Abstract
Background: understanding the determinants of health burden after a fracture in ageing populations is important.
Objective: assess the effect of clinical vertebral and other osteoporotic fractures on function and the subsequent risk of hospitalisation.
Design: individuals from the prospective population-based cohort study Age, Gene/Environment Susceptibility (AGES)-Reykjavik study were examined between 2002 and 2006 and followed up for 5.4 years.
Subjects: a total of 5,764 individuals, 57.7% women, born 1907–35, mean age 77.
Method: four groups with a verified fracture status were used; vertebral fractures, other osteoporotic fractures excluding vertebral, non-osteoporotic fractures and not-fractured were compared and analysed for the effect on mobility, strength, QoL, ADL, co-morbidity and hospitalisation.
Results: worst performance on functional tests was in the vertebral fracture group for women (P < 0.0001) and the other osteoporotic fractures group for men (P < 0.05). Both vertebral and other osteoporotic fractures, showed an increased risk of hospitalisation, HR = 1.4 (95% CI: 1.3–1.7) and 1.2 (95% CI: 1.1–1.2) respectively (P < 0.0001). Individuals with vertebral fractures had 50% (P < 0.0001) longer hospitalisation than not-fractured and 33% (P < 0.002) longer than the other osteoporotic fractures group.
Conclusion: individuals with a history of clinical vertebral fracture seem to carry the greatest health burden compared with other fracture groups, emphasising the attention which should be given to those individuals.
Keywords: vertebral fracture, health burden, osteoporotic fracture, strength, ADL, quality of life, mobility, elderly
Introduction
Osteoporotic fractures have a major impact on global public health [1]. Fractures are one of the most common causes of disability and represent a considerable cost in the health care system [2]. In ageing populations, an understanding of the determinants of a decline in function after these fractures may be important for reducing costs and improving quality of life (QoL). However, the literature has mostly focused on the epidemiology and health care burden of hip fractures, reflecting the high individual hospital cost, the personal burden of these fractures in terms of limited mobility and the ease of identifying these fractures since treatment generally requires hospitalisation. On the other hand, since hip fractures represent less than 50% of all fractures even in the elderly over 80 years of age, the burden of other osteoporotic fractures might be underestimated [3, 4]. Research into the epidemiology of other clinical fractures is then necessary to develop a better understanding of the health burden, hospitalisation and determinants of decline of function after fracture [5].
Vertebral fractures (Vfrs) are the most frequent complication of osteoporosis and incident clinical Vfrs were estimated to have affected 1.4 million individuals worldwide in 2000 [6]. The lifetime risk of sustaining a Vfr is 15.4% for women older than 45 years and 8.6% for men [7]. The prevalence of these fractures increase with age among both sexes [8]. Vfrs are significantly under-reported [9] and the personal burden of these fractures may therefore be underestimated.
Only a few studies have addressed the question of whether or not Vfrs are associated with other key outcomes including hospitalisation [10, 11] with the increased cost of health care [12]. It is important to obtain better information on the health burden of vertebral and other osteoporotic fractures [5] for the potential of preventive interventions.
The population-based Ages Gene/Environment Susceptibility (AGES)-Reykjavik study [13] gives a unique opportunity to examine the health burden of those who have suffered fractures as well as the epidemiological association with factors that may make it possible to improve such things as mobility, strength, activities of daily living (ADL) and QoL.
Methods
Participants
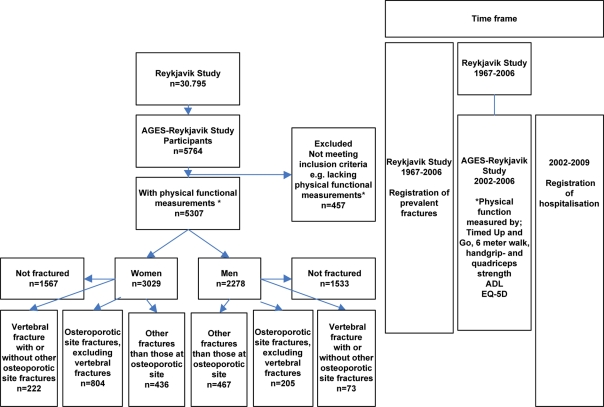
The AGES-Reykjavik study is an extension of the population-based Reykjavik study which started in 1967 [14, 15]. Participants were invited for examinations through 1996 with a response rate of 71.8%. In 2002, survivors of the Reykjavik study, a total of 8.030 participants, were randomly selected and invited, and 5,764 agreed to participate; of these 457 were excluded as they did not have functional measurements or did not give permission to connect their data with their hospital files (n = 18). Recruitment into the study was carried out between September 2002 and January 2006. Participants were grouped into one of four mutually exclusive groups: (i) Vfrs, may have had other fractures (Ofrs), (ii) other osteoporotic site fractures excluding vertebral (oOSfr), (iii) Ofrs not osteoporotic site and (iv) not-fractured (Nfr) (Figure 1).
