Abstract
Camptothecin (CPT), a plant alkaloid with antitumor activity, is a specific inhibitor of eukaryotic DNA topoisomerase I. We have previously isolated and characterized a CPT-resistant topoisomerase I isolated from a CPT-resistant human leukemia cell line, CPT-K5. cDNA clones of topoisomerase I were isolated from the CPT-resistant and the parental CPT-sensitive cell lines, respectively. Sequencing of the clones identified two mutations in the cDNA isolated from the resistant cells, which cause amino acid changes from aspartic acid to glycine at residues 533 and 583 of the parental topoisomerase I. When the CPT-K5 topoisomerase I was expressed in E. coli as a fusion protein with Staphylococcal Protein A fragment, the activity was resistant to CPT at a dose level up to 125 microM, whereas the parental fusion protein was sensitive to CPT as low as 1 microM. The resistance index (greater than 125) of the CPT-K5 fusion topoisomerase I is similar to that of the native CPT-K5 topoisomerase I. These results indicate that either or both of the two amino acid changes identified in the mutant enzyme is responsible for the resistance to CPT.
Full text
PDF

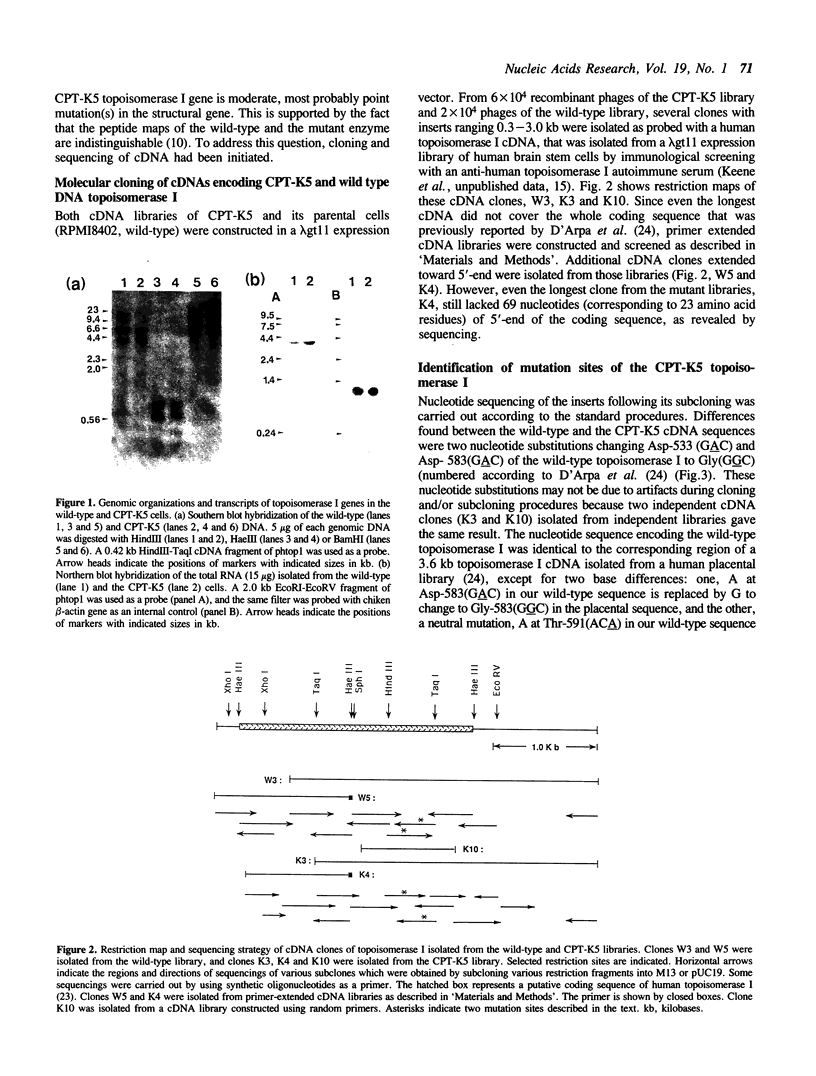
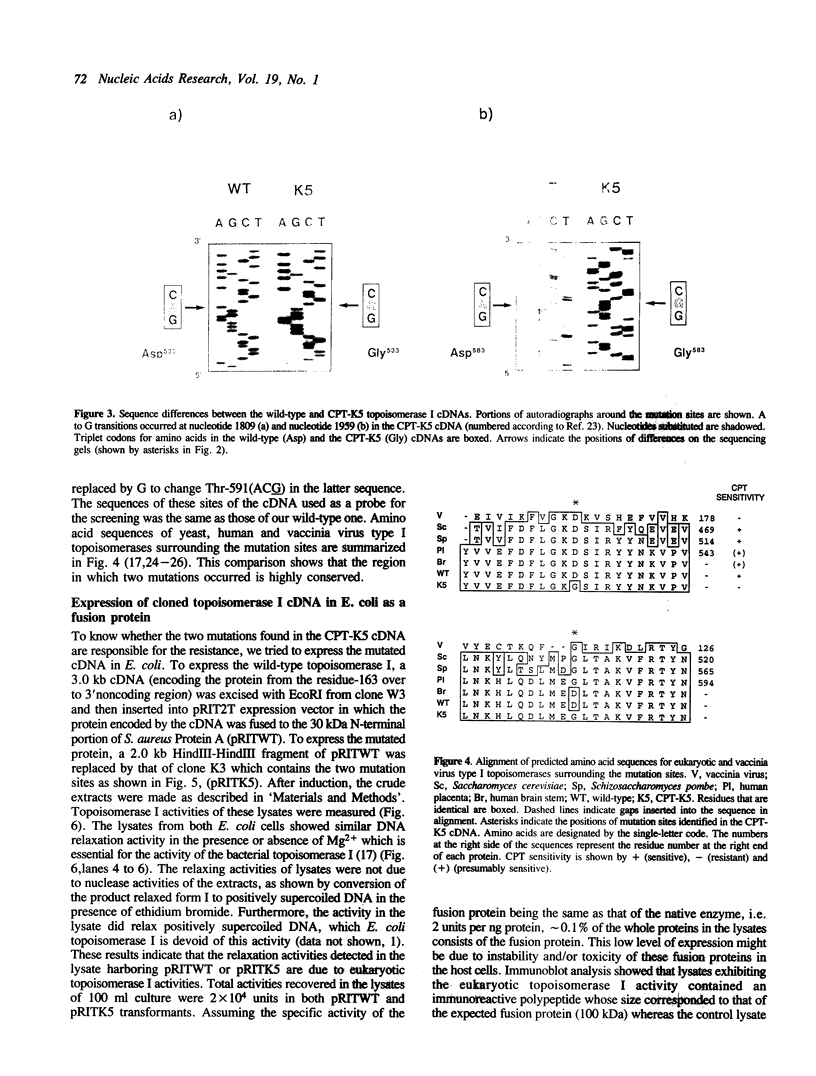
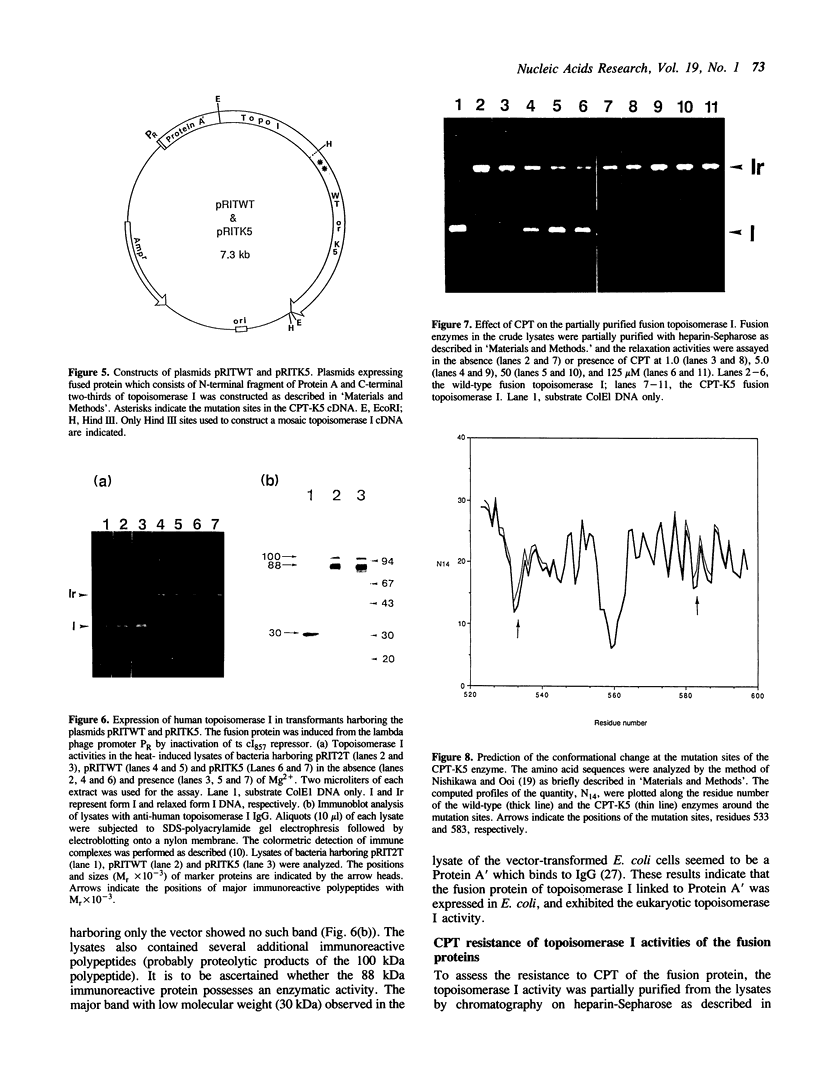


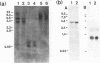
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abelson H. T., Penman S. Selective interruption of high molecular weight RNA synthesis in HeLa cells by camptothecin. Nat New Biol. 1972 May 31;237(74):144–146. doi: 10.1038/newbio237144a0. [DOI] [PubMed] [Google Scholar]
- Andoh T., Ishii K., Suzuki Y., Ikegami Y., Kusunoki Y., Takemoto Y., Okada K. Characterization of a mammalian mutant with a camptothecin-resistant DNA topoisomerase I. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5565–5569. doi: 10.1073/pnas.84.16.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornsti M. A., Wang J. C. Expression of yeast DNA topoisomerase I can complement a conditional-lethal DNA topoisomerase I mutation in Escherichia coli. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8971–8975. doi: 10.1073/pnas.84.24.8971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- D'Arpa P., Machlin P. S., Ratrie H., 3rd, Rothfield N. F., Cleveland D. W., Earnshaw W. C. cDNA cloning of human DNA topoisomerase I: catalytic activity of a 67.7-kDa carboxyl-terminal fragment. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2543–2547. doi: 10.1073/pnas.85.8.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. S., Gupta R., Eng B., Lock R. B., Ross W. E., Hertzberg R. P., Caranfa M. J., Johnson R. K. Camptothecin-resistant mutants of Chinese hamster ovary cells containing a resistant form of topoisomerase I. Cancer Res. 1988 Nov 15;48(22):6404–6410. [PubMed] [Google Scholar]
- Hsiang Y. H., Hertzberg R., Hecht S., Liu L. F. Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. J Biol Chem. 1985 Nov 25;260(27):14873–14878. [PubMed] [Google Scholar]
- Hsiang Y. H., Liu L. F. Identification of mammalian DNA topoisomerase I as an intracellular target of the anticancer drug camptothecin. Cancer Res. 1988 Apr 1;48(7):1722–1726. [PubMed] [Google Scholar]
- Huff A. C., Leatherwood J. K., Kreuzer K. N. Bacteriophage T4 DNA topoisomerase is the target of antitumor agent 4'-(9-acridinylamino)methanesulfon-m-anisidide (m-AMSA) in T4-infected Escherichia coli. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1307–1311. doi: 10.1073/pnas.86.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii K., Hasegawa T., Fujisawa K., Andoh T. Rapid purification and characterization of DNA topoisomerase I from cultured mouse mammary carcinoma FM3A cells. J Biol Chem. 1983 Oct 25;258(20):12728–12732. [PubMed] [Google Scholar]
- Kessel D., Bosmann H. B., Lohr K. Camptothecin effects on DNA synthesis in murine leukemia cells. Biochim Biophys Acta. 1972 May 10;269(2):210–216. doi: 10.1016/0005-2787(72)90429-7. [DOI] [PubMed] [Google Scholar]
