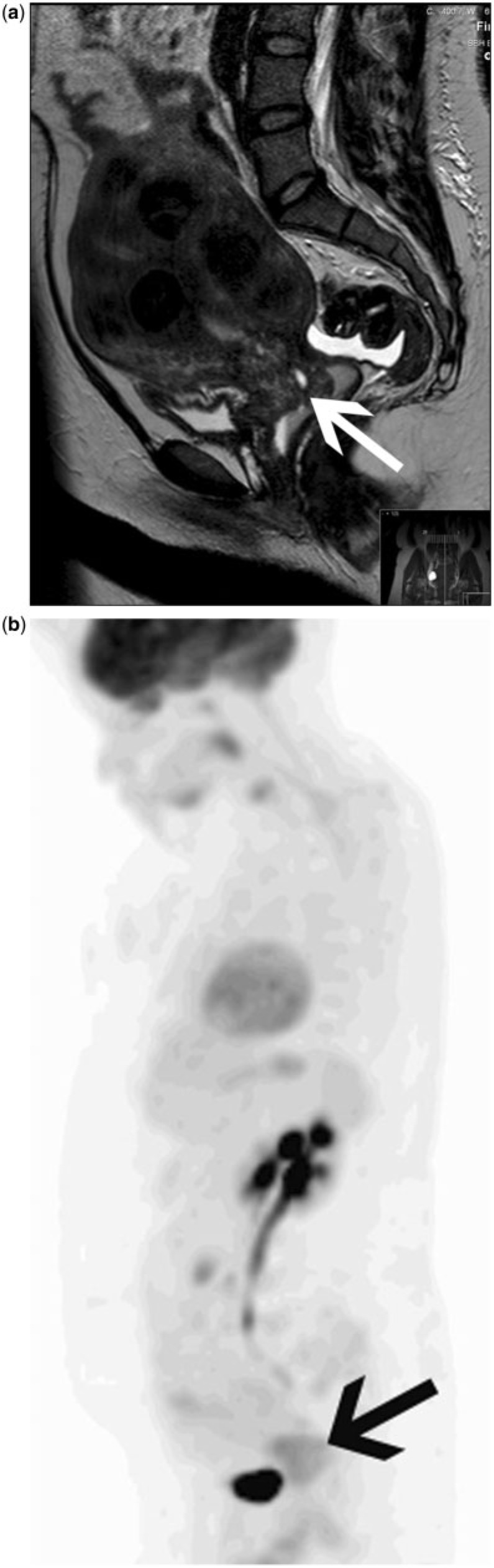
Figure 3.
Patient with CA-125 relapse but no clear evidence of recurrence on CT imaging. Fused FDG-PET/CT (a) and accompanying contrast-enhanced CT (b) demonstrate a serosal deposit along a loop of bowel within the stoma (arrow) that is very difficult to appreciate on CT. Repeat imaging after the first cycle of chemotherapy demonstrates a significant reduction in the metabolic activity of the serosal deposit, highly suggestive of a likely responder to treatment.
In 2001, a study using FDG-PET in 24 women reported a diagnostic accuracy for detecting recurrent ovarian cancer of 79.2%[21]. The accuracy increased to 94.4% when combined with conventional imaging modalities. The findings were confirmed by several further studies[22–25].
A recent study including 51 patients found that FDG-PET/CT provided a statistically significant improvement in accuracy from 83% to 92% in the diagnosis of ovarian cancer recurrence compared with CT alone[22]. The co-registered functional and anatomic information from PET/CT is particularly helpful in the abdomen and pelvis. A study from Pittsburgh found a sensitivity of 94.5% and a specificity of 100% for FDG-PET/CT in the detection of ovarian cancer recurrence[23]. The authors concluded that FDG-PET/CT had the greatest utility in the clinical setting of increasing CA-125 levels but negative or indeterminate conventional CT imaging.
However, it is important to note that the reference standard used for evaluation of imaging modalities is of crucial importance. In a direct comparison with second look laparotomy in 31 women, the lesion-based sensitivity was only 45.3% for FDG-PET and increased to 58.2% for FDG-PET/CT[26]. In another study, Sironi et al.[27] studied 31 women prior to second look laparotomy and found that FDG-PET/CT correctly identified 32 of the 41 lesions that were positive at histologic analysis. The overall patient-based sensitivity and specificity were 53% and 86%, respectively. All lesions missed with PET/CT were equal to or smaller than 0.5 cm in maximum diameter. This study highlights an important limitation of PET imaging in that the metabolic activity of small tumour deposits is often not sufficient for positive identification, particularly in areas with higher background activity such as in the abdomen and pelvis.
In 43 patients, FDG-PET/CT had a sensitivity and specificity of 88.4% and 88.2%, respectively, for detection of recurrent ovarian cancer using histology and clinical follow-up as the reference[24]. More specifically they found a slightly lower sensitivity of 80.9% for pelvic disease and of 93.5% for extrapelvic disease; specificity was 93.7% for both. A recent meta-analysis compared FDG-PET, FDG-PET/CT, MRI and the tumour marker CA-125 for detection of recurrent disease[20]. The authors concluded that FDG-PET alone seemed to be particularly useful for the diagnosis of recurrence when CA-125 levels are increasing and conventional imaging (CT or MR) is inconclusive or negative (Fig. 3). They also found no significant differences between PET results interpreted with or without the use of CT. Nevertheless, the anatomic information is often crucial to differentiate physiologic from pathologic FDG uptake and to precisely localize the disease.
Simcock et al.[28], in a study of 56 women, found that the addition of FDG-PET to CT altered the known disease distribution in 61% of scans leading to a change in management in 57% of patients. In 32 patients with suspected ovarian cancer recurrence, PET/CT showed a higher sensitivity (91%) than contrast-enhanced CT (62%)[25]. With the addition of FDG-PET/CT, 44% of patients received different treatment; only 2% of patients were managed expectantly compared with 22% after CT alone. The percentage of patients who were referred for chemotherapy increased from 31% to 50% when FDG-PET/CT results were considered. Thrall et al.[23] noted that FDG-PET/CT revealed unsuspected disease either outside the abdomen or in surgically inaccessible areas in 28.6% of cases and thus the treatment plan was changed. In another study, FDG-PET combined with contrast-enhanced CT resulted in a change of management in 39% of the cases compared with 12% for contrast-enhanced CT alone and 2% for FDG-PET combined with a low-dose CT[29]. This indicates an advantage of using contrast-enhanced CT with FDG-PET/CT, particularly for recurrent ovarian cancer.
FDG-PET/CT for monitoring response in ovarian cancer
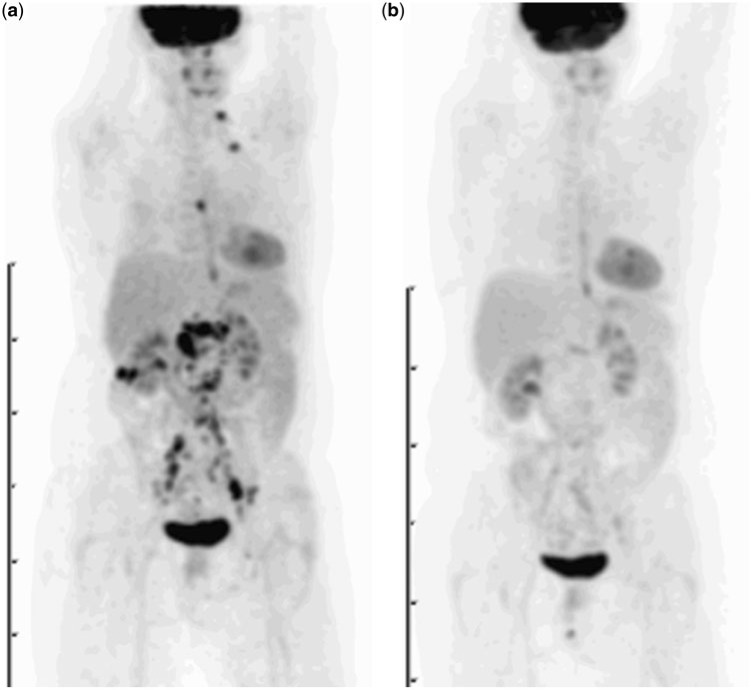
Although a substantial number of patients with ovarian cancer respond to initial chemotherapy, non-responding patients generally have a poor prognosis. The concept of individualizing cancer treatment is very appealing and would involve predicting the response to treatment early in the course of therapy. The use of FDG-PET in this capacity is based on the fact that tumours show early changes in glucose utilization and that changes in uptake closely correlate with response to treatment[30,31]. In a variety of tumours it has been shown that changes in glucose metabolism precede changes in tumour size and reflect treatment response. To predict treatment response, 2 sequential FDG-PET scans are required; one baseline FDG-PET before treatment and a second after initiation of chemotherapy (Figs. 3 and 4). The change in level of tumour metabolic activity after one or two cycles of chemotherapy can then be compared with treatment response after completion of the treatment course.