Figure 1.
Flow chart of timeline and participants categorised by sex and previous fractures. Fractures sustained before the entry into the AGES-Reykjavik Study were recorded, verified and confirmed from medical and radiological records as described [16]. The following were defined as osteoporotic sites: vertebral (S12.1, S12.2, S22.0, S22.1, S32.0, T08), pelvic (S32.1, S32.3, S32.4, S32.5), proximal humerus (S42.2, S42.4), distal forearm (S52.5, S52.6), hip (S72.0, S72.1, S72.2), proximal tibia (S82.1).
Fractures
Fracture data were ascertained from the Reykjavik study fracture registry and verified by medical and radiological records from 1966 to 2006 [16]. The fracture status used in the study was recorded at the participant's entry into the AGES-Reykjavik study 2002–06. Osteoporotic site fractures were defined according to ICD10 diagnostic codes.
Baseline examination
ADL was assessed by asking the participants how difficult it was to: dress, eat, bathe, transfer out of bed/chair or walk from room to room. A score of 0–5 was given with a greater score associated with dependency. Health-related QoL was assessed by EQ-5D, [17] where lower score was associated with poorer outcome. Basic mobility defined as the result of a Timed Up and Go test [18, 19] and a six-metre walk test [20]. Strength was measured by a maximal isometric muscle strength of the hand and quadriceps [21].
Co-morbidity and hospitalisation
Information of the participant's first hospital admission, diagnosis (ICD10 code) and duration were obtained from the database of Landspitalinn University Hospital, the main hospital in Reykjavik. The hospitalisations were grouped into nine mutually exclusive groups using the diagnostic codes in Supplementary data are available in Age and Ageing online, Table S1 from the participant's entry into the AGES-Reykjavik study until 31 December 2009. The Charlson score was used to assess co-morbidity [22].
Statistical analyses
Functional outcomes and QoL measures were compared by sex according to subgroups defined from fractures status. General linear models were used with adjustment for age and a four-level categorical predictor for fracture status. The assumption of normally distributed residuals was inspected graphically using normal quantile plots. The six-metre walk and the Timed Up and Go variables were log-transformed. A comparison of the relative effect of fracture on function and strength outcomes (normalised for thigh length) between men and women was made by analysing men and women together using log-transformed outcomes with an interaction term for sex. The effect of fracture on function was analysed by dividing fracture status into two groups; fracture within 10 years before entry into the study and more than 10 years before entry.
The time to first hospital admission after entry into the study was analysed using the Cox proportional hazards regression model, by sex with age adjustment and a four-level categorical predictor for fracture status. Analyses were done with and without adjustment for mobility, strength and co-morbidity. Men and women were also analysed together and interaction between sex and fractures status was tested using a product term in the regression model.
The survival analysis of hospital admissions was also performed by estimating the effect of fracture by time since the fracture occurred before entry into the study (within 10 years or more than 10 years).
The proportional hazards assumption was assessed graphically (log(-log(survival)) versus log(time)) and tested formally using the cox.zph function of the survival package in R version 2.10.1. Violations from proportionality were not found to be significant, neither visually or statistically.
The duration of hospitalisation was analysed for the individuals with hospital admission by a Poisson regression model with log-length of follow-up as an offset. The analysis was performed separately by sex with age adjustment and fracture status as an explanatory variable.
