Abstract
A new scalarane-type pentacyclic sesterterpene, sesterstatin 6 (6) was isolated in 8.3 × 10−7 % yield from the Republic of Maldives marine sponge Hyrtios erecta. The structure was elucidated by analyses of HRMS and high field 2-D NMR spectra. Sesterstatin 6 showed significant cancer cell growth inhibition against the murine P388 lymphocytic leukemia and a series of human tumor cell lines (ED50 0.17 μg/mL, GI50 1.8 ~ 8.9 × 10−1 μg/mL), and proved to be the most inhibitory of the series.
Introduction
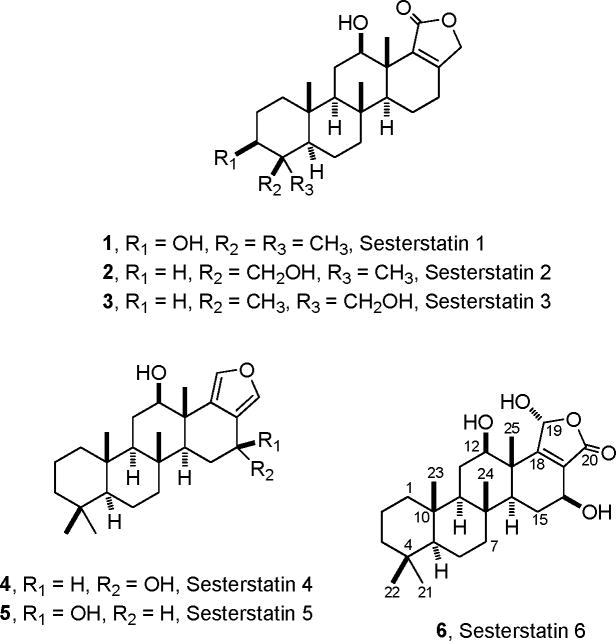
The marine poriferia are proving to be an exceptionally productive source of new terpenes exhibiting a great variety of novel structural modifications and biological activities. Illustrative are the recent isolation and structural determination of cyclosmenospongine, a sesquiterpenoid aminoquinone from Spongia sp.,2a yardenones A and B, cytotoxic triterpenes from an Axinella sp.,2b cytotoxic furanoterpenoids from Sarcotragus sp.,2c a sesquiterpenoid from Axinyssa sp.,2d cancer cell line inhibitory sesterterpenes from Thorectandra sp.,2e the cancer cell line active theopederins K and L from Discodermia sp.,2f and especially pertinent here, the cancer cell line active salmahyrtisol A from the Red Sea sponge Hyrtios erecta.2g We had earlier discovered in a Republic of Maldives collection of H. erecta the cancer cell inhibitory pentacyclic sesterterpene sesterstatins 1–5 (1–5).3,4 Both 3β-acetyl and 21-acetyl derivatives of compounds 1 and 3, respectively, were also isolated from a Red Sea collection of the sponge H. erecta.2g The present study was directed at investigating remaining cancer cell line inhibitory (murine P388 lymphocytic leukemia), albeit very minor, fractions from a scaleup recollection (600 kg) of the Republic of Maldives H. erecta. That endeavor resulted in isolation and structural elucidation of a new cancer cell line inhibitory (P388 ED50 0.17 μg/mL) pentacyclic sesterterpene designated sesterstatin 6 (6).
Results and Discussion
A fraction prepared from the CH2Cl2 – CH3OH extract of H. erecta (600 kg, wet weight) collected in the Republic of Maldives in 1994 was obtained by bioassay-directed (P388 leukemia cell line) fractionation of the extract by a sequence of solvent partitioning (between 9:1 CH3OH – H2O and n-hexane, then diluted to 3:2 CH3OH – H2O and extracted with CH2Cl2) to afford an active (P388 0.31 μg/mL) fraction. An extensive series of column gel-permeation and partition chromatographic separations on Sephadex LH-20 followed by reversed-phase HPLC separations and final purification on a C18 column with acetonitrile-H2O (1:1) as mobile phase, gave sesterstatin 6 (6) as colorless fine needles (5.0 mg, 8.3 × 10−7 % yield), m.p. 253–255 °C.