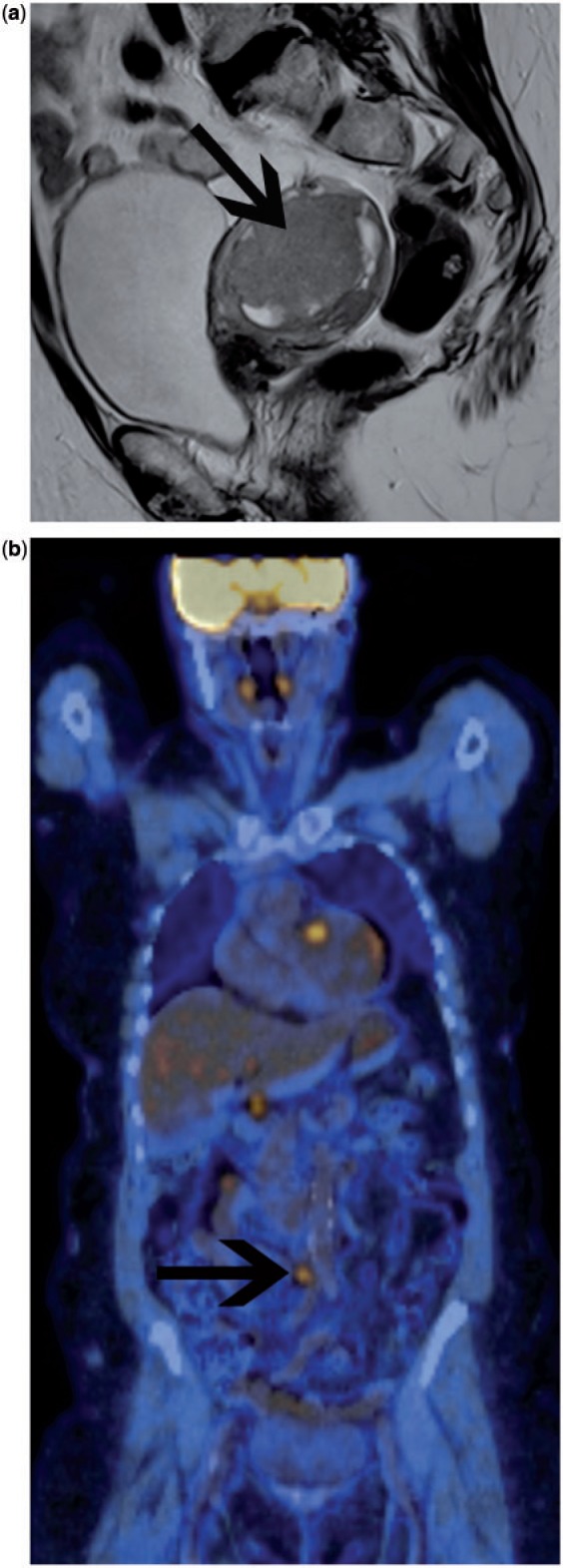
Figure 4.
Patient with recurrent ovarian cancer. Maximum intensity projection (MIP) image of FDG-PET study prior to treatment (a) shows widespread avid uptake at multiple sites of metastatic disease in the neck, chest and abdomen. Two months after the start of chemotherapy, follow-up imaging demonstrates no residual FDG-avid tumour sites, consistent with a complete metabolic response.
In advanced stage ovarian cancer, a significant correlation was found between the metabolic response after the first and third cycles of chemotherapy and overall survival using FDG-PET. This was found to be superior to clinical response, CA-125 measurements and histopathology[32]. Median overall survival was higher in those patients found to be metabolic responders after the first and third cycles of chemotherapy. After the first cycle of chemotherapy, metabolic responders were defined as a decrease in SUV from baseline to 20%. Patients with a metabolic response had a median overall survival of 38.3 months compared with 23.1 months in metabolic non-responders. After the third cycle of chemotherapy, metabolic responders were defined as a 55% decrease in SUV. Metabolic responders had a median overall survival of 38.9 months compared with 19.7 months in metabolic non-responders.
The use of sequential FDG-PET to predict early response to systemic therapy is an appealing application of metabolic imaging. However, further clinical trials would have to validate defined FDG-PET criteria for which treatment can be safely changed.
Endometrial cancer
FDG-PET/CT in primary endometrial cancer
Endometrial cancer is the most common gynaecological cancer in most European countries and in the United States, having recently overtaken the incidence of ovarian cancer (http://www.cancerresearch.org.uk). Definitive staging of the disease is performed surgically, according to the FIGO staging classification[1]. Thus, the role of preoperative imaging for staging evaluation remains controversial.
Primary endometrial cancer usually demonstrates an increased uptake of FDG. However, FDG-PET/CT cannot reliably determine the depth of myometrial invasion. This is due to the approximately 4–5 mm spatial resolution of current PET scanner technology as well as to an inherent limitation to precisely define tumour borders on PET images. Nodal status is of clinical importance, having a marked impact on prognosis. Currently, the decision to undertake lymphadenectomy remains controversial. Early studies evaluating the use of FDG-PET for the identification of nodal metastases showed little promise[33,34]. Two more recent studies preoperatively assessed the lymph node status with FDG-PET/CT in 40 and 12 patients, respectively, with histology as the reference standard[29,35,36]. Based on nodal diagnosis, both studies found a comparable sensitivity of 53% and a specificity of 99% (Fig. 5). In the larger study by Kitajima et al.[29,35], the accuracy of PET/CT was 97.8%. The sensitivity for detecting metastatic lesions 4 mm or less in diameter was 16.7%, 66.7% for lesions between 5 and 9 mm and 93.3% for lesions 10 mm or larger. These findings have led to the conclusion that although FDG-PET/CT is superior to conventional imaging techniques, it is only moderately sensitive in predicting lymph node metastasis preoperatively and it is inadequate for local staging of patients with endometrial cancer (Table 1).
Figure 5.
Preoperative imaging assessment in a patient with high-grade endometrial carcinoma. Sagittal T2-weighted MRI (a) demonstrates a large tumour mass in the endometrial cavity (arrow), which demonstrated deep myometrial invasion. No enlarged nodes could be seen on MRI in the pelvis or para-aortic positions. The stage on MRI alone was FIGO IB. Coronal fused FDG-PET/CT image (b) demonstrates focal uptake within a para-aortic lymph node, up-staging the patient to FIGO IIIC2.
Table 1.
Diagnostic performance of FDG-PET in the detection of nodal metastases in primary endometrial carcinoma
| Study | Technique | Patients/ nodes | Sensitivity (%) | Specificity (%) | Negative predictive value (%) | Comment |
|---|---|---|---|---|---|---|
| Horowitz et al.[33] | PET | Patients: 19 | 67 | 94 | In patients with moderate or high-grade histology | |
| Nodes | 60 | 98 | ||||
| Suzuki et al.[34] | PET | Patients: 30 | Did not identify any metastatic node that was < 10 mm in diameter | |||
| Kitajima et al.[35] | PET/CT | Patients: 40 | 50 | 86.7 | ||
| Nodes: 1484 | 53 | 99.6 | ||||
| Nayot et al.[36] | PET/CT | Patients: 12 | ||||
| Nodes | 53 | 99 | ||||
| Park et al.[74] | PET/CT | Patients: 53 | No difference when compared with MRI (P = 0.25 (sensitivity)) | |||
| Nodal site | 69.2 | 90 | ||||
| Picchio et al.[37] | PET/CT | Patients: 32 | 57 | 100 | 86 | In patients with high-grade histology; 5 cases of additionally detected extranodal metastatic disease |
| Nodes: not available | ||||||
| Signorelli et al.[38] | PET/CT | Patients: 37 | 77.8 | 100 | 93.3 | 11 patients with grade 2/deep invasion and 26 patients with grade 3 histology |
| Nodal site | 66.7 | 99.4 | 97.2 |
Picchio et al.[37] recently published a study on the value of FDG-PET/CT in preoperative staging specifically in high-grade patients. Thirty-two patients with grade 3 endometrial cancers were assessed preoperatively with FDG-PET/CT. Although the sensitivity for lymph node metastasis was comparable with previous studies (57.1%), FDG-PET/CT was found to have a greater sensitivity for distant metastatic lesions in the abdomen and thorax compared with conventional imaging, when compared with the surgical findings or follow-up imaging. This study underlines the potential for FDG-PET/CT in assessing patients for distant metastases and emphasizes its advantages over conventional imaging as a whole-body diagnostic tool. A smaller study by Signorelli et al.[38] also evaluated high-grade patients. Although the sensitivity for lymph node site metastases was somewhat higher (66.7%), the important outcome was the high negative predictive value for lymph node involvement on a nodal site basis (97.2%) and on a patient basis (93.1%). This suggests that in the high-grade group of patients, surgical lymphadenectomy may be avoided in selected cases after FDG-PET/CT assessment.