The Charlson score was analysed using a four level response variable: (i) A score of 0, (ii) a score of 1, (iii) a score of 2–5, (iv) a score higher than 5. A proportional odds polytomous regression model with a generalised logit link was used to test for significant association with fracture status and interaction between sex and fracture status. The model included adjustment for age. SAS/STAT version 8.02 and R version 2.10.1 was used to analyse the data.
Results
There were 5,307 participants with baseline functional measurements available for the analysis (Figure 1), 3,029 women (57%) with a mean age of 76 years (range 66–95) and 2,278 men (43%) with a mean age of 77 years (range 67–96). The prevalence at entry into the study of clinical Vfrs increased with age in both sexes. It was significantly higher in women (P < 0.0001). The prevalence peaked at 13.2% in the oldest age group (>85 years) for women but levelled off in men at 3.5% at the age of 80 years (Supplementary data are available in Age and Ageing online, Figure S1).
Functional capability and quality of life
Table 1 shows function and QoL by fracture status at entry into the study. Having sustained a fracture was generally associated with a worse performance and QoL. Women with Vfr had significantly poorer performance and self-reported function compared with Ofr groups and Nfr (P ≤ 0.01). For men with Vfr poorer performance was seen for quadriceps-strength, mobility (TUG) and ADL (P < 0.01). A previous oOSfr had an effect both in men and women, but more clearly seen in men.
Table 1.
Functional measurements and health quality of life by fracture status
| Not fractured (Nfr);
men n = 1,533;
women n = 1,567 |
Other fracture than those at osteoporotic
site (Ofr);
men n = 485;
women n = 538 |
Osteoporotic fracture excluding vertebral (oOSfr); men n = 187;
women n = 702 |
Vertebral fracture (Vfr);
men n = 73;
women n = 222 |
Test of difference between fractures and control group P-valuesa |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Vfr versus oOSfr | Vfr versus Nfr | oOSfr versus Nfr | Vfr versus Ofr | oOSfr versus Ofr | Ofr versus Nfr | |
| Men | ||||||||||||||
| Grip strength, average (kg) | 39.4 | 9.5 | 39.4 | 9.9 | 36.8 | 8.9 | 36.7 | 8.2 | — | –— | 0.002 | — | 0.03 | — |
| Quadriceps strength, average (kg) | 41.3 | 11.1 | 40.6 | 11.0 | 38.3 | 10.8 | 37.7 | 10.0 | — | 0.02 | 0.001 | — | — | 0.02 |
| Timed Up and Go, average (s)b | 12.2 | 3.2 | 12.6 | 3.6 | 12.8 | 3.8 | 13.3 | 3.8 | — | 0.04 | — | — | — | 0.01 |
| Six-metre walk, average (s)b | 6.48 | 1.94 | 6.64 | 2.06 | 6.86 | 2.13 | 6.84 | 1.87 | — | — | 0.03 | — | — | 0.01 |
| Activity of daily living | 0.33 | 0.77 | 0.36 | 0.81 | 0.5 | 1.01 | 0.64 | 1.16 | — | 0.003 | 0.02 | 0.02 | — | — |
| EQ-5D score | 0.88 | 0.16 | 0.87 | 0.17 | 0.83 | 0.18 | 0.85 | 0.17 | — | — | 0.001 | — | — | — |
| Women | ||||||||||||||
| Grip strength, average (kg) | 24.2 | 5.9 | 24.0 | 6.1 | 22.9 | 5.6 | 21.3 | 5.3 | 0.002 | <0.0001 | 0.02 | 0.0002 | — | — |
| Quadriceps strength, average (kg) | 26.7 | 7.7 | 26.5 | 8.3 | 25.6 | 7.5 | 22.6 | 7.02 | <0.0001 | <0.0001 | — | <0.0001 | — | — |
| Timed Up and Go, average (s)b | 12.4 | 3.7 | 12.4 | 3.7 | 13.2 | 4.2 | 14.3 | 5.6 | 0.01 | <0.0001 | 0.02 | <0.0001 | 0.02 | — |
| Six-metre walk, average (s)b | 6.88 | 1.95 | 6.79 | 1.77 | 7.21 | 2.09 | 7.72 | 2.34 | 0.005 | <0.0001 | — | <0.0001 | 0.02 | — |
| Activity of daily living | 0.48 | 0.96 | 0.51 | 0.96 | 0.58 | 1.09 | 0.86 | 1.34 | 0.001 | <0.0001 | — | 0.001 | — | — |
| EQ-5D score | 0.81 | 0.2 | 0.78 | 0.21 | 0.8 | 0.2 | 0.72 | 0.25 | <0.0001 | <0.0001 | — | 0.003 | — | 0.03 |
—, Not significant.
aComparisons are adjusted for age.
bAnalysed on logarithmic scale.