- Kessel D. Effects of camptothecin on RNA synthesis in leukemia L1210 cells. Biochim Biophys Acta. 1971 Aug 26;246(2):225–232. doi: 10.1016/0005-2787(71)90131-6. [DOI] [PubMed] [Google Scholar]
- Kjeldsen E., Bonven B. J., Andoh T., Ishii K., Okada K., Bolund L., Westergaard O. Characterization of a camptothecin-resistant human DNA topoisomerase I. J Biol Chem. 1988 Mar 15;263(8):3912–3916. [PubMed] [Google Scholar]
- Kunze N., Yang G. C., Jiang Z. Y., Hameister H., Adolph S., Wiedorn K. H., Richter A., Knippers R. Localization of the active type I DNA topoisomerase gene on human chromosome 20q11.2-13.1, and two pseudogenes on chromosomes 1q23-24 and 22q11.2-13.1. Hum Genet. 1989 Dec;84(1):6–10. doi: 10.1007/BF00210661. [DOI] [PubMed] [Google Scholar]
- Liu L. F. DNA topoisomerase poisons as antitumor drugs. Annu Rev Biochem. 1989;58:351–375. doi: 10.1146/annurev.bi.58.070189.002031. [DOI] [PubMed] [Google Scholar]
- Liu L. F., Miller K. G. Eukaryotic DNA topoisomerases: two forms of type I DNA topoisomerases from HeLa cell nuclei. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3487–3491. doi: 10.1073/pnas.78.6.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn R. M., Bjornsti M. A., Caron P. R., Wang J. C. Peptide sequencing and site-directed mutagenesis identify tyrosine-727 as the active site tyrosine of Saccharomyces cerevisiae DNA topoisomerase I. Proc Natl Acad Sci U S A. 1989 May;86(10):3559–3563. doi: 10.1073/pnas.86.10.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson B., Holmgren E., Josephson S., Gatenbeck S., Philipson L., Uhlen M. Efficient secretion and purification of human insulin-like growth factor I with a gene fusion vector in Staphylococci. Nucleic Acids Res. 1985 Feb 25;13(4):1151–1162. doi: 10.1093/nar/13.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa K., Ooi T. Radial locations of amino acid residues in a globular protein: correlation with the sequence. J Biochem. 1986 Oct;100(4):1043–1047. doi: 10.1093/oxfordjournals.jbchem.a121783. [DOI] [PubMed] [Google Scholar]