Few studies have compared the tumour metabolic activity measured as the SUV with biological features such as proliferation, grade and expression of glucose transporters. Recently, the first study of correlation of SUV with histologic grade in endometrial cancer has been published[39]. The findings suggest that the level of FDG uptake is correlated to aggressive biological characteristics in endometrial cancer, namely FIGO grade, size of primary tumour and glucose transporter-1 expression (all P < 0.001). The strongest correlation was with FIGO grade.
FDG-PET/CT in recurrent endometrial cancer
FDG-PET/CT has been reported to be highly accurate in the assessment of patients suspected of having recurrent endometrial cancer (Fig. 6). In one study, 31 women underwent FDG-PET/CT for suspected recurrence, 20 of whom underwent surgical biopsy and 11 had imaging follow-up[40]. Twelve patients had a documented recurrence by surgical biopsy or clinical follow-up and 19 patients had no evidence of recurrence. Overall sensitivity, specificity, and accuracy of FDG-PET/CT were 100%, 94.7%, 92.3%, respectively. PET/CT results modified the treatment plan in 7 (22.6%) patients, resulting in 5 patients undergoing previously unplanned therapeutic procedures and eliminating previously planned diagnostic procedures in 2 patients (6.5%). Patients with negative PET/CT showed significantly better progression-free survival than those presenting with positive tumour FDG uptake.
Figure 6.
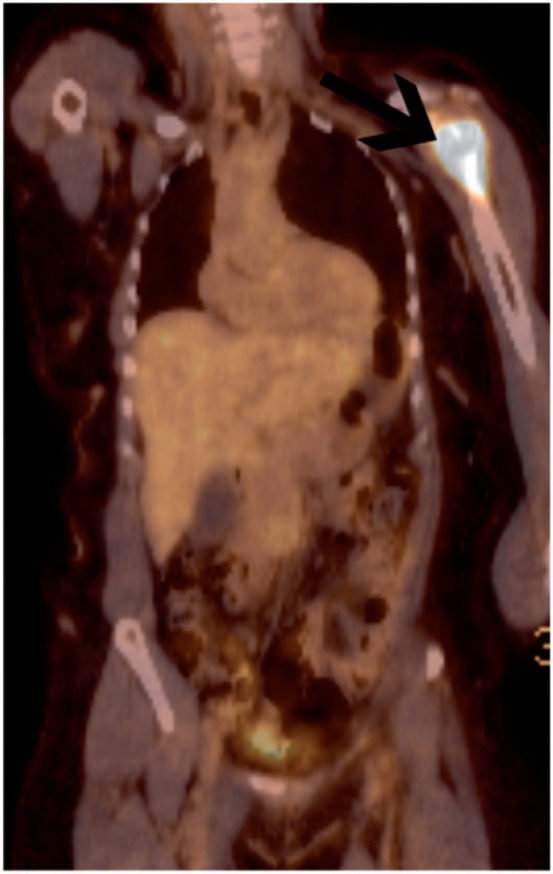
Patient with a history of high-grade endometrioid endometrial cancer treated 2 years previously presented with a painful left shoulder and suspicious radiograph. FDG-PET/CT confirmed metabolically active disease confined to the left shoulder (arrow).
Park et al.[41] evaluated FDG-PET or PET/CT in 24 women with suspected recurrent endometrial cancer and 64 patients undergoing routine surveillance, using histopathology or 6 months follow-up as the reference standard. They reported very high sensitivity (100%), specificity (83.3%) and positive predictive values (95%) in cases of suspected recurrence and very impressive results in patients under routine surveillance (Table 2)[41].
Table 2.
Diagnostic performance of FDG-PET in the detection of recurrent endometrial cancer
| Study | Technique | Patients | Sensitivity (%) | Specificity (%) | Negative predictive value (%) |
|---|---|---|---|---|---|
| Chung et al.[40] | PET/CT | 31a | 100 | 94.7 | 96.8 |
| Park et al.[41] | PET and PET/CT | 24 suspected recurrence | 100 | 83.3 | 100 |
| PET and PET/CT | 64 asymptomatic surveillance | 100 | 100 | 100 | |
| Kitajima et al.[42] | PET/low-dose CT | 100b | 83 | 94 | |
| PET/contrast-enhanced CT | 100b | 90 | 97 |
a20 with histologic confirmation, 11 with imaging follow-up.
b55 women with cervix cancer and 45 women with endometrial cancer.
Kitajima et al.[42] compared the use of FDG-PET with integrated low-dose CT versus contrast-enhanced CT in 100 patients with either cervical (55 women) or endometrial (45 women) cancer who underwent FDG-PET/CT scanning with and without iodinated contrast. Sensitivity was not significantly different between the groups (Table 2). However, the non-contrast group showed 4 sites graded as equivocal for recurrence, whereas the contrast group recorded no equivocal results while recording the same number of negatives.
Cervical cancer
FDG-PET/CT in primary cervical cancer
Accurate pretreatment staging of cervical carcinoma has a significant impact on planning the optimal primary treatment modality. The selection of patients suitable for surgery requires the accurate ruling out of disease beyond the cervix into the parametrium and regional or distant nodal metastases (Fig. 7a,b). Primary cervical carcinoma is typically highly metabolically active, although the presence of FDG within the urinary tract can create diagnostic difficulty, now largely overcome by PET/CT co-registration. An early FDG-PET study identified all 21 primary tumours when patients voided just prior to imaging but identified only 16 (76%) of the primary tumours when there was activity in the bladder[43]. In a series of 101 patients, FDG-PET detected 99% of primary cervical cancers, using hydration, diuretics and bladder drainage for reducing urinary activity[44]. Similar results were shown in several smaller series[45–47]. The level of metabolic activity of primary cervical tumours has been reported to be a predictor of survival (Fig. 8)[48]. However, since the FDG uptake and subsequent SUV measurements are influenced by many factors such as tumour size, blood glucose level, time interval and mode of PET data acquisition as well as PET image reconstruction and analysis, the use of SUV measurements is not yet in widespread clinical practice.
Figure 7.
Patient with FIGO stage IIB squamous cell carcinoma of the cervix. (a) Initial pretreatment MRI (axial T2-weighted image) demonstrates primary cervical tumour with parametrial invasion (long arrow) and left pelvic node involvement (short arrow). No para-aortic lymphadenopathy was demonstrated on abdominal sequences. Fused FDG-PET/CT confirmed highly FDG-avid primary tumour and a single pelvic nodal metastasis (b). Therefore the patient was not suitable for radical surgery and underwent primary chemoradiotherapy treatment. FDG-PET/CT was performed 3 months after completion of treatment (c,d). This demonstrates complete response to the irradiated tumour in the pelvis (c). However, there is disease relapse above the radiotherapy field in the para-aortic region (arrow, d). This finding indicates an adverse prognosis.
Figure 8.
Stage IIa adenocarcinoma of the cervix. Sagittal T2-weighted MRI (a) demonstrates an ill-defined tumour in the cervix (arrow). MIP image of an FDG-PET study (b) demonstrates intermediate- to low-grade FDG uptake in the primary tumour (arrow). The level of tumour metabolic activity has been reported to be a predictor of survival.
Quantitative assessment of tumour volume by FDG-PET has been found to correlate with prognosis[49]. However, this information is also available by MRI, which in contrast to FDG-PET provides detailed anatomic information that allows assessment of local invasion and radiation treatment planning. Although FDG-PET is generally positive in patients with primary cervical cancer, the lack of anatomic information, even in conjunction with CT, limits the clinical utility for initial staging of the local extent of disease.