The HRFABMS of sesterstatin 6 displayed an intense [M + H]+ ion at m/z 419.2793 corresponding to molecular formula C25H38O5 requiring seven sites of unsaturation. A combination of 1H-NMR, 13C-APT, and HMQC revealed five singlet methyls, seven methylenes, six methines, four quaternary carbons, two olefinic carbons, and one carbonyl, which counted for two unsaturations. Though interrupted by quaternary carbons and overlapping signals, a sophisticated TOCSY and 1H, 1H-COSY analysis led to assignment of the following spin systems: CH2-CH2-CH2 (C1-C3), CH-CH2-CH2 (C5-C7), CH2-CH (O) (C11-C12), and CH-CH2-CH (O) (C14-C16). On the basis of the HMBC information, the four quaternary carbons (δC 33.1 C-4, 37.1 C-8, 37.3 C-10, and 45.2 C-13) were found bonded to these alkyl fragments by a series of cross peaks between the carbons and adjacent protons (Table 1) to form a substructure comprising three six-membered rings with one hydroxyl each at C-12 and C-16. The fourth ring was deduced by HMBC interpretations of olefinic carbons at C-18 (δC 167.4) with H-12 (δH 3.68), H-16 (δH 4.47), H-25 (δH 1.15) and C-17 (δC128.72) with H-16 (δH 4.47), H-15a (δH 2.11). The following NMR data analysis revealed a γ-hydroxyl, α, β-unsaturated lactone unit linked to the C-17 and C-18: (1) the 1H, 1H-COSY relationship of a hydroxyl (δH 7.80) with H-19 (δH 6.11); (2) the HMBC coupling between H-19 and a carbonyl (δC-20 170.8). The 13C shifts (δC-17 128.7, δC-18 167.4) showed that the carbonyl was connected to C-17 rather than C-18, which made the lactone ring of sesterstatin 6 (6) opposite from that of compounds 1–3.3a
Table 1.
| Positions | δC | δH | 1H, 1H-COSY | HMBC |
|---|---|---|---|---|
| 1-CH2 | 39.6 | 1.64 (1H, m) | H-1b | C-3, C-5 |
| 0.74 (1H, m) | H-1a, H-2b | |||
| 2-CH2 | 18.0 | 1.54 (1H, m) | H-2b | |
| 1.32 (1H, m) | H-1b, H-2a | C-1, C-4 | ||
| 3-CH2 | 41.9 | 1.30 (1H, m) | H-2a, H-3b | C-1, C-4, C-5 |
| 1.04 (1H, m) | H-3a | |||
| 4-C | 33.1 | |||
| 5-CH | 56.5 | 0.74 (1H, m) | H-6b | |
| 6-CH2 | 18.5 | 1.58 (1H, m) | H-6b, H-7b | C-8 |
| 1.35 (1H, m) | H-5, H-6a | |||
| 7-CH2 | 41.4 | 1.77 (1H, m) | H-7b | C-5 |
| 0.84 (1H, m) | H-6a, H-7a | |||
| 8-C | 37.1 | |||
| 9-CH | 58.4 | 0.84 (1H, m) | H-11b | C-8, C-14 |
| 10-C | 37.3 | |||
| 11-CH2 | 25.4 | 1.73 (1H, m) | H-11b | C-12, C-13, C-8 |
| 1.48 (1H, m) | H-9, H-11a, H-12 | C-12 | ||
| 12-CH | 73.7 | 3.68 (1H, dd, 11.0, 4.0) | H-11b | C-18, C-25 |
| 13-C | 45.2 | |||
| 14-CH | 53.2 | 1.10 (1H, m) | H-15a | |
| 15-CH2 | 26.0 | 2.11 (1H, dd, 12.5, 6.5) | H-15b, H-16 | C-8, C-13, C-14, C-16, C-17 |