The difference in the relative effect of fracture status on function between men and women was found to be insignificant for strength, TUG and ADL. In the case of the six-metre walk and QoL, women had significantly worse result than men (P < 0.05). Adjustment for the prevalence of hip fractures did not change the estimated effect of fractures on TUG, strength and ADL.
The time since fracture impacted the participants function and QoL. Compared with Nfr, those who fractured less than 10 years ago had worse function than those who fractured more than 10 years ago (Supplementary data are available in Age and Ageing online, Table S3).
Co-morbidity and hospitalisation
During the follow-up with a median of 5.4 years (inter-quartile range 4.7–6.3), 3,457 (65%) participants were hospitalised at least once; 68% men and 63% women. For men, the percentages hospitalised by fracture status were Vfr 82; oOSfr 70; Ofr 69 and not-fractured 67%. For women, Vfr 76; oOSfr 68; Ofr 63 and Nfr 58%.
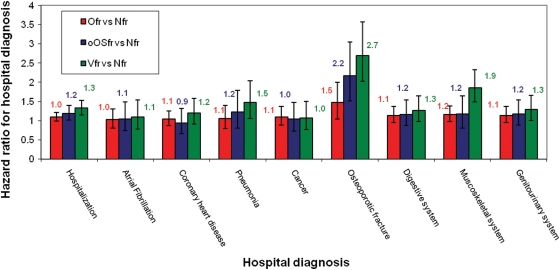
The most common hospital diagnoses (80%), during follow-up, were the same in the fracture group as in the Nfr group; atrial fibrillation, coronary heart disease, pneumonia, cancer, osteoporotic fracture, digestive system diseases, muscoskeletal and genitourinary system. After adjustment for age, sex, TUG, quadriceps-strength and Charlson score, the risk of hospitalisation was still greater for those with previous fracture (Figure 2). Generally the Vfr group was worse off depending on the hospital diagnosis; the risk (HR) for a new osteoporotic fracture was 2.7 (95% CI: 2.0–3.6), musculoskeletal diseases HR1.9 (95% CI: 1.5–2.3), pneumonia HR 1.5 (95% CI: 1.1–2.0) and genitourinary diseases HR 1.3 (95% CI: 1.0–1.7). On the other hand, the oOSfr group was only hospitalised more often because of a new osteoporotic fracture compared with the Nfr after adjustment for confounders.
Figure 2.
The 80% of the most common hospital diagnosis according to the study groups. Hazard ratios (95% CI) of clinical vertebral and other fractures group compared with the control group. Adjustment made for age and sex, quadriceps strength, six-metre walk and co-morbidity. Vfr: vertebral fracture, oOSfr: osteoporotic fracture excluding vertebral, Ofr, fracture excluding all osteoporotic fracture; Nfr, not-fractured.
To examine if hospitalisation was influenced by the time since fracture occurred, individuals with fractures were grouped into subgroups; those sustaining fracture less or more than10 years ago (Supplementary data are available in Age and Ageing online, Table S2). A greater risk of hospitalisation was seen in the group with the shorter time since the last fracture, especially in the Vfr group. Relative to the Nfr group, the Vfr group had HR of 1.4 (95% CI: 1.2–1.6 (P = 0.0002)), the oOSfr; HR1.2 (95% CI: 1.1–1.4 (P = 0.001)); the Ofr; HR1.2 (95% CI: 1.2–1.3 (P = 0.02)). The HR for the Vfr group that had fractured more than 10 years ago was 1.2 (95% CI: 1.0–1.6 (P = NS)), the oOSfr; HR1.2 (95% CI: 1.0–1.3 (P = 0.02)), the Ofr; HR1.2 (95% CI: 0.9–1.2 (P = NS)). Adjustment was made for age, sex, six-metre walk, quadriceps-strength and co-morbidity.