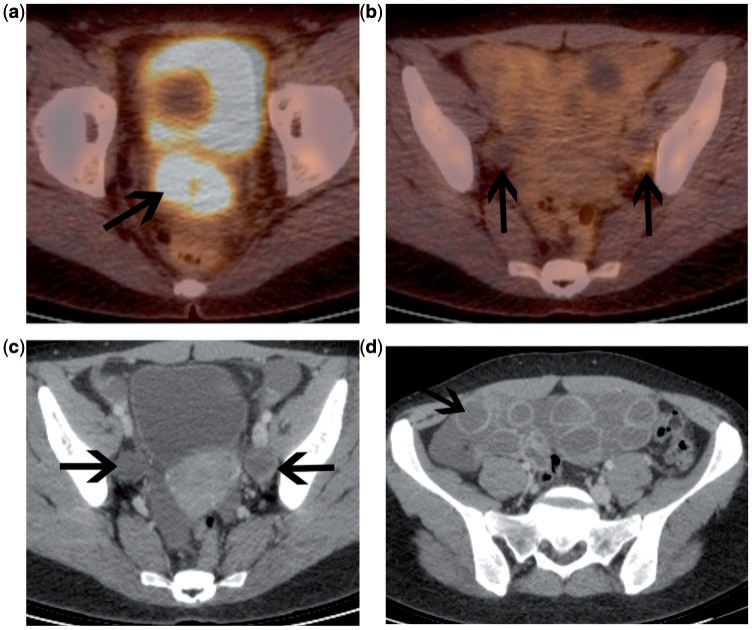
One area for which cross-sectional anatomic imaging performs poorly is in the detection of nodal metastatic disease. In 35 patients, Reinhart et al.[47] compared the diagnostic accuracy of MRI with FDG-PET for detection of metastatic lymph node involvement prior to radical hysterectomy and pelvic lymphadenectomy. Histology revealed pN0-stage cancer in 24 patients and pN1-stage cancer in 11 patients. FDG-PET had a sensitivity of 91% with a corresponding specificity of 100%, compared with 73% and 83%, respectively, for MRI. In another study of 22 patients, FDG-PET found 9 unsuspected extrapelvic nodal sites (6 para-aortic, 2 mediastinal and 1 supraclavicular node)[45]. However, FDG-PET missed 8 microscopic pelvic nodal metastases. Although FDG-PET can visualize increased metabolic activity in tumour-involved lymph nodes of normal size, it cannot detect microscopic tumour deposits, a limitation that holds true for any imaging modality. Nodal necrosis is another potential cause of false-negative nodal staging on FDG-PET (Fig. 9). However, conventional CT and MRI are highly accurate in the diagnosis of nodal necrosis, which has an extremely high positive predictive value for nodal involvement[50].
Figure 9.
Patient with advanced cervical carcinoma at presentation. Fused FDG-PET/CT demonstrates a highly metabolically active primary tumour (a). Nodal evaluation is difficult on the fused image (b). However, on the contrast-enhanced CT, there is bilateral nodal necrosis (arrows). These could not be mistaken for the ovaries in this case, as there was ovarian hyperstimulation prior to egg retrieval (d).
Narayan et al.[46] assessed whether FDG-PET or MRI could obviate the need for nodal sampling in patients with locally advanced cervical carcinoma prior to radiotherapy. Imaging findings were compared with surgical staging in 27 patients. In 24 patients evaluable for pelvic nodal status, sensitivity and specificity for FDG-PET were 83% and 92%, respectively. MRI detected only 6 of 12 (50%) patients with confirmed pelvic nodal disease, all of which were also seen by CT. PET detected 4 of 7 cases with confirmed para-aortic nodal involvement. All of the histologically confirmed sites of nodal involvement not identified on PET were less than 1 cm in diameter. FDG-PET had an overall accuracy of 75%. As more data are acquired and reported, there appears to be a distinction in diagnostic performance between patients with advanced stage disease, for which FDG-PET has performed reasonably well, and early stage disease, for which PET sensitivity is limited[51,52]. Chou et al.[51] evaluated FDG-PET in 60 patients with early stage disease with tumour less than 4 cm in long axis with no nodal enlargement on MRI. FDG-PET only identified 1 of the 10 histologically confirmed nodal metastases.
The lymph node status as assessed by FDG-PET can be a predictor of disease-free survival[44]. For pelvic lymph node status, the 2-year disease-free survival was 84% for CT and FDG-PET negative patients, 64% for CT negative and FDG-PET positive patients and 48% for CT and FDG-PET positive patients. The para-aortic lymph node status as assessed by FDG-PET was the strongest predictor of survival in a multivariate regression analysis. No patients with positive supraclavicular lymph nodes on FDG-PET survived for 2 years[54]. The cause-specific survival for patients with FIGO stage IIIb carcinoma was found to be highly dependent on the extent of lymph node metastasis demonstrated by FDG-PET at initial presentation. Three-year estimates of cause-specific survival were 73% for patients with no lymph node metastasis, 58% for those with only pelvic lymph node metastasis, 29% for those with pelvic and para-aortic lymph node metastasis and 0% for those with pelvic and para-aortic and supraclavicular lymph node metastasis[54].
Quantification of metabolic activity within nodes has also been shown to be a prognostic biomarker outcome in patients with advanced stage disease[55]. In patients with pelvic nodal SUV >4.3, the chance of responding to treatment was lower, the recurrence rate was higher and the survival rate was reduced.
In summary, FDG-PET/CT is a reasonably sensitive method of detecting pelvic and para-aortic lymph nodal disease in cervical cancer in patients with advanced stage disease, and seems to be superior to MRI and CT. It is currently recommended by the National Comprehensive Cancer Network (NCCN) for the initial staging of cervical cancer stage IB2 or higher[56]. Despite the limitations of FDG-PET in identifying small foci of disease, there seems to be an important role for FDG in the selection of patients for radiation therapy without the need for histologic confirmation of lymph node involvement. Prognosis is also correlated to the presence and extent of FDG-avid lymphadenopathy.
FDG-PET/CT in radiotherapy planning
The delineation of metabolically active disease by FDG-PET/CT has the potential to improve the accuracy of three-dimensional radiotherapy planning. The technique of co-registration of FDG-PET images with the radiotherapy planning CT has been evaluated predominantly in patients with lung or head and neck cancer and an expert report outlining the use of PET and PET/CT in radiation therapy planning has been published by the International Atomic Energy Agency[57]. In patients with cervical cancer limited to the pelvis, the irradiated tumour volume should include the pelvis only. In patients in whom para-aortic nodal metastases are suspected, the irradiated volume should include the pelvis as well as the para-aortic region. The extent of nodal metastases demonstrated on FDG-PET may be used to help delineate the radiotherapy plan.
However, the need for accurate geographic delineation of the tumour volume has become of particular relevance with the advent of conformal radiotherapy and intensity-modulated radiotherapy (IMRT) whereby the shape of the radiotherapy field can be planned to encompass the tumour and metastatic deposits while avoiding sensitive normal tissues, such as small bowel and bladder. In addition, the radiotherapy dose may be adjusted in order to deliver a boost dose to appropriate regions. Nodal irradiation can be accurately contoured for IMRT using established guidelines[58].
In order to use FDG-PET-guided IMRT for cervical cancer, patients must undergo PET/CT radiotherapy simulation. This involves positioning the patient in the PET scanner in the same position as that used during the radiotherapy treatment. This includes the use of a flat-bed insert, standard skin markers and immobilization devices. Using the PET portion of the study, the metabolic tumour volume can be contoured specifically to encompass the metabolically active primary tumour as well as sites of nodal disease[59]. In addition, standard coverage of nodal volumes is planned and then the final planning target volume is delineated, providing a safe margin to allow for slight movements of the tumour during the treatment.
Theoretically, this technique has the potential to allow higher doses to be delivered to the active tumour tissues, while avoiding irradiation of adjacent normal structures. The outcome may be reduced toxicity with greater tumour control. However, further research is needed in order to establish whether the potential benefits are demonstrated in patients.
FDG-PET/CT in recurrent cervical cancer
Approximately one-third of women diagnosed with cervical cancer develop recurrent disease. Recurrent disease typically occurs relatively early; about 90% of recurrences are detected within 3 years of initial treatment (http://www.cancerresearch.org.uk). Accurate and timely detection of recurrent disease is vital and may ultimately improve overall survival[60]. CT and MRI are widely used in the detection of local and distant recurrence. However, several limitations of these anatomic techniques are recognized. Benign post-treatment appearances and fibrosis may mimic residual or recurrent disease following chemoradiotherapy, which may be better characterized on PET/CT (Fig. 10). One important advantage of PET imaging is the inclusion of the whole body, allowing detection of metastatic disease in unexpected locations.
Figure 10.