| 1.49 (1H, m) | H-14, H-15a, H-16 | C-8, C-13, C-14, C-16 | ||
| 16-CH | 65.0 | 4.47 (1H, dd, 18.0, 2.0) | H-15a, H-15b | C-15, C-17, C-18 |
| 17-C | 128.7 | |||
| 18-C | 167.4 | |||
| 19-CH | 96.3 | 6.11 (1H, s) | C-17, C-20 | |
| 19-OH | 7.80 (1H, br, s) | |||
| 20-CO | 170.8 | |||
| 21-CH3 | 33.1 | 0.78 (3H, s) | C-3, C-4, C-5, C-22 | |
| 22-CH3 | 21.1 | 0.76 (3H, s) | C-3, C-4, C-5, C-21 | |
| 23-CH3 | 16.0 | 0.78 (3H, s) | C-1, C-5, C-9 | |
| 24-CH3 | 17.5 | 0.84 (3H, s) | C-7, C-9 | |
| 25-CH3 | 16.8 | 1.15 (3H, s) | C-12, C-18 |
500 MHz for 1H NMR, 100 MHz for 13C NMR, CDC13-DMSO-d6 in 5:1.
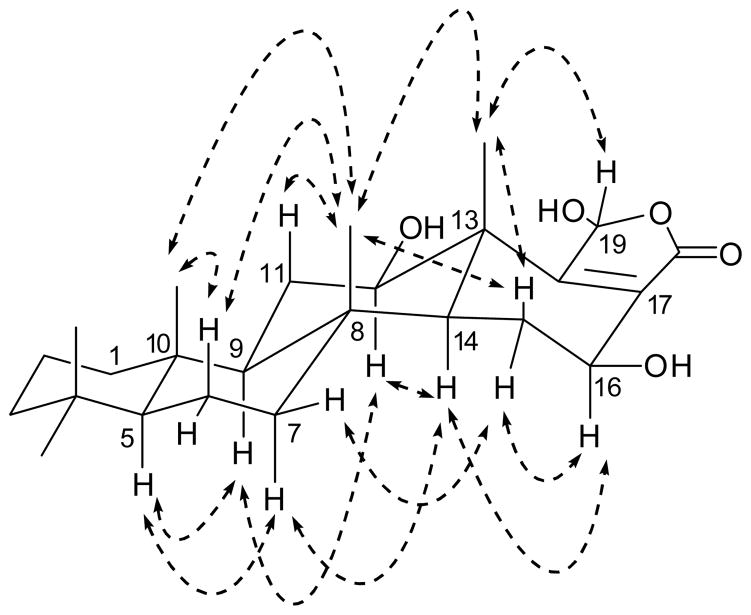
The all trans-fused A-B-C-D ring system of sesterstatin 6 (6) was suggested by the ROESY correlation (Figure 1) including the following key cross signals: H-5 (δH 0.74) with H-7b (δH 0.84); H-7b with H-14 (δH 1.10); H-23 (δH 0.78) with H-24 (δH 0.84); and H-24 with H-25 (δH 1.15). The option of a cis-fused A-B ring was excluded by the impossibility of a ROESY correlation between H-5 (or H-1; both at δH 0.74) and H-7 (or H-9, both at δH 0.84). The stereochemistry of the 12β-OH, 16β-OH and 19α-OH groups was determined by the ROESY correlations (Fig 1): H-12α (δH 3.68) with H-9α (δH 0.84) and H-14α (δH 1.10); H-16α (δH 4.47) with H-14α and H-15α (δH 2.11); and H-19β (δH 6.11) with H-25 (δH 1.15). The α-orientation of the 19-OH was further confirmed via GOESY (Gradient Overhauser Enhancement Spectroscopy) NMR experiments, which caused the signal of 25-H to be increased by irradiation at H-19. Sesterstatin 6 was therefore assigned structure 6.
Figure 1.
Key ROESY correlations of sesterstatin 6 (6).
After the structure assignment of sesterstatin 6 was completed, its 16α-hydroxy epimer was reported from the Okinawan variety of H. erectus, and named hyrtiolide by Yamada,5a as well as the 12α-acetoxy, 16α-hydroxy epimer by Yan5b and coworkers from a South China Sea collection of H. erecta. The different axial and equatorial orientations of the 16-H in sesterterstatin 6 and hyrtiolide produced, as expected, different coupling constants: the C-16 β-epimer (6), δH 4.47 (dd, J = 18, and 2 Hz), and the α-epimer, δH 4.42 (d, br, J = 2.9 Hz).