- Richards F. M. Areas, volumes, packing and protein structure. Annu Rev Biophys Bioeng. 1977;6:151–176. doi: 10.1146/annurev.bb.06.060177.001055. [DOI] [PubMed] [Google Scholar]
- Rose G. D., Gierasch L. M., Smith J. A. Turns in peptides and proteins. Adv Protein Chem. 1985;37:1–109. doi: 10.1016/s0065-3233(08)60063-7. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shero J. H., Bordwell B., Rothfield N. F., Earnshaw W. C. High titers of autoantibodies to topoisomerase I (Scl-70) in sera from scleroderma patients. Science. 1986 Feb 14;231(4739):737–740. doi: 10.1126/science.3003910. [DOI] [PubMed] [Google Scholar]
- Shuman S., Golder M., Moss B. Characterization of vaccinia virus DNA topoisomerase I expressed in Escherichia coli. J Biol Chem. 1988 Nov 5;263(31):16401–16407. [PubMed] [Google Scholar]
- Shuman S., Moss B. Identification of a vaccinia virus gene encoding a type I DNA topoisomerase. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7478–7482. doi: 10.1073/pnas.84.21.7478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrash C., Bankier A. T., Barrell B. G., Sternglanz R. Cloning, characterization, and sequence of the yeast DNA topoisomerase I gene. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4374–4378. doi: 10.1073/pnas.82.13.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Gordon J. Immunoblotting and dot immunobinding--current status and outlook. J Immunol Methods. 1984 Sep 4;72(2):313–340. doi: 10.1016/0022-1759(84)90001-2. [DOI] [PubMed] [Google Scholar]
- Uemura T., Morino K., Uzawa S., Shiozaki K., Yanagida M. Cloning and sequencing of Schizosaccharomyces pombe DNA topoisomerase I gene, and effect of gene disruption. Nucleic Acids Res. 1987 Dec 10;15(23):9727–9739. doi: 10.1093/nar/15.23.9727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. C. DNA topoisomerases. Annu Rev Biochem. 1985;54:665–697. doi: 10.1146/annurev.bi.54.070185.003313. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]