Individuals with Vfr had an average 50% (95% CI: 27–77% (P < 0.0001)) longer stay in hospital than those with Nfr and 33% (95% CI: 11–60% (P = 0.005)) longer than the oOSfr group. Men without previous fractures had the shortest hospital stay, on average/year, 6.5 days (range 6.6–7.7) but men in the Vfr group had the longest, 12.7 days, (range 12.3–13.0). Generally, individuals with Vfr had more co-morbidity than Nfr (P = 0.005) and men were more often hospitalised and had longer hospital stay than women with Vfr (P < 0.0001).
Discussion
This population-based study of elderly men and women, with 5.4 years of follow-up, indicates that individuals with Vfr were associated with a greater health burden than those with other osteoporotic fractures. This was shown by more co-morbidity, hospitalisations and longer hospital stay for both sexes in those with previous Vfrs in comparison with Nfr and Ofr groups. Men were significantly worse off than women and a shorter time since fracture, increased the risk of worse function and hospitalisation.
Our elderly men with fractures seem to represent an especially frail part of the population. The increased risk of hospitalisation in the fracture groups of men as well as the high co-morbidity, especially in the vertebral group, supports this. We observed that pneumonia and muscoskeletal system diseases were significantly increased in the post-Vfr group. The former may be caused by impaired chest function and the latter, because of a possible increased use of pain killers.
Our results confirm the increased prevalence of Vfr with increasing age and the expected difference between the sexes previously reported [4]. However, our study shows the prevalence to level out in the oldest age groups of men. The reason might be an effect of survival but it has previously been shown that individuals suffering from Vfrs have high mortality risk [23]. This may have been more pronounced in our vertebral group of men as men have been shown to have a higher rate of fracture-related mortality compared with women [24, 25] which might reflect a poor health status.
Tests like muscle strength and mobility such as the six-metre walk, Timed Up and Go which measure the actual physical performance enhance our understanding of the impact of previous fractures on the individual's body function. These objective measurements have been used in an epidemiological context to examine fracture risk and appear to be of importance [26]. These measurements have also been used to measure functional recovery after hip fractures and it has been recognised that many individuals do not achieve their pre-fracture level of function 1 year after a fracture [27]. Similar information on Vfrs is scarce. Our results showed that the relative effect size of a clinical Vfr on function performance to be mostly similar in both sexes even though women had an overall worse function than men. A possible explanation might be that baseline absolute values are higher in men as well as the skeletal muscle fatigue resistance [28]. The fact that osteoporotic fractures, other than Vfr had more impact on men than women might reflect poorer health in the men who survive a pelvic or hip fracture.
The results from the QoL measurements showed that women were significantly worse off than men. A partial explanation might be that women are known to rate their QoL lower than men [29] and do have more clinical fractures as well as twice the amount of morphometric deformities in the spine than come to clinical attention [9]. These silent fractures are not without symptoms [30] and may therefore be of clinical significance.
Our study has several strengths but also limitations. This is a population representative study of free-living elderly people, including both sexes from a large verified fracture registry. The study is prospective for hospitalisation, but the functions were only measured at entry and not assessed again during the follow-up. The main weakness consists of a potential selection bias caused by non-responders who are likely to be sicker and may have more of deleterious fractures such as hip fractures and not make it to the study. However, although this may underestimate the total effect of fractures on function and hospitalisation, it should not affect the importance of this study results with respect to the impact of fracture on the participant's function and hospitalisation.
In conclusion, this study adds important information on the consequences of osteoporotic fractures in older people. Individuals with these fractures have diminished performance in all functional tests, a worse QoL, more co-morbidity and a greater need of hospitalisation as well as longer hospital stays. This group of elderly people with a history of Vfr needs special attention to maintain their function and prevent further hospitalisations.
Key points.
Knowledge is missing if an impact of Vfrs on the individual's health differs from other osteoporotic fractures.
Individuals with history of Vfrs carried more of a health burden than individuals with other osteoporotic fractures.
This was reflected in significantly increased co-morbidity, more hospitalisations and longer hospital stays.
Men with previous history of Vfr had significantly more of a health burden than women.
Time since fracture impacts the individuals function, hospitalisation and QoL.