The cancer cell growth inhibitory properties of sesterstatin 6 was evaluated using a minipanel of human cancer cell lines (Table 2) and the murine P388 lymphocytic leukemia (Table 2). Sesterstatin 6 was clearly more inhibitory (10 x) than sesterstatins 4 (4) and 5 (5) against the human cancer cell lines. The activity against the P388 lymphocytic leukemia cell line was superior to the sesterterpenes (2–5) we isolated previously from H. erecta, but similar to sesterstatin 1 (1, Table 2). The significant cancer cell growth inhibitory properties of sesterstatins 1 (1) and 6 (6) merit further study; investigations to ascertain molecular targets and also to discover whether associated microorganisms are the source of the sesterstatins should provide useful results.
Table 2.
Cancer Cell Growth Inhibitory Activity Comparison of Sesterstatins 1–6 (1–6) Against the Murine P388 Lymphocytic Leukemia (ED50)a and a Selection of Human Cancer Cell Lines (GI50)a
| Cell Type | Cell Line | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|---|
| Murine Leukemia | P388 | 0.46 | 4.2 | 4.3 | 4.9 | >10 | 0.17 |
| Pancreas-a | BXPC-3 | 1.6 | 2.2 | 0.44 | |||
| Melanoma | RPMI-7951 | 2.1 | |||||
| CNS | U251 | 1.9 | |||||
| Thyroid ca | KAT-4 | 2.0 | 0.40 | ||||
| Thyroid ca | SW1736 | 2.1 | 0.87 | ||||
| Lung-NSC | NCI-H460 | 1.8 | 2.5 | 0.18 | |||
| Pharynx-sq | FADU | 2.0 | 1.9 | 0.89 | |||
| Prostate | DU-145 | 1.6 | 1.9 | 0.37 |
ED50 and GI50 in μg/mL
Experimental Section
General Methods
Except as recorded in the following experimental introduction, the general experimental methods have been summarized in reference 3a. Solvents used for chromatographic procedure were redistilled. A specific rotation result was determined using a Perkin-Elmer 241 polorimeter. Ultraviolet spectrum was observed using a Perkin- Elmer Lambda 3β UV/VIS spectrophotometer equipped with a Hewlett-Packard Laser Jet 2000 plotter. IR spectrum was obtained with an AVATAR 360 FT-IR instrument and the sample was prepared as a CHCl3 film. High resolution mass spectrum was obtained using a JEOL LCMate magnetic sector instrument.
Hyrtios erecta Collection and Extraction
The experiments summarized here emanated from the 600 kg (wet weight) of a 1994 collection of H. erecta in the Republic of Maldives.6a The H. erecta was identified by Dr. John N. A. Hooper and corresponds to Queensland Museum voucher number QMG304492. The CH3OH-H2O extract was initially separated as summarized in reference 3a and as summarized for our earlier discovery of spongistatins 1–3 in H. erecta.6a
Isolation of Sesterstatin 6
A CH2Cl2 P388 active fraction prepared earlier3a from 600 kg (wet weight) of H. erecta was used to provide several very minor and partially separated active (P388 leukemia) fractions. The active fraction selected for further investigation was chromatographed on a silica gel column with n-hexane-CH2Cl2 –CH3OH (6:9:1) as eluent. The third fraction (0.34g) was further separated employing a preparative reversed-phase HPLC column (Prepex C 8, 10 × 250 mm) with a gradient of CH3CN-H2O (48.5% to 52%, 6 mL/min.) to give ten fractions. HPLC purification (LiChrospher 100 RP-18 column, 4.6 × 250 mm, 50% CH3CN-H2O, a flow rate of 1 mL/min.) of the fourth fraction yielded sesterstatin 6 (6, 5.0 mg, 8.3 × 10−7% yield).
Sesterstatin 6 (6)
Colorless fine needles, mp 253–255 °C; [α]24D +11.3° (c 0.08, CHCl3); IR (film) υmax 3387, 2931, 1749, 1099 and 756 cm−1; 1H and 13C NMR data see Table 1; EIMS m/z (%) 418 [M]+ (3), 400 (13), 385 (10), 374 (26), 356 (21), 275 (19), 235 (28), 191 (100), 148 (69); HRFABMS m/z 419.2793 [M+H]+, (calcd for C25H39O5 419.2797).