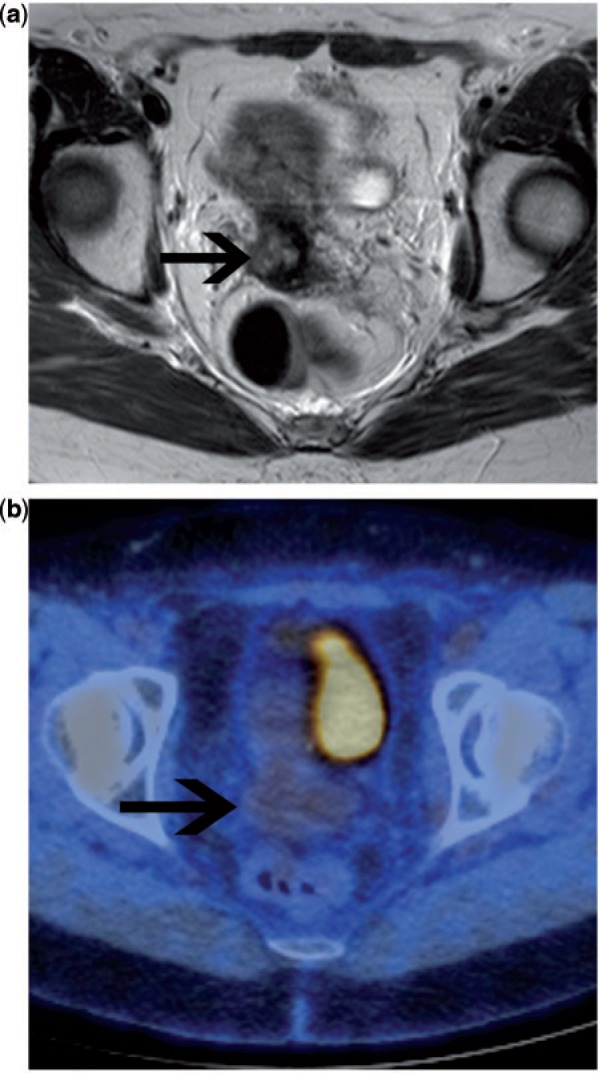
Patient with advanced carcinoma of the cervix, imaged 6 months after treatment with primary chemoradiation. Axial T2-weighted MRI (a) demonstrates an area of intermediate T2 signal intensity at the site of the original tumour. This was suspected to be residual disease. Fused FDG-PET/CT (b) demonstrates no evidence of FDG-avid disease. The patient has remained well on clinical follow-up.
A number of early studies have investigated the role of FDG-PET in recurrent cervical cancer, reporting relatively high sensitivity and specificity. In a large study of 249 patients with previously treated cervical cancer, sensitivity and specificity of FDG-PET for detection of recurrent disease were 90% and 76%, respectively[61]. Most recurrences were detected at 9–12 months after completion of treatment with curative intent, suggesting its value may lie in the detection of early recurrence. However, it should be noted that false-positive FDG uptake from a wide variety of sources including infection, post-radiation inflammation in the immediate post-treatment period or in patients with fistulas can all make interpretation slightly more difficult. All these factors need to be taken into account when deciding the appropriate time interval between completion of treatment and follow-up PET.
The introduction of integrated PET/CT, with its improved anatomic localization of suspicious areas of increased metabolic activity, reduces false-positive PET findings, thereby increasing specificity. The role of integrated PET/CT in recurrent cervical carcinoma has been evaluated in a number of recent studies[62–65] (Table 3). Although most of these were small retrospective studies, they show sensitivity and specificity values above 80% and endorse the belief that FDG-PET/CT has favourable efficacy for the detection of recurrence after initial treatment with curative intent. The Scottish Guidelines of Management of Cervical Cancer state that for detection of relapsed disease, whole-body PET or PET/CT should be performed on all patients in whom recurrent or persistent disease has been demonstrated on MRI or CT and for whom salvage therapy (either pelvic exenteration or radiotherapy) is being considered[66]. This guideline recommends performance of PET/CT at 9 months after completion of treatment in all women who have had primary chemoradiotherapy[66].
Table 3.
Studies evaluating the role of integrated PET/CT in recurrent cervical cancer
| Study | No. of patients | Sensitivity (%) | Specificity (%) | Accuracy (%) |
|---|---|---|---|---|
| Chung et al.[62] | 52 | 90.3 | 81 | 86.5 |
| Sironi et al.[63] | 25 | 92.9 | 100 | 96 |
| Kitajima et al.[65] | 52 | 92 | 92.6 | 92.3 |
| Mittra et al.[64] | 30 | 93 (local recurrence) | 93 (local recurrence) | 93 (local recurrence) |
| 96 (distant metastasis) | 95 (distant metastasis) | 95 (distant metastasis) |
FDG-PET/CT for monitoring therapy response in cervical cancer
The role of FDG-PET in monitoring response has been evaluated in several different tumour types. This has been based on in vitro studies that associate decreases in tumour cell glucose uptake with decreases in the fraction of viable tumour cells[67].
A number of retrospective studies have demonstrated that the use of FDG-PET in the post-therapy evaluation of patients with cervical carcinoma is predictive of survival outcome[68,69]. In one such study of 152 patients by Grigsby et al.[68], post-therapy FDG-PET was performed 1–12 months (mean 3 months) after completion of treatment. Five-year overall survival rates were 92% for those with no abnormal FDG uptake, 46% for those with persistent FDG uptake and 0% for those with new FDG uptake (the corresponding 5-year cause-specific survival rates were 80%, 32% and 0%, respectively) (Fig. 7). These findings were validated in a clinically important follow-up prospective cohort study by the same group[70]. Ninety-two women with cervical carcinoma were treated with concurrent chemoradiation and post-therapy FDG-PET/CT was performed 2–4 months (mean 3 months) after completion of therapy. Complete metabolic response was evident in 65 patients (70%), a partial metabolic response in 15 (16%) and progressive disease in 12 (13%). Their 3-year progression-free survival rates were 78%, 33% and 0%, respectively. This study validates the use of post-treatment FDG-PET as a metabolic biomarker of tumour response in cervical carcinoma. It also provides long-term prognostic information only 3 months after treatment with curative intent and may help to select patients for additional treatment where necessary.
FDG-PET/CT imaging of vulvar cancer
There are scant studies reporting PET results in vulval cancer staging[71–73]. As described for other gynaecological malignancies, FDG-PET/CT can visualize metastatic lymph nodes when they are still of normal size according to conventional cross-sectional imaging, thus showing promising results also for vulval cancer lymph node staging, which requires further investigation and validation (Fig. 11).
Figure 11.
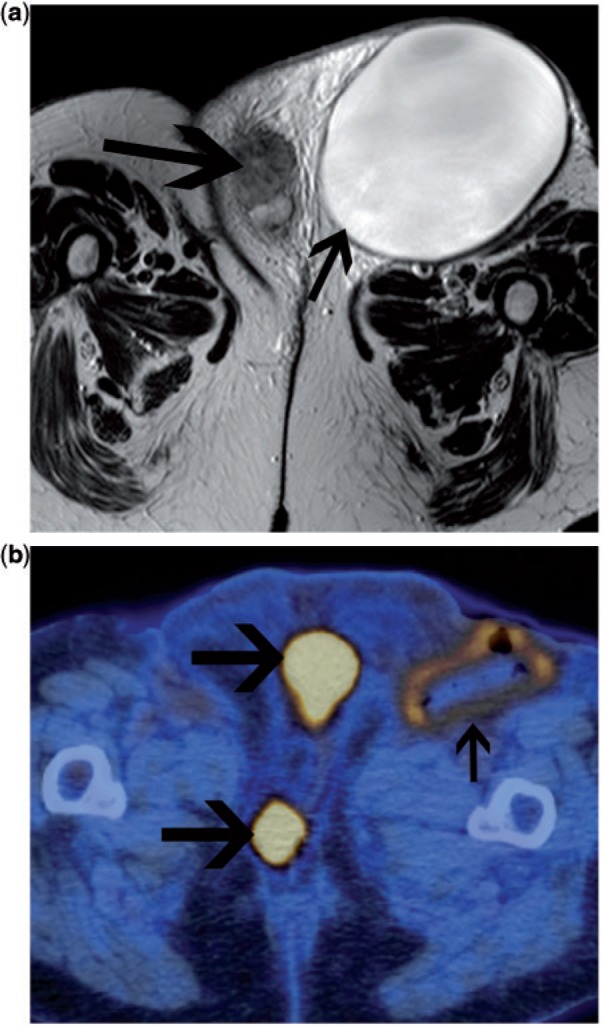
Patient with recurrent vulval carcinoma. On the axial T2-weighted MRI (a), there is a large lymphocyst in the left groin due to previous groin node dissection (small arrow). The recurrent tumour is seen in the vulva (large arrow). The patient underwent percutaneous drainage of the lymphocyst. Following this, FDG-PET/CT was undertaken to exclude other sites of disease prior to planning further surgery. Fused FDG-PET/CT confirmed multifocal disease in the vulva (small arrows). Low-grade FDG uptake is seen in the wall of the drained lymphocyst, consistent with probable inflammatory change. No FDG-avid disease was seen elsewhere.