Supplementary Material
Acknowledgements
We are grateful to Olafur Grimur Bjornsson, MD. PhD and David Vilmundarson for helpful comments on the manuscript and to the staff of the Icelandic Heart Association Research Institute for their contribution.
Conflicts of interest
None declared.
Ethical approval
Informed consent was obtained and the study was reviewed and approved by the National Bioethics Committee in Iceland (VSN 00-063) and the National Institute on Aging Intramural Institutional Review Board.
Funding
This study was funded by the National Institutes of Health, USA contract N01-AG-12100, the National Institute on Aging Intramural Research Program, Hjartavernd (The Icelandic Heart Association) and the Althingi (The Icelandic Parliament).
Supplementary data
Supplementary data mentioned in the text is available to subscribers in Age and Ageing online.
References
- 1.Cole ZA, Dennison EM, Cooper C. The impact of methods for estimating bone health and the global burden of bone disease. Salud Publica Mex. 2009;51(Suppl. 1):S38–45. doi: 10.1590/s0036-36342009000700007. [DOI] [PubMed] [Google Scholar]
- 2.Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet. 2002;359:1761–7. doi: 10.1016/S0140-6736(02)08657-9. [DOI] [PubMed] [Google Scholar]
- 3.Jones G, Nguyen T, Sambrook PN, Kelly PJ, Gilbert C, Eisman JA. Symptomatic fracture incidence in elderly men and women: the Dubbo Osteoporosis Epidemiology Study (DOES) Osteoporos Int. 1994;4:277–82. doi: 10.1007/BF01623352. [DOI] [PubMed] [Google Scholar]
- 4.Melton LJ, III, Crowson CS, O'Fallon WM. Fracture incidence in Olmsted County, Minnesota: comparison of urban with rural rates and changes in urban rates over time. Osteoporos Int. 1999;9:29–37. doi: 10.1007/s001980050113. [DOI] [PubMed] [Google Scholar]
- 5.O'Neill TW, Roy DK. How many people develop fractures with what outcome? Best Pract Res Clin Rheumatol. 2005;19:879–95. doi: 10.1016/j.berh.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17:1726–33. doi: 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- 7.Kanis JA, Johnell O, Oden A, et al. Long-term risk of osteoporotic fracture in Malmo. Osteoporos Int. 2000;11:669–74. doi: 10.1007/s001980070064. [DOI] [PubMed] [Google Scholar]
- 8.Sakuma M, Endo N, Oinuma T, et al. Incidence and outcome of osteoporotic fractures in 2004 in Sado City, Niigata Prefecture, Japan. J Bone Miner Metab. 2008;26:373–8. doi: 10.1007/s00774-007-0841-1. [DOI] [PubMed] [Google Scholar]
- 9.Majumdar SR, Kim N, Colman I, et al. Incidental vertebral fractures discovered with chest radiography in the emergency department: prevalence, recognition, and osteoporosis management in a cohort of elderly patients. Arch Intern Med. 2005;165:905–9. doi: 10.1001/archinte.165.8.905. [DOI] [PubMed] [Google Scholar]
- 10.Strom O, Borgstrom F, Zethraeus N, et al. Long-term cost and effect on quality of life of osteoporosis-related fractures in Sweden. Acta Orthop. 2008;79:269–80. doi: 10.1080/17453670710015094. [DOI] [PubMed] [Google Scholar]
- 11.Bouza C, Lopez T, Palma M, Amate JM. Hospitalised osteoporotic vertebral fractures in Spain: analysis of the national hospital discharge registry. Osteoporos Int. 2007;18:649–57. doi: 10.1007/s00198-006-0292-x. [DOI] [PubMed] [Google Scholar]
- 12.Puffer S, Torgerson DJ, Sykes D, Brown P, Cooper C. Health care costs of women with symptomatic vertebral fractures. Bone. 2004;35:383–6. doi: 10.1016/j.bone.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 13.Harris TB, Launer LJ, Eiriksdottir G, et al. Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol. 2007;165:1076–87. doi: 10.1093/aje/kwk115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bjornsson O, Davidsson D, Olafsson H, et al. Reykjavik: The Icelandic Heart Association; 1979. Report ABC XVIII Health Survey in the Reykjavik Area—Men Stages I–III 1967–1969 1970–1971 and 1974–1976 Participants Invitation Response etc. [Google Scholar]