Acknowledgments
We are pleased to acknowledge the very important financial support provided by grant RO1 CA 90441-01-04 with the Division of Cancer Treatment and Diagnosis, NCI, DHHS; the Arizona Disease Control Research Commission; the Fannie E. Rippel Foundation; the Robert B. Dalton Endowment Fund; Dr. Alec D. Keith; J. W. Kieckhefer Foundation; and the Margaret T. Morris Foundation. We also thank for other assistance Drs. J.-C. Chapuis, F. Hogan, J. N. A. Hooper, J. C. Knight, N. Melody, and J. M. Schmidt, as well as M. Dodson and L. Williams.
References and Notes
- 1.Contribution 542 in the series Antineoplastic Agents: for part 541 refer to: Pettit GR, Tan R. J Nat Prod. 2005;68:60–63. doi: 10.1021/np040092w.
- 2.(a) Utkina NK, Denisenko VA, Scholokova OV, Virovaya MV, Prokof’eva NG. Tetrahedron Lett. 2003;44:101–102. [Google Scholar]; (b) Carletti I, Long C, Funel C, Amade P. J Nat Prod. 2003;66:25–29. doi: 10.1021/np020208t. [DOI] [PubMed] [Google Scholar]; (c) Liu Y, Hong J, Lee CO, Im KS, Kim ND, Choi JS, Jung JH. J Nat Prod. 2002;65:1307–1314. doi: 10.1021/np020145e. [DOI] [PubMed] [Google Scholar]; (d) Iwashima M, Terada I, Iguchi K, Yamori T. Chem Pharm Bull. 2002;50:1286–1289. doi: 10.1248/cpb.50.1286. [DOI] [PubMed] [Google Scholar]; (e) Charan RD, McKee TC, Boyd MR. J Nat Prod. 2002;65:492–495. doi: 10.1021/np010439k. [DOI] [PubMed] [Google Scholar]; (f) Paul GK, Gunasekera SP, Longley RE, Pomponi SA. J Nat Prod. 2002;65:59–61. doi: 10.1021/np0103766. [DOI] [PubMed] [Google Scholar]; (g) Youssef DTA, Yamaki RK, Kelly M, Scheuer PJ. J Nat Prod. 2002;65:2–6. doi: 10.1021/np0101853. [DOI] [PubMed] [Google Scholar]
- 3.(a) Pettit GR, Cichacz ZA, Tan R, Hoard MS, Melody N, Pettit RK. J Nat Prod. 1998;61:13–16. doi: 10.1021/np970203+. [DOI] [PubMed] [Google Scholar]; (b) Pettit GR, Cichacz ZA, Tan R, Herald DL, Melody N, Hoard MS, Doubek DL, Hooper JNA. Collect Czech Chem Commun. 1998;63:1671–1677. [Google Scholar]
- 4.Pettit GR, Tan R, Melody N, Cichacz ZA, Herald DL, Hoard MS, Pettit RK, Chapuis JC. Bioorg Med Chem Lett. 1998;8:2093–2098. doi: 10.1016/s0960-894x(98)00373-4. [DOI] [PubMed] [Google Scholar]
- 5.(a) Miyaoka H, Nishijima S, Mitome H, Yamada Y. J Nat Prod. 2000;63:1369–1372. doi: 10.1021/np000115g. [DOI] [PubMed] [Google Scholar]; (b) Yan Q, Deng Z, Pei Y, Fu H, Li J, Proksch P, Lin W. J Nat Prod. 2004;67:921–924. doi: 10.1021/np030457x. [DOI] [PubMed] [Google Scholar]
- 6.(a) Pettit GR, Cichacz ZA, Gao F, Herald CL, Boyd MR, Schmit JM, Hooper JNN. J Org Chem. 1993;58:1302–1304. [Google Scholar]; (b) Pettit GR, Cichacz ZA, Gao F, Herald CL, Boyd MR. J Chem Soc Chem Commun. 1993;14:1166–1168. [Google Scholar]