Summary
-
1.
Ovarian cancer:
-
a.
Current indications:
-
•
In patient with CA-125 relapse and negative conventional imaging, FDG-PET may identify the site of relapse. This may allow prompt treatment including surgical resection of isolated recurrence or chemotherapy/trial entry.
-
b.
Likely indications in the near future:
-
•
In monitoring treatment response, allowing early identification of metabolic responders vs non-responders.
-
•
FDG-PET/CT may become the initial imaging modality in patients with CA-125 relapse, obviating the current initial step of conventional CT. This would very likely improve detection of sites of relapse as well as being used as the baseline for subsequent early prediction of response to chemotherapy.
-
c.
Possible indications in the future:
-
•
FDG-PET/CT may potentially be used as the initial imaging modality in staging ovarian cancer. This would very likely enhance initial staging accuracy as well as provide a baseline for assessment of response for patients undergoing neoadjuvant chemotherapy, allowing early change to alternative therapy in non-responders.
-
d.
Unlikely to be of benefit:
-
•
In the evaluation of indeterminate adnexal masses.
-
2.
Endometrial cancer:
-
a.
Unlikely to be of benefit:
-
•
FDG-PET has not been shown to be of value in the initial staging of most patients with endometrial cancer or the evaluation of endometrial masses. Diagnostic performance for the detection of nodal metastases is not sufficiently high to add any value to staging with MRI.
-
b.
Of potential benefit:
-
•
FDG-PET may be advocated in selected patients with high-risk disease (advanced local disease and/or high-grade histology) for the identification of extranodal metastatic disease in the abdomen or thorax.
-
•
There is some evidence to support FDG-PET in patients clinically suspected of recurrent disease that has not been identified on conventional imaging.
-
3.
Cervix cancer:
-
a.
Current indications:
-
•
At presentation, FDG-PET or PET/CT is recommended as a staging investigation in patients with FIGO stage IIB or above, in order to facilitate optimal radiotherapy planning and for prognostication. Patients with earlier stage tumours that are greater than 4 cm may also be considered for staging with FDG-PET.
-
•
Following treatment with primary chemoradiation, imaging at 3–6 months after the end of treatment may be used to evaluate response. Patients with residual metabolically active tumour may be offered salvage therapy, ideally in the context of a clinical trial. The extent of residual FDG uptake may help to determine the appropriate salvage therapy; surgery may be possible if metabolic activity is limited to the primary site, or alternative chemotherapy may be offered if FDG uptake is identified beyond the cervix.
-
•
FDG-PET is indicated in detection of relapse, prior to consideration of surgical exenteration.
-
b.
Unlikely to be of benefit:
-
•
There is currently no evidence to support the use of FDG-PET or PET/CT in staging patients who present with early stage disease clinically, FIGO stage IB1 or less. Diagnostic performance for the detection of nodal metastases in this group of patients is not sufficient to exclude nodal metastases.
-
c.
Possible future role:
-
•
The use of FDG-PET/CT in the assessment of treatment response in clinical trials of novel chemotherapy agents is a potential future role which requires further study.
Footnotes
This paper is available online at http://www.cancerimaging.org. In the event of a change in the URL address, please use the DOI provided to locate the paper.
References
- 1.Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105:103–4. doi: 10.1016/j.ijgo.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 2.Fletcher JW, Djulbegovic B, Soares HP, et al. Recommendations on the use of 18F-FDG PET in oncology. J Nucl Med. 2008;49:480–508. doi: 10.2967/jnumed.107.047787. [DOI] [PubMed] [Google Scholar]
- 3.Weber WA, Avril N, Schwaiger M. Relevance of positron emission tomography (PET) in oncology. Strahlenther Onkol. 1999;175:356–73. doi: 10.1007/s000660050022. [DOI] [PubMed] [Google Scholar]
- 4.Short S, Hoskin P, Wong W. Ovulation and increased FDG uptake on PET: potential for a false-positive result. Clin Nucl Med. 2005;30:707. doi: 10.1097/01.rlu.0000178248.98702.9e. [DOI] [PubMed] [Google Scholar]
- 5.Subhas N, Patel PV, Pannu HK, Jacene HA, Fishman EK, Wahl RL. Imaging of pelvic malignancies with in-line FDG PET-CT: case examples and common pitfalls of FDG PET. Radiographics. 2005;25:1031–43. doi: 10.1148/rg.254045155. [DOI] [PubMed] [Google Scholar]
- 6.Stahl A, Weber WA, Avril N, Schwaiger M. Effect of N-butylscopolamine on intestinal uptake of fluorine-18-fluorodeoxyglucose in PET imaging of the abdomen. Nuklearmedizin. 2000;39:241–5. [PubMed] [Google Scholar]
- 7.Castellucci P, Perrone AM, Picchio M, et al. Diagnostic accuracy of 18F-FDG PET/CT in characterizing ovarian lesions and staging ovarian cancer: correlation with transvaginal ultrasonography, computed tomography, and histology. Nucl Med Commun. 2007;28:589–95. doi: 10.1097/MNM.0b013e3281afa256. [DOI] [PubMed] [Google Scholar]
- 8.Fenchel S, Grab D, Nuessle K, et al. Asymptomatic adnexal masses: correlation of FDG PET and histopathologic findings. Radiology. 2002;223:780–8. doi: 10.1148/radiol.2233001850. [DOI] [PubMed] [Google Scholar]
- 9.Grab D, Flock F, Stohr I, et al. Classification of asymptomatic adnexal masses by ultrasound, magnetic resonance imaging, and positron emission tomography. Gynecol Oncol. 2000;77:454–9. doi: 10.1006/gyno.2000.5768. [DOI] [PubMed] [Google Scholar]
- 10.Nam EJ, Yun MJ, Oh YT, et al. Diagnosis and staging of primary ovarian cancer: correlation between PET/CT, Doppler US, and CT or MRI. Gynecol Oncol. 2010;116:389–94. doi: 10.1016/j.ygyno.2009.10.059. [DOI] [PubMed] [Google Scholar]
- 11.Rieber A, Nussle K, Stohr I, et al. Preoperative diagnosis of ovarian tumors with MR imaging: comparison with transvaginal sonography, positron emission tomography, and histologic findings. AJR Am J Roentgenol. 2001;177:123–9. doi: 10.2214/ajr.177.1.1770123. [DOI] [PubMed] [Google Scholar]
- 12.Kawahara K, Yoshida Y, Kurokawa T, et al. Evaluation of positron emission tomography with tracer 18-fluorodeoxyglucose in addition to magnetic resonance imaging in the diagnosis of ovarian cancer in selected women after ultrasonography. J Comput Assist Tomogr. 2004;28:505–16. doi: 10.1097/00004728-200407000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Risum S, Hogdall C, Loft A, et al. The diagnostic value of PET/CT for primary ovarian cancer–a prospective study. Gynecol Oncol. 2007;105:145–9. doi: 10.1016/j.ygyno.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 14.Kim SK, Kang KW, Roh JW, Sim JS, Lee ES, Park SY. Incidental ovarian 18F-FDG accumulation on PET: correlation with the menstrual cycle. Eur J Nucl Med Mol Imaging. 2005;32:757–63. doi: 10.1007/s00259-005-1771-6. [DOI] [PubMed] [Google Scholar]
- 15.Nishizawa S, Inubushi M, Okada H. Physiological 18F-FDG uptake in the ovaries and uterus of healthy female volunteers. Eur J Nucl Med Mol Imaging. 2005;32:549–56. doi: 10.1007/s00259-004-1703-x. [DOI] [PubMed] [Google Scholar]
- 16.Lerman H, Metser U, Grisaru D, Fishman A, Lievshitz G, Even-Sapir E. Normal and abnormal 18F-FDG endometrial and ovarian uptake in pre- and postmenopausal patients: assessment by PET/CT. J Nucl Med. 2004;45:266–71. [PubMed] [Google Scholar]
- 17.Yoshida Y, Kurokawa T, Kawahara K, et al. Incremental benefits of FDG positron emission tomography over CT alone for the preoperative staging of ovarian cancer. AJR Am J Roentgenol. 2004;182:227–33. doi: 10.2214/ajr.182.1.1820227. [DOI] [PubMed] [Google Scholar]
- 18.Kitajima K, Murakami K, Yamasaki E, et al. Diagnostic accuracy of integrated FDG-PET/contrast-enhanced CT in staging ovarian cancer: comparison with enhanced CT. Eur J Nucl Med Mol Imaging. 2008;35:1912–20. doi: 10.1007/s00259-008-0890-2. [DOI] [PubMed] [Google Scholar]
- 19.De Rosa V, Mangoni di Stefano ML, Brunetti A, et al. Computed tomography and second-look surgery in ovarian cancer patients. Correlation, actual role and limitations of CT scan. Eur J Gynaecol Oncol. 1995;16:123–9. [PubMed] [Google Scholar]
- 20.Gu P, Pan LL, Wu SQ, Sun L, Huang G. CA 125, PET alone, PET-CT, CT and MRI in diagnosing recurrent ovarian carcinoma: a systematic review and meta-analysis. Eur J Radiol. 2009;71:164–74. doi: 10.1016/j.ejrad.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 21.Nakamoto Y, Saga T, Ishimori T, et al. Clinical value of positron emission tomography with FDG for recurrent ovarian cancer. AJR Am J Roentgenol. 2001;176:1449–54. doi: 10.2214/ajr.176.6.1761449. [DOI] [PubMed] [Google Scholar]
- 22.Sebastian S, Lee SI, Horowitz NS, et al. PET-CT vs. CT alone in ovarian cancer recurrence. Abdom Imaging. 2008;33:112–18. doi: 10.1007/s00261-007-9218-0. [DOI] [PubMed] [Google Scholar]
- 23.Thrall MM, DeLoia JA, Gallion H, Avril N. Clinical use of combined positron emission tomography and computed tomography (FDG-PET/CT) in recurrent ovarian cancer. Gynecol Oncol. 2007;105:17–22. doi: 10.1016/j.ygyno.2006.10.060. [DOI] [PubMed] [Google Scholar]
- 24.Iagaru AH, Mittra ES, McDougall IR, Quon A, Gambhir SS. 18F-FDG PET/CT evaluation of patients with ovarian carcinoma. Nucl Med Commun. 2008;29:1046–51. doi: 10.1097/MNM.0b013e32831089cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mangili G, Picchio M, Sironi S, et al. Integrated PET/CT as a first-line re-staging modality in patients with suspected recurrence of ovarian cancer. Eur J Nucl Med Mol Imaging. 2007;34:658–66. doi: 10.1007/s00259-006-0306-0. [DOI] [PubMed] [Google Scholar]
- 26.Cho SM, Ha HK, Byun JY, Lee JM, Kim CJ, Nam-Koong SE. Usefulness of FDG PET for assessment of early recurrent epithelial ovarian cancer. AJR Am J Roentgenol. 2002;179:391–5. doi: 10.2214/ajr.179.2.1790391. [DOI] [PubMed] [Google Scholar]
- 27.Sironi S, Messa C, Mangili G, et al. Integrated FDG PET/CT in patients with persistent ovarian cancer: correlation with histologic findings. Radiology. 2004;233:433–40. doi: 10.1148/radiol.2332031800. [DOI] [PubMed] [Google Scholar]
- 28.Simcock B, Neesham D, Quinn M, Drummond E, Milner A, Hicks RJ. The impact of PET/CT in the management of recurrent ovarian cancer. Gynecol Oncol. 2006;103:271–6. doi: 10.1016/j.ygyno.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 29.Kitajima K, Murakami K, Yamasaki E, et al. Performance of integrated FDG-PET/contrast-enhanced CT in the diagnosis of recurrent ovarian cancer: comparison with integrated FDG-PET/non-contrast-enhanced CT and enhanced CT. Eur J Nucl Med Mol Imaging. 2008;35:1439–48. doi: 10.1007/s00259-008-0776-3. [DOI] [PubMed] [Google Scholar]
- 30.Weber WA. Positron emission tomography as an imaging biomarker. J Clin Oncol. 2006;24:3282–92. doi: 10.1200/JCO.2006.06.6068. [DOI] [PubMed] [Google Scholar]
- 31.Avril NE, Weber WA. Monitoring response to treatment in patients utilizing PET. Radiol Clin North Am. 2005;43:189–204. doi: 10.1016/j.rcl.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 32.Avril N, Sassen S, Schmalfeldt B, et al. Prediction of response to neoadjuvant chemotherapy by sequential F-18-fluorodeoxyglucose positron emission tomography in patients with advanced-stage ovarian cancer. J Clin Oncol. 2005;23:7445–53. doi: 10.1200/JCO.2005.06.965. [DOI] [PubMed] [Google Scholar]
- 33.Horowitz NS, Dehdashti F, Herzog TJ, et al. Prospective evaluation of FDG-PET for detecting pelvic and para-aortic lymph node metastasis in uterine corpus cancer. Gynecol Oncol. 2004;95:546–51. doi: 10.1016/j.ygyno.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki R, Miyagi E, Takahashi N, et al. Validity of positron emission tomography using fluoro-2-deoxyglucose for the preoperative evaluation of endometrial cancer. Int J Gynecol Cancer. 2007;17:890–6. doi: 10.1111/j.1525-1438.2007.00859.x. [DOI] [PubMed] [Google Scholar]
- 35.Kitajima K, Murakami K, Yamasaki E, et al. Accuracy of 18F-FDG PET/CT in detecting pelvic and paraaortic lymph node metastasis in patients with endometrial cancer. AJR Am J Roentgenol. 2008;190:1652–8. doi: 10.2214/AJR.07.3372. [DOI] [PubMed] [Google Scholar]
- 36.Nayot D, Kwon JS, Carey MS, Driedger A. Does preoperative positron emission tomography with computed tomography predict nodal status in endometrial cancer? A pilot study. Curr Oncol. 2008;15:123–5. doi: 10.3747/co.v15i3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Picchio M, Mangili G, Samanes Gajate AM, et al. High-grade endometrial cancer: value of [(18)F]FDG PET/CT in preoperative staging. Nucl Med Commun. 2010;31:506–12. doi: 10.1097/MNM.0b013e328337cb47. [DOI] [PubMed] [Google Scholar]
- 38.Signorelli M, Guerra L, Buda A, et al. Role of the integrated FDG PET/CT in the surgical management of patients with high risk clinical early stage endometrial cancer: detection of pelvic nodal metastases. Gynecol Oncol. 2009;115:231–5. doi: 10.1016/j.ygyno.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 39.Nakamura K, Kodama J, Okumura Y, Hongo A, Kanazawa S, Hiramatsu Y. The SUVmax of 18F-FDG PET correlates with histological grade in endometrial cancer. Int J Gynecol Cancer. 2010;20:110–15. doi: 10.1111/IGC.0b013e3181c3a288. [DOI] [PubMed] [Google Scholar]
- 40.Chung HH, Kang WJ, Kim JW, et al. The clinical impact of [(18)F]FDG PET/CT for the management of recurrent endometrial cancer: correlation with clinical and histological findings. Eur J Nucl Med Mol Imaging. 2008;35:1081–8. doi: 10.1007/s00259-007-0687-8. [DOI] [PubMed] [Google Scholar]
- 41.Park JY, Kim EN, Kim DY, et al. Clinical impact of positron emission tomography or positron emission tomography/computed tomography in the posttherapy surveillance of endometrial carcinoma: evaluation of 88 patients. Int J Gynecol Cancer. 2008;18:1332–8. doi: 10.1111/j.1525-1438.2008.01197.x. [DOI] [PubMed] [Google Scholar]
- 42.Kitajima K, Suzuki K, Nakamoto Y, et al. Low-dose non-enhanced CT versus full-dose contrast-enhanced CT in integrated PET/CT studies for the diagnosis of uterine cancer recurrence. Eur J Nucl Med Mol Imaging. 2010;37:1490–8. doi: 10.1007/s00259-010-1440-2. [DOI] [PubMed] [Google Scholar]
- 43.Sugawara Y, Eisbruch A, Kosuda S, Recker BE, Kison PV, Wahl RL. Evaluation of FDG PET in patients with cervical cancer. J Nucl Med. 1999;40:1125–31. [PubMed] [Google Scholar]
- 44.Grigsby PW, Siegel BA, Dehdashti F. Lymph node staging by positron emission tomography in patients with carcinoma of the cervix. J Clin Oncol. 2001;19:3745–3749. doi: 10.1200/JCO.2001.19.17.3745. [DOI] [PubMed] [Google Scholar]
- 45.Belhocine T, Thille A, Fridman V, et al. Contribution of whole-body (18)FDG PET imaging in the management of cervical cancer. Gynecol Oncol. 2002;87:90–97. doi: 10.1006/gyno.2002.6769. [DOI] [PubMed] [Google Scholar]
- 46.Narayan K, Hicks RJ, Jobling T, Bernshaw D, McKenzie AF. A comparison of MRI and PET scanning in surgically staged loco-regionally advanced cervical cancer: potential impact on treatment. Int J Gynecol Cancer. 2001;11:263–71. doi: 10.1046/j.1525-1438.2001.011004263.x. [DOI] [PubMed] [Google Scholar]
- 47.Reinhardt MJ, Ehritt-Braun C, Vogelgesang D, et al. Metastatic lymph nodes in patients with cervical cancer: detection with MR imaging and FDG PET. Radiology. 2001;218:776–82. doi: 10.1148/radiology.218.3.r01mr19776. [DOI] [PubMed] [Google Scholar]
- 48.Xue F, Lin LL, Dehdashti F, Miller TR, Siegel BA, Grigsby PW. F-18 fluorodeoxyglucose uptake in primary cervical cancer as an indicator of prognosis after radiation therapy. Gynecol Oncol. 2006;101:147–51. doi: 10.1016/j.ygyno.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 49.Miller TR, Grigsby PW. Measurement of tumor volume by PET to evaluate prognosis in patients with advanced cervical cancer treated by radiation therapy. Int J Radiat Oncol Biol Phys. 2002;53:353–9. doi: 10.1016/s0360-3016(02)02705-0. [DOI] [PubMed] [Google Scholar]
- 50.Yang WT, Lam WW, Yu MY, Cheung TH, Metreweli C. Comparison of dynamic helical CT and dynamic MR imaging in the evaluation of pelvic lymph nodes in cervical carcinoma. AJR Am J Roentgenol. 2000;175:759–66. doi: 10.2214/ajr.175.3.1750759. [DOI] [PubMed] [Google Scholar]
- 51.Chou HH, Chang HP, Lai CH, et al. 18)F-FDG PET in stage IB/IIB cervical adenocarcinoma/adenosquamous carcinoma. Eur J Nucl Med Mol Imaging. 37:728–35. doi: 10.1007/s00259-009-1336-1. [DOI] [PubMed] [Google Scholar]
- 52.Magne N, Chargari C, Vicenzi L, et al. New trends in the evaluation and treatment of cervix cancer: the role of FDG-PET. Cancer Treat Rev. 2008;34:671–81. doi: 10.1016/j.ctrv.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 53.Tran BN, Grigsby PW, Dehdashti F, Herzog TJ, Siegel BA. Occult supraclavicular lymph node metastasis identified by FDG-PET in patients with carcinoma of the uterine cervix. Gynecol Oncol. 2003;90:572–6. doi: 10.1016/S0090-8258(03)00402-5. [DOI] [PubMed] [Google Scholar]
- 54.Singh AK, Grigsby PW, Dehdashti F, Herzog TJ, Siegel BA. FDG-PET lymph node staging and survival of patients with FIGO stage IIIb cervical carcinoma. Int J Radiat Oncol Biol Phys. 2003;56:489–93. doi: 10.1016/S0360-3016(02)04521-2. [DOI] [PubMed] [Google Scholar]
- 55.Kidd EA, Siegel BA, Dehdashti F, Grigsby PW. Pelvic lymph node F-18 fluorodeoxyglucose uptake as a prognostic biomarker in newly diagnosed patients with locally advanced cervical cancer. Cancer. 2010;116:1469–75. doi: 10.1002/cncr.24972. [DOI] [PubMed] [Google Scholar]
- 56.Cervical cancer 1.2011. NCCN clinical practice guidelines in oncology (NCCN Guidelines™) http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#site (accessed 30 January 2012; login required) [Google Scholar]
- 57.MacManus M, Nestle U, Rosenzweig KE, et al. Use of PET and PET/CT for radiation therapy planning: IAEA expert report 2006–2007. Radiother Oncol. 2009;91:85–94. doi: 10.1016/j.radonc.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 58.Taylor A, Rockall AG, Reznek RH, Powell ME. Mapping pelvic lymph nodes: guidelines for delineation in intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2005;63:1604–12. doi: 10.1016/j.ijrobp.2005.05.062. [DOI] [PubMed] [Google Scholar]
- 59.Grigsby PW. PET/CT imaging to guide cervical cancer therapy. Future Oncol. 2009;5:953–958. doi: 10.2217/fon.09.70. [DOI] [PubMed] [Google Scholar]
- 60.Babar S, Rockall A, Goode A, Shepherd J, Reznek R. Magnetic resonance imaging appearances of recurrent cervical carcinoma. Int J Gynecol Cancer. 2007;17:637–45. doi: 10.1111/j.1525-1438.2007.00849.x. [DOI] [PubMed] [Google Scholar]
- 61.Ryu SY, Kim MH, Choi SC, Choi CW, Lee KH. Detection of early recurrence with 18F-FDG PET in patients with cervical cancer. J Nucl Med. 2003;44:347–52. [PubMed] [Google Scholar]
- 62.Chung HH, Jo H, Kang WJ, et al. Clinical impact of integrated PET/CT on the management of suspected cervical cancer recurrence. Gynecol Oncol. 2007;104:529–34. doi: 10.1016/j.ygyno.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 63.Sironi S, Picchio M, Landoni C, et al. Post-therapy surveillance of patients with uterine cancers: value of integrated FDG PET/CT in the detection of recurrence. Eur J Nucl Med Mol Imaging. 2007;34:472–9. doi: 10.1007/s00259-006-0251-y. [DOI] [PubMed] [Google Scholar]
- 64.Mittra E, El-Maghraby T, Rodriguez CA, et al. Efficacy of (18)F-FDG PET/CT in the evaluation of patients with recurrent cervical carcinoma. Eur J Nucl Med Mol Imaging. 2009;36:1952–9. doi: 10.1007/s00259-009-1206-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kitajima K, Murakami K, Yamasaki E, Domeki Y, Kaji Y, Sugimura K. Performance of FDG-PET/CT for diagnosis of recurrent uterine cervical cancer. Eur Radiol. 2008;18:2040–7. doi: 10.1007/s00330-008-0979-9. [DOI] [PubMed] [Google Scholar]
- 66.Scottish Intercollegiate Guidelines Network. Management of cervical cancer. 2008. http://www.sign.ac.uk/guidelines/fulltext/99/index.html (accessed 30 January 2012) [Google Scholar]
- 67.Spaepen K, Stroobants S, Dupont P, et al. [(18)F]FDG PET monitoring of tumour response to chemotherapy: does [(18)F]FDG uptake correlate with the viable tumour cell fraction? Eur J Nucl Med Mol Imaging. 2003;30:682–8. doi: 10.1007/s00259-003-1120-6. [DOI] [PubMed] [Google Scholar]
- 68.Grigsby PW, Siegel BA, Dehdashti F, Rader J, Zoberi I. Posttherapy [18F] fluorodeoxyglucose positron emission tomography in carcinoma of the cervix: response and outcome. J Clin Oncol. 2004;22:2167–71. doi: 10.1200/JCO.2004.09.035. [DOI] [PubMed] [Google Scholar]
- 69.Grigsby PW. The role of FDG-PET/CT imaging after radiation therapy. Gynecol Oncol. 2007;107:S27–9. doi: 10.1016/j.ygyno.2007.07.034. [DOI] [PubMed] [Google Scholar]
- 70.Schwarz JK, Siegel BA, Dehdashti F, Grigsby PW. Association of posttherapy positron emission tomography with tumor response and survival in cervical carcinoma. JAMA. 2007;298:2289–95. doi: 10.1001/jama.298.19.2289. [DOI] [PubMed] [Google Scholar]
- 71.Imperiale A, Heymann S, Claria M, et al. F-18 FDG PET-CT in a rare case of Bartholin's gland undifferentiated carcinoma managed with chemoradiation and interstitial brachytherapy. Clin Nucl Med. 2007;32:498–500. doi: 10.1097/RLU.0b013e318053783d. [DOI] [PubMed] [Google Scholar]
- 72.Husain A, Akhurst T, Larson S, Alektiar K, Barakat RR, Chi DS. A prospective study of the accuracy of 18Fluorodeoxyglucose positron emission tomography (18FDG PET) in identifying sites of metastasis prior to pelvic exenteration. Gynecol Oncol. 2007;106:177–80. doi: 10.1016/j.ygyno.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 73.De Hullu JA, Pruim J, Que TH, et al. Noninvasive detection of inguinofemoral lymph node metastases in squamous cell cancer of the vulva by L. Int J Gynecol Cancer. 1999;9:141–6. doi: 10.1046/j.1525-1438.1999.09909.x. [DOI] [PubMed] [Google Scholar]
- 74.Park JY, Kim EN, Kim DY, et al. Comparison of the validity of magnetic resonance imaging and positron emission tomography/computed tomography in the preoperative evaluation of patients with uterine corpus cancer. Gynecol Oncol. 2008;108:486–92. doi: 10.1016/j.ygyno.2007.11.044. [DOI] [PubMed] [Google Scholar]