- 15.Bjornsson G, Bjornsson O, Davidsson D, et al. Reykjavik: Icelandic Heart Association; 1982. Report abc XXIV Health Survey in the Reykjavik Area—Women Stages I–III 1968–1969 1971–1972 and 1976–1978 Participants Invitation Response etc. [Google Scholar]
- 16.Siggeirsdottir K, Aspelund T, Sigurdsson G, et al. Inaccuracy in self-report of fractures may underestimate association with health outcomes when compared with medical record based fracture registry. Eur J Epidemiol. 2007;22:631–9. doi: 10.1007/s10654-007-9163-9. [DOI] [PubMed] [Google Scholar]
- 17.EuroQol—a new facility for the measurement of health-related quality of life. The EuroQol Group. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 18.Podsiadlo D, Richardson S. The timed ‘Up & Go’: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–8. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 19.Siggeirsdottir K, Jonsson BY, Jonsson H, Jr, Iwarsson S. The timed ‘Up & Go’ is dependent on chair type. Clin Rehabil. 2002;16:609–16. doi: 10.1191/0269215502cr529oa. [DOI] [PubMed] [Google Scholar]
- 20.Ostir GV, Markides KS, Black SA, Goodwin JS. Lower body functioning as a predictor of subsequent disability among older Mexican Americans. J Gerontol A Biol Sci Med Sci. 1998;53:M491–5. doi: 10.1093/gerona/53a.6.m491. [DOI] [PubMed] [Google Scholar]
- 21.Era P. Maximal isometric muscle strength and anthropometry in 75-year-old men and women in three Nordic localities. Scand J Med Sci Sports. 1994;4:26–31. [Google Scholar]
- 22.Armitage JN, van der Meulen JH. Identifying co-morbidity in surgical patients using administrative data with the Royal College of Surgeons Charlson Score. Br J Surg. 2010;97:772–81. doi: 10.1002/bjs.6930. [DOI] [PubMed] [Google Scholar]
- 23.Kanis JA, Johnell O, Oden A, et al. The risk and burden of vertebral fractures in Sweden. Osteoporos Int. 2004;15:20–6. doi: 10.1007/s00198-003-1463-7. [DOI] [PubMed] [Google Scholar]
- 24.Center JR, Nguyen TV, Schneider D, Sambrook PN, Eisman JA. Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet. 1999;353:878–82. doi: 10.1016/S0140-6736(98)09075-8. [DOI] [PubMed] [Google Scholar]
- 25.Bliuc D, Nguyen ND, Milch VE, Nguyen TV, Eisman JA, Center JR. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA. 2009;301:513–21. doi: 10.1001/jama.2009.50. [DOI] [PubMed] [Google Scholar]
- 26.Stel VS, Pluijm SM, Deeg DJ, Smit JH, Bouter LM, Lips P. Functional limitations and poor physical performance as independent risk factors for self-reported fractures in older persons. Osteoporos Int. 2004;15:742–50. doi: 10.1007/s00198-004-1604-7. [DOI] [PubMed] [Google Scholar]
- 27.Ganz SB, Peterson MG, Russo PW, Guccione A. Functional recovery after hip fracture in the subacute setting. Hss J. 2007;3:50–7. doi: 10.1007/s11420-006-9022-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wust RC, Morse CI, de Haan A, Jones DA, Degens H. Sex differences in contractile properties and fatigue resistance of human skeletal muscle. Exp Physiol. 2008;93:843–50. doi: 10.1113/expphysiol.2007.041764. [DOI] [PubMed] [Google Scholar]
- 29.Kirchengast S, Haslinger B. Gender differences in health-related quality of life among healthy aged and old-aged Austrians: cross-sectional analysis. Gend Med. 2008;5:270–8. doi: 10.1016/j.genm.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 30.Nevitt MC, Thompson DE, Black DM, et al. Effect of alendronate on limited-activity days and bed-disability days caused by back pain in postmenopausal women with existing vertebral fractures. Fracture Intervention Trial Research Group. Arch Intern Med. 2000;160:77–85. doi: 10.1001/archinte.160.1.77